Abstract
The recrystallization of an amorphous drug in a solid dispersion system could lead to a loss in the drug solubility and bioavailability. The primary objective of the current research was to use an improved kinetic model to evaluate the recrystallization kinetics of amorphous structures and to further understand the factors influencing the physical stability of amorphous solid dispersions. Amorphous solid dispersions of fenofibrate with different molecular weights of hydroxypropylcellulose, HPC (Klucel™ LF, EF, ELF) were prepared utilizing hot-melt extrusion technology. Differential scanning calorimetry was utilized to quantitatively analyze the extent of recrystallization in the samples stored at different temperatures and relative humidity (RH) conditions. The experimental data were fitted into the improved kinetics model of a modified Avrami equation to calculate the recrystallization rate constants. Klucel LF, the largest molecular weight among the HPCs used, demonstrated the greatest inhibition of fenofibrate recrystallization. Additionally, the recrystallization rate (k) decreased with increasing polymer content, however exponentially increased with higher temperature. Also k increased linearly rather than exponentially over the range of RH studied.
Keywords: Avrami equation, hot-melt extrusion, kinetics model, physical stability, recrystallization
Introduction
A major concern during formulation development has been to enhance the dissolution rate and bioavailability of poorly water-soluble drugs1,2. The amorphous forms of active pharmaceutical ingredient (APIs) have attracted considerable attention as the amorphous forms tend to exhibit significantly higher levels of supersaturation in aqueous media when compared to the crystalline forms3,4. For drugs which bioavailability is limited by aqueous solubility (biopharmaceutical classification system (BCS) II drugs), which is a significant number of recently discovered drug candidates, the improved solubility may lead to enhanced bioavailability5–7. Oral absorption of APIs depends on two broad, but crucial, events, namely drug solubilization and gastrointestinal permeation8. Amorphous solid dispersions have been widely investigated to increase the gastrointestinal permeability, and the roles played by polymers and other excipients were also studied9,10.
However, even after 40 years of active research there have not been many commercial products that have reached the market based on amorphous solid dispersion technology11,12. The primary reason for this is a combination of the stability and scale-up issues associated with this approach, which has been reported by several authors13–15. Hot-melt extrusion currently stands as the most promising approach for solving the scale-up issues associated with amorphous solid dispersion systems16. Unfortunately, amorphous solid dispersion systems still face the issue that the amorphous form is, in general, thermodynamically unstable relative to its crystalline counterpart(s)17,18, and therefore may ultimately result in unacceptable changes in the API’s physical properties19. The most common approach of preserving the solubility/bioavailability enhancement imparted by the high-energy amorphous form is to kinetically stabilize the drug substance in the amorphous state for the duration of the anticipated shelf life. Amorphous solid dispersions can be stabilized as such if there exists a sufficient energy barrier provided by the polymer(s) with a high glass transition temperature, or by way of molecular interactions between the API and the polymeric carrier, such as hydrogen bonding.
The most commonly used methods of evaluating the physical stability of these dispersed systems are short- and long-term stability tests in accordance to ICH guidance20. However, during the research and development process of a new solid dispersion drug product, hundreds of formulations need to be evaluated, which is neither costly nor time efficient. Fortunately, the recrystallization process of the drug–polymer system can be quantitatively described by kinetics models21–23. Among these models, the Avrami model/equation is most frequently used to express and predict the solid-state recrystallization processes.
| (1) |
where the α(t) is relative crystallinity, k represents the recrystallization rate constant and n is the Avrami exponent.
The kinetic description of crystallization processing is composed of two main independent procedures: nucleation and crystal growth. The Avrami equation has been used to model crystallization kinetics for decades; however, one of the primary assumptions inherent in this equation is that the nucleation rate is a constant. Treating the nucleation process as such leads to an over-prediction in the recrystallization rate. This becomes particularly apparent during the later stages of the process. In our previous study24, an improved description of the nucleation rate was proposed that is proportional to the amorphous fraction.
| (2) |
where the J(t) term is the nucleation rate of sample at time of t, J0 is the initial nucleation rate, and 1−a(t) is a function of the time of the experiment.
The improved kinetic equation enables enhanced predictability by using the experimental date of formulations under high temperature and high RH conditions to predict the performance of formulations under ambient conditions, which could, in turn, save a great deal of time and expense in terms of the formulation screening.
In this study, the improved Avrami equation was utilized to evaluate the recrystallization kinetics for several amorphous solid dispersion systems including fenofibrate with different grades of hydroxypropylcellulose (Klucel™ LF/EF/ELF). Fenofibrate is an agonist of the nuclear per-oxisome proliferator-activated receptor α. It reduces the blood triglyceride levels and, to a lesser extent, the total cholesterol and low-density lipoprotein (LDL) cholesterol levels25. Fenofibrate is practically insoluble in water which as a result of being a neutral and lipophilic molecule with a log P value of 5.226,27. Hydroxypropylcellulose (Klucel HPC) is a non-ionic water-soluble cellulose ether, with a remarkable combination of properties including organic solvent solubility, thermoplasticity, and surface activity26. Klucel HPC has been widely used in solid dispersion formulations to control drug release28, inhibit drug recrystallization 29, and also serve as a granulation binder30.
The solid dispersion systems of fenofibrate with different grades of HPC (Klucel) were compared to investigate the influence of polymer molecular weight on the physical stability of the amorphous systems. Compared to the original Avrami equation, the improved Avrami equation model performance was examined and validated by comparing the predicted values with experimental data. The effects of polymer content, temperature and relative humidity (RH) on the recrystallization rate were also investigated by the improved Avrami equation. To the best of our knowledge, this is the first study using this kinetics equation to evaluate the physical stability of amorphous solid dispersions prepared by hot-melt extrusion.
Materials and methods
Materials
Hydroxypropylcellulose (Klucel; grades LF/EF/ELF) was kindly donated by ASHLAND Specialty Products (Wayne, NJ) and fenofibrate was purchased from Aurobindo Pharma Ltd. (Hyderabad, India). Reagent grade methanol was purchased from Sigma–Aldrich (St. Louis, MO).
Methods
Preparation of amorphous solid dispersions using hot-melt extrusion
Amorphous solid dispersions of fenofibrate and Klucel LF/EF/ELF were prepared using hot-melt extrusion technology. The extrusion temperature range was determined by Thermal gravimetric analysis (TGA). The API and polymer were mixed in a V-cone blender (MaxiBlendTM, GlobePharma, New Brunswick, NJ) at 30 rpm for 20 min and then extruded with a co-rotating twin-screw extruder (Process 11, ThermoFisher Scientific, Waltham, MA) into uniform rod extrudates at an extrusion temperature range of 120 °C to 130 °C based on the molecular weight of Klucel and a screw speed of 100 rpm. The feed rate was 12 g/min so that the extruder % torque indicator was in a safe range of 40–50%. The extrudates were milled using a comminuting Fitz Mill (Model#L1A, Fitzpatrick Company, Elmhurst, IL) at a speed of 3600 rpm using a 0.0232 inch-sieve. Each formulation and its process parameters are shown in Table 1.
Table 1.
Composition of solid dispersions and hot melt extrusion parameters for each formulation.
| Content of fenofibrate (%) | Polymer carrier
|
Temp. (°C) | Screw speed (rpm) | Torque (%) | |
|---|---|---|---|---|---|
| Content (%) | Grade | ||||
| 20 | 80 | ELF | 120 | 100 | 35–45 |
| 15 | 85 | ELF | 120 | 100 | 35–45 |
| 10 | 90 | ELF | 120 | 100 | 40–50 |
| 10 | 90 | LF | 130 | 100 | 40–50 |
| 10 | 90 | EF | 125 | 100 | 40–50 |
Thermal gravimetric analysis
TGA studies were performed on Perkin Elmer Pyris 1 TGA with the Pyris software. Samples weighing 3–5 mg were heated at heating rate of 20 °C /min from 20 °C to 300 °C.
Differential scanning calorimetry (DSC)
DSC was utilized to measure the melting enthalpy of the solid dispersions. Samples were weighed (3–5 mg) in an aluminum sample pan and hermetically sealed at each time point using a heating rate of 20 °C /min from −40 °C to 180 °C (Perkin Elmer, Diamond DSC, Shelton, CT). The nitrogen purge rate was 20 mL/min. An empty pan was used as reference. Measurements were repeated three times. An Indium standard was used for calibration.
HPLC-UV analysis
A Waters HPLC-UV system (Waters Corp, Milford, MA), equipped with a Luna 5um C18 100Å column (Phenomenex, Torrance, CA), was used at a detection wavelength of 286 nm. The mobile phase consisted of acetonitrile and phosphoric acid in water (pH =2.5) at a ratio of 85:15 (v/v). The flow rate was maintained at 1.0 mL/min. The retention time of fenofibrate was 6 min. The injection volume was 20 μL. All of the HPLC data were analyzed using Empower V. software (Waters Corp, Milford, MA).
Evaluation of crystallinity on hot-melt extrudates
The freshly extruded samples were stored in stability chambers with different temperature (±0.1 °C) and RH (±1%) conditions, which were predetermined for different formulations (Temperature: 40, 50, 60 °C and %RH: 10, 40, 70). Samples were removed from the chambers at each sampling time point and the degree of recrystallization was measured by DSC. The absolute percentage of crystallinity, x (t), could be determined by the ratio of melting enthalpies from recrystallized fenofibrate over the melting enthalpies of the physical mixture of fenofibrate and polymeric carrier. The relative crystallinity, α(t), was determined by the ratios of the melting enthalpies of sample with the final melting enthalpy without any change. The equations31 are listed below:
| (3) |
where ΔH(t) is the melting enthalpy of the recrystallized solid dispersion sample and the ΔH(P.M.) represents the melting enthalpies of physical mixtures of the API and polymers for each formulation.
The relative crystallinity, α(t) was calculated using the following equation:
| (4) |
where ΔH∞ is the final melting enthalpy of solid dispersion sample for a certain stress condition (this value was constant and did not change during testing), α(t) varies from 0 to 1.
Improved kinetic model
An improved kinetics model was developed in a previous study24 to quantify the physical stability of each amorphous dispersion system 32. All the experimental data were fitted into the improved Avrami equation using a multivariate non-linear regression method to get the optimized parameters (k values and ΔEA) by MatLab software (version R2011b, MathWorks Inc., Natick, MA).
| (5) |
| (6) |
24,33 where n represents the dimensionality of crystal growth and k is the recrystallization rate constant, which can expressed as Equation (6) where k0 describes the pre-exponential factor, ΔEA is activation energy, R is the universal gas constant and T is the absolute temperature (in kelvin).
Results and discussion
Preparation of solid dispersions utilizing hot-melt extrusion
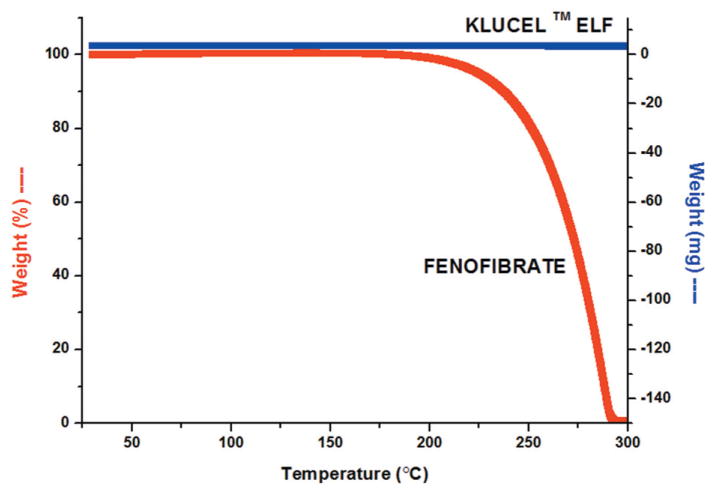
All three grades of Klucel™ HPC with fenofibrate showed excellent extrudability under the utilized processing parameters. The TGA figure indicated both API and polymer were stable in the extrusion temperature range (Figure 1). Higher molecular weight grades of Klucel™ (LF and EF) required higher extrusion temperatures to decrease the torque on the extruder. Polymers with higher molecular weights usually have a higher glass transition temperature, which requires higher energy input to soften the polymer34. Milling of the extrudates was difficult due to the polymer’s high degree of thermoplasticity. Cryomilling, keeping the extrudate in −80 °C for several hours before milling, was utilized to resolve this issue.
Figure 1.
TGA figure for fenofibrate and Klucel ELF.
Currently, many different polymers are used for hot-melt extrusion processing, and for a certain type of polymer, there might be multiple grades with different molecular weights or different substitution ratios (e.g. PVP, PEG, etc.). The improved Avrami equation could be a powerful screening tool to arrive at the most suitable polymer for hot-melt extrusion processing. The same evaluation process should be applicable to determine the effects of other excipients in a given formulation.
HPLC analysis
HPLC analysis of the freshly extruded solid dispersions showed no reduction of drug content nor was the appearance of a degradation peak from fenofibrate observed, which indicated that the extrudates were very stable during the processing conditions. All of the extrudates were well within the specifications (85–115%) of content uniformity standards. The results obtained in this study showed that the hot-melt extrusion process with Klucel™ and fenofibrate could have very high homogeneity regardless of the molecular weight of HPC used.
Physical stability of solid dispersions determined by DSC
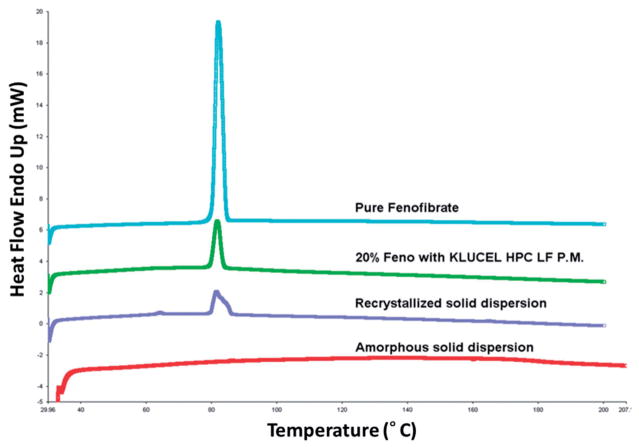
The freshly extruded samples were tested by DSC and the absence of melting peaks indicated that the solid dispersion were in the amorphous state as shown in Figure 2 (red curve). As the stress test was conducted, the API in the dispersions transformed back into its crystalline phase, which was indicated by the increasing integration values of the melting peaks as shown in Figure 2 (purple curve). The peak area on the DSC thermogram is the ΔH(t), which is the melting enthalpies of the recrystallized solid dispersion sample. The peak area of physical mixture thermogram indicates the ΔH(P.M.), which represents the melting enthalpies of the physical mixture of the API and polymer. The ratio of these peak areas can be used to evaluate how much amorphous API recrystallized35. In the early stage of recrystallization processing, different formulations had significant different ΔH(t) which indicated different relative crystalinity. This phenomenon might due to the specific recrystallization activation energy of each formulation that caused different nucleation energy barrier for the API molecular. While in the late stage of recrystallization processing, nucleation rate decreased as the portion of amorphous API decreased, the crystal growth rate turned into the governing factor24.
Figure 2.
Evaluation of crystalinity using DSC: pure fenofibrate (blue curve), physical mixture of 20% fenofibrate with Klucel LF (green curve), recrystallized solid dispersion (purple curve), amorphous solid dispersion (red curve).
Assessment of the improved kinetic model
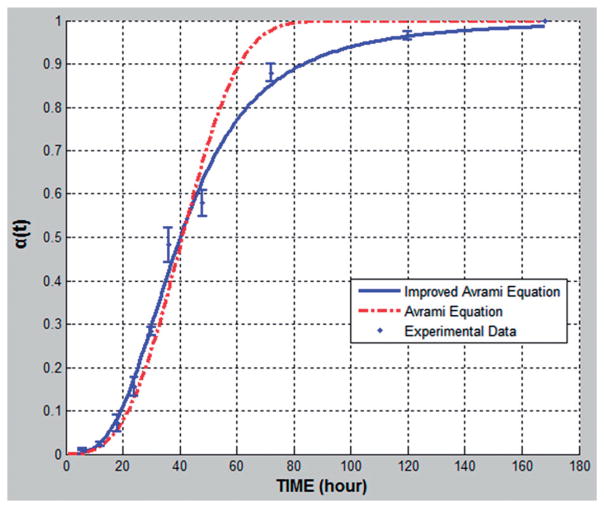
The recrystallization predictions based on the original Avrami model and the new improved kinetic model is shown in Figure 3. The experimental data were fitted into the new modeling equation by a multivariate non-linear regression method using MatLab software. Initial values of k = 0.1 were given to compute the optimized values of model parameters by minimizing the sum of residual squares using a successive linear programming method. Correlation coefficient, r2, for improved Avrami equation is 0.986, while r2 for the Avrami equation is 0.971, which suggests that the improved model provides a better fit than the Avrami equation. However, this is especially true in the late stages of recrystallization (after 50 h) (Figure 3). This is attributed to the changing of the nucleation rate in the improved Avrami equation, which is decreasing with the decline of amorphous content in the system. The Avrami exponent n describs dimensionality of crystal growth in the solid dispersion, when n equals to 2, 3, 4, indicate, respectively, the rod, plate, spherical geometry for nucleation. For this study and most situations for small molecular API, n euqals to 3.
Figure 3.
Comparison of Avrami equation (r2 =0.986) and Improved Avrami equation (r2 =0.971) on recrystallization prediction based on relative crystallinity α(t) plotted against time for 15% fenofibrate with 85% Klucel EL Funder 60 °C /10% RH stability condition.
The effect of polymeric carrier on amorphous solid dispersions stability
Three different formulations of fenofibrate and Klucel™ ELF were prepared with different drug loadings of 10, 15, and 20%. The experimental data showed a significant decrease in the recrystallization rate constant (k) with an increase in polymer content. With lower drug loading, the density of API in the polymeric matrix is lower, and the change in nucleation, along with the amount of molecules to nucleate in later stages, are both lower. Two formulations of fenofibrate with Klucel™ LF and EF, respectively, were produced at a fixed drug loading of 10%. For the different grades of HPC (Klucel™ LF/EF/ELF), under the same drug loading and same stress test condition (60 °C /10%RH), LF exhibited a better recrystallization inhibitory effect than was observed for EF and ELF (LF>EF>ELF) (Table 2).
Table 2.
Recrystallization prediction results by the improved Avrami equation for fenofibrate amorphous solid dispersion with different grades of Klucel HPC under storage condition of 60°C/10%RH.
| Content of fenofibrate (%) | Polymer carrier
|
|
Final time (hours) | k (10−6) | ||
|---|---|---|---|---|---|---|
| Content (%) | Grade | |||||
| 10 | 90 | LF | 61.1% | ~168 | 8.24 | |
| 10 | 90 | EF | 67.7% | ~144 | 14.5 | |
| 10 | 90 | ELF | 73.3% | ~144 | 16.7 | |
Concerning the recrystallization rate constant (k) values, ELF>EF>LF, these data indicated that under the specified conditions, the API in ELF recrystallized faster than that with EF, or LF. The data also revealed from the ratio between ΔH∞ and ΔH(P.M.), that more API was remaining in the amorphous phase in the API/LF system. This difference might due to the difference of molecular weight between these three grades of HPC (Table 3). Klucel™ LF is a higher molecular weight polymer compared to EF and ELF. Klucel™ ELF, with the lowest molecular weight, possesses comparatively lower viscosity due to smaller chains, which could not provide the same energy barrier as EL or LF to prevent the recrystallization of the API. The polymer with the higher molecular weight could have a higher Tg36,37, which is a commonly accepted indication of physical stability improvement of amorphous solid dispersions.
Table 3.
Molecular weight of Klucel LF/EF/ELF.
| Polymer | Klucel LF | Klucel EF | Klucel ELF |
|---|---|---|---|
| Molecular Weight(Da) | 90 000 | 80 000 | 45 000 |
To develop a stable, high-quality amorphous solid dispersion system for one API, the best polymeric carrier can be chosen by calculating the solubility parameters38, miscibility39 and molecular interactions40. However, final physical and chemical stability assessment of a formulation is confirmed by long-term stability testing according to ICH regulations. The improved Avrami equation could be an accurate tool to evaluate the physical stability of various formulations within an abbreviated interval, thus saving valuable research and development time.
The effect of temperature on the recrystallization rate constant
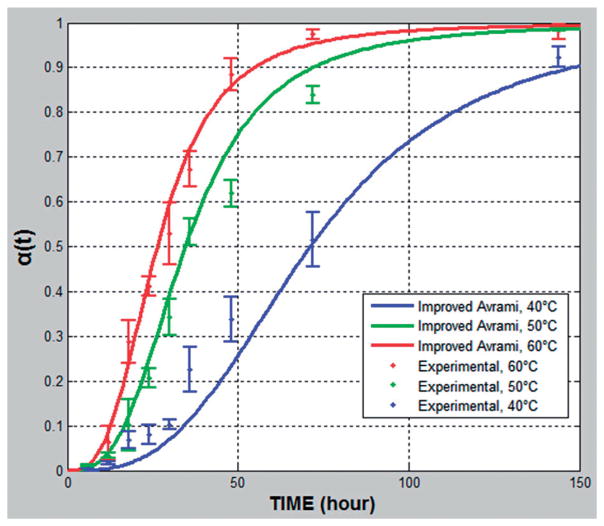
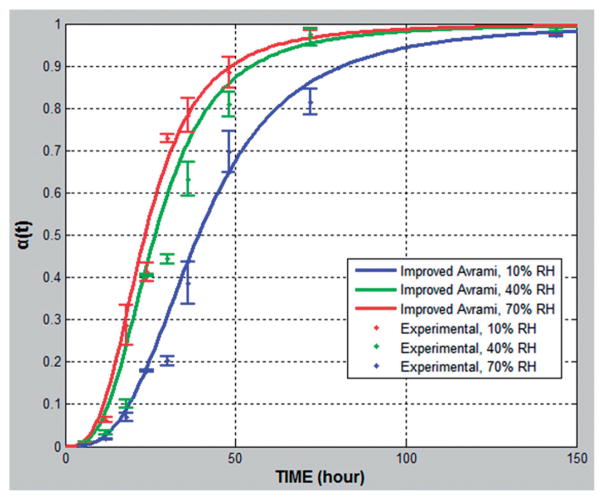
The samples were stored in stability chambers with fixed RH and three different temperatures (10%RH and 40, 50, 60 °C). The selected storage temperatures were higher than those normally used for pharmaceutical stability testing in order to promote rapid recrystallization within a short time according to the ICH 2AQR guidance for stability testing. Under all of these conditions the characteristic S-shaped curve was noted again, which was observed in “Assessment of the improved kinetic model”, with faster recrystallization at increasing temperatures (Figure 5).
Figure 5.
Relative crystallinity α(t) plotted against time for 10% fenofibrate with 90% Klucel ELF under different temperature conditions (40, 50 and 60 °C /10%RH).
The semi-log plot of k versus 1/T reveals that the crystallization rate constant increased exponentially with temperature within the temperature range studied (Figure 6). According to (Equation 4), the recrystallization activation energy was determined by the slope of the linear regression of the lnk and 1/T since k0 and R are both constant. The formulation with a greater slope indicates that the system has lower activation energy and could have faster recrystallization rate. Based on the value of the activation energy, the equation allows the prediction of recrystallization kinetics at temperatures outside the experimental range.
Figure 6.
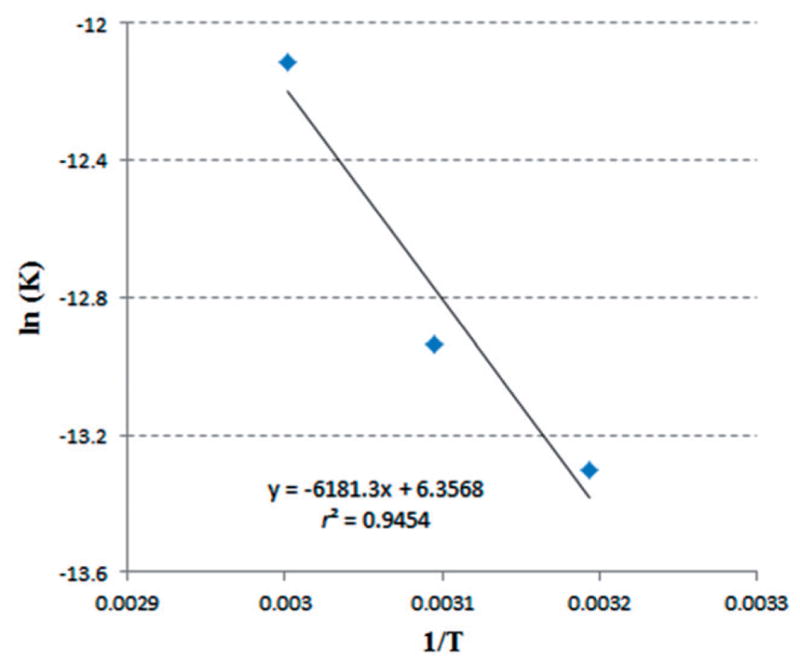
An Arrhenius plot of recrystallization constant against temperature for 10% fenofibrate with 90% Klucel ELF under different temperature conditions 40, 50 and 60 °C /10%RH.
Some samples stored in high temperature/low RH conditions (60 °C /10%RH) deliquesced in the late stages of the experiments. This event is due to the fact that the high temperature could decrease the critical RH for deliquescing by enhancing water uptake rate whereby the samples could reach the equilibrium weight gain more quickly41. Consequently, the higher moisture content of the system and increased molecular mobility triggered more recrystallization of API.
The effect of relative humidity on the recrystallization rate constant
Initial amorphous solid dispersions samples were stored in the stability chambers with fixed temperature and three different RH (60 °C and 10, 40, 70%RH) to determine the influence of humidity on the recrystallization process. Rapid recrystallization rates were observed under high RH (Figure 7).
Figure 7.
Relative crystallinity α(t) plotted against time for 10% fenofibrate with 90% Klucel ELF under different RH conditions (10, 40 and 70%RH/6 °C).
Linear regression of the experimental data (Figure 8) suggests that k increases linearly rather than exponentially over the range of RH studied, which confirmed Yang’s results for PVP-efavirenz solid dispersion systems24. Different polymer–API combinations would have regression equations with different sloped and the slopes could be a quantitative factor to evaluate how sensitive the system is to the RH condition. On the other hand, the Y-intercept of the regression equation could serve as an indicator to quantify the other factors’ contribution to the recrystallization rate under 0% RH, such as polymer and temperature. Moisture potentially acted as a plasticizer and hence lowered the glass transition temperature of the solid dispersion system, which increased the recrystallization rate constant42. The rapid recrystallization might also be due to the increased molecular mobility of APIs in the systems caused by high RH which can increase the molecular rotation and movement subsequently to a lower glass transition temperature of the dispersion system43. Since the recrystallization rate constant has a liner relationship with the RH, we can assume that the moisture content of the solid dispersion system, which is determined by the polymer’s hydroscopic nature, is proportional to the recrystallization rate of the API during stability testing. This indicates that characterizing the water uptake ability of the polymer carrier and other excipients is very important when developing a solid dispersion system.
Figure 8.
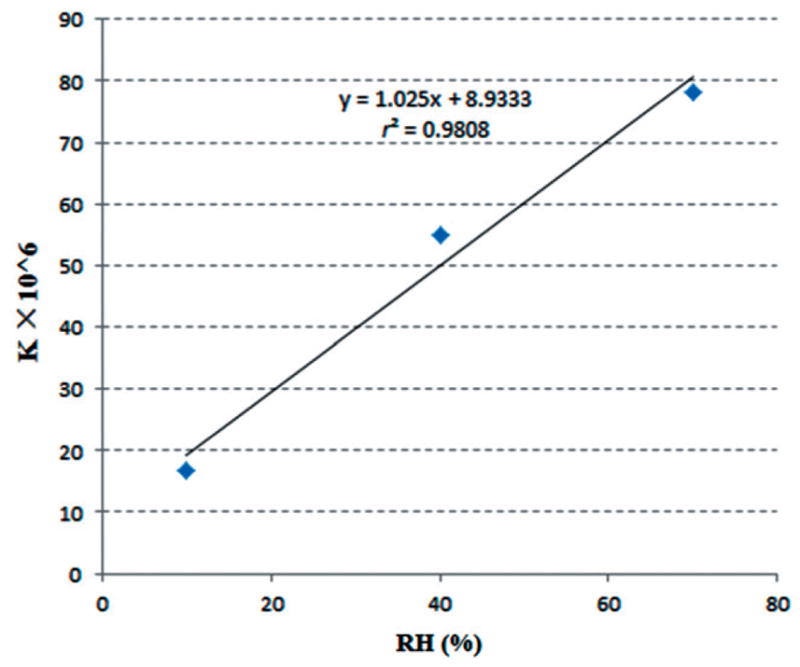
Recrystallization rate constant plotted against RH for 10% fenofibrate with 90% Klucel ELF under different RH conditions (10, 40 and 70%RH/60 °C).
The deliquescence phenomenon was also observed under high RH conditions. Study of the consequence of deliquescence on amorphous solid dispersion samples and hygroscopicity of polymer carriers will be helpful in developing solid dispersion system with better physical stability. Surface properties (e.g. surface free energy, surface area, etc.), water uptake ability and mechanisms of the polymer carrier44, might be the causes of different recrystallization inhibition abilities for the amorphous solid dispersion system under the same RH conditions, which will be the focus of our future studies.
Conclusion
The improved Avrami equation demonstrated more accurate evaluation for all of the solid dispersion systems investigated in this study. This is particularly evident for the late stages of the recrystallization process, which provides a novel approach for early stage formulation development of amorphous solid dispersion systems. This can be viewed as both time and cost effective when compared to the conventional ICH stability tests. By resolving the relationships between the recrystallization rate constant, temperature, RH and formulation, an accurate and reliable prediction can be obtained in reference to recrystallization kinetics. The polymers (Klucel EF/LF/ELF) inhibited the recrystallization process of the amorphous API and HPC grades with a higher molecular weight exhibited more favorable results. The method utilized in this study would also be useful for screening the most suitable polymeric carrier and other excipients in solid dispersion systems.
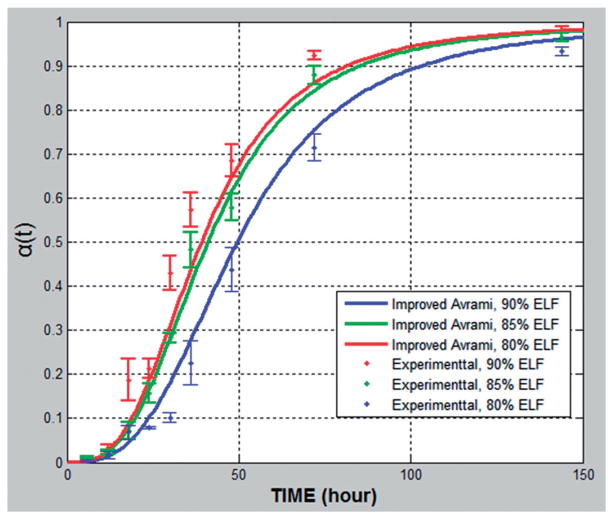
Figure 4.
Relative crystallinity α(t) plotted against time for formulations with different polymer content (90, 85 and 80% of Klucel ELF) under 60 °C /10%RH stability condition.
Acknowledgments
This project was partially supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Shah S, Maddineni S, Lu J, Repka MA. Melt extrusion with poorly soluble drugs. Int J Pharm. 2013;453:233–52. doi: 10.1016/j.ijpharm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Palanisamy M, Khanam J. Solid dispersion of prednisolone: solid state characterization and improvement of dissolution profile. Drug Dev Indus Pharm. 2011;37:373–86. doi: 10.3109/03639045.2010.513984. [DOI] [PubMed] [Google Scholar]
- 3.Jang DJ, Sim T, Oh E. Formulation and optimization of spray-dried amlodipine solid dispersion for enhanced oral absorption. Drug Dev Indus Pharm. 2013;39:1133–41. doi: 10.3109/03639045.2012.723218. [DOI] [PubMed] [Google Scholar]
- 4.Sarode AL, Wang P, Obara S, Worthen DR. Supersaturation, nucleation, and crystal growth during single- and biphasic dissolution of amorphous solid dispersions: polymer effects and implications for oral bioavailability enhancement of poorly water soluble drugs. Eur J Pharm Biopharm. 2014;86:351–60. doi: 10.1016/j.ejpb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Newman A, Knipp G, Zografi G. Assessing the performance of amorphous solid dispersions. J Pharm Sci. 2012;101:1355–77. doi: 10.1002/jps.23031. [DOI] [PubMed] [Google Scholar]
- 6.Sotthivirat S, McKelvey C, Moser J, et al. Development of amorphous solid dispersion formulations of a poorly water-soluble drug, MK-0364. Int J Pharm. 2013;452:73–81. doi: 10.1016/j.ijpharm.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Marsac PJ, Li T, Taylor LS. Estimation of drug-polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2009;26:139–51. doi: 10.1007/s11095-008-9721-1. [DOI] [PubMed] [Google Scholar]
- 8.Khan S, Elshaer A, Rahman AS, et al. Systems biology approach to study permeability of paracetamol and its solid dispersion. Int J Pharm. 2011;417:272–9. doi: 10.1016/j.ijpharm.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Cornaire G, Woodley J, Hermann P, et al. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int J Pharm. 2004;278:119–31. doi: 10.1016/j.ijpharm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Johnson BM, Charman WN, Porter CJ. An in vitro examination of the impact of polyethylene glycol 400, pluronic P85, and vitamin E da-tocopheryl polyethylene glycol 1000 succinate on P-glycoprotein efflux and enterocyte-based metabolism in excised rat intestine. AAPS Pharmsci. 2002;4:193–205. doi: 10.1208/ps040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Dai W-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2014;4:18–25. doi: 10.1016/j.apsb.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Repka MA, Battu SK, Upadhye SB, et al. Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Indus Pharm. 2007;33:1043–57. doi: 10.1080/03639040701525627. [DOI] [PubMed] [Google Scholar]
- 13.Bhugra C, Pikal MJ. Role of thermodynamic, molecular, and kinetic factors in crystallization from the amorphous state. J Pharm Sci. 2008;97:1329–49. doi: 10.1002/jps.21138. [DOI] [PubMed] [Google Scholar]
- 14.Yoshihashi Y, Iijima H, Yonemochi E, Terada K. Estimation of physical stability of amorphous solid dispersion using differential scanning calorimetry. J Therm Anal Calorim. 2006;85:689–92. [Google Scholar]
- 15.Crowley KJ, Zografi G. The effect of low concentrations of molecularly dispersed poly (vinylpyrrolidone) on indomethacin crystallization from the amorphous state. Pharm Res. 2003;20:1417–22. doi: 10.1023/a:1025706110520. [DOI] [PubMed] [Google Scholar]
- 16.Repka MA, Majumdar S, Kumar Battu S, et al. Applications of hot-melt extrusion for drug delivery. Expert Opin Drug Deliv. 2008;5:1357–76. doi: 10.1517/17425240802583421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahlneck C, Zografi G. The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state. Int J Pharm. 1990;62:87–95. [Google Scholar]
- 18.Hancock BC. Disordered drug delivery: destiny, dynamics and the Deborah number. J Pharm Pharmacol. 2002;54:737–46. doi: 10.1211/0022357021778989. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki T, Yoshioka S, Aso Y, Kawanishi T. Crystallization rate of amorphous nifedipine analogues unrelated to the glass transition temperature. Int J Pharm. 2007;336:191–5. doi: 10.1016/j.ijpharm.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 20.ICH I, editor. harmonised tripartite guideline, stability testing of new drug substances and products Q1A (R2). International Conference on Harmonisation; 2003; Geneva, Switzerland. [Google Scholar]
- 21.Avrami M. Kinetics of phase change. I: general theory. J Chem Phys. 1939;7:1103–12. [Google Scholar]
- 22.Avrami M. Kinetics of phase change. II: transformation-time relations for random distribution of nuclei. J Chem Phys. 1940;8:212–24. [Google Scholar]
- 23.Avrami M. Granulation, phase change, and microstructure kinetics of phase change. III. J Chem Phys. 1941;9:177–84. [Google Scholar]
- 24.Yang J, Grey K, Doney J. An improved kinetics approach to describe the physical stability of amorphous solid dispersions. Int J Pharm. 2010;384:24–31. doi: 10.1016/j.ijpharm.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 25.Van Speybroeck M, Mellaerts R, Mols R, et al. Enhanced absorption of the poorly soluble drug fenofibrate by tuning its release rate from ordered mesoporous silica. Eur J Pharm Sci. 2010;41:623–30. doi: 10.1016/j.ejps.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Mohammed NN, Majumdar S, Singh A, et al. Klucel EF and ELF polymers for immediate-release oral dosage forms prepared by melt extrusion technology. AAPS PharmSciTech. 2012;13:1158–69. doi: 10.1208/s12249-012-9834-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogt M, Kunath K, Dressman JB. Dissolution enhancement of fenofibrate by micronization, cogrinding and spray-drying: comparison with commercial preparations. Eur J Pharm Biopharm. 2008;68:283–8. doi: 10.1016/j.ejpb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Repka MA, Gutta K, Prodduturi S, Munjal M, Stodghill SP. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur J Pharm Biopharm. 2005;59:189–96. doi: 10.1016/j.ejpb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Deng W, Majumdar S, Singh A, et al. Stabilization of fenofibrate in low molecular weight hydroxypropylcellulose matrices produced by hot-melt extrusion. Drug Dev Indus Pharm. 2013;39:290–8. doi: 10.3109/03639045.2012.679280. [DOI] [PubMed] [Google Scholar]
- 30.Picker-Freyer KM, Dürig T. Physical mechanical and tablet formation properties of hydroxypropylcellulose: in pure form and in mixtures. AAPS PharmSciTech. 2007;8:82–90. doi: 10.1208/pt0803063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao C, Prasanth Chamarthy S, Byrn SR, Pinal R. A calorimetric method to estimate molecular mobility of amorphous solids at relatively low temperatures. Pharm Res. 2006;23:2269–76. doi: 10.1007/s11095-006-9071-9. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, McCoy BJ, Madras G. Distribution kinetics of polymer crystallization and the Avrami equation. J Chem Phys. 2005;122:122–32. doi: 10.1063/1.1844373. [DOI] [PubMed] [Google Scholar]
- 33.Yoshioka M, Hancock BC, Zografi G. Crystallization of indomethacin from the amorphous state below and above its glass transition temperature. J Pharm Sci. 1994;83:1700–5. doi: 10.1002/jps.2600831211. [DOI] [PubMed] [Google Scholar]
- 34.Sathigari SK, Radhakrishnan VK, Davis VA, et al. Amorphous-state characterization of efavirenz–polymer hot-melt extrusion systems for dissolution enhancement. J Pharm Sci. 2012;101:3456–64. doi: 10.1002/jps.23125. [DOI] [PubMed] [Google Scholar]
- 35.Baird JA, Taylor LS. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv Drug Deliv Rev. 2012;64:396–421. doi: 10.1016/j.addr.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Meena A, Parikh T, Gupta SS, Serajuddin ATM. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion – II: cellulosic polym0065rs. J Excip Food Chem. 2014;5:46–55. [Google Scholar]
- 37.Parikh T, Gupta SS, Meena A, Serajuddin A. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion – III: polymethacrylates and polymethacrylic acid based polymers. J Excip Food Chem. 2014;5:32–45. [Google Scholar]
- 38.Just S, Sievert F, Thommes M, Breitkreutz J. Improved group contribution parameter set for the application of solubility parameters to melt extrusion. Eur J Pharm Biopharm. 2013;85:1191–9. doi: 10.1016/j.ejpb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Djuris J, Nikolakakis I, Ibric S, Djuric Z, Kachrimanis K. Preparation of carbamazepine-Soluplus solid dispersions by hot-melt extrusion, and prediction of drug-polymer miscibility by thermodynamic model fitting. Eur J Pharm Biopharm. 2013;84:228–37. doi: 10.1016/j.ejpb.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Maniruzzaman M, Boateng JS, Chowdhry BZ, Snowden MJ, Douroumis D. A review on the taste masking of bitter APIs: hot-melt extrusion (HME) evaluation. Drug Dev Indus Pharm. 2014;40:145–56. doi: 10.3109/03639045.2013.804833. [DOI] [PubMed] [Google Scholar]
- 41.Baird JA, Olayo-Valles R, Rinaldi C, Taylor LS. Effect of molecular weight, temperature, and additives on the moisture sorption properties of polyethylene glycol. J Pharm Sci. 2010;99:154–68. doi: 10.1002/jps.21808. [DOI] [PubMed] [Google Scholar]
- 42.Good D, Miranda C, Rodríguez-Hornedo N. Dependence of cocrystal formation and thermodynamic stability on moisture sorption by amorphous polymer. CrystEngComm. 2011;13:1181–9. [Google Scholar]
- 43.Heljo VP, Nordberg A, Tenho M, et al. The effect of water plasticization on the molecular mobility and crystallization tendency of amorphous disaccharides. Pharm Res. 2012;29:2684–97. doi: 10.1007/s11095-011-0658-4. [DOI] [PubMed] [Google Scholar]
- 44.Bianco S, Tewes F, Tajber L, et al. Bulk, surface properties and water uptake mechanisms of salt/acid amorphous composite systems. Int J Pharm. 2013;456:143–52. doi: 10.1016/j.ijpharm.2013.07.076. [DOI] [PubMed] [Google Scholar]