Abstract
Aims
To assess the hemodynamic effects of organic vs. inorganic nitrate administration among patients with heart failure with preserved ejection fraction (HFpEF).
Methods and Results
We assessed carotid and aortic pressure-flow relations non-invasively before and after the administration of 0.4 mg of sublingual-nitroglycerin (NTG; n=26), and in a separate sub-study, in response to 12.9 mmol of inorganic nitrate (n=16). NTG did not consistently reduce wave reflections arriving at the proximal aorta (change in real part of reflection coefficient, 1st harmonic:-0.09; P=0.01; 2nd harmonic:-0.045, P=0.16; 3rd harmonic:+0.087; P=0.05), but produced profound vasodilation in the carotid territory, with a significant reduction in systolic blood pressure (133.6 vs 120.5 mmHg; P=0.011) and a marked reduction in carotid bed vascular resistance (19580 vs. 13078 dynes·s/cm5; P=0.001) and carotid characteristic impedance (3440 vs. 1923 dynes·s/cm5; P=0.002). Inorganic nitrate, in contrast, consistently reduced wave reflections across the first 3 harmonics (change in real part of reflection coefficient, 1st harmonic: -0.12; P=0.03; 2nd harmonic:-0.11, P=0.01; 3rd harmonic:-0.087; P=0.09) and did not reduce blood pressure, carotid bed vascular resistance or carotid characteristic impedance (P=NS).
Conclusions
NTG produces marked vasodilation in the carotid circulation, with a pronounced reduction in blood pressure and inconsistent effects on central wave reflections. Inorganic nitrate, in contrast, produces consistent reductions in wave reflections, and unlike NTG, it does so without significant hypotension or cerebrovascular dilatation. These hemodynamic differences may underlie the different effects on exercise capacity and side effect profile of inorganic vs. organic nitrate in HFpEF.
Keywords: Heart failure with preserved ejection fraction, inorganic nitrate, organic nitrates, arterial load, cerebrovascular input impedance
Introduction
Heart failure (HF) affects ∼2% of the western population and is the most common cause of hospitalization in adults >65 years of age. Approximately half of patients with HF have a preserved left ventricular (LV) ejection fraction (HFpEF). Multiple effective pharmacologic therapies that result in substantial clinical benefit in HF with reduced ejection fraction are available. In contrast, there are currently no proven effective pharmacologic therapies to improve outcomes in HFpEF.
Pulsatile arterial load exerts important effects on LV function and remodeling. In particular, wave reflections originating at the periphery and conducted back to the heart, have been shown to cause LV diastolic dysfunction, hypertrophy and fibrosis in experimental models, a concept supported by an increasing body of human studies.(1-6) Acute administration of organic nitrates has been shown to reduce wave reflections arriving at the central aorta in hypertensive or healthy subjects in some, but not all studies.(7-11) The effects of organic nitrates on pulsatile arterial hemodynamics have not been well characterized in HFpEF. In recent trials in patients with HFpEF, (12, 13) organic nitrates have been poorly tolerated; important side effects of organic nitrate therapy included hypotension and headaches, suggesting adverse effects on the cerebrovascular territory.
In contrast to the unfavorable effects of organic nitrates in HFpEF, recent randomized controlled trials have demonstrated that inorganic nitrate, administered as a single dose(14) or after 1 week of sustained administration,(15) improves exercise capacity in patients with HFpEF. Inorganic nitrate undergoes a 2-step reduction to nitric oxide via the nitrate-nitrite-NO pathway.(16, 17) The conversion of nitrite to NO occurs in conditions of hypoxia and acidosis, but a recent report indicates that it also occurs via paradoxical normoxic activation in conduit arteries,(18) indicating potential effects on pulsatile arterial hemodynamic function and arterial wave reflections. Furthermore, in contrast to the frequent occurrence of headache with organic nitrate, the administration of inorganic nitrate has not been associated with headaches or other side effects in two recent trials in this population.(14, 15)
We aimed to assess the effect of organic and inorganic nitrate in HFpEF on: (1) Wave reflections arriving at the central aorta, and (2) Carotid arterial hemodynamics (i.e., to characterize cerebrovascular effects).
Methods
We performed 2 sub-studies. In the first sub-study, we studied aortic and carotid hemodynamics at baseline and after the administration of 0.4 mg of sublingual nitroglycerin (NTG). In the second sub-study, we analyzed aortic and carotid pressure-flow data from our previous randomized controlled trial of inorganic nitrate administration in HFpEF.(14) Protocols were approved by the University of Pennsylvania and Philadelphia VA Medical Center Institutional Review Boards, as appropriate. All subjects provided written informed consent.
Study population
For sub-study 1, we included subjects with HFpEF who met the following criteria: (1) Symptomatic HF with a LV ejection fraction>50%; (2) At least one of the following within 1 year prior to consent: hospitalization for decompensated HF, acute treatment for HF with intravenous diuretics or hemofiltration, chronic treatment with a loop diuretic for control of HF symptoms, or chronic diastolic dysfunction evidenced by left atrial enlargement (left atrial volume index>34 ml/m2) or Doppler signs of increased left atrial pressure, as defined by the European Society of Cardiovascular Imaging/American Society of Echocardiography;(19) (4) Stable medical therapy. Key exclusion criteria were: (1) Clinically significant valve disease (more than mild aortic/mitral stenosis or more than moderate aortic/mitral regurgitation); (2) Atrial fibrillation/flutter; (3) Current nitrate therapy; (4) significant ischemia on stress testing within the past year that was not revascularized; (5) Other clinically important causes of dyspnea; (6) Hypertrophic, infiltrative or inflammatory cardiomyopathy; (7) Pericardial disease; (8) Primary pulmonary arteriopathy; (9) Blood pressure <110/40 mmHg or >180/100 mmHg; (10) Resting heart rate>100 bpm; (11) LV ejection fraction<50% in the past; (12) Adverse reactions to organic nitrates or phosphodiesterase inhibitor use; (13) Severe renal dysfunction (glomerular filtration rate<30 ml/min/1.73m2) or liver disease. In sub-study 2, we utilized very similar inclusion/exclusion criteria, as previously described.(14)
Study Protocol
Sub-study 1
Twenty-six subjects participated in this sub-study. After >10 minutes of rest in the supine position, blood pressure was taken in the right arm with a validated oscillometric device (Omron HEM-705CP, Omron Corporation, Kyoto, Japan). Carotid pressure (Figure-1) was recorded via applanation tonometry, using a SphygmoCor-CPV System (AtCor Medical; Itasca, IL) equipped with a high-fidelity Millar tonometer (Millar Instruments; Houston, TX).
Figure 1.
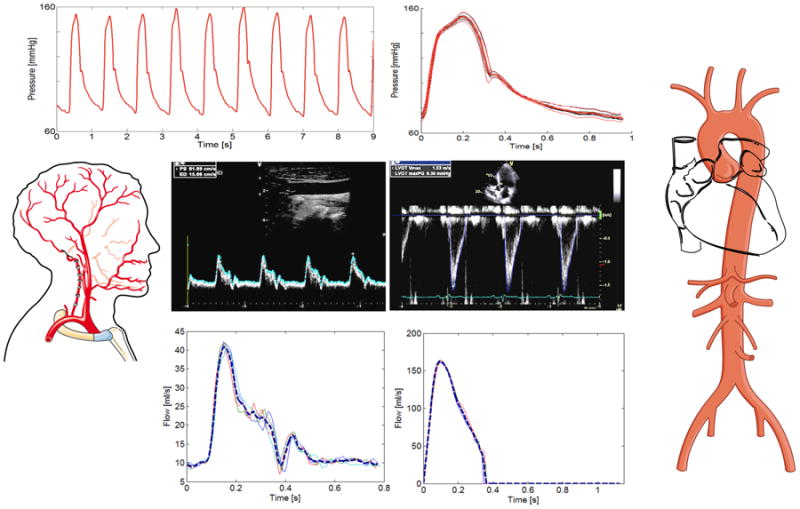
Example of pressure-flow analyses. Tonometric pressure data is shown at the top (left), along with the signal-averaged pressure waveform (top right, red). Doppler flow interrogations were performed, as shown for the carotid (left) and LV outflow (right) locations. Tracing and signal averaging of flow waveforms (bottom blue curves) was subsequently performed. The carotid pressure waveform is used for both carotid pressure-flow analyses and aortic pressure-flow analyses, since the carotid pressure is a good surrogate of the aortic pressure waveform.
Pulsed-wave Doppler measurements of flow velocities in the LV outflow tract (Figure-1) were performed using a GE-e9 ultrasound machine (GE Healthcare; Fairfield, CT), with the Doppler sample immediately proximal to the aortic valve leaflets within the centerline of the LV outflow tract. We computed LV outflow tract cross sectional area from its radius measured in the parasternal long axis view (area=πr2). Carotid diameters and blood velocities were also acquired, using a vascular linear probe. Carotid flow was computed as flow-velocity multiplied by lumen cross-sectional area (area=πr2). After baseline measurements were obtained, a single dose (0.4 mg) of NTG was administrated sublingually, and measurements were repeated starting 2 minutes after administration. Comparisons are made between the values measurements obtained pre- vs. post-NTG administration.
Sub-study 2
Seventeen subjects participated a randomized, double-blind, crossover study of a single dose of inorganic nitrate given as concentrated nitrate-rich beetroot juice (NO3−, BEET-IT Sport, James-White Drinks Ltd, Ipswich, UK) containing 12.9 mmol NO3− in 140 mL, versus an otherwise identical nitrate-depleted placebo juice (James White Drinks, Ltd). The interventions were separated by a washout period of at least 5 days. We measured aortic and carotid hemodynamics using identical methods as in sub-study 1, ∼2.5 hours after juice ingestion. Comparisons were made between measurements obtained after administration of nitrate-rich vs. nitrate-depleted beetroot juice. One subject was excluded from these analyses due to lack of carotid flow data during one of the study visits.
Pressure-flow analyses
Pressure and Doppler flow velocity files were processed off-line using custom-designed software written in Matlab (The Mathworks, Natick, MA) as previously described.(20) A representative example of pressure-flow data processing is shown in Figures 1-2. Time-alignment of pressure and LV outflow curves was performed to maximize: (1) the rapid systolic upstroke of pressure and flow; (2) concordance of the pressure dicrotic notch and cessation of flow in the LV outflow tract, or the flow dicrotic notch in the carotid; (3) Linearity of the early systolic pressure-flow relationship.
Figure 2.
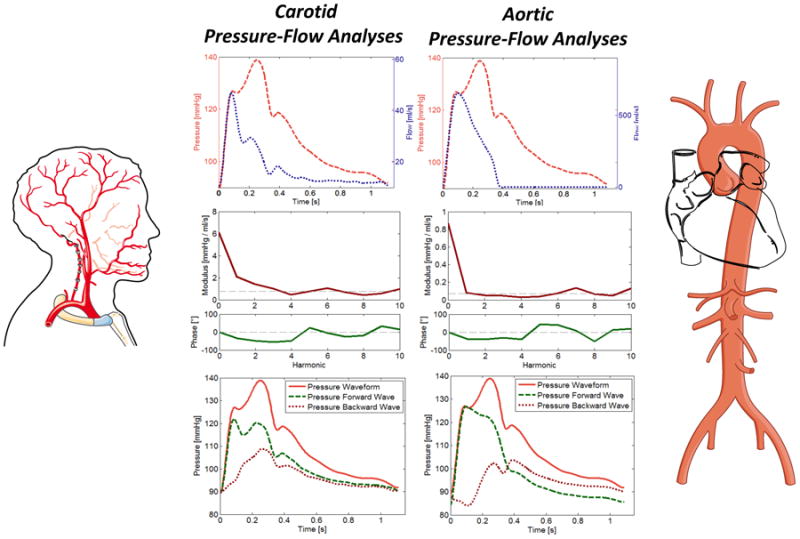
Carotid (left panels) and aortic (right panels) pressure-flow pairs are processed via mathematical analyses in the frequency domain. The input impedance spectrum, which consists of modulus (red) and phase (blue) is obtained. Characteristic impedance (Zc, dashed line in modulus plot) is computed based on the average modulus of higher harmonics of input impedance modulus. Zc is a local vascular property of the aortic root or carotid artery, which governs the local pressure-flow relation in the absence of wave reflections. Once Zc is known, the modulus and phase of the reflection coefficient in the frequency domain (at each harmonic) can be computed (not shown), along with the net contribution of reflections to pressure at each harmonic (i.e., the real part of the reflection coefficient). Reflection coefficients in the frequency domain are derived purely from the input impedance spectrum and thus depend purely on arterial load. The first 3 harmonics contain the vast majority of the pulsatile energy and are the relevant harmonics. We also performed wave separation in the time domain, as shown in the bottom panels. This approach yields forward (green dashed line) and backward (red dotted line) waves reconstructed in the time domain. At the aorta, forward and backward wave amplitude and morphology in the time domain are not purely dependent on arterial load, but depend also on re-reflections in the heart and the LV contraction pattern.
Pressure-flow analyses
We computed aortic input impedance (Figure-2), which characterizes the ratio of pulsatile pressure over flow in each harmonic of heart rate. In this analysis, the fundamental frequency, or 1st harmonic, is the heart rate, and higher harmonics are multiples of that frequency. Proximal aortic characteristic impedance (Zc) was computed in the frequency domain. Each pressure and flow harmonic was separated into forward and backward components using wave separation analysis (21, 22). We assessed the reflection coefficient in the first 3 harmonics. These are the relevant harmonics for assessing wave reflections because: (1) they contain the vast majority of the pulsatile energy in pressure and flow signals; (2) at higher frequencies, reflections cancel out at random, and the input impedance spectrum hovers around aortic root characteristic impedance, mimicking a reflection-free system.
As the reflection coefficient is derived from the ratio of two sine waves, it is a complex number with an amplitude and phase-angle, which can correspond to different degrees of destructive or constructive interference between forward and backward waves. Therefore, the net-effect of reflections was expressed as the real part of the reflection coefficient, which becomes increasingly positive as pressure from wave reflections increases (constructive interference), and negative when destructive interference leads to a net decrease in pressure by wave reflections at a given harmonic.(23)
We also reconstructed the forward and backward pressure waves in the time domain. The sum of forward and backward pressure harmonics yields the forward and backward waves, respectively (Figure-2). The time of the onset of the reflected wave was defined as the time at which the reflected wave starts adding to mean pressure. We computed the Buckberg index (also known as the sub-endocardial viability ratio), as the ratio of diastolic/systolic pressure-time integrals (i.e., areas under the pressure curve). This index provides an assessment of the effect of pulsatile hemodynamics on myocardial oxygen demand (systolic load) vs. supply (perfusion pressure).(24)
We also performed analyses of carotid pressure-flow relations. Hemodynamic analysis commonly assumes a parabolic or flat flow velocity profile to convert velocity measured in a sample volume into a volumetric flow. However, this simplification may be inadequate for the carotid artery, as the flow velocity profile is neither of both. We therefore implemented a conversion accounting for the Womersley number (a well-established dimensionless fluid-dynamics parameter for oscillatory flow), as previously described.(25)
In our laboratory, repeated measurements of all indices from aortic and carotid pressure-flow analyses yielded coefficients of variation of 17% or less.
Statistical analysis
Descriptive data are presented as mean ± standard deviation (SD) for continuous variables, or counts (%) for categorical variables. Comparisons between pre- and post-NTG values for sub-study 1, and between values corresponding to nitrate-rich vs. nitrate-depleted beetroot juice administration for sub-study 2, were performed using paired t-tests. Physiologic indices were expressed as absolute values at each time point, as well as absolute differences between measurements (with 95% confidence intervals). A two-tailed P value< 0.05 was considered statistically significant. Statistical analyses were performed using SPSS v-15 for Mac-OS (SPSS Inc., Chicago, IL).
Results
General characteristics of the study populations for sub-studies 1 and 2 are shown in Table 1. The characteristics of study subjects in both sub-studies were very similar. In both sub-studies, subjects were obese, with a high prevalence of hypertension and diabetes, as well as left atrial enlargement and a high mitral E/e' ratio. Both study samples were composed predominantly of males, with a high proportion of African-Americans. All subjects in both sub-studies had NYHA class II-III symptoms.
Table 1. General Characteristics of Study Subjects.
| Variable | NTG Substudy (SubStudy 1) (n=26) | BeetRoot Study (SubStudy 2) (n=16) | P value |
|---|---|---|---|
| Age, median (IQR) | 60 (56, 65) | 65 (62.5, 70.5) | 0.10 |
| Male, n (%) | 20 (76.9) | 14 (87.5) | 0.68 |
| Race | 0.15 | ||
| African-American, n (%) | 16 (61.5) | 14 (87.5) | |
| Caucasian, n (%) | 9 (34.6) | 2 (12.5) | |
| BMI (kg/m2), mean (SD) | 36.5 (6.5) | 34.4 (3.5) | 0.24 |
| Obesity (BMI>30 kg/m2), n (%) | 22 (84.6) | 15 (93.8) | 0.63 |
| Current Smoker, n (%) | 4 (15.4) | 1 (6.3) | 0.63 |
| Hypertension, n (%) | 24 (92.3) | 16 (100) | 0.52 |
| Diabetes, n (%) | 17 (65.4) | 11 (68.8) | 0.82 |
| Coronary Artery Disease, n (%) | 8 (30.8) | 3 (18.8) | 0.49 |
| Chronic Kidney Disease (eGFR<60 ml/min/1.73m2) | 9 (34.6) | 5 (31.3) | 0.82 |
| Drug Therapy, n (%) | |||
| Beta Blocker | 14 (53.9) | 10 (62.5) | 0.58 |
| ACE-Inhibitor/ARB | 18 (69.2) | 10 (62.5) | 0.65 |
| Calcium-Channel Blocker | 14 (53.9) | 7 (43.8) | 0.53 |
| Mineralocorticoid Receptor Antagonist | 0 (0) | 1 (6.3) | 0.38 |
| Statin | 15 (57.7) | 9 (56.3) | 0.93 |
| Aspirin | 17 (65.4) | 14 (87.5) | 0.16 |
| Thiazide | 14 (53.9) | 4 (25.0) | 0.07 |
| Loop Diuretics | 13 (50.0) | 6 (37.5) | 0.43 |
| Laboratory Data | |||
| eGFR (mL/min/1.73 m2), median (IQR) | 74.1 (53.5, 95.4) | 65.5 (52.4, 89.5) | 0.95 |
| Echocardiography | |||
| Left Ventricular Ejection Fraction (%), median (IQR) | 57.4 (55.0, 65.5) | 62.4 (57.5, 69.8) | 0.30 |
| Left Atrial Volume Index (mL/m2), mean (SD) | 30.3 (10.9) | 35.7 (10.9) | 0.13 |
| Mitral E-wave velocity (cm/s), mean (SD) | 81.8 (24.9) | 71.7 (16.4) | 0.16 |
| Mitral A-wave velocity (cm/s), mean (SD) | 79.2 (24.6) | 73.3 (24.2) | 0.45 |
| Mitral Septal Tissue Doppler Velocity (e', cm/s), mean (SD) | 6.6 (2.2) | 6.5 (1.7) | 0.88 |
| Mitral E/e' Ratio, median (IQR) | 12.8 (11.0, 14.4) | 11.4 (9.2, 13.3) | 0.17 |
| Tricuspid Annular Systolic Excursion (cm), median (IQR) | 2.3 (2.1, 2.6) | 2.6 (2.1, 2.8) | 0.44 |
| TAPSE <1.6 cm, n (%) | 0 (0) | 0 (0) | |
| Tricuspid Regurgitant Jet Peak Gradient (mmHg), mean (SD) † | 31.8 (3.7) | 27.9 (8.9) | 0.054 |
| LVEDd (cm), mean (SD) | 4.8 (0.6) | 4.5 (0.6) | 0.12 |
| LV mass (g), median (IQR) | 243.7 (214.1, 318.3) | 236.6 (183.2, 275.2) | 0.62 |
| LV mass indexed to height (g/m1.7) | 96.9 (71.7,114.1) | 91.6 (70.7,102.8) | 0.39 |
| LV mass indexed to BSA (g/m2) | 109 (23.5) | 106.3 (37.6) | 0.77 |
| Relative wall thickness, median (IQR) | 0.52 (0.47, 0.58) | 0.59 (0.54, 0.66) | 0.041 |
| NT-pro BNP (pg/mL), median (IQR) | 250.5 (90.5, 510.6) | 148.0 (61.7, 272.5) | 0.16 |
| Ejection duration (ms) | 318 (28) | 330 (35) | 0.22 |
eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) Study equation
Could be assessed reliably from the tricuspid regurgitation envelope in only 9 subjects in sub-study 1 and 7 subjects in sub-study 2.
Sub-study 1: Effects of Sublingual NTG on Aortic and Carotid Hemodynamics
Key hemodynamic parameters measured before and after the administration of NTG are shown in Table 2 and Figure 3. NTG reduced central systolic and mean pressure. NTG did not have any significant effect on the amplitude of forward or backward waves, the ratio of backward/forwards wave amplitudes, or the time to reflected wave onset. NTG tended to delay the peak of the reflected wave, but did not improve the Buckberg index. NTG did not significantly reduce aortic root characteristic impedance.
Table 2. Aortic and carotid pulsatile hemodynamics before (Pre) and after (Post) administration of sublingual NTG.
| Variable | Pre-NTG | Post-NTG | P value |
|---|---|---|---|
| Central systolic blood pressure, mmHg | 133.6 (27.6) | 120.5 (29.8) | 0.011 |
| Central pulse pressure, mmHg | 59.5 (23.6) | 50 (19.4) | 0.06 |
| Central mean Arterial pressure, mmHg | 96.8 (17) | 90.5 (20.4) | 0.019 |
| Central diastolic blood pressure, mmHg | 74 (15.4) | 70.5 (15.3) | 0.18 |
| Aortic pressure-flow relations | |||
| Aortic Characteristic Impedance, dynes·s/cm5 | 172 (69) | 174 (119) | 0.93 |
| Reflection coefficient, 1st harmonic | 0.093 (26) | 0.002 (26) | 0.017 |
| Reflection coefficient, 2nd harmonic | -0.071 (26) | -0.117 (26) | 0.16 |
| Reflection coefficient, 3rd harmonic | -0.012 (26) | 0.058 (26) | 0.054 |
| Forward wave amplitude, mmHg* | 52.6 (20.2) | 47.6 (16.6) | 0.26 |
| Backward wave amplitude, mmHg* | 19.1 (9.1) | 16.9 (7.5) | 0.21 |
| Backward/Forward wave amplitude* | 0.36 (0.07) | 0.35 (0.08) | 0.33 |
| Time to reflected wave onset, ms | 78 (30) | 89 (44) | 0.22 |
| Buckberg index, % | 130 (33) | 129 (33) | 0.81 |
| Carotid pressure-flow relations | |||
| Carotid Characteristic Impedance, dynes·s/cm5 | 3440 (2757) | 1923 (1277) | 0.002 |
| Carotid Cross-sectional area, cm2 | 0.38 (0.09) | 0.43 (0.1) | <0.0001 |
| Carotid Bed Vascular resistance, dynes·s/cm5 | 19580 (13402) | 13078 (8974) | 0.001 |
Computed in the time domain.
Figure 3.
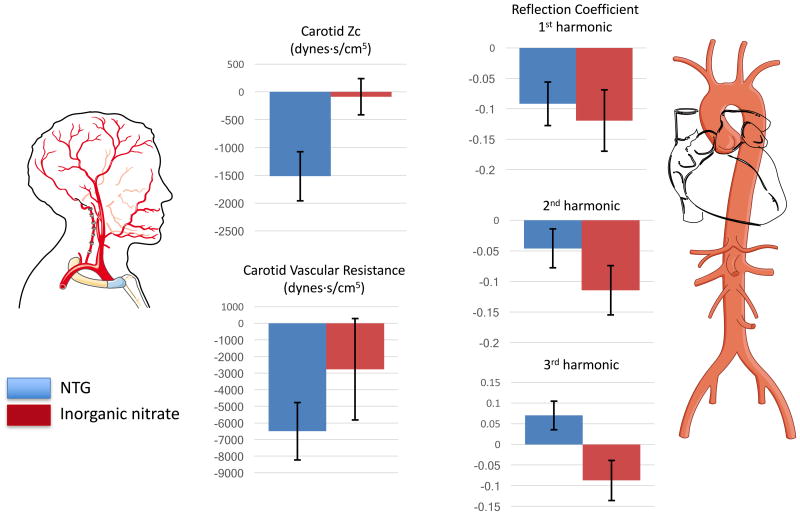
Changes in key pulsatile hemodynamic indices in the aorta (right) and the carotid artery (left), in sub-study 1 (sublingual NTG, solid blue bars) and sub-study 2 (inorganic nitrate, solid red bars). Mean changes ± standard errors are shown.
Table 2 and Figure 3 show the effect of NTG on the real part of the reflection coefficient in the first 3 harmonics. NTG reduced the reflection coefficient of the 1st harmonic, but did not reduce it in the 2nd harmonic and actually tended to increase the reflection coefficient of the 3rd harmonic.
Despite these inconsistent effects on systemic wave reflections, NTG markedly reduced carotid artery Zc, increased carotid cross-sectional area and reduced carotid bed vascular resistance.
Effects of Inorganic nitrate on Aortic and Carotid Hemodynamics
Key comparisons of hemodynamic parameters obtained after administration of nitrate-rich beetroot juice vs. placebo juice are shown in Table 3 and Figure 3. Inorganic nitrate did not significantly reduce central systolic or mean pressure. Similar to NTG, inorganic nitrate did not have any significant effect on the amplitude of forward or backward waves or the ratio of backward/forwards wave amplitudes, and did not reduce aortic root characteristic impedance. However, it significantly delayed the systolic onset of the reflected wave, moved its peak well into diastole, and tended to improve the Buckberg index.
Table 3.
Aortic and carotid pulsatile hemodynamics after administration of 12.9 mmol of inorganic nitrate (nitrate-rich beetroot juice, NO3) vs. placebo juice (nitrate-depleted beetroot juice).
| Variable | Placebo | NO3 | P value |
|---|---|---|---|
| Central systolic blood pressure, mmHg | 130 (20.8) | 126.6 (24.2) | 0.50 |
| Central pulse pressure, mmHg | 55.3 (16.7) | 51 (18) | 0.34 |
| Central diastolic blood pressure, mmHg | 74.7 (12.6) | 75.6 (9.8) | 0.70 |
| Central mean Arterial pressure, mmHg | 97.6 (14.8) | 96.4 (15.2) | 0.70 |
| Aortic pressure-flow relations | |||
| Aortic Characteristic Impedance, dynes·s/cm5 | 163 (74) | 185 (64) | 0.20 |
| Reflection coefficient, 1st harmonic | 0.119 (0.15) | -0.001 (0.21) | 0.032 |
| Reflection coefficient, 2nd harmonic | -0.03 (0.13) | -0.144 (0.15) | 0.012 |
| Reflection coefficient, 3rd harmonic | 0.046 (0.16) | -0.042 (0.16) | 0.091 |
| Forward wave amplitude, mmHg* | 47.2 (13.8) | 47.1 (14.1) | 0.98 |
| Backward wave amplitude, mmHg* | 16.8 (6) | 18.8 (7.9) | 0.30 |
| Backward/Forward wave amplitude* | 0.35 (0.05) | 0.39 (0.08) | 0.08 |
| Time to reflected wave onset, ms | 64 (30) | 93 (30) | 0.016 |
| Buckberg index, % | 128 (29) | 141 (25) | 0.053 |
| Carotid pressure-flow relations | |||
| Carotid Characteristic Impedance, dynes·s/cm5 | 3013 (1867) | 2928 (1279) | 0.80 |
| Carotid Cross-sectional area, cm2 | 0.37 (0.08) | 0.40 (0.12) | 0.15 |
| Carotid Bed Vascular resistance, dynes·s/cm5 | 19391 (13557) | 16624 (6800) | 0.38 |
Computed in the time domain.
Table 3 and Figure 3 show the effect of inorganic nitrate on the real part of the reflection coefficient in harmonics 1-3. Inorganic nitrate significantly reduced the reflection coefficient of the 1st and 2nd harmonics, and tended to reduce the reflection coefficient of the 3rd harmonic.
In contrast to NTG, inorganic nitrate did not reduce reduced carotid artery Zc, carotid cross-sectional area or carotid bed vascular resistance.
Discussion
We assessed the effects of organic and inorganic nitrate on aortic and carotid pulsatile hemodynamics in HFpEF. We demonstrate that organic nitrate substantially reduced blood pressure, but reduced arterial wave reflections inconsistently across the first 3 harmonics of the pressure-flow relation (in which most of the pulsatile energy is contained). NTG did not significantly improve the Buckberg index and produced profound vasodilation in the carotid territory, with a reduction in cerebrovascular resistance and carotid characteristic impedance. In contrast, inorganic nitrate produced consistent reductions in wave reflections across the first 3 harmonics, with a delay in the reflected wave and a trend for improvement in the Buckberg index, without significant cerebrovascular dilatation. These hemodynamic differences likely underlie the differential clinical effects of organic vs. inorganic nitrate observed in recent clinical trials.
While both organic and inorganic nitrate/nitrite ultimately act by increasing NO bioavailability, biochemical differences exist between the two classes of drugs that lead to important differences in their action. Inorganic nitrate is reduced to nitrite and NO via the nitrate-nitrite-NO pathway,(16, 17) which involves the reduction of nitrate to nitrite upon ingestion and when nitrate is subsequently excreted by the salivary glands (enterosalivary circulation).(26) Subsequent reduction of nitrite to NO occurs via: (1) A hypoxia/acidosis-dependent mechanism (which enhances reductions in microvascular resistance during exercise)(14), and; (2) A “paradoxic” normoxia-dependent mechanism operating in muscular conduit arteries,(18) which explains the effect on arterial wave reflections. (14, 18) There also appears to be non-enzymatic nitrite reduction to NO in the acid gastric medium.(27) In contrast, organic nitrates require activation in the cytochrome-P450 system, leading to tonic NO release.(28) Alternative activation via mitochondrial aldehyde-dehydrogenase for NTG and other organic nitrates also occurs.(28)
Headache is a common side effect of organic nitrates, and can limit compliance with these medications. Hypotension can be seen, and may result in syncope.(12, 16) In contrast, inorganic nitrate has been well tolerated in HFpEF, with no limiting side effects, as reported in 2 recent trials.(14, 15) No significant hypotension and in particular, no vasoactive symptoms (such as headache) were reported. These side effect differences are consistent with the observed hemodynamic effects observed in our study. The profound carotid bed vasodilation seen in response to NTG, but not inorganic nitrate, is likely due to differences in the activation of these compounds. The high mitochondrial content of neurons may facilitate the activation of NTG by mitochondrial aldehyde-dehydrogenase in the brain, thus reducing microvascular resistance in the cerebrovascular bed. In contrast, inorganic nitrite (produced via reduction of inorganic nitrate) is reduced to NO in the microvasculature, but this conversion occurs preferentially in conditions of hypoxia/acidosis.(16, 17) Such conditions are not present in the brain, a highly aerobic organ which demonstrates relatively preserved microvascular oxygenation(29) due to its low resistance and high arterial flow. Therefore, conditions of hypoxia and acidosis are not present in the cerebral microvasculature, explaining the lack of significant effects of inorganic nitrate in our study.
Wave reflections originate at sites of impedance-mismatch throughout the arterial tree and return to the heart during ejection, increasing pulsatile load and affecting the LV loading sequence (early vs. late-systolic load).(22, 30) Wave reflections and late-systolic load have been shown to cause diastolic dysfunction and myocardial remodeling in animal models.(1, 2) Human studies demonstrated a relationship between increased wave reflections/late systolic load and worse longitudinal LV function,(3) LV hypertrophy,(4) and a higher risk of incident new-onset HF(5) and readmission after an episode of established acute decompensated HF.(6)
In our study, both NTG and inorganic nitrate reduced wave reflections. However, inorganic nitrate produced numerically greater and more consistent reductions in the real part of the reflection coefficient across the first 3 harmonics, which contain most of the pulsatile energy. In contrast, NTG reduced the real component of the reflection coefficient only in the 1st harmonic, without an effect in the 2nd harmonic, and an increase in the 3rd harmonic. The real part of the reflection coefficient characterizes the net-effect of wave reflections on the pressure-flow relation in the aorta at a given harmonic.(23) Inorganic nitrate, but not NTG, delayed the reflected wave and tended to improve the Buckberg index (which characterizes the effect of pulsatile hemodynamics on LV systolic load vs. diastolic perfusion pressure). The reduction in wave reflections with inorganic nitrate occurred without reductions in SVR or blood pressure. This clearly indicates that the effects of inorganic nitrate on microvascular resistance/blood pressure and those on wave reflections are not necessarily linked. Reductions in wave reflections can thus be achieved in the absence of significant hypotension. Although these hemodynamic effects are unexpected from the well-known hypoxia-mediated reduction of nitrite in the microvasculature, they are consistent with the recently described paradoxic normoxia-dependent activation of inorganic nitrite in the wall of muscular conduit arteries (18), because muscular arteries are known modify the magnitude and phase of wave reflections returning to the proximal aorta.(22, 24)
The effects of inorganic nitrate on pulsatile load from wave reflections demonstrated in our study, along with the absence of cerebrovascular effects and side effects, is helpful not only to interpret its demonstrated short-term clinical effects (i.e., improvements in exercise tolerance and the absence of side effects such as headache), but may also have implications for its long term clinical effects. By virtue of reducing wave reflections, which are deleterious for the LV, inorganic nitrate may exert long-term “disease-modifying” effects in HFpEF, potentially reducing LV diastolic dysfunction and remodeling. We are currently assessing the efficacy of sustained administration of inorganic nitrate (oral potassium nitrate) in a randomized cross-over phase IIb trial funded by the National Heart, Lung and Blood Institute (KNO3CK OUT HFPEF trial; clinicaltrials.gov NCT02840799). A single-dose study with inorganic nitrite yielded promising results in HFpEF, (31) and a larger study with inhaled sodium nitrite (INDIE HFPEF; clinicaltrials.gov NCT02742129) is under way.
An interesting observation is the absence of significant effects of either NTG or inorganic nitrate on the ratio of amplitudes of backward/forward waves, which is a commonly used index of wave reflections. It should be noted that this ratio does not account for the time-resolved shape of the waveforms (which can be different despite identical amplitudes). Similarly, this ratio does not account for the time at which the reflected wave exerts its effects on central pressure (late systole vs. diastole). Furthermore, the amplitudes of both forward and backward waves are not purely a function of arterial properties, but are heavily dependent on ventricular contraction dynamics. In addition, the ratio of backward/forward waves is confounded by rectified reflections (i.e., re-reflections in the heart), which substantially contribute to forward wave amplitude.(32) Despite the lack of change in reflection magnitude, detailed analyses in the time domain demonstrated favorable changes (delay in the onset of the reflected wave after ejection, and a trend towards improvement in the Buckberg index) with inorganic nitrate, but not with NTG.
Our study should be interpreted in the context of its strengths and limitations. Strengths include the careful assessment of pulsatile carotid and aortic hemodynamics using state-of-the-art non-invasive pressure-flow analyses, rather than pressure-only approaches. Input impedance assessments distinctly characterize arterial properties distal to the point of measurement, whereas pressure-only approaches (such as assessments of augmentation index) can be confounded by changes in ventricular contraction or preload. Additional strengths of our study include the use of identical methods to measure hemodynamics after organic and inorganic nitrate administration, facilitating the interpretation of differential hemodynamic effects. Our study also has limitations. Our study populations were relatively small; however, the paired nature of the analyses reduced measurement variability and enhanced detection of drug effects in each sub-study. Due to the characteristics of the patient population at the VA Medical Center, where most subjects were enrolled, our study populations were composed primarily of men. Our population was predominantly African-American. Larger studies will be required to assess whether the hemodynamic effects of inorganic nitrate differ by ethnicity. Enrollment in the ongoing KNO3CK OUT HFpEF trial is stratified based on gender and ethnicity; this study is also assessing detailed hemodynamic phenotypes and will clarify this issue. The exclusion of patients with atrial fibrillation reduces generalizability of the findings to the important subject of patients who have atrial fibrillation in the setting of HFpEF. We performed 2 separate sub-studies with different designs. We tested the effects of NTG compared to the values before drug administration, whereas the effects of inorganic nitrate were tested in a crossover blinded design. This was an acute administration study, and the chronic effects of these drugs on the carotid and peripheral circulations could be different. The patient population had relatively mild HFpEF as evidenced by the relatively low use of loop diuretics; therefore, these results may not apply to patients who have more severe or advanced HFpEF.
Conclusions
We compared the effects of organic and inorganic nitrate on aortic and carotid pulsatile hemodynamics in patients with HFpEF. We demonstrate that organic nitrate administration reduced arterial wave reflections less consistently than observed with inorganic nitrate. Organic nitrate also produced profound vasodilation in the carotid territory, with a reduction in cerebrovascular resistance and carotid characteristic impedance while inorganic nitrate did not produce significant cerebrovascular dilatation. These important hemodynamic differences are likely related to the differential clinical effects of these agents documented in recent clinical trials.
Acknowledgments
Funding information: This study was supported by NIH grants 5-R21-AG-043802-02 (JAC), R01 HL-121510-01A1 (JAC), R56 HL-124073-01A1 (JAC) and a grant from the VA Health Network - VISN-4 (JAC).
J.A.C. has received consulting honoraria from Bristol-Myers-Squibb, OPKO Healthcare, Fukuda Denshi, Microsoft, Merck and Vital Labs. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol-Myers-Squibb, Microsoft and CVRx Inc., and device loans from Atcor Medical. He is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction.
Footnotes
Disclosures: Other authors have no disclosures.
References
- 1.Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation. 1996 Dec 15;94(12):3362–3368. doi: 10.1161/01.cir.94.12.3362. [DOI] [PubMed] [Google Scholar]
- 2.Gillebert TC, Lew WY. Influence of systolic pressure profile on rate of left ventricular pressure fall. Am J Physiol. 1991 Sep;261(3 Pt 2):H805–813. doi: 10.1152/ajpheart.1991.261.3.H805. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 3.Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC, Asklepios I. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: the Asklepios study. Hypertension. 2013 Feb;61(2):296–303. doi: 10.1161/HYPERTENSIONAHA.111.00530. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O'Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008 May;26(5):1017–1024. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]
- 5.Chirinos JA, Kips JG, Jacobs DR, Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis) J Am Coll Cardiol. 2012 Nov 20;60(21):2170–2177. doi: 10.1016/j.jacc.2012.07.054. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung SH, Yu WC, Cheng HM, Lee CW, Lin MM, Chuang SY, Chen CH. Excessive wave reflections on admission predict post-discharge events in patients hospitalized due to acute heart failure. Eur J Heart Fail. 2012 Dec;14(12):1348–1355. doi: 10.1093/eurjhf/hfs124. [DOI] [PubMed] [Google Scholar]
- 7.Fok H, Guilcher A, Li Y, Brett S, Shah A, Clapp B, Chowienczyk P. Augmentation pressure is influenced by ventricular contractility/relaxation dynamics: novel mechanism of reduction of pulse pressure by nitrates. Hypertension. 2014 May;63(5):1050–1055. doi: 10.1161/HYPERTENSIONAHA.113.02955. [DOI] [PubMed] [Google Scholar]
- 8.Latson TW, Hunter WC, Katoh N, Sagawa K. Effect of nitroglycerin on aortic impedance, diameter, and pulse-wave velocity. Circ Res. 1988 May;62(5):884–890. doi: 10.1161/01.res.62.5.884. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 9.Fitchett DH, Simkus GJ, Beaudry JP, Marpole DG. Reflected pressure waves in the ascending aorta: effect of glyceryl trinitrate. Cardiovascular research. 1988 Jul;22(7):494–500. doi: 10.1093/cvr/22.7.494. [DOI] [PubMed] [Google Scholar]
- 10.Yaginuma T, Avolio A, O'Rourke M, Nichols W, Morgan JJ, Roy P, Baron D, Branson J, Feneley M. Effect of glyceryl trinitrate on peripheral arteries alters left ventricular hydraulic load in man. Cardiovascular research. 1986 Feb;20(2):153–160. doi: 10.1093/cvr/20.2.153. [DOI] [PubMed] [Google Scholar]
- 11.Stokes GS, Barin ES, Gilfillan KL. Effects of isosorbide mononitrate and AII inhibition on pulse wave reflection in hypertension. Hypertension. 2003 Feb;41(2):297–301. doi: 10.1161/01.hyp.0000049622.07021.4f. [Clinical Trial Randomized Controlled Trial] [DOI] [PubMed] [Google Scholar]
- 12.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E, Network NHFCR Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2015 Dec 10;373(24):2314–2324. doi: 10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamani P, Akers S, Soto-Calderon H, Beraun M, Koppula MR, Varakantam S, Rawat D, Shiva-Kumar P, Haines PG, Chittams J, Townsend RR, Witschey WR, Segers P, Chirinos JA. Isosorbide Dinitrate, With or Without Hydralazine, Does Not Reduce Wave Reflections, Left Ventricular Hypertrophy, or Myocardial Fibrosis in Patients With Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2017 Feb 20;6(2) doi: 10.1161/JAHA.116.004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015 Jan 27;131(4):371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. discussion 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One Week of Daily Dosing With Beetroot Juice Improves Submaximal Endurance and Blood Pressure in Older Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2016 Feb 2; doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirinos JA, Zamani P. The Nitrate-Nitrite-NO Pathway and Its Implications for Heart Failure and Preserved Ejection Fraction. Curr Heart Fail Rep. 2016 Feb;13(1):47–59. doi: 10.1007/s11897-016-0277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanderpool R, Gladwin MT. Harnessing the nitrate-nitrite-nitric oxide pathway for therapy of heart failure with preserved ejection fraction. Circulation. 2015 Jan 27;131(4):334–336. doi: 10.1161/CIRCULATIONAHA.114.014149. [DOI] [PubMed] [Google Scholar]
- 18.Omar SA, Fok H, Tilgner KD, Nair A, Hunt J, Jiang B, Taylor P, Chowienczyk P, Webb AJ. Paradoxical normoxia-dependent selective actions of inorganic nitrite in human muscular conduit arteries and related selective actions on central blood pressures. Circulation. 2015 Jan 27;131(4):381–389. doi: 10.1161/CIRCULATIONAHA.114.009554. discussion 389. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009 Mar;10(2):165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 20.Chirinos JA, Segers P, Gupta AK, Swillens A, Rietzschel ER, De Buyzere ML, Kirkpatrick JN, Gillebert TC, Wang Y, Keane MG, Townsend R, Ferrari VA, Wiegers SE, St John Sutton M. Time-varying myocardial stress and systolic pressure-stress relationship: role in myocardial-arterial coupling in hypertension. Circulation. 2009 Jun 02;119(21):2798–2807. doi: 10.1161/CIRCULATIONAHA.108.829366. [DOI] [PubMed] [Google Scholar]
- 21.Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovascular research. 1972 Nov;6(6):648–656. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- 22.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension. 2010 Oct;56(4):563–570. doi: 10.1161/HYPERTENSIONAHA.110.157339. [DOI] [PubMed] [Google Scholar]
- 23.Swillens A, Lanoye L, De Backer J, Stergiopulos N, Verdonck PR, Vermassen F, Segers P. Effect of an abdominal aortic aneurysm on wave reflection in the aorta. IEEE Trans Biomed Eng. 2008 May;55(5):1602–1611. doi: 10.1109/TBME.2007.913994. [DOI] [PubMed] [Google Scholar]
- 24.Nichols WWORM, Vlachopoulos C. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. Hodder Arnold. (6) 2011 [Google Scholar]
- 25.Ponzini R, Vergara C, Rizzo G, Veneziani A, Roghi A, Vanzulli A, Parodi O, Redaelli A. Womersley number-based estimates of blood flow rate in Doppler analysis: in vivo validation by means of phase-contrast MRI. IEEE Trans Biomed Eng. 2010 Jul;57(7):1807–1815. doi: 10.1109/TBME.2010.2046484. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008 Feb;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 27.Montenegro MF, Sundqvist ML, Larsen FJ, Zhuge Z, Carlstrom M, Weitzberg E, Lundberg JO. Blood Pressure-Lowering Effect of Orally Ingested Nitrite Is Abolished by a Proton Pump Inhibitor. Hypertension. 2016 Oct 31; doi: 10.1161/HYPERTENSIONAHA.116.08081. [DOI] [PubMed] [Google Scholar]
- 28.Omar SA, Artime E, Webb AJ. A comparison of organic and inorganic nitrates/nitrites. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2012 May 15;26(4):229–240. doi: 10.1016/j.niox.2012.03.008. [Review] [DOI] [PubMed] [Google Scholar]
- 29.Thavasothy M, Broadhead M, Elwell C, Peters M, Smith M. A comparison of cerebral oxygenation as measured by the NIRO 300 and the INVOS 5100 Near-Infrared Spectrophotometers. Anaesthesia. 2002 Oct;57(10):999–1006. doi: 10.1046/j.1365-2044.2002.02826.x. [DOI] [PubMed] [Google Scholar]
- 30.Chirinos JA, Segers P, Gillebert TC, Gupta AK, De Buyzere ML, De Bacquer D, St John-Sutton M, Rietzschel ER, Asklepios I. Arterial properties as determinants of time-varying myocardial stress in humans. Hypertension. 2012 Jul;60(1):64–70. doi: 10.1161/HYPERTENSIONAHA.112.190710. [DOI] [PubMed] [Google Scholar]
- 31.Borlaug BA, Koepp KE, Melenovsky V. Sodium Nitrite Improves Exercise Hemodynamics and Ventricular Performance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2015 Oct 13;66(15):1672–1682. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 32.Phan TS, Li JK, Segers P, Chirinos JA. Misinterpretation of the Determinants of Elevated Forward Wave Amplitude Inflates the Role of the Proximal Aorta. J Am Heart Assoc. 2016 Feb 19;5(2) doi: 10.1161/JAHA.115.003069. [DOI] [PMC free article] [PubMed] [Google Scholar]