Abstract
Mounting evidence suggests that therapeutic cell and drug delivery strategies designed to actively harness the regenerative potential of the inflammatory response have great potential in regenerative medicine. In particular, macrophages have emerged as a primary target because of their critical roles in regulating multiple phases of tissue repair through their unique ability to rapidly shift phenotypes. Herein, we review macrophage-based therapies, focusing on the translational potential for cell delivery of ex vivo-activated macrophages and delivery of molecules and biomaterials to modulate accumulation and phenotype of endogenous macrophages. We also review current obstacles to progress in translating basic findings to therapeutic applications, including the need for improved understanding of context-dependent macrophage functions and the myriad factors that regulate macrophage phenotype; potential species-specific differences (e.g. humans versus mice); quality control issues; and the lack of standardized procedures and nomenclature for characterizing macrophages. Looking forward, the inherent plasticity of macrophages represents a daunting challenge for harnessing these cells in regenerative medicine therapies but also great opportunity for improving patient outcomes in a variety of pathological conditions.
Graphical Abstract
1. Introduction
1.1 Overview
Tissue repair and regeneration following injury is critical for the survival of all organisms. In response to tissue damage, the integrated actions of diverse cell types and molecular pathways regulate overlapping phases of inflammation, tissue formation, and remodeling [1–3]. A properly regulated inflammatory response is important for efficient healing, not only for preventing infection and clearing necrotic tissue but also for regulating tissue formation and remodeling. Indeed, the pharmacologic depletion of immune cells causes impaired tissue healing and regeneration in experimental models of injury [4, 5], and dysregulation of immune cell activation results in encapsulation of biomaterials in fibrous tissue in the foreign body response [6–8].
In contrast, recent studies have indicated that harnessing the inflammatory response can be an effective strategy for improving tissue healing and regeneration. In particular, macrophages, the primary effector cells of the innate immune system, have emerged as a major target in regenerative medicine because of their critical roles in regulating all stages of tissue repair through their unique plasticity. The objectives of this review are to briefly describe the current state of knowledge of macrophage plasticity from the perspective of researchers in regenerative medicine, to highlight key therapeutic strategies to manipulate macrophage behavior, and to describe critical areas in which more research is needed in order to advance translation of macrophage-based therapies.
1.2 Diverse roles of macrophage phenotype in tissue repair and regeneration
During each phase of healing, macrophages accumulate in the damaged tissue and orchestrate diverse processes in tissue repair and regeneration. Recent studies using selective depletion of macrophages during repair of skin, liver, kidney, heart, skeletal muscle and other tissues have demonstrated multiple critical functions, including regulation of angiogenesis, granulation tissue formation and extracellular matrix assembly [4, 9–12]. In addition, dysregulated macrophage responses have been linked to the development of chronic skin ulcers, fibrosis, atherosclerosis, heart failure and impaired healing of various tissues [13–16].
The varied functions of macrophages during tissue regeneration are realized through the tremendous plasticity of these cells. Throughout the normal healing process, macrophages adopt phenotypes ranging from an aggressive pro-inflammatory or “M1” phenotype to a less inflammatory or “M2” phenotype that is associated with the resolution of inflammation and healing [16–20]. It is now generally accepted that a wide variety of stimuli can influence macrophage phenotype, resulting in practically infinite numbers of phenotypes and a spectrum of diverse behaviors, particularly in vivo [21, 22]. In addition, multiple phenotypes with an M2-like designation have been described, including M2a, M2b, M2c, and M2d, among others [23, 24], even though many of these subtypes may share very little phenotypic traits in common [7]. Nonetheless, the M1/M2 paradigm remains useful for describing the divergent effects of relatively pro-inflammatory and anti-inflammatory macrophages as they progress through the stages of tissue repair. It is important to note, however, that the functions of these multiple phenotypes are very context-dependent, and this oversimplification brings its own challenges (discussed in section 4); thus in this review we use the terms “M1-like” and “M2-like” to describe macrophage that exhibit features of each phenotype.
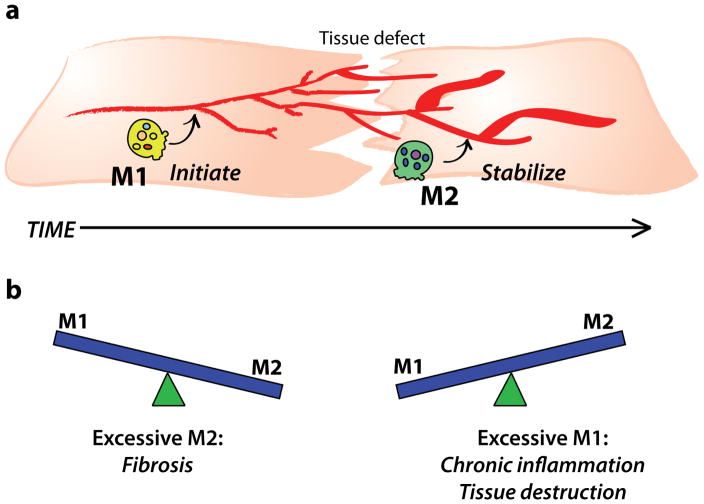
The precise roles of each macrophage phenotype in tissue repair processes are poorly understood, but studies suggest that the chronological appearance of M1-like and M2-like macrophages in tissue repair correspond with their sequential and complementary functions, in that M1-like macrophages initiate the healing process while M2-like macrophages promote stabilization and tissue maturation (Figure 1a). M1-like macrophages and pro-inflammatory cytokines have been shown to stimulate angiogenesis [7, 25, 26], while M2-like macrophages have been shown to stabilize angiogenesis, promote fibroblast proliferation and orchestrate extracellular matrix assembly [26–29]. For example, during skeletal muscle regeneration in mice, temporally controlled depletion of macrophages using CD11b-diptheria toxin receptor mice showed that depletion of M1-like macrophages at early stages of injury completely inhibited muscle regeneration, while depletion of M2-like macrophages at later stages of injury (5 days) reduced the diameter of regenerating fibers [18]. In mouse studies that utilized genetic deletion of interleukin-4 (IL-4), a primary M2-promoting cytokine, the reduction in M2-like macrophages led to fibrotic skin repair with deformed collagen fibrils and vascular structures [28], as well as reduced diameters and function of muscle fibers in response to normally regenerative biomaterials [30]. Collectively, these studies show that sequential activation of M1-like and M2-like macrophages is critical for optimal tissue repair.
Figure 1. Balance of macrophage phenotypes in tissue repair.
(a) M1-like and M2-like macrophages act sequentially in normal tissue repair to initiate and stabilize multiple repair processes, respectively. (b) An imbalance of either phenotype (in terms of either numbers or timing) impairs tissue healing.
Indeed, insufficient accumulation of either M1-like macrophages at early stages of tissue repair [31–33] or of M2-like macrophages at later stages [17, 32] both have been associated with ineffective healing characteristic of pathologic conditions like diabetic wounds. By the same token, excessive accumulation of either phenotype is also detrimental, with excessive M1 behavior leading to chronic inflammation and tissue destruction [13, 34] and excessive M2 behavior promoting fibrosis [35, 36] (Figure 1b). These studies highlight the importance of properly regulated transitions in macrophage phenotype for tissue regeneration.
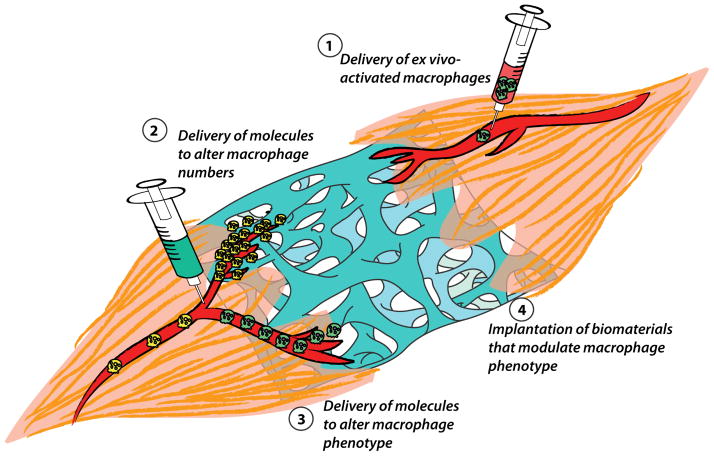
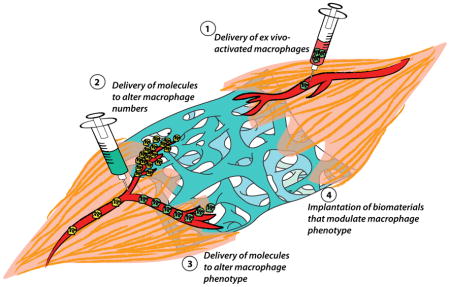
In the normal tissue repair process, macrophage phenotype is regulated at multiple stages during the lifespan of these cells. Immediately following injury, damage- and pathogen-associated molecular patterns and other pro-inflammatory molecules induce the pro-inflammatory M1-like phenotype [37–39]. At later stages, lipid mediators such as resolvins, anti-inflammatory cytokines and cellular processes such as efferocytosis (the phagocytosis of apoptotic cells) downregulate the M1-like phenotype and induce an M2-like phenotype [40–44]. Less well understood is the role of preprogramming of cells prior to the tissue repair response, which may include biasing of phenotype via differentiation of monocytes and/or epigenetic marking of progenitor cells that is passed down to daughter cells [45–48]. Although the precise mechanisms that dictate macrophage phenotype during tissue regeneration are likely context- and time-dependent, the pathways involved provide opportunity for manipulating macrophage phenotype at multiple stages to improve healing. Strategies under investigation to manipulate macrophage behavior in regenerative medicine can be grossly divided into four categories: those that administer exogenously activated macrophages, those that aim to increase or decrease macrophage numbers, those that employ local or systemic delivery of bioactive factors to alter the phenotype of the macrophages that accumulate following injury, and those that modulate macrophage phenotype through the design of immunomodulatory biomaterial implants (Figure 2; Table 1).
Figure 2.
Summary of strategies to manipulate macrophage numbers or phenotype in regenerative medicine.
Table 1.
Examples of strategies that target macrophages to improve tissue regeneration
| Delivery of ex vivo activated macrophages | ||
|---|---|---|
| Approach | Tissue | Outcome |
| Macrophages activated by hypo-osmotic shock (M1-like; local) | Skin | Accelerated healing of pressure ulcers in humans [50] |
| Macrophages activated by IL-4 or IL-10 (M2-like; local) | Skin | Impaired wound healing in mice [65] |
| Mixture of mesenchymal stromal cells and macrophages (M2-like; local) | Heart | Reduced cardiac events in humans with dilated cardiomyopathy [53] |
| Macrophages activated by IL-4+IL-13 or IL-10+TGF-b (M2-like) | Kidney | Protected against adriamycin-induced kidney injury in mice [60] |
| Macrophages activated by IFN-γ+ LPS or TNF-α (M1-like; local) | Skeletal muscle | Reduced fibrosis following traumatic or ischemic injury [63, 64] |
| Macrophages activated by LPS (M1-like; local) | Kidney | Increased fibrosis following adriamycin-induced kidney injury [62] |
| Delivery of molecules to alter macrophage numbers | ||
| Approach | Tissue | Outcome |
| Recombinant M-CSF (local) to increase macrophage accumulation | Skeletal muscle | Accelerated recovery from atrophy in mice [66] |
| Bone | Improved fracture repair in mice [67] | |
| Recombinant M-CSF (systemic) to increase macrophage accumulation | Kidney | Improved repair following ischemia in mice [68] |
| Spinal cord | Improved remyelination in mice [69] | |
| CCR2 siRNA in nanparticles to reduce monocyte infiltration | Heart | Improved repair following ischemia in mice [70] |
| MCP-1 RNA oligonucleotides reduce monocyte infiltration | Liver | Reduced steatohepatitis in mice [71] |
| Recombinant MCP-1 (local) to increase macrophage accumulation | Skin | Accelerated wound closure in mice [31] |
| CCR5 blocking antibody (systemic) to reduce macrophage accumulation | Spinal cord | Improved recovery after injury in mice [72] |
| Delivery of molecules to alter macrophage phenotype | ||
| Approach | Tissue | Outcome |
| Inflammasome inhibitors (local) to downregulate M1-like macrophages | Skin | Increased granulation, accelerated wound closure in mice [13, 77] |
| IL-17 blocking antibody (local) to downregulate M1-like macrophages | Skin | Accelerated wound closure in mice [79] |
| TNF-α blocking antibody (systemic) to downregulate M1-like macrophages | Skin | Accelerated wound closure in mice [80] |
| IRF-5 siRNA (systemic) to downregulate M1-like macrophages | Heart | Improved repair after ischemia in mice [81] |
| Recombinant IL-4 (systemic) to upregulate M2-like macrophages | Heart | Improved repair after ischemia in mice [29] |
| Recombinant IL-4 (local) to upregulate M2-like macrophages* | Skin | Accelerated wound closure in mice [82] |
| Recombinant IL-33 (local) to upregulate M2-like macrophages* | Skin | Accelerated wound closure in mice [84] |
| DHA derivatives (local) to upregulate M2-like macrophages | Skin | Accelerated wound closure in mice [85, 86] |
| PPAR-γ agonists (local) to upreguate M2-like macrophages | Skin | Accelerated wound closure in mice [42] |
| Design of biomaterials to alter macrophage phenotype | ||
| Approach | Tissue | Outcome |
| Vascular graft with human bone marrow cells and poly(glycolic acid) scaffold to increase macrophages | Blood vessel | Mouse macrophages replaced human cells and formed functional blood vessels in mice [93] |
| Vascular graft with MCP-1 microspheres and poly(glycolic acid) scaffold to increase macrophages | Blood vessel | Mouse macrophages infiltrated graft and formed functional blood vessels in mice [93] |
| S1P3 agonist in poly(lactic-co-glycolic acid) scaffold to increase M2-like Macrophages | Skeletal muscle | Increased arteriogenesis following ischemic injury [94] |
| IFN-γ in decellularized bone graft to increase M1-like macrophages | Subcutaneous | Increased vascularization in subcutaneous |
| Patterned biomaterials to increase M2-like polarization | Subcutaneous | Reduced fibrous capsule encapsulation [100] |
| Iron-oxide loaded phosphatidyl serine liposomes to increase M2-like macrophages and allow tracking | Heart | Reduced infarct size and increased tissue vascularization following ischemia [104] |
2. Macrophage-based therapies in regenerative medicine
2.1. Delivery of ex vivo-activated macrophages
The delivery of ex vivo-activated pro-inflammatory macrophages as a cell therapy for the treatment of cancers has been investigated extensively since the 1980s, although with limited success [49]. More recently, the delivery of macrophages activated with diverse phenotypes has been explored in regenerative medicine. For example, in a clinical trial of 100 patients with stage III–IV pressure ulcers, it was found that wound administration of allogeneic macrophages activated by hypo-osmotic shock promoted healing of chronic pressure ulcers to a greater extent than the standard of care [50]. The hypo-osmotic shock method likely induces a pro-inflammatory M1-like activation state, based on increased secretion of IL-1 and IL-6 by the macrophages in vitro [51]. Another macrophage-based cell therapy, which is under clinical investigation for the treatment of congestive heart failure and critical limb ischemia, is Ixmyelocel-T, a mixture of autologous macrophages and mesenchymal stem cells (MSCs) derived from a patient’s bone marrow mononuclear cells and expanded in culture over 14 days [52–54]. The expansion and co-culture process is believed to promote an M2-like phenotype in the macrophages, as demonstrated by expression of the cell surface markers CD206, CD163, and MerTK, and reduced secretion of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) even after stimulation with pro-inflammatory endotoxin [55]. In a phase 2B clinical trial of 125 patients treated for dilated cardiomyopathy (ischemic heart failure), transendocardial administration of ixmyelocel-T led to a 37% reduction in adverse cardiac events compared with the placebo group over 12 months of follow up [53]. By contrast, intramyocardial delivery of non-expanded autologous bone marrow mononuclear cells failed to show clinical improvement in a separate study of patients treated for ischemic cardiomypathy [56], although the two treatments have not been directly compared in the same study. Ixmyelocel-T also has not been compared directly to treatment with a pure population of MSCs, which is also believed to promote cardiac tissue repair through the actions of host macrophages [57, 58], so the contribution of exogenous macrophages to the beneficial effects of the MSC-macrophage mixture are not known. Finally, the mechanisms of action behind the therapeutic efficacy of ixmeylocel-T remain to be identified, although results from a rat hindlimb ischemia model and in vitro studies suggest that the cells in ixmyelocel-T promote angiogenesis [59].
In animal models, ex vivo-activated macrophages have shown potential for promoting repair of multiple tissue types. Lu et al. polarized splenocyte-derived macrophages to phenotypes denoted as M2a (stimulated with IL-4 and IL-13) or M2c (stimulated with IL-10 and TGF-β) and compared to resting (M0) macrophages [60]. A single tail vein injection of either M2a or M2c macrophages at 5 days after adriamycin-induced nephropathy in a murine model of chronic kidney disease protected against renal injury after 28 days, while unactivated macrophages had no effect. Interestingly, the protective effects were more pronounced for M2c macrophages than M2a macrophages unless the animals were depleted of regulatory T cells [60], suggesting a role for adaptive immune cell modulation in the mechanism of action [61] and highlighting the importance of discerning the differences between multiple distinct M2-like subsets of macrophages. In contrast to a study that showed increased fibrosis with infusion of M1-like macrophages in renal injury [62], other studies have reported decreased fibrosis with administration of M1-like macrophages in muscle injury [63, 64], demonstrating the need for tailoring macrophage-based therapies to their specific contexts. In addition, wound administration of murine macrophages that were activated to M2-like phenotypes ex vivo with IL-4 or IL-10 actually impaired skin wound healing in a diabetic mouse model compared to unactivated macrophages [65]. The authors attributed this result to inhibition of the important inflammatory phase at early times after injury, further suggesting that the proper M1-to-M2 sequence of macrophage activation is critical for successful wound healing. Thus, in order for macrophage-based cell therapies to be clinically viable, it will be critical to understand and control the time- and context-dependent effects of macrophage phenotypes on tissue repair.
2.2. Delivery of molecules to target macrophage accumulation
Instead of delivering exogenous macrophages, a number of studies have exerted control over the accumulation of endogenous macrophages via pharmacologic targeting of chemokines and receptors that regulate tissue infiltration. Local injection of macrophage colony stimulating factor (M-CSF or CSF-1) into the soleus muscle of mice after hindlimb suspension-induced atrophy increased macrophage accumulation and accelerated recovery of muscle fiber area and force production [66]. In addition, local treatment with M-CSF increased macrophage numbers and improved fracture healing of the mouse tibia [67]. Finally, systemic M-CSF has been reported to increase macrophage numbers and improve repair following ischemic kidney injury in mice [68] and promoted remyelination following spinal cord injury in mice [69]. All of these improvements were due at least in part to macrophage-dependent effects.
Monocyte chemotactic protein (MCP)-1 (CCL2) and its receptor CC-chemokine receptor (CCR)-2 also regulate recruitment of monocytes, which differentiate into macrophages as they infiltrate damaged tissue [25]. In hyper-inflammatory apolipoprotein E-deficient mice, monocyte-directed siRNA against CCR2 encapsulated in nanoparticles reduced inflammatory (Ly6Chi) monocytes and improved myocardial infarct healing [70]. In addition, systemic treatment with MCP-1-targeting L-enantiomeric RNA oligonucleotides reduced inflammatory monocyte infiltration and steatohepatitis following chemical- and diet-induced liver injury in mice [71]. Similarly, recovery from spinal cord injury in mice improved following treatment with a blocking antibody against another important monocyte chemokine receptor, CCR5, concomitant with reduced total macrophage numbers but increased proportion of those that expressed CD206, an M2 marker [72]. Interestingly, although wounds in diabetic mice exhibit prolonged inflammation associated with persistent elevations in MCP-1 and CCR2 [73], the very early stage of diabetic wound healing has been associated with reduced levels of this chemokine system as well as reduced levels of CD68+ cells [31]. Wound administration of MCP-1 immediately after injury rescued macrophage accumulation and accelerated wound closure in diabetic mice compared to non-diabetic mice [31]. This study suggests that recruiting endogenous macrophages may be advantageous even in pathological conditions that suffer from impaired macrophage infiltration. However, an important point to consider is that monocytes and macrophages may be less responsive to inflammatory stimuli in the elderly and in patients with immune disorders, suggesting that macrophage-recruiting strategies may be less effective in some patients [74, 75]. It also may be possible to induce macrophage proliferation at the target site as opposed to recruitment of additional macrophages [76], but this approach has not yet been sufficiently evaluated in regenerative medicine.
2.3. Delivery of molecules to target macrophage phenotype
As mentioned earlier, the appropriate phenotype dynamics of macrophages are arguably more important for proper tissue repair than overall numbers of macrophages. Thus, strategies to modulate macrophage phenotype have proven effective in regenerative medicine. Attempts to inhibit the inflammatory M1-like macrophage phenotype, including blocking activity of inflammatory cytokines and transcription factors involved in inducing this phenotype, have been investigated for the treatment of inflammatory tissue diseases. For example, local administration of an IL-1β blocking antibody [77] or inhibitors of the Nod-like receptor (NLRP)-3 inflammasome, which is required for activating IL-1β [13], downregulated the M1-like macrophage phenotype, allowed a switch to an M2-like phenotype, and improved healing in diabetic mice. Importantly, in these studies, treatments were started on day 3 after injury to allow the initial inflammatory response to proceed normally [78]. Similarly, local administration of a blocking antibody against IL-17 [79] or systemic treatment with neutralizing antibody against TNF-α [80] inhibited M1-like macrophage activity and accelerated wound closure in diabetic mice. Finally, nanoparticle delivery of siRNA against the transcription factor insulin response factor (IRF)-5, which has been associated with the M1-like phenotype, reduced expression of inflammatory genes in macrophages, reduced protease activity and promoted the resolution of inflammation after myocardial infarction in mice, with reduced subsequent left ventricular dilation [81].
In addition to the delivery of M1-inhibiting molecules, many studies have investigated the administration of M2-promoting molecules to enhance tissue repair. For example, intraperitoneal injection of IL-4 increased the number of M2-like (CD206+F4/80+) macrophages in cardiac tissue following myocardial infarction in mice, and was associated with increased fibroblast activation, deposition of supportive fibrous tissues, and improved prognosis [29]. Similarly, topical administration of IL-4 to excisional mouse wounds accelerated the rate of healing [82], although its effect on macrophages was not assessed and IL-4 could influence cells other than macrophages. In addition, systemic administration of IL-4 to rats early (to day 5) after ligament injury promoted healing but did not alter the number of CD163+ cells, a marker of M2-like activation, suggesting that IL-4 may promote ligament healing through mechanisms other than macrophage polarization, or that this single marker was insufficient to capture changes in M2 polarization [83]. Finally, IL-33, a member of the IL-1 family, was reported to increase the number of M2-like (CD206+F4/80+) macrophages in wound tissue and to accelerate wound closure in healthy mice [84].
Intradermal injection of lipid mediators derived from docosahexaenoic acid (DHA) including resolvins and protectins have been shown to induce an M2-like macrophage phenotype, promote resolution of inflammation, and accelerate wound healing in diabetic mice [85, 86]. Another anti-inflammatory lipid mediator, lipoxin-A4 has been shown to reduce inflammation and promote corneal wound healing in mice [87] and has been suggested as a potential anti-inflammatory therapy for tendon inflammation [88]. Peroxisome proliferator-activated receptor (PPAR)-γ is activated by lipid mediators and is known to promote an M2-like macrophage phenotype [89]. Importantly, PPAR-γ activity is impaired in wound macrophages of diabetic mice and humans, and topical application of PPAR-γ agonists induced an M2-like wound macrophage phenotype and improved healing in diabetic mice [42]. In these studies, the role of PPAR-γ was confirmed using myeloid cell specific knockout mice.
2.4. Design of biomaterials to manipulate macrophage phenotype
Biomaterials are often used as scaffolds to facilitate the growth of new tissues, and the role of macrophage phenotype in the success or failure of implanted biomaterials is increasingly apparent [90]. Thus, the design of biomaterials that actively modulate the accumulation and phenotype of infiltrating macrophages has emerged as a new strategy in regenerative medicine [91, 92]. For example, when tissue-engineered vascular grafts formed from human bone mononuclear cells and poly(glycolic acid) scaffolds were implanted into immunocompromised mice, murine macrophages replaced the human cells within the grafts, later repopulating them with murine endothelial cells and smooth muscle cells and transforming them into patent, functional blood vessels over 24 weeks [93]. Testing the hypothesis that the functionality of the implanted cells was through the actions of host macrophages, the authors replaced the human cells with hydrogel microspheres that released MCP1 over 3 days to recruit macrophages; these grafts were also populated by host macrophages and transformed into patent blood vessels that were identical in functionality to the cell-seeded scaffolds and far superior to control scaffolds without the macrophage-recruiting cytokine [93].
Following this landmark study, biomaterials have been designed to recruit certain phenotypes of host macrophages or that actively modulate the phenotype of recruited macrophages. For example, Awojoodu et al. [94] found that the release of the S1P3 receptor agonist FTY720 from poly(lactic-co-glycolic acid) (PLGA) scaffolds increased local recruitment of a monocyte subset that preferentially polarized into M2-like macrophages, as measured by CD206 expression, to the site of ischemic muscle injury in mice, resulting in increased arteriogenesis. Follow-up studies highlighted the utility of recruiting particular monocyte subsets in order to target M2-like macrophage phenotype [95–97].
Other studies have delivered macrophage-polarizing cytokines to directly modulate the phenotype of local macrophages. In a study of biomaterial vascularization for bone regeneration, the release of the pro-inflammatory cytokine interferon-γ (IFN-γ) from decellularized bone scaffolds caused M1 polarization of seeded human macrophages in vitro, as measured by gene expression and protein secretion of a variety of pro-inflammatory markers, and resulted in increased vascularization in a murine subcutaneous implantation model [98]. These two reports further support the theory that both M1 and M2 macrophages contribute to angiogenesis in different ways, so that strategies that promote transient increases in either phenotype may be tailored to enhance angiogenesis.
Physical properties of biomaterials themselves may also modulate macrophage phenotype, in the absence of encapsulated drugs or proteins. For example, McWhorter et al. [99] observed that murine bone marrow-derived M2-like macrophages activated in vitro by IL-4 and IL-13 display a more elongated morphology than M1-like macrophages activated by lipopolysaccharide (LPS) and IFN-γ. When macrophages were forced to adapt an elongated morphology by seeding on micropatterned substrates with adhesive lines, they increased expression of M2 markers Arg1, CD206, and Ym1 in the absence of polarizing cytokines. This effect extended to the in vivo environment, with patterned materials showing increased Arg1 expression and reduced fibrous encapsulation in a murine subcutaneous implantation model [100]. However, it should be noted that human macrophages activated with IL-4 have been reported to adopt a more circular morphology [101], so it is unclear if these findings will translate to clinical human applications.
Finally, the delivery of microparticles and nanoparticles represents a major opportunity to modulate macrophage behavior, because macrophages are professional phagocytic cells that readily take up these particles [102, 103]. Microparticles and nanoparticles also can be functionalized for use as contrast agents in imaging modalities, enabling a dual-functionality or theranostic approach. For example, Harel-Adar et al. [104] designed iron oxide-loaded liposomes decorated with phosphatidylserine (PS), a cell surface ligand displayed on apoptotic cells that triggers macrophage uptake and a subsequent phenotypic switch to an M2-like phenotype, enabling macrophage phenotype manipulation as well as imaging and tracking by magnetic resonance imaging. Upon phagocytosis of the PS-presenting liposomes, murine macrophages increased expression of the M2 marker CD206 and decreased expression of the M1 marker CD86 [104]. The macrophages also increased secretion of anti-inflammatory cytokines IL-10 and TGF-β. Following myocardial infarction, delivery of the PS-presenting liposomes caused a significant reduction in the infarct size and increased tissue vascularization compared to control liposomes, further highlighting the potential for macrophage-targeted therapies in regenerative medicine [104]. In addition, because macrophages can home to the site of injury, researchers are investigating the possibility of targeting drugs to the sites of injury by using macrophages as carriers, which has been reviewed in Ref. [49]. Such a strategy has been explored for the delivery of therapeutic agents across the blood brain barrier in mouse models of neuroAIDS [105] and Parkinson’s disease [106–109].
In summary, drug delivery and biomaterials design strategies that target macrophage accumulation and phenotype hold great potential in regenerative medicine. To achieve successful clinical translation of these strategies, several obstacles must be overcome, discussed below.
4. Unresolved questions: Obstacles and Opportunities
4.1. Context-dependent macrophage functions
Limited understanding of context-dependent macrophage functions represents the major obstacle to the translation of macrophage-based therapies. For example, M1-like macrophages impair regeneration in some situations [13, 77] and promote regeneration in others [33, 63]. The same is true for M2-like macrophages, which have been associated with both tissue regeneration [5, 110] and fibrosis [35, 36]. With respect to biomaterial-mediated tissue repair, both M1- and M2-like macrophages have been separately associated with either vascular integration or fibrous encapsulation [6, 7, 90, 111, 112]. We propose that these apparently conflicting reports are related to the timing of macrophage activation, with any aberrations from the natural M1-to-M2 sequence resulting in impaired healing, as discussed throughout this review.
Another potential reason for the apparently conflicting reports may be that there is no standard nomenclature for describing macrophage phenotype [113] (Table 2), especially for macrophages derived from the in vivo environment, potentially resulting in misidentification of very different phenotypes. For example, at least two different phenotypes of macrophages are commonly referred to as M2, including those stimulated in vitro with IL-4 and those stimulated with IL-10, even though these macrophages display distinct phenotypes with different effects on angiogenesis, fibrosis, and tissue repair [7, 114–117]. Moreover, it is widely acknowledged that the terms “M1” and “M2” are oversimplified, and that macrophages frequently exist as hybrid phenotypes as well as phenotypes that share no apparent resemblance to the M1 and M2 macrophages that have been described in vitro [113]. Finally, the selection of M2 markers is more often based on which antibodies are convenient to use rather than which marker would be most appropriate for the application. This is especially problematic considering that M1 and M2 markers are not on/off switches, but are rather up- or down-regulated with changes in activation, and all M1 or M2 markers do not necessarily change expression in the same direction upon activation, so that merely counting cells that stain positively for one or even a few markers likely misclassifies macrophages [7]. To advance our understanding of the context-dependent functions of macrophages in vivo, descriptions of macrophage phenotype must be as thorough as possible and methods used to generate these descriptions must be clearly explained and carefully considered when interpreting data from each study. For example, one recent study used whole genome gene expression signatures to compare the phenotype of macrophages extracted from the injured spinal cord of mice to 19 publicly available data sets of macrophages polarized with different stimuli [118]. The found that the spinal cord macrophages most closely resembled foam cells found in atherosclerotic mice, and follow-up studies confirmed that inhibiting foam cell formation improved recovery from spinal cord injury [118]. While this technique of macrophage phenotyping is very thorough, it may not be technically or financially possible in many applications. At the very least, multiple markers of M1 and M2 phenotypes should be employed. In addition, although many studies assess phenotype markers in tissue homogenates or for populations of cells dissociated from tissue, using assays that can identify multiple markers on single cells (e.g. flow cytometry, single cell genomics/proteomics) will provide critical information about heterogeneity of macrophage populations in vivo, in both physiological and pathological situations.
Table 2.
Advantages and disadvantages of different macrophage phenotype nomenclature systems
| Nomenclature | Example | Advantages | Disadvantages |
|---|---|---|---|
| Based On historical characterization | Classically activated vs. alternatively activated | Simple; Agnostic about role of phenotype, Which is context-dependent | Does not account for multiple phenotypes; implies mode of activation that may not reflect in vivo reality |
| Based on function | Pro-inflammatory, pro-healing | Simple; may reflect in vivo function | Implies functions that might be out-of-context |
| Based On Th1/Th2 paradigm, and expansions thereof | M1, M2, M2a, M2b, M2c | Agnostic about role of phenotype, which is Context-dependent | Differences in activation protocols lead to differences in behavior, which may not be reflected in nomenclature; implies mode of activation that may not reflect in vivo reality |
| Based On activation protocol in vitro [113] | M(IFNg, LPS), M(IL4, IL13) | Avoids confusion about how researchers define an activation state | Cannot incorporate all experimental details that may affect macrophage phenotype; not useful for macrophage derived from in vivo environment |
| Based On multiple protein markers [113] | Arg1hiRetnlahipST AT6+pSTAT1− | Avoids Confusion about How phenotype conclusions were drawn | Small number of markers not adequate for describing complex macrophage activation states; classification will be antibody-dependent |
| Based on comparison to reference gene expression signatures [21, 118] | Co-expression or gene set enrichment analysis with whole genome data sets of polarized Mp | Highly Specific about phenotype | Requires gene sequencing and bioinformatic techniques that can be expensive and technically challenging; reference signatures may not reflect in vivo reality |
4.2. Important considerations for preclinical models
While the gap between animal models and humans has long been recognized as a major barrier to translation of biomedical discoveries, overcoming mouse-human differences may be particularly challenging for therapies that target the immune system. Major differences have been described between the mouse and human inflammatory response to infection and injury [119]. The study of macrophage phenotype and function appears to be particularly susceptible to mouse-human differences, as several critical genes are differentially regulated in mouse and human macrophages in response to standard activation stimuli such as LPS (M1 stimulus) and IL-4 (M2 stimulus) [117, 120–122]. For example, the popular murine M1 and M2 markers iNOS and Arg1 are not expressed at appreciable levels by human macrophages at all [122, 123], and the critical anti-inflammatory factors IL-10 and TGF-β1 are regulated in opposite directions in mouse and human M1 and M2 macrophages in vitro [117].
Other factors such as ageing and sex are also important considerations. For example, axon regeneration following peripheral nerve grafting was impaired in aged rats compared to young rats, and was associated with delayed macrophage infiltration, decreased phagocytosis, and decreased secretion of both pro- and anti-inflammatory cytokines [124]. Similarly, another study reported that macrophages in aged mice secreted lower levels of pro-angiogenic factors than macrophages from young mice, resulting in impaired wound healing [125]. In addition, inhibiting M-CSF receptor kinase activity reduced macrophage numbers and induced a trend of increased bone formation in old but not young mice [126]. These data indicate that the function of macrophages may change with age, and thus age and other contextual factors should be considered when designing macrophage-targeted approaches. Furthermore, macrophages may exhibit sex-dependent differences in behavior during tissue regeneration [127], but these sex differences have not yet been thoroughly explored.
4.3. Quality control issues
The remarkable plasticity of macrophages makes therapies that target them particularly susceptible to quality control issues. For example, it is not clear if exogenously stimulated macrophages retain their phenotype after in vivo administration. Interestingly, Cao et al. [128] showed that ex vivo-polarized splenic macrophages but not bone marrow-derived macrophages retained an M2-like phenotype and protected against injury for weeks after administration in a murine renal injury model. This stark difference in the therapeutic effects of macrophages derived from the two different sources was found to result from the ability of the bone marrow-derived macrophages to proliferate in vivo, unlike splenic macrophages, which are relatively mature and differentiated. In another study, Lavin et al. [45] showed that transplantation of peritoneal macrophages, which are also a relatively mature macrophage population, into the lung caused them to take on the epigenetic landscape and gene expression profile that is characteristic of lung macrophages, although they did retain features of peritoneal macrophages. Thus, before macrophage-based cell therapies can be translated into clinical application, it will be necessary to devise strategies that ensure their functional stability in vivo or at least be able to predict changes in phenotype that may occur after administration.
For biomaterial-based strategies, timing and dose will be a major quality control issue. Jiang et al. [129] showed that long term (90 days) effects could be achieved by just 10–30 days of controlled release of dexamethasone, an anti-inflammatory glucocorticoid, from synthetic scaffolds encapsulating pancreatic islet cells for cell transplantation therapy of diabetes. However, release of dexamethasone from the scaffold in this study was characterized in vitro, which may be different than in vivo conditions [129, 130]. Another critical quality control issue for drug-releasing implants will be dose. In the islet transplantation study, low doses of released dexamethasone improved graft survival, while high doses excessively suppressed macrophage infiltration, thus hindering vascularization and graft integration and reducing islet cell survival [129]. Presumably, too low of a dose would fail to sufficiently modulate macrophage behavior. These results highlight the importance of carefully characterizing release profiles in vivo and tailoring to the application at hand.
4.4 Additional issues and opportunities
Patient-to-patient variability will also be a problem for the translation of macrophage-targeted therapies, considering that a patient’s immune cells may respond differently to inflammatory stimuli based on the patient’s individual medical history. Chronic, pro-inflammatory disease conditions can influence the phenotype of macrophages at all levels from progenitors in the bone marrow to mature effector cells in tissue, in part through epigenetic programming. In a recent study, wound macrophages from diabetic db/db mice exhibited a more M1-like phenotype than those from non-diabetic mice both at days 4 and 7 post-injury [47]. In addition, cultured bone marrow-derived macrophages from diabetic db/db mice showed a more M1-like phenotype than macrophages from non-diabetic control mice, even under identical culture conditions [47]. This phenotype difference persisted after adoptive transfer of these macrophages into wounds of non-diabetic mice, indicating that macrophages from diabetic mice are intrinsically primed to be more pro-inflammatory than those from non-diabetic mice [47]. Similar results were produced using pre-diabetic high fat diet (HFD)-fed mice, as wound macrophages exhibited a more M1-like phenotype on days 3 and 7 post-injury, and this phenotype difference was transmitted by bone marrow transfer from HFD or lean donor mice to lean recipient mice [48]. An epigenetic mechanism may explain the intrinsic programming of diabetic macrophages, as repressive histone methylation is decreased at the promoter of the IL-12 gene in bone marrow progenitors, resulting in increased IL-12 production in progeny wound macrophages [48].
On the other hand, some studies have shown that despite higher baseline levels of inflammatory activity, macrophages in patients with chronic inflammatory disorders may actually be hypo-responsive to inflammatory stimuli. For example, Malaponte et al. [131] showed that monocytes isolated from patients undergoing dialysis secreted higher levels of pro-inflammatory cytokines at baseline compared to monocytes from healthy volunteers, but upon stimulation with endotoxin the monocytes secreted lower levels than those from healthy volunteers. Moreover, this impairment in the inflammatory response to endotoxin was directly proportional to the amount of time the patient had been on dialysis, suggesting that dialysis caused chronic stress that led to monocyte tolerance in a dose-responsive manner. In another study, patients with Crohn’s disease exhibited reduced secretion of inflammatory cytokines following acute injury to the bowel or in response to injection of bacteria, compared to healthy controls, suggesting that the disease state led to suppressed inflammatory responses to acute stimuli [74]. Consideration of the state of macrophage behavior upon treatment will be particularly important for the design of biomaterials, which alter the phenotype of infiltrating macrophages with resulting effects on tissue repair whether or not manipulation of macrophage behavior is an intentional aspect of their design [112]. Indeed, the inflammatory response to biomaterials is different in healthy and diseased animal models [132]. Better understanding of the influence of the patient’s disease status, including epigenetic programming, on macrophage phenotypes will provide insight into how the disease environment may impact therapies designed to modulate macrophage phenotype and may provide additional targets for modulating macrophage phenotype. Such understanding may also help to interpret experiments designed to assess the mechanisms by which the phenotype of exogenous macrophages are regulated following ex vivo activation and therapeutic administration in various disease conditions.
Other understudied areas are the relative effects of proliferation, cell death and migration into/out of the target tissue on macrophage accumulation and phenotype. Most studies in regenerative medicine assume that macrophage accumulation is dominated by persistent recruitment of blood borne monocytes, but the influence of cell proliferation as well as the role of tissue-resident macrophages are becoming better appreciated [133–135]. In addition, the fate of macrophages after they have performed their functions in the target tissue is poorly understood and could have important implications both for the local and systemic inflammatory response, the latter potentially via trafficking through lymph nodes and even reverse migration to the bone marrow [136, 137]. Better understanding of these processes should lead to improved targeting of macrophage responses in translational regenerative medicine approaches.
5. Conclusions/outlook
Strategies that harness the inflammatory response, specifically macrophage behavior, may prove to be particularly effective in regenerative medicine compared to other diseases because of the critical roles of macrophages in regulating multiple tissue repair processes. The most effective therapies may involve delivery of molecules or biomaterials designed to modulate accumulation and phenotype of host macrophages and/or regulate the phenotype and survival of ex vivo-activated macrophages in a temporally and spatially dependent manner. As an example translational paradigm, it is well known that many of the positive effects of therapies involving MSCs are mediated by secretion of molecules that act in a paracrine fashion to regulate other cells involved in tissue repair and regeneration [138–140]. Since macrophages are known to be the primary source of a large number of cytokines and growth factors, macrophage-based therapies may compare favorably with MSC-based therapies. Better understanding of the context-dependent macrophage functions and the myriad factors that regulate macrophage phenotype along with rigorous quality control measures will provide great opportunity for improving patient outcomes in a variety of pathological conditions.
Acknowledgments
The authors gratefully acknowledge funding support from NHLBI (R01 HL130037 to KLS) and NIGMS (R01 GM092850 to TJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 2.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxena A, Russo I, Frangogiannis NG. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res. 2016;167(1):152–66. doi: 10.1016/j.trsl.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175(6):2454–62. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110(23):9415–20. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu T, Wang W, Nassiri S, Kwan T, Dang C, Liu W, et al. Temporal and spatial distribution of macrophage phenotype markers in the foreign body response to glutaraldehyde-crosslinked gelatin hydrogels. J Biomater Sci Polym Ed. 2016;27(8):721–42. doi: 10.1080/09205063.2016.1155881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–88. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170(3):818–29. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6(7):e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Wu C, Yang Q, Gao J, Li L, Yang D, et al. Macrophages Mediate the Repair of Brain Vascular Rupture through Direct Physical Adhesion and Mechanical Traction. Immunity. 2016;44(5):1162–76. doi: 10.1016/j.immuni.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63(3):1103–14. doi: 10.2337/db13-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55(15):1629–38. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125(2):364–74. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44(3):450–62. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine. 2011;56(2):256–64. doi: 10.1016/j.cyto.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93(6):875–81. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183(5):1352–63. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40(2):274–88. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Nassiri S, Graney PL, Spiller KL. Manipulation of macrophages to enhance bone repair and regeneration. In: Zreiqat H, Rosen V, Dunstan CR, editors. A Tissue Regeneration Approach to Bone Repair. Springer; 2014. [Google Scholar]
- 25.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120(3):613–25. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 26.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184(7):3964–77. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 27.Ploeger DT, Hosper NA, Schipper M, Koerts JA, de Rond S, Bank RA. Cell plasticity in wound healing: paracrine factors of M1/M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun Signal. 2013;11(1):29. doi: 10.1186/1478-811X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knipper JA, Willenborg S, Brinckmann J, Bloch W, Maab T, Wagener R, et al. Interleukin-4 Receptor α Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity. 2015;43:803–16. doi: 10.1016/j.immuni.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest. 2016;126(6):2151–66. doi: 10.1172/JCI85782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352(6283):366–70. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood S, Jayaraman V, Huelsmann EJ, Bonish B, Burgad D, Sivaramakrishnan G, et al. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One. 2014;9(3):e91574. doi: 10.1371/journal.pone.0091574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nassiri S, Zakeri I, Weingarten MS, Spiller KL. Relative Expression of Proinflammatory and Antiinflammatory Genes Reveals Differences between Healing and Nonhealing Human Chronic Diabetic Foot Ulcers. J Invest Dermatol. 2015;135(6):1700–3. doi: 10.1038/jid.2015.30. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–91. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985–97. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furukawa S, Moriyama M, Tanaka A, Maehara T, Tsuboi H, Iizuka M, et al. Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz’s disease. Clin Immunol. 2015;156(1):9–18. doi: 10.1016/j.clim.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011;43(1):154–62. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8(4):279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 39.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–6. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 40.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27(3):200–15. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, et al. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165(5):2798–808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 42.Mirza RE, Fang MM, Novak ML, Urao N, Sui A, Ennis WJ, et al. Macrophage PPARgamma and impaired wound healing in type 2 diabetes. J Pathol. 2015 doi: 10.1002/path.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bratton DL, Henson PM. Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol. 2011;32(8):350–7. doi: 10.1016/j.it.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norris PC, Gosselin D, Reichart D, Glass CK, Dennis EA. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc Natl Acad Sci U S A. 2014;111(35):12746–51. doi: 10.1073/pnas.1404372111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–26. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345(6204):1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bannon P, Wood S, Restivo T, Campbell L, Hardman MJ, Mace KA. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Dis Model Mech. 2013;6(6):1434–47. doi: 10.1242/dmm.012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallagher KA, Joshi A, Carson WF, Schaller M, Allen R, Mukerjee S, et al. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes. 2015;64(4):1420–30. doi: 10.2337/db14-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S, Kivimae S, Dolor A, Szoka FC. Macrophage-based cell therapies: The long and winding road. J Control Release. 2016 doi: 10.1016/j.jconrel.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuloff-Shani A, Adunsky A, Even-Zahav A, Semo H, Orenstein A, Tamir J, et al. Hard to heal pressure ulcers (stage III–IV): efficacy of injected activated macrophage suspension (AMS) as compared with standard of care (SOC) treatment controlled trial. Arch Gerontol Geriatr. 2010;51(3):268–72. doi: 10.1016/j.archger.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Frenkel O, Shani E, Ben-Bassat I, Brok-Simoni F, Rozenfeld-Granot G, Kajakaro G, et al. Activated macrophages for treating skin ulceration: gene expression in human monocytes after hypo-osmotic shock. Clin Exp Immunol. 2002;128(1):59–66. doi: 10.1046/j.1365-2249.2002.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartel RL, Cramer C, Ledford K, Longcore A, Parrish C, Stern T, et al. The Aastrom experience. Stem Cell Res Ther. 2012;3(4):26. doi: 10.1186/scrt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, et al. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet. 2016;387(10036):2412–21. doi: 10.1016/S0140-6736(16)30137-4. [DOI] [PubMed] [Google Scholar]
- 54.Powell RJ, Marston WA, Berceli SA, Guzman R, Henry TD, Longcore AT, et al. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther. 2012;20(6):1280–6. doi: 10.1038/mt.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ledford KJ, Zeigler F, Bartel RL. Ixmyelocel-T, an expanded multicellular therapy, contains a unique population of M2-like macrophages. Stem Cell Res Ther. 2013;4(6):134. doi: 10.1186/scrt345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307(16):1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, et al. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 2015;125(8):3147–62. doi: 10.1172/JCI81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ben-Mordechai T, Holbova R, Landa-Rouben N, Harel-Adar T, Feinberg MS, Abd Elrahman I, et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62(20):1890–901. doi: 10.1016/j.jacc.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 59.Ledford KJ, Murphy N, Zeigler F, Bartel RL, Tubo R. Therapeutic potential of ixmyelocel-T, an expanded autologous multicellular therapy for treatment of ischemic cardiovascular diseases. Stem Cell Res Ther. 2015;6:25. doi: 10.1186/s13287-015-0007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu JY, Cao Q, Zheng D, Sun Y, Wang C, Yu X, et al. Discrete functions of M and M macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int. 2013 doi: 10.1038/ki.2013.135. [DOI] [PubMed] [Google Scholar]
- 61.Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, et al. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21(6):933–42. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72(3):290–9. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 63.Novak ML, Weinheimer-Haus EM, Koh TJ. Macrophage activation and skeletal muscle healing following traumatic injury. J Pathol. 2014;232(3):344–55. doi: 10.1002/path.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rybalko V, Hsieh PL, Merscham-Banda M, Suggs LJ, Farrar RP. The Development of Macrophage-Mediated Cell Therapy to Improve Skeletal Muscle Function after Injury. PLoS One. 2015;10(12):e0145550. doi: 10.1371/journal.pone.0145550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jetten N, Roumans N, Gijbels MJ, Romano A, Post MJ, de Winther MP, et al. Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PLoS One. 2014;9(7):e102994. doi: 10.1371/journal.pone.0102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dumont NA, Frenette J. Macrophage colony-stimulating factor-induced macrophage differentiation promotes regrowth in atrophied skeletal muscles and C2C12 myotubes. Am J Pathol. 2013;182(2):505–15. doi: 10.1016/j.ajpath.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26(7):1517–32. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 68.Alikhan MA, Jones CV, Williams TM, Beckhouse AG, Fletcher AL, Kett MM, et al. Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am J Pathol. 2011;179(3):1243–56. doi: 10.1016/j.ajpath.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doring A, Sloka S, Lau L, Mishra M, van Minnen J, Zhang X, et al. Stimulation of monocytes, macrophages, and microglia by amphotericin B and macrophage colony-stimulating factor promotes remyelination. J Neurosci. 2015;35(3):1136–48. doi: 10.1523/JNEUROSCI.1797-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127(20):2038–46. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61(3):416–26. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 72.Li F, Cheng B, Cheng J, Wang D, Li H, He X. CCR5 blockade promotes M2 macrophage activation and improves locomotor recovery after spinal cord injury in mice. Inflammation. 2015;38(1):126–33. doi: 10.1007/s10753-014-0014-z. [DOI] [PubMed] [Google Scholar]
- 73.Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115(2):245–53. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 74.Marks DJ, Harbord MW, MacAllister R, Rahman FZ, Young J, Al-Lazikani B, et al. Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet. 2006;367(9511):668–78. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 75.Yoon P, Keylock KT, Hartman ME, Freund GG, Woods JA. Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mech Ageing Dev. 2004;125(2):137–43. doi: 10.1016/j.mad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62(7):2579–87. doi: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weinheimer-Haus EM, Mirza R, Koh TJ. Nod-like receptor protein-3 inflammasome plays an important role in early stages of wound healing. PLoS One. 2015 doi: 10.1371/journal.pone.0119106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodero MP, Hodgson SS, Hollier B, Combadiere C, Khosrotehrani K. Reduced Il17a Expression Distinguishes a Ly6c(lo)MHCII(hi) Macrophage Population Promoting Wound Healing. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.368. [DOI] [PubMed] [Google Scholar]
- 80.Goren I, Muller E, Schiefelbein D, Christen U, Pfeilschifter J, Muhl H, et al. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatol. 2007;127(9):2259–67. doi: 10.1038/sj.jid.5700842. [DOI] [PubMed] [Google Scholar]
- 81.Courties G, Heidt T, Sebas M, Iwamoto Y, Jeon D, Truelove J, et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol. 2014;63(15):1556–66. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salmon-Ehr V, Ramont L, Godeau G, Birembaut P, Guenounou M, Bernard P, et al. Implication of interleukin-4 in wound healing. Lab Invest. 2000;80(8):1337–43. doi: 10.1038/labinvest.3780141. [DOI] [PubMed] [Google Scholar]
- 83.Chamberlain CS, Leiferman EM, Frisch KE, Wang S, Yang X, Brickson SL, et al. The influence of interleukin-4 on ligament healing. Wound Repair Regen. 2011;19(3):426–35. doi: 10.1111/j.1524-475X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yin H, Li X, Hu S, Liu T, Yuan B, Gu H, et al. IL-33 accelerates cutaneous wound healing involved in upregulation of alternatively activated macrophages. Mol Immunol. 2013;56(4):347–53. doi: 10.1016/j.molimm.2013.05.225. [DOI] [PubMed] [Google Scholar]
- 85.Hong S, Tian H, Lu Y, Laborde JM, Muhale FA, Wang Q, et al. Neuroprotectin/protectin D1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am J Physiol Cell Physiol. 2014;307(11):C1058–67. doi: 10.1152/ajpcell.00270.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62(2):618–27. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kakazu A, He J, Kenchegowda S, Bazan HE. Lipoxin A(4) inhibits platelet-activating factor inflammatory response and stimulates corneal wound healing of injuries that compromise the stroma. Exp Eye Res. 2012;103:9–16. doi: 10.1016/j.exer.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dakin SG, Martinez FO, Yapp C, Wells G, Oppermann U, Dean BJ, et al. Inflammation activation and resolution in human tendon disease. Sci Transl Med. 2015;7(311):311ra173. doi: 10.1126/scitranslmed.aac4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14(11):1835–42. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 91.Brown BN, Ratner BD, Goodman SB, Amar SA, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33(15):3792–802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garash R, Bajpai A, Marcinkiewicz BM, Spiller KL. Drug delivery strategies to control macrophages for tissue repair and regeneration. Exp Biol Med (Maywood) 2016;241(10):1054–63. doi: 10.1177/1535370216649444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grayson WL, Frohlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107(8):3299–304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, et al. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A. 2013;110(34):13785–90. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, et al. Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci Rep. 2017;7(1):447. doi: 10.1038/s41598-017-00477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krieger JR, Ogle ME, McFaline-Figueroa J, Segar CE, Temenoff JS, Botchwey EA. Spatially localized recruitment of anti-inflammatory monocytes by SDF-1alpha-releasing hydrogels enhances microvascular network remodeling. Biomaterials. 2016;77:280–90. doi: 10.1016/j.biomaterials.2015.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.San Emeterio CL, Olingy CE, Chu Y, Botchwey EA. Selective recruitment of non-classical monocytes promotes skeletal muscle repair. Biomaterials. 2017;117:32–43. doi: 10.1016/j.biomaterials.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spiller KL, Nassiri S, Witherel CE, Anfang R, Ng J, Nakazawa K, et al. Sequential delivery of cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. 2013;110(43):17253–8. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang T, Luu TU, Chen A, Khine M, Liu WF. Topographical modulation of macrophage phenotype by shrink-film multi-scale wrinkles. Biomater Sci. 2016;4(6):948–52. doi: 10.1039/c6bm00224b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vogel DY, Glim JE, Stavenuiter AW, Breur M, Heijnen P, Amor S, et al. Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology. 2014;219(9):695–703. doi: 10.1016/j.imbio.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 102.Patel SK, Janjic JM. Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases. Theranostics. 2015;5(2):150–72. doi: 10.7150/thno.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pentecost AE, Lurier EB, Spiller KL. Nanoparticulate Systems for Controlling Monocyte/Macrophage Behavior. In: Singh A, Gaharwar AK, editors. Microscale Technologies for Cell Engineering. Switzerland: Springer International Publishing; 2016. pp. 291–302. [Google Scholar]
- 104.Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci U S A. 2011;108(5):1827–32. doi: 10.1073/pnas.1015623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J, et al. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol. 2009;183(1):661–9. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao Y, Haney MJ, Mahajan V, Reiner BC, Dunaevsky A, Mosley RL, et al. Active Targeted Macrophage-mediated Delivery of Catalase to Affected Brain Regions in Models of Parkinson’s Disease. J Nanomed Nanotechnol. 2011:S4. doi: 10.4172/2157-7439.S4-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao Y, Haney MJ, Klyachko NL, Li S, Booth SL, Higginbotham SM, et al. Polyelectrolyte complex optimization for macrophage delivery of redox enzyme nanoparticles. Nanomedicine (Lond) 2011;6(1):25–42. doi: 10.2217/nnm.10.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haney MJ, Zhao Y, Li S, Higginbotham SM, Booth SL, Han HY, et al. Cell-mediated transfer of catalase nanoparticles from macrophages to brain endothelial, glial and neuronal cells. Nanomedicine (Lond) 2011;6(7):1215–30. doi: 10.2217/nnm.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Biju KC, Santacruz RA, Chen C, Zhou Q, Yao J, Rohrabaugh SL, et al. Bone marrow-derived microglia-based neurturin delivery protects against dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. Neurosci Lett. 2013;535:24–9. doi: 10.1016/j.neulet.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33(34):8793–801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8(3):978–87. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–91. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu J, Cao Q, Zheng D, Sun Y, Wang C, Yu X, et al. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int. 2013;84(4):745–55. doi: 10.1038/ki.2013.135. [DOI] [PubMed] [Google Scholar]
- 115.Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E, et al. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85(5):779–87. doi: 10.1189/jlb.0908579. [DOI] [PubMed] [Google Scholar]
- 116.Lurier EB, Dalton D, Dampier W, Nassiri S, Ferraro NM, Raman P, et al. Transcriptome analysis of IL10-stimulated (M2c) macrophages by next generation sequencing. Immunobiology. 2016 doi: 10.1016/j.imbio.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spiller KL, Wrona EA, Romero-Torres S, Pallotta I, Graney PL, Witherel CE, et al. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Exp Cell Res. 2016;347(1):1–13. doi: 10.1016/j.yexcr.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 118.Zhu Y, Lyapichev K, Lee DH, Motti D, Ferraro NM, Zhang Y, et al. Macrophage Transcriptional Profile Identifies Lipid Catabolic Pathways That Can Be Therapeutically Targeted after Spinal Cord Injury. J Neurosci. 2017;37(9):2362–76. doi: 10.1523/JNEUROSCI.2751-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood. 2013;121(9):e57–69. doi: 10.1182/blood-2012-06-436212. [DOI] [PubMed] [Google Scholar]
- 121.Schroder K, Irvine KM, Taylor MS, Bokil NJ, Le Cao KA, Masterman KA, et al. Conservation and divergence in Toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages. Proc Natl Acad Sci U S A. 2012;109(16):E944–53. doi: 10.1073/pnas.1110156109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Raes G, Van den Bergh R, De Baetselier P, Ghassabeh GH, Scotton C, Locati M, et al. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol. 2005;174(11):6561. doi: 10.4049/jimmunol.174.11.6561. author reply -2. [DOI] [PubMed] [Google Scholar]
- 123.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011;89(4):557–63. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scheib JL, Hoke A. An attenuated immune response by Schwann cells and macrophages inhibits nerve regeneration in aged rats. Neurobiol Aging. 2016;45:1–9. doi: 10.1016/j.neurobiolaging.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 125.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79(12):1479–87. [PubMed] [Google Scholar]
- 126.Slade Shantz JA, Yu YY, Andres W, Miclau T, 3rd, Marcucio R. Modulation of macrophage activity during fracture repair has differential effects in young adult and elderly mice. J Orthop Trauma. 2014;28(Suppl 1):S10–4. doi: 10.1097/BOT.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4(5):554–63. doi: 10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cao Q, Wang Y, Zheng D, Sun Y, Wang C, Wang XM, et al. Failed renoprotection by alternatively activated bone marrow macrophages is due to a proliferation-dependent phenotype switch in vivo. Kidney Int. 2014;85(4):794–806. doi: 10.1038/ki.2013.341. [DOI] [PubMed] [Google Scholar]
- 129.Jiang K, Weaver JD, Li Y, Chen X, Liang J, Stabler CL. Local release of dexamethasone from macroporous scaffolds accelerates islet transplant engraftment by promotion of anti-inflammatory M2 macrophages. Biomaterials. 2017;114:71–81. doi: 10.1016/j.biomaterials.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 130.Sundararaj SC, Al-Sabbagh M, Rabek CL, Dziubla TD, Thomas MV, Puleo DA. Comparison of sequential drug release in vitro and in vivo. J Biomed Mater Res B Appl Biomater. 2016;104(7):1302–10. doi: 10.1002/jbm.b.33472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Malaponte G, Bevelacqua V, Fatuzzo P, Rapisarda F, Emmanuele G, Travali S, et al. IL-1β, TNF-α and IL-6 release from monocytes in haemodialysis patients in relation to dialytic age. Nephrology Dialysis Transplantation. 2002;17(11):1964–70. doi: 10.1093/ndt/17.11.1964. [DOI] [PubMed] [Google Scholar]
- 132.Oliva N, Carcole M, Beckerman M, Seliktar S, Hayward A, Stanley J, et al. Regulation of dendrimer/dextran material performance by altered tissue microenvironment in inflammation and neoplasia. Sci Transl Med. 2015;7(272):272ra11. doi: 10.1126/scitranslmed.aaa1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, et al. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013;4:1886. doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19(9):1166–72. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103(10):3781–6. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leiriao P, del Fresno C, Ardavin C. Monocytes as effector cells: activated Ly-6C(high) mouse monocytes migrate to the lymph nodes through the lymph and cross-present antigens to CD8+ T cells. Eur J Immunol. 2012;42(8):2042–51. doi: 10.1002/eji.201142166. [DOI] [PubMed] [Google Scholar]
- 138.Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316(14):2213–9. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]