Abstract
Introduction
A rapid increase in awareness of androgen deficiency has led to substantial increases in prescribing of testosterone therapy (TTh), with the benefits of improvements in mood, libido, bone density, muscle mass, body composition, energy and cognition. However, TTh may be limited by its side effects, particularly erythrocytosis. This review examines the literature regarding testosterone-induced erythrocytosis and polycythemia.
Aim
To review the available literature on testosterone-induced erythrocytosis, discuss possible mechanisms for pathophysiology, determine the significance of formulation, and elucidate the potential thromboembolic risk.
Methods
A literature review was performed using PubMed for articles addressing TTh, erythrocytosis, and polycythemia.
Main Outcome Measures
Mechanism, pharmacologic contribution, and risk of testosterone induced erythrocytosis.
Results
For men undergoing TTh, the risk of developing erythrocytosis compared to controls is well established, with short acting injectable formulations having the highest associated incidence. Potential mechanisms explaining the relationship between TTh and erythrocytosis include the role of hepcidin, iron sequestration and turnover, erythropoietin production, bone marrow stimulation, and genetic factors. High blood viscosity increases the risk for potential vascular complications involving the coronary, cerebrovascular, and peripheral vascular circulation, though there is limited evidence supporting a relationship between TTh and vascular complications.
Conclusions
Short acting injectable testosterone is associated with greater risk of erythrocytosis when compared with other formulations. The mechanism of the pathophysiology and its role on thromboembolic events remains unclear, though few data support an increased risk of CV events resulting from testosterone-induced erythrocytosis.
Keywords: Hypogonadism, testosterone, hormone replacement, erythrocytosis, polycythemia
Introduction
With an increasing awareness of men’s health issues, including androgen deficiency, the use of testosterone therapy (TTh) is on the rise. Testosterone prescription sales for men over 40 years old have tripled during the last decade, and quadrupled in men 18–45 years old [1, 2]. Furthermore, direct to consumer marketing campaigns by drug manufacturers have introduced the concepts of “andropause” and “low T” to the general population, as physicians have established terminology such as late-onset hypogonadism and androgen deficiency in the aging male.
Hypogonadism is defined as “biochemically low testosterone levels in the setting of a cluster of clinical symptoms, which may include reduced sexual desire (libido) and activity, decreased spontaneous erections, decreased energy and depressed mood” [3]. Men may also present with reductions in muscle mass and strength, increased fat mass, decreased bone mineral density, and anemia [3, 4]. Symptomatic hypogonadism is a pathologic disruption of the hypothalamic-pituitary-testicular axis. There are two widely accepted forms of hypogonadism: primary (testicular failure) and secondary (hypothalamic or pituitary failure). Primary hypogonadism represents failure of testosterone production, characterized by low serum testosterone and elevated gonadotropins. Secondary hypogonadism results from a failure of testicular stimulation, characterized by low serum gonadotropin and low serum testosterone levels. The Sexual Medicine Society of North America (SMSNA) described the clinical scenario of men presenting with signs and symptoms of low testosterone, distinct from the classical picture of primary (testicular failure) or secondary (pituitary or hypothalamic failure) hypogonadism, as Adult Onset Hypogonadism (AOH) [5]. The SMSNA suggested that the term AOH could be applied to most men with hypogonadism, many of whom have concomitant metabolic disease (obesity, type 2 diabetes, metabolic syndrome, etc.). In an official statement, the organization proposed an algorithm for TTh in patients with AOH [5].
The Hypogonadism in Males (HIM) study found that the prevalence of hypogonadism in men more than 45 years old is > 38%, with a 17% increase in risk for every 10 year increase in age; however, the HIM study did not include symptoms in its definition of hypogonadism [6]. In the European Male Aging Study (EMAS), the overall prevalence of low testosterone (total T < 10.5 nmol/L without symptoms) was somewhat lower at 23.3% [7]. Further assessment of the cohort with an evaluation of nine candidate symptoms in addition to low testosterone levels found a prevalence of 2.1% for symptomatic hypogonadism (low T with at least 3 symptoms) [8]. The authors acknowledged the lower prevalence of hypogonadism in consideration of both serum testosterone levels and symptoms, noting that “this finding underscores the paramount importance of using not only biochemical measures but also stringently defined, symptom-based criteria to prevent over diagnosis…”[8]. While the above studies demonstrated that the incidence of hypogonadism differs as a function of patient age and definition of hypogonadism, the Food and Drug Administration has concluded that available evidence does not support an indication for TTh in the setting of “age-related hypogonadism” [9]. Given increased testing for low testosterone levels and the large increase in testosterone prescribing, as well as incompletely defined indications for therapy, it is paramount that we thoroughly understand the risks and benefits of TTh.
Despite its positive effects, TTh has several common side effects, including increases in estrogen levels, gynecomastia, and erythrocytosis [10–15]. Much recent attention has been focused on the effects of TTh on the cardiovascular system. Extensive debate has surrounded high impact publications with questionable methodologies and controversial conclusions that suggested significant cardiovascular risk for men on TTh with alternative studies suggesting benefit [16–20]. In light of this controversy, the American Urological Association (AUA) issued a policy statement stating that, based upon current evidence, definitive answers on the cardiovascular risks of TTh are not currently available [3].
Testosterone-induced elevations in hemoglobin (Hb) and hematocrit (Hct) may lead to erythrocytosis, generally defined clinically as Hb > 18.5 g/dl or Hct > 52% in males, though this definition varies [21]. Physiologically, erythrocytosis is defined by an erythrocyte mass that exceeds 125% of that predicted for sex and body mass [22]. This is the most common dose-limiting adverse effect of TTh [10, 12, 21, 23]. Much of the concern surrounding increases in blood viscosity resulting from increased red blood cell mass centers on the potential increased risk for venous thromboembolism (VTE), myocardial infarction (MI), and cerebrovascular accidents (CVA) [24]. However, little evidence supports an increased risk of these negative sequelae in men on TTh [25]. Here, we review the literature examining testosterone-induced erythrocytosis and summarize proposed mechanisms and risks of thromboembolic sequelae.
Hypogonadism and Testosterone Therapy
Testosterone levels decrease by 1–2% per year after age 35, correlating to a decrease of 110 ng/dL per decade of life [26, 27]. These age-related decreases in testosterone are often attributed to a combination of decreasing gonadotropin levels and testicular hypofunction. Most professional society guidelines recommend treatment for testosterone levels <300 ng/dL in men with concomitant hypogonadal symptoms [8, 28–30].
The primary treatment for hypogonadism is TTh, which can improve insulin resistance, increase bone and muscle mass, decrease subcutaneous fat, lower low-density lipoprotein (LDL) cholesterol, triglycerides, blood glucose, HbA1c, and blood pressure, increase high-density lipoprotein (HDL) cholesterol, and improve erectile function and life parameters (i.e., increased energy, friendliness, decreased anger, anxiety, etc.) [31, 32]. While improvement in physiologic parameters is enough to warrant therapy, it is the improvement in physical and mental symptoms that drives the patient satisfaction. Kovac et al. longitudinally evaluated patient satisfaction of men treated with different formulations of TTh (52.5% injection, 30.6% gels, 16.9% pellets) with overall satisfaction rates ranging from 62.8% with <6 months of therapy to over 79% between 25 and 36 months [33].
Presently, numerous testosterone formulations are available, including short and long acting injections, topical gels and creams, transdermal and buccal patches, and implantable pellets. As early as the 1940s, subcutaneous testosterone pellets were available. Relatively short acting intramuscular (IM) injections, such as testosterone enanthate (TE), an ester metabolized over 4 to 5 days, and testosterone cypianote (TC), a longer acting testosterone metabolized over 7 to 8 days, were introduced in the 1950s. Oral testosterone undecanoate (TU) was developed in the 1970s, though this formulation is not currently approved for use in the United States. Transdermal patches were developed in the 1990s, and soon after, topical gels, buccal patches, and extended release IM formulations (TU) became available [34, 35]. While all testosterone formulations are effective, each formulation’s unique adverse effect profile is determined by dosage, pharmacokinetics, and method of administration [33, 36].
Testosterone-Induced Erythrocytosis
Polycythemia and erythrocytosis are used interchangeably to refer to an abnormal elevation of hemoglobin (Hb) or hematocrit (Hct). Whereas stimulation of erythropoiesis is therapeutic in the treatment of anemias, an unclear understanding of the thromboembolic potential of testosterone-induced elevations in Hb and Hct necessitates vigilant screening. In the clinical setting, erythrocytosis generally translates to Hb > 18.5g/dl or Hct > 52% in males, though this definition varies. The Endocrine Society uses a Hct > 50% as a relative contraindication to initiation of testosterone therapy, and Hct > 54% as a reason to stop therapy[4]. Other professional societies utilize Hct ranging from 52–55% as thresholds to modify or discontinue TTh [37]. When obtaining laboratory studies on patient, we recommend utilizing a single laboratory to longitudinally track results for each patient. Such an approach provides consistency and reproducibility of results. Physiologic erythrocytosis can be subdivided into primary and secondary subtypes, with primary erythrocytosis arising from a bone marrow-mediated proliferation of erythrogenic precursors, and secondary erythrocytosis resulting from an external alteration to which erythroid hyperplasia is a compensatory response. This compensatory response may be physiologically appropriate (i.e. compensation for hypoxia), or inappropriate (i.e. secondary to TRT) [38].
The erythrogenic effect of testosterone has been well established since prior to the recent surge in prescribing [23, 39]. Increased Hct is associated with increased blood viscosity, reduced venous return and increased platelet adhesiveness [40–42]. Clinical and academic interest currently resides with persistently elevated Hb and Hct and the potential increased risk for thromboembolic events and ischemic sequelae due to blood hyperviscosity, particularly in the setting of TTh induced erythrocytosis [43–45].
Erythrocytosis and Thromboembolic Risk
Several studies have attempted to evaluate the relationship between erythrocytosis and endothelial dysfunction. In 1978, Pearson and Wetherley-Mein observed a positive correlation between packed red cell volume and vascular venocclusive episodes [45]. Though not testosterone-induced, an increased thromboembolic risk from elevated Hct was demonstrated. In 2010, Braekkan et. al., in a large, prospective, population-based study found that a 5% increase in Hct in men resulted in an increased risk of VTE (HR 1.46 (95% CI: 1.15–1.84)); this relationship remained significant in the multivariable model adjusted for age, smoking, and BMI (HR 1.33, 95% CI: 1.05–1.70) [24]. Unfortunately, smoking was assessed as a dichotomous variable and the authors acknowledged this as a limitation, as they were unable to take into account the potential dose-dependent effect. Also, there were limited data on underlying medical diseases which may have acted as cofounding variables. In a 2013 study, Marchioli et al. randomized 365 adults (62% males) with polycythemia vera to a more intensive (target Hct <45%) or less intensive (target Hct 45–50%) treatment group with primary endpoint of time until death from cardiovascular causes or major thromboembolic event. After 31 months of follow up, the less intensive treatment group experienced significantly more events (HR 3.91; 95% CI: 1.45–10.53; p=0.007) than the more intensive treatment group [46].
Conversely, numerous studies have conflicting results, observing no increased risk of thromboembolism with persistent Hct elevations. Tsai et al. used prospective data from the Atherosclerosis Risk in Communities Study and the Cardiovascular Health Study and assessed cardiovascular risk factors and VTE incidence [47]. Among the variables investigated, an elevated Hct did not correlate with increased risk (hazard ratio: 1.03, 95% CI: 0.69–1.53). Unfortunately, the upper Hct threshold was >43.5%, which included many subjects with normal Hct levels. It is unclear whether an increased risk would have been observed in subjects with hematocrits above a higher threshold value. Shibata et al. investigated the relationship between thromboembolic complications and erythrocytosis (Hct >85%) in a transgenic mouse model, observing no evidence of thromboembolic complications [48]. The authors hypothesized that reduced clot strength and slowed clot formation kinetics, possibly secondary to high erythrocyte concentration, resulted in mechanical interference of platelet and fibrin interactions with the endothelium.
Testosterone-Induced Erythrocytosis Risk
The above studies highlight a potential link between blood hyperviscosity and thromboembolic complications. However, to date, no randomized or prospective studies have observed a direct relationship between TTh-induced erythrocytosis and thromboembolic events. One small retrospective study by Krauss et al. examined a 15-subject cohort of men receiving short acting IM testosterone enanthate every 3 weeks, and observed a correlation between elevated Hct and transient ischemic attacks when the men were separated into groups with a mean Hct of greater or less than 48% [44]. However, the small sample size precluded the ability to draw significant conclusions. Other studies that directly assess TTh-induced erythrocytosis and cardiovascular risk are not available. Widely debated studies by Basaria et al., Vigen et al., and Finkle et al. have observed an increased cardiovascular risk related to TTh, but these studies do not specifically correlate cardiovascular events to T-induced erythrocytosis, irrespective of their methodological flaws [16, 17, 19]. More recently, in a meta-analysis of all randomized controlled trials related to TTh and CV risk, Corona et al. concluded that the available evidence “does not support a causal role between testosterone supplementation and adverse CV events when hypogonadism is properly diagnosed and replacement therapy correctly performed” [49]. With regard to the risk of cardiovascular events, despite the lack of evidence, the FDA has mandated that testosterone manufacturers add a warning to testosterone labels indicating “a possible increased risk of heart attacks and strokes in patients taking testosterone” [9].
Pathophysiology of Testosterone-Induced Erythrocytosis
Multiple explanations for the mechanism of testosterone-induced erythrocytosis have been offered, with the proposed mechanisms outlined in a 2015 review by Jones et al. [50]. Initial hypotheses posited increased production of erythropoietin (EPO) by the kidneys, and subsequent studies suggested direct stimulation of erythroid progenitor cells; however, more recent studies in humans have not supported this mechanism [11, 23, 51–55]. In a study by Maggio et al., 108 men >65 years old with T <475 ng/dl were randomized to 36 months of testosterone patch vs. placebo in a double-blind fashion. Of these, 67 men (43 treatment, 24 placebo) ultimately had serum available for testosterone, hemoglobin and EPO assays using samples from before and after treatment [53]. Mean testosterone and hemoglobin levels increased significantly in the treatment group, but no significant changes in EPO were observed between the treatment or placebo groups (Treatment-by-time: β = −0.24, SE = 2.16, p = 0.9).
Bachman et al. proposed a mechanism of testosterone-induced erythrocytosis focused on the suppression of hepcidin, the master iron regulatory peptide, which subsequently results in increased iron absorption, increased systemic iron transport, and erythropoiesis [56]. Graded doses of testosterone were used to assess dose-dependent changes in hepcidin levels during 20 weeks of treatment, with findings that testosterone potently suppressed hepcidin in a dose-dependent manner. This study was followed by further work by Bachman that hypothesized a multifactorial model suggesting that “testosterone stimulates EPO transiently, along with suppression of hepcidin, and these two mechanisms result in a new EPO “set point” at a higher physiologic level of hemoglobin” [57]. In this study, 166 subjects from the randomized, double-blind, placebo controlled Testosterone in Older Men with Mobility Limitation Trial who had undergone at least 6 months of study intervention were examined. The subjects were >65 years old with limited mobility and total testosterone levels 100–350 ng/dl with no contraindications to therapy. Subjects were randomized to placebo or 10g testosterone gel. Serum hepcidin and EPO were both measured in conjunction with the study design, and assessed at baseline, 1, 3 and 6 months after randomization. EPO levels increased 58% from baseline at 1 month of testosterone treatment and remained significantly elevated at 3 months. EPO levels then trended toward baseline at 6 months. No significant changes in EPO level were observed in the placebo cohort. The authors further noted that there was a shift in the EPO-hemoglobin relationship curve that suggested “testosterone administration had reset the ‘set point’ for EPO in relation to hemoglobin”, these findings being based upon EPO levels remaining elevated following an increase in Hb, thus suggesting a lack of negative feedback. Furthermore, testosterone was associated with a 49% suppression of hepcidin, supporting the findings of the authors’ prior study. Suppression persisted at 1 and 3 months, but return to baseline at 6 months. Serum soluble transferrin receptor (sTR) concentration reflects erythroid activation and signifies plasma iron turnover and erythroid transferrin uptake. The authors further noticed increased sTR levels in the testosterone treatment group but not the placebo group. The observed hematologic changes suggest that testosterone increases iron utilization for erythropoiesis, hypothesizing a mechanism for testosterone induced increases in hematocrit.
In contrast to the above studies, which focused on testosterone, Calado et al. focused on estradiol as a causative factor for erythrocytosis, basing their hypothesis on known stimulation of hematopoietic cells by sex hormones [58]. Estradiol is produced by aromatization of testosterone. Calado et al. observed that in vitro exposure of peripheral blood lymphocytes and bone marrow to androgens increased the activity of telomerase, an enzyme involved in cell replication. Mutated cells with low telomerase activity exhibited normal telomerase levels upon exposure to androgens, and estradiol treatment resulted in similar effects on restoration of telomerase activity. Downregulation of the estrogen receptor-α (ERα), but not ERβ, inhibited telomerase function, thus isolating the target for estradiol mediated telomerase expression, which may lead to increased hematopoetic cell proliferation.
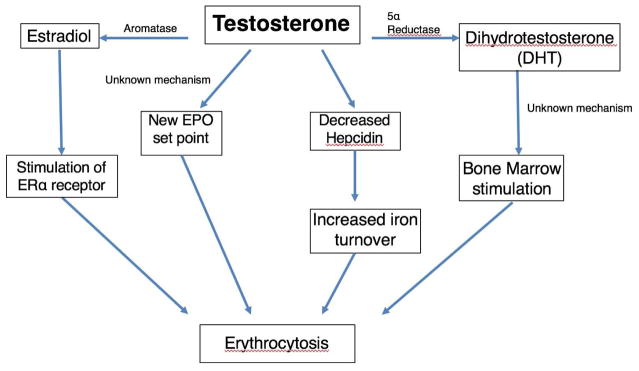
Other studies have correlated dihydrotestosterone (DHT) with increased hematocrit, independent of testosterone and free T levels, implicating DHT in testosterone-induced erythrocytosis [59–61]. Several randomized control trials have attempted to further elucidate a relationship between DHT and testosterone-induced erythrocytosis by examining whether patients on 5α-reductase inhibitors and TTh were less likely to develop erythrocytosis [62]. While one study showed a 4.7% increase in Hct, another showed no difference in post-treatment Hct [63–65]. However, all of these studies suggested indirect effects of testosterone levels on bone marrow hyperplasia without describing a clear mechanism. Figure 1 illustrates the proposed direct and indirect effects of testosterone on erythropoiesis.
Figure 1.
Impact of testosterone on erythropoiesis
A proposed genetic correlation between TTh and elevations in Hb and Hct was investigated by Zitzmann et al. who showed that the erythropoetic response to testosterone is inversely related to androgen receptor (AR) CAG repeats, which have been associated with AR activity [66]. The authors observed that men with <20 CAG repeats had the highest incidence of blood hyperviscosity.
Effects of Testosterone Formulation
Of the available testosterone formulations, short acting IM injections (T cypionate and enanthate) have the highest incidence of erythrocytosis, approaching 40% [14]. Recent studies support a unified hypothesis in which testosterone formulation, dose, and pharmacokinetics collectively determine the risk of erythrocytosis by establishing the duration of supraphysiological testosterone levels [52]. Testosterone formulations that result in stable serum concentrations (pellets, transdermal gels and patches, and extended release IM TU) result in a low incidence of erythrocytosis that is dependent on dose and serum level, and independent of duration of therapy [11, 52, 67]. The relationship of individual T formulations and associated effects on average T levels and incidence of erythrocytosis is summarized in Table 1.
Table 1.
Testosterone formulations and their impact on erythrocytosis
| Testosterone Formulation | Dosing Regimen | (N) | Rate of Erythrocytosis (>50%) | Mean. T level (ng/dL) (converted units) | Reference |
|---|---|---|---|---|---|
| T enanthate and cypionate (short acting IM) | 100–200 mg IM qW | 57 | 66.7% | 306 ± 164 | 70 |
| T undecanoate (extended release IM) | 1g q6 wks x3, then 1g q9 wks | 347 | 7% | 467 ± 32 | 78 |
| Transdermal (Testim® gel, Androgel® 1.62%) | 50–100 mg T, one to two packets applied to the shoulder area daily (Testim® gel), 20.25–80.1 mg T, two to four pumps applied to the skin daily (Androgel®) | 47 | 12.8% | 300 ± 89 | 70 |
| Oral testosterone undecanoate | N/A | 1343 | 0.003% (Hct>52%) | N/A | 77 |
| Testosterone pellets | 75 mg crystalline T/pellet implanted with 10–14 pellets q 3–6 months | 74 | 35.1% | 268 ± 167 | 70 |
In patients treated using subcutaneous testosterone pellets for an average of 8 years, increasing trough testosterone levels linearly correlated with increases in Hb and Hct [52]. Pharmacokinetic studies in patients on transdermal TTh demonstrate that Hb and Hct levels increase for first 5–6 months of therapy and then plateau [68, 69]. Discontinuation of TTh results in a return to baseline Hb and Hct levels in 3–12 months [57, 69]. In their comparison of the effects of testosterone on hematocrit, Pastuszak et. al found that hematocrits exceeded the study threshold of > 50% significantly earlier with injectable testosterone when compared with both gels and pellets (10.5±9.1 months vs. 14.0±12.6 months vs. 16.4±10.7 months, respectively, p=0.01) [70]. Wang et al. found a direct relationship between testosterone dose and the rate of erythrocytosis, which increased from 11.3% to 17.9% when increasing testosterone gel dose from 50 to 100 mg/day [71].
Short acting intramuscular testosterone formulations (T cypionate and enanthate) are associated with the most rapid and significant increases in serum testosterone levels, with supraphysiological testosterone levels achieved within days of an injection and a return to baseline by 10–14 days, followed by a decrease to subphysiological levels within 3 weeks if not re-dosed [14, 72]. In contrast, other testosterone formulations result in more stable serum testosterone levels, with extended release injectable TU maintaining stable serum levels within the normal range for approximately 12 weeks, and transdermal options maintaining stable levels with daily dosing [32, 73, 74]. For subcutaneous pellets, total testosterone levels peak within 2–4 weeks after implantation [75].
While initially described by Dobs et al., several subsequent studies have examined the higher incidence of erythrocytosis associated with short acting injectable testosterone, particularly T cypionate and enanthate over transdermal formulations [14, 76]. Dobs et al. evaluated 58 hypogonadal men randomly assigned to either transdermal or IM TTh 4–6 weeks after IM TTh was stopped [76]. Both therapies proved effective, though IM administration was associated with 43.8% of patients developing erythrocytosis (Hct>52%) compared to 15.4% of the transdermal cohort (p=0.025). Dobs et al.’s findings were the basis of the 2004 review by Rhoden et al. which addressed risks of TTh and recommendations for monitoring [14]. Pastuszak et al. expanded the comparison to include subcutaneous testosterone pellets (75 mg crystalline T/pellet implanted with 10–14 pellets to achieve a peak serum T level of 500–800 ng/dL every 3–6 months) [70]. The authors found that erythrocytosis, defined as a Hct > 50%, was more common with injectable (100–200 mg of T cypionate or enanthate intramuscularly weekly) than with transdermal (Testim® 50–100 mg T, one to two packets applied to the shoulder area daily, Androgel® 1.62% 20.25–80.1 mg T, two to four pumps applied to the skin daily) or pellet formulations (66.7% vs. 12.8% vs. 35.1%, respectively; p<0.0001) [70]. Though not approved for use in the U.S., oral TU was compared to short acting IM testosterone cypionate and enanthate injections in a study of 5,813 men included in the polycythemia cohort of United Kingdom General Practice Research Database [77]. The study used a hematocrit of >52% to define erythrocytosis, and found 3.4% of men on IM injections and 0.003% of men on oral testosterone therapy developed erythrocytosis. However, the authors noted inconsistent recording of hematocrits during the study period, likely resulting in an underestimate of the true incidence of erythrocytosis.
In a prospective observational study by Middleton et al., the adverse effects of extended release TU, including secondary polycythemia, were examined [78]. 347 patients received a total of 3,022 TU injections over 3.5 years, with 25 patients (7%) developing a Hct>50%, and 14 patients (4%) a Hct>52%. The study showed a contrast in the rates of erythrocytosis between short acting IM formulations, such as T cypionate and T enanthate (up to 40%), and extended release IM T formulation, such as TU (up to 7%)[78]. Previous pharmacokinetic studies comparing TU (1000 mg every 6 weeks, followed by 1000 mg every 9 weeks) and T enanthate (250 mg every 3 weeks) also showed higher, stable trough T concentration for TU at the time of injections when compared to T enanthate (14.9 ± 5.2 to 16.5 ±8.0 nmol/L for TU vs <10 nmol/L for T enanthate) [73].
Clinical Recommendations
Testosterone dosing should generally follow manufacturer recommendations. For at risk populations (type 2 diabetics, smokers, obese men), injectable testosterone formulations should be considered only after potential adverse hematological responses are discussed with the patient. Additional factors for selecting testosterone formulations in consideration of hematologic effects include age, which is an independent risk factor for erythrocytosis in the setting of TTh [11]. Additional risk factors to consider prior to initiation of TTh include thrombophilias such as Factor V Leiden, antiphospholipid antibody syndrome (APLAS), and prothrombin gene mutations, high Factor VIII levels, and high homocysteine levels. Transdermal, or subcutaneous formulations should be strongly considered in at risk populations in order to minimize significant alterations in Hb and Hct.
For patients who meet criteria for and desire TTh, a baseline Hb and Hct should be assessed. After initiation of therapy, the Sexual Medicine Society of North America advises that men should be “monitored regularly” for erythrocytosis [5]. In our practice, that consists of laboratory evaluation of Hb and Hct levels every 3–6 months. Consensus ISA, ISSAM, EAU, European Academy of Andrology, and American Society of Andrology guidelines advise that Hb and Hct should be checked after 3–4 months, then after 1 year, and annually thereafter [37]. Based on Endocrine Society Clinical Practice Guidelines, once a Hct > 54% is reached, TTh should either be discontinued, or therapeutic phlebotomy offered to reduce the risk of potential future thromboembolic events [4]. In our practice, therapeutic phlebotomy is recommended at a Hct of ≥50%. In the event of a thromboembolic episode for patients on TTh, we recommend discontinuing TTh and beginning anticoagulation per recommended guidelines.
Considerations for Future Research
A complete understanding of the molecular mechanisms of testosterone-induced erythrocytosis is essential to prevention and treatment of this common and significant adverse effect of TTh. Furthermore, the clinical implications of testosterone-induced erythrocytosis must be further elucidated to identify any actual risks associated with this condition. Alternative options for management of hypogonadal men, such as clomiphene citrate, human chorionic gonadotropin or aromatase inhibitors, may represent treatment options that can provide symptomatic benefit with rare supraphysiological T levels and low rates of erythrocytosis, though these therapies need further study in this context. Finally, randomized controlled trials are still needed in order to rigorously determine the effects of TTh on erythrocytosis, and the potential thromboembolic sequela that may result.
Conclusions
Erythrocytosis is often a limiting variable in patients on TTh. Direct and indirect effects related to supraphysiologic T levels are thought to mediate the effects on erythrocytosis. The true mechanism of erythrocytosis and its role on thromboembolic events remains unclear, though few data support an increased risk of CV events resulting from testosterone-induced erythrocytosis. Large multicenter randomized controlled trials are required to study TTh, its effects on Hb and Hct, and the clinical significance of treatment induced elevations in red blood cell mass.
Acknowledgments
AWP is a K12 scholar supported by a Male Reproductive Health Research (MRHR) Career Development Physician-Scientist Award (Grant # HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Program
Footnotes
Conflict of Interest Statement
Dr. Pastuszak is an advisory board member and consultant for Endo Pharmaceuticals Dr. Ohlander and Mr. Varghese report no conflicts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baillargeon J, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465–6. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao PK, et al. Trends in Testosterone Replacement Therapy Use Among Reproductive-Age US Men, 2003–2013. J Urol. 2016 doi: 10.1016/j.juro.2016.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AUA. AUA Position Statement on Testosterone Therapy 2014. 2015 Aug; [cited 2016 December 01]; Available from: http://www.auanet.org/education/testosterone-therapy.cfm.
- 4.Bhasin S, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 5.SMSNA. Consensus Statement and White Paper1 Executive Summary: Adult Onset Hypogonadism (AOH) 2015 [cited 2016 November 13]; Available from: http://www.smsna.org/V1/about/position-statements.
- 6.Mulligan T, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762–9. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corona G, et al. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS) J Sex Med. 2010;7(4 Pt 1):1362–80. doi: 10.1111/j.1743-6109.2009.01601.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu FC, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–35. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 9.FDA. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. 2015 doi: 10.1016/j.juro.2015.06.058. [cited 2016 November 13]; Available from: http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm. [DOI] [PubMed]
- 10.Calof OM, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451–7. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 11.Coviello AD, et al. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab. 2008;93(3):914–9. doi: 10.1210/jc.2007-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Balsells MM, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95(6):2560–75. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 13.Haddad RM, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350(5):482–92. doi: 10.1056/NEJMra022251. [DOI] [PubMed] [Google Scholar]
- 15.Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int J Endocrinol. 2012;2012:625434. doi: 10.1155/2012/625434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basaria S, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkle WD, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muraleedharan V, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725–33. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 19.Vigen R, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–36. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Moon du G, Park JK. Effect of testosterone undecanoate on hematological profiles, blood lipid and viscosity and plasma testosterone level in castrated rabbits. Can Urol Assoc J. 2013;7(3–4):E221–5. doi: 10.5489/cuaj.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahidi NT. Androgens and erythropoiesis. N Engl J Med. 1973;289(2):72–80. doi: 10.1056/NEJM197307122890205. [DOI] [PubMed] [Google Scholar]
- 22.Keohane C, McMullin MF, Harrison C. The diagnosis and management of erythrocytosis. BMJ. 2013;347:f6667. doi: 10.1136/bmj.f6667. [DOI] [PubMed] [Google Scholar]
- 23.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75(4):1092–8. doi: 10.1210/jcem.75.4.1400877. [DOI] [PubMed] [Google Scholar]
- 24.Braekkan SK, et al. Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica. 2010;95(2):270–5. doi: 10.3324/haematol.2009.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreijer AJ, Reitsma PH, Cannegieter SC. High hematocrit as a risk factor for venous thrombosis. Cause or innocent bystander? Haematologica. 2010;95(2):182–4. doi: 10.3324/haematol.2009.017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harman SM, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 27.Morley JE, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–3. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 28.Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84(2):573–7. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 29.Blute M, et al. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108–22. doi: 10.1159/000176048. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57(2):M76–99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- 31.Okada K, et al. Comprehensive evaluation of androgen replacement therapy in aging Japanese men with late-onset hypogonadism. Aging Male. 2014;17(2):72–5. doi: 10.3109/13685538.2014.888052. [DOI] [PubMed] [Google Scholar]
- 32.Yassin DJ, et al. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11(6):1567–76. doi: 10.1111/jsm.12523. [DOI] [PubMed] [Google Scholar]
- 33.Kovac JR, et al. Patient satisfaction with testosterone replacement therapies: the reasons behind the choices. J Sex Med. 2014;11(2):553–62. doi: 10.1111/jsm.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meikle AW, et al. Enhanced transdermal delivery of testosterone across nonscrotal skin produces physiological concentrations of testosterone and its metabolites in hypogonadal men. J Clin Endocrinol Metab. 1992;74(3):623–8. doi: 10.1210/jcem.74.3.1740497. [DOI] [PubMed] [Google Scholar]
- 35.Nieschlag E. VII. If Testosterone, Which Testosterone? Which Androgen Regimen Should be Used for Supplementation in Older Men? Formulatin, Dosing, and Monitoring Issues. The Journal of Clinical Endocrinology and Metabolism. 1998;83(10):3443–3445. doi: 10.1210/jcem.83.10.5060-1. [DOI] [PubMed] [Google Scholar]
- 36.Tenover JL. The androgen-deficient aging male: current treatment options. Rev Urol. 2003;5(Suppl 1):S22–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159(5):507–14. doi: 10.1530/EJE-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golde DW, et al. Polycythemia: mechanisms and management. Ann Intern Med. 1981;95(1):71–87. doi: 10.7326/0003-4819-95-1-71. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy BJ. Stimulation of erythropoiesis by androgenic hormones. Ann Intern Med. 1962;57:917–36. doi: 10.7326/0003-4819-57-6-917. [DOI] [PubMed] [Google Scholar]
- 40.Guyton AC, Richardson TQ. Effect of hematocrit on venous return. Circ Res. 1961;9:157–64. doi: 10.1161/01.res.9.1.157. [DOI] [PubMed] [Google Scholar]
- 41.Hellem AJ, Borchgrevink CF, Ames SB. The role of red cells in haemostasis: the relation between haematocrit, bleeding time and platelet adhesiveness. Br J Haematol. 1961;7:42–50. doi: 10.1111/j.1365-2141.1961.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 42.Wells RE, Jr, Merrill EW. Influence of flow properties of blood upon viscosity-hematocrit relationships. J Clin Invest. 1962;41:1591–8. doi: 10.1172/JCI104617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin YZ, et al. Relationship Between Hematocrit Level and Cardiovascular Risk Factors in a Community-Based Population. J Clin Lab Anal. 2015;29(4):289–93. doi: 10.1002/jcla.21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krauss DJ, et al. Risks of blood volume changes in hypogonadal men treated with testosterone enanthate for erectile impotence. J Urol. 1991;146(6):1566–70. doi: 10.1016/s0022-5347(17)38168-5. [DOI] [PubMed] [Google Scholar]
- 45.Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet. 1978;2(8102):1219–22. doi: 10.1016/s0140-6736(78)92098-6. [DOI] [PubMed] [Google Scholar]
- 46.Marchioli R, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22–33. doi: 10.1056/NEJMoa1208500. [DOI] [PubMed] [Google Scholar]
- 47.Tsai AW, et al. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162(10):1182–9. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 48.Shibata J, et al. Hemostasis and coagulation at a hematocrit level of 0.85: functional consequences of erythrocytosis. Blood. 2003;101(11):4416–22. doi: 10.1182/blood-2002-09-2814. [DOI] [PubMed] [Google Scholar]
- 49.Corona G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13(10):1327–51. doi: 10.1517/14740338.2014.950653. [DOI] [PubMed] [Google Scholar]
- 50.Jones SD, Jr, et al. Erythrocytosis and Polycythemia Secondary to Testosterone Replacement Therapy in the Aging Male. Sex Med Rev. 2015;3(2):101–112. doi: 10.1002/smrj.43. [DOI] [PubMed] [Google Scholar]
- 51.Mirand EA, Gordon AS, Wenig J. Mechanism of testosterone action in erythropoiesis. Nature. 1965;206(981):270–2. doi: 10.1038/206270a0. [DOI] [PubMed] [Google Scholar]
- 52.Ip FF, et al. Trough serum testosterone predicts the development of polycythemia in hypogonadal men treated for up to 21 years with subcutaneous testosterone pellets. Eur J Endocrinol. 2010;162(2):385–90. doi: 10.1530/EJE-09-0717. [DOI] [PubMed] [Google Scholar]
- 53.Maggio M, et al. Is the haematopoietic effect of testosterone mediated by erythropoietin? The results of a clinical trial in older men. Andrology. 2013;1(1):24–8. doi: 10.1111/j.2047-2927.2012.00009.x. [DOI] [PubMed] [Google Scholar]
- 54.Rishpon-Meyerstein N, et al. The effect of testosterone on erythropoietin levels in anemic patients. Blood. 1968;31(4):453–60. [PubMed] [Google Scholar]
- 55.Palacios A, et al. Effect of testosterone enanthate on hematopoiesis in normal men. Fertil Steril. 1983;40(1):100–4. doi: 10.1016/s0015-0282(16)47185-2. [DOI] [PubMed] [Google Scholar]
- 56.Bachman E, et al. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95(10):4743–7. doi: 10.1210/jc.2010-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bachman E, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci. 2014;69(6):725–35. doi: 10.1093/gerona/glt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calado RT, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114(11):2236–43. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Idan A, et al. Long-term effects of dihydrotestosterone treatment on prostate growth in healthy, middle-aged men without prostate disease: a randomized, placebo-controlled trial. Ann Intern Med. 2010;153(10):621–32. doi: 10.7326/0003-4819-153-10-201011160-00004. [DOI] [PubMed] [Google Scholar]
- 60.Kunelius P, et al. The effects of transdermal dihydrotestosterone in the aging male: a prospective, randomized, double blind study. J Clin Endocrinol Metab. 2002;87(4):1467–72. doi: 10.1210/jcem.87.4.8138. [DOI] [PubMed] [Google Scholar]
- 61.Ly LP, et al. A double-blind, placebo-controlled, randomized clinical trial of transdermal dihydrotestosterone gel on muscular strength, mobility, and quality of life in older men with partial androgen deficiency. J Clin Endocrinol Metab. 2001;86(9):4078–88. doi: 10.1210/jcem.86.9.7821. [DOI] [PubMed] [Google Scholar]
- 62.Aghazadeh M, et al. Elevated Dihydrotestosterone is Associated with Testosterone Induced Erythrocytosis. J Urol. 2015;194(1):160–5. doi: 10.1016/j.juro.2015.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amory JK, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89(2):503–10. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 64.Gruntmanis U. The role of 5alpha-reductase inhibition in men receiving testosterone replacement therapy. JAMA. 2012;307(9):968–70. doi: 10.1001/jama.2012.259. [DOI] [PubMed] [Google Scholar]
- 65.Page ST, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90(3):1502–10. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 66.Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92(10):3844–53. doi: 10.1210/jc.2007-0620. [DOI] [PubMed] [Google Scholar]
- 67.Delev DP, et al. Effect of testosterone propionate on erythropoiesis after experimental orchiectomy. Folia Med (Plovdiv) 2013;55(2):51–7. doi: 10.2478/folmed-2013-0017. [DOI] [PubMed] [Google Scholar]
- 68.Swerdloff RS, Wang C. Three-year follow-up of androgen treatment in hypogonadal men: preliminary report with testosterone gel. Aging Male. 2003;6(3):207–11. [PubMed] [Google Scholar]
- 69.Wang C, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89(5):2085–98. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- 70.Pastuszak AW, et al. Comparison of the Effects of Testosterone Gels, Injections, and Pellets on Serum Hormones, Erythrocytosis, Lipids, and Prostate-Specific Antigen. Sex Med. 2015;3(3):165–73. doi: 10.1002/sm2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839–53. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- 72.Bhasin S, et al. Issues in testosterone replacement in older men. J Clin Endocrinol Metab. 1998;83(10):3435–48. doi: 10.1210/jcem.83.10.5060-1. [DOI] [PubMed] [Google Scholar]
- 73.Schubert M, et al. Intramuscular testosterone undecanoate: pharmacokinetic aspects of a novel testosterone formulation during long-term treatment of men with hypogonadism. J Clin Endocrinol Metab. 2004;89(11):5429–34. doi: 10.1210/jc.2004-0897. [DOI] [PubMed] [Google Scholar]
- 74.Swerdloff RS, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85(12):4500–10. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- 75.Pastuszak AW, et al. Pharmacokinetic evaluation and dosing of subcutaneous testosterone pellets. J Androl. 2012;33(5):927–37. doi: 10.2164/jandrol.111.016295. [DOI] [PubMed] [Google Scholar]
- 76.Dobs AS, et al. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84(10):3469–78. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 77.Jick SS, Hagberg KW. The risk of adverse outcomes in association with use of testosterone products: a cohort study using the UK-based general practice research database. Br J Clin Pharmacol. 2013;75(1):260–70. doi: 10.1111/j.1365-2125.2012.04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Middleton T, et al. Complications of injectable testosterone undecanoate in routine clinical practice. Eur J Endocrinol. 2015;172(5):511–7. doi: 10.1530/EJE-14-0891. [DOI] [PubMed] [Google Scholar]