Abstract
Individual differences in arousal experience have been linked to differences in resting-state salience network connectivity strength. In this study, we investigated how adding task-related skin conductance responses (SCR), a measure of sympathetic autonomic nervous system activity, can predict additional variance in arousal experience. Thirty-nine young adults rated their subjective experience of arousal to emotionally evocative images while SCRs were measured. They also underwent a separate resting-state fMRI scan. Greater SCR reactivity (an increased number of task-related SCRs) to emotional images and stronger intrinsic salience network connectivity independently predicted more intense experiences of arousal. Salience network connectivity further moderated the effect of SCR reactivity: In individuals with weak salience network connectivity, SCR reactivity more significantly predicted arousal experience, whereas in those with strong salience network connectivity, SCR reactivity played little role in predicting arousal experience. This interaction illustrates the degeneracy in neural mechanisms driving individual differences in arousal experience and highlights the intricate interplay between connectivity in central visceromotor neural circuitry and peripherally expressed autonomic responses in shaping arousal experience.
INTRODUCTION
Neuroscientists, psychologists, and philosophers agree that affect is a basic property of consciousness (Barrett & Bliss-Moreau, 2009; Wundt, 2009; Edelman & Tononi, 2000). As a component of affect, arousal (ranging from calm and relaxed to highly activated) is a fundamental element of mood and emotion (Barrett, Mesquita, Ochsner, & Gross, 2007; Barrett, 2006; Russell, 1980, 1991, 2003; Russell & Barrett, 1999) as well as other psychological phenomena such as perception and memory (Kensinger & Schacter, 2008; Phelps, 2006; Phelps, Ling, & Carrasco, 2006; Vuilleumier, 2005; Cahill & McGaugh, 1998). Arousal can also be considered a teaching signal that indicates an opportunity for learning, especially within the context of aversive learning (for a review, see Öhman & Mineka, 2001) Therefore, as arousal is a fundamental property of our experiences as we engage with the world around us, it is crucial to better understand its neural bases.
Several decades of research in psychophysiology have demonstrated that the experience of arousal can be related to electrodermal activity (EDA), a peripherally expressed index of the autonomic nervous system function, where larger and more numerous skin conductance responses (SCRs) are usually associated with more intense experiences of arousal (e.g., Mauss & Robinson, 2009; Lang, Bradley, & Cuthbert, 1998; Lang, Greenwald, Bradley, & Hamm, 1993; Greenwald, Cook, & Lang, 1989). The water, electrolytes, and sweat composing EDA are secreted by eccrine sweat glands located in all outer layers of the skin, and the activity of these glands is regulated by a series of neurons originating in the hypothalamus that make various connections in the brainstem, spinal cord, and sympathetic paravertebral ganglia before reaching and innervating the eccrine sweat glands (Critchley, 2002).
EDA provides a widely used index of sympathetic autonomic nervous system activity related to human emotions (Boucsein et al., 2012; Dawson, Schell, & Filion, 2000; Boucsein, 1992; Venables & Christie, 1980); various brain stimulation, lesion, and functional imaging studies have shown EDA to be subserved by a neural matrix involved in controlling sympathetic arousal that includes limbic structures such as amygdala, the insula, and the anterior cingulate (Tranel, 2000; Zahn, Grafman, & Tranel, 1999; Mangina & Beuzeron-Mangina, 1996; Tranel & Damasio, 1994; for a review, see Critchley, 2002). Specifically with regard to fMRI, greater task-evoked BOLD response in the amygdala, anterior insula, and ACC predicts greater magnitude or frequency of SCRs (Nagai, Critchley, Featherstone, Trimble, & Dolan, 2004; Williams et al., 2001; Critchley, Elliott, Mathias, & Dolan, 2000; Fredrikson et al., 1998; for a meta-analysis, see Beissner, Meissner, Bär, & Napadow, 2013).
Given activity in these regions is associated with EDA, and EDA itself is intimately linked to subjective arousal, it comes as no surprise that increased task-evoked activation measured by fMRI BOLD signals in these same regions also predicts more intense experiences of arousal when participants view emotionally evocative images or simulate emotionally evocative scenarios (Wilson-Mendenhall, Barrett, & Barsalou, 2013; Hermans et al., 2011; Moriguchi et al., 2011; Weierich, Wright, Negreira, Dickerson, & Barrett, 2010; Nielen et al., 2009; Lewis, Critchley, Rotshtein, & Dolan, 2007). These regions including the amygdala, anterior insula, anterior and mid cingulate cortex, OFC, and striatum are part of a large-scale intrinsic network known as the “salience network” (Touroutoglou, Hollenbeck, Dickerson, & Feldman Barrett, 2012; Seeley et al., 2007). In addition to task-evoked activation, resting-state fMRI studies show that individuals with greater intrinsic connectivity in the salience network will also report more intense experiences of arousal, as compared with individuals with weaker connectivity who report less intense experiences under the same conditions (Touroutoglou, Bickart, Barrett, & Dickerson, 2014; Touroutoglou et al., 2012). Furthermore, the connectivity strength between major nodes of this network has been implicated in different aspects of arousal such as anxiety (Seeley et al., 2007) and cortisol responsivity (Hermans et al., 2011).
These studies demonstrate that task-evoked and resting-state CNS function as well as task-evoked peripheral manifestation of autonomic nervous system activity all predict subjective arousal. However, no study to our knowledge has examined whether distinct neural substrates provide independent or redundant contributions. Recent work from our laboratory on task-evoked and resting-state CNS demonstrated that task-evoked and resting-state CNS provide independent contributions to arousal experience (Touroutoglou et al., 2014). Given that amygdala activation is linked to EDA, we may hypothesize that EDA, a peripheral manifestation of autonomic activity, and resting-state salience network connectivity will also independently predict arousal.
In this study, we tested this hypothesis by investigating both central and peripheral nervous system contributions to the experience of arousal. We studied healthy young adults and recorded their event-related SCRs while they viewed evocative images and reported their arousal experiences. Participants also underwent fMRI scanning at rest during which we measured the strength of intrinsic functional connectivity within the salience network. Specifically, we examined whether these peripheral and CNS measures reflect a common neural substrate for arousal (i.e., are strongly correlated with each other) or whether a larger number of task-related SCRs and stronger salience network connectivity each independently predict the intensity of arousal experience, and if so, whether salience network connectivity exerts its effect on arousal through SCR reactivity (a mediational relationship) or whether salience network connectivity influences the magnitude of the SCR reactivity contribution to arousal (a moderator relationship).
METHODS
Participants
Thirty-nine participants (ages 18–32 years, SD = 3.59; 20 women) were right-handed, native English speakers and had normal or corrected-to-normal vision. None reported any history of neurological or psychiatric condition, learning disability, or serious head trauma. Participants did not smoke or take medications or other psychoactive substances that could interfere with autonomic responsiveness such as beta-blockers or anticholinergic medications. All participants gave written informed consent in accordance with guidelines established by the Massachusetts General Hospital/Partners Human Research Committee.
Affective Image Task and EDA Monitoring
Ninety full-color images were selected from the International Affective Picture System (IAPS) and used to induce affective experiences (Lang, Bradley, & Cuthbert, 2008). They represented images that have been normed to evoke five combinations of valence and arousal experiences (i.e., negative valence–high arousal, positive valence–high arousal, neutral valence–low arousal, negative valence–low arousal, positive valence–low arousal). Normative valence and arousal ratings as well as examples of picture content are provided in Table 1.
Table 1.
Normative Ratings and Content Examples of Images
| Type of Image | Valencea Mean (SD) | Arousala Mean (SD) | Examples of Picture Content |
|---|---|---|---|
| Negative valence-high arousal (n = 15) | 1.42 (0.19) | 4.18 (0.21) | Mutilated human body parts, acts of violence toward humans or animals, public disasters |
| Positive valence-high arousal (n = 15) | 4.28 (0.37) | 4.22 (0.23) | Erotic acts, extreme sports, amusement park rides |
| Neutral valence-low arousal (n = 30) | 3.32 (0.15) | 2.27 (0.37) | Portraits of humans with neutral expressions, familiar home objects (e.g., wrenches, spoon), food items (e.g., crackers), mushrooms |
| Negative valence-low arousal (n = 15) | 2.68 (0.22) | 2.38 (0.29) | Humans expressing boredom or mild discomfort, objects associated with soiling (e.g., garbage cans, dirty cleaning utilities) |
| Positive valence-low arousal (n = 15) | 4.51 (0.32) | 2.43 (0.21) | Nonthreatening animals, pleasant human activities (e.g., family meals, picnics), flowers, landscapes |
Normative valence and arousal ratings displayed have been adjusted from the original 9-point scale to the 5-point scale used in this study.
Following sensor attachment for electrodermal monitoring, participants sat upright in a comfortable chair and completed the image task in a dimly lit room. They viewed each of the 90 IAPS images sequentially on a 120 × 75 cm high-definition (Sharp, Aquos) screen placed 2 m from the participant. Images were grouped into three blocks of 30 each, with images within each block fully randomized during stimulus presentation. For each stimulus, participants viewed the IAPS image for 6 sec and then rated the valence and arousal of the image using the Self-Assessment Manikin (Bradley & Lang, 1994). Only the arousal ratings are reported here, which ranged from “Very calm,” the lowest level, to “Very activated,” the highest. A variable intertrial interval of 10–15 sec during which participants viewed a small fixation cross on the screen followed the rating before presentation of the next image. Before beginning the task, participants were familiarized with the Self-Assessment Manikin rating procedure and practiced by rating five images. The images and rating scales were administered via E-Prime software (Psychology Software Tools, Pittsburgh, PA)
SCRs were recorded using disposable electrodermal electrodes (containing isotonic paste) affixed to the thenar and hypothenar eminences of the left hand, collected using Mindware’s data acquisition software (BioLab Acquisition Software version 3.0.13; Mindware Technologies, Gahanna, OH), and analyzed using Mindware’s data reduction software for EDA (Electrodermal Activity/Skin Conductance Analysis version 3.0.21; Mindware Technologies). For each 6-sec image, we measured the number of event-related phasic SCRs. We considered an SCR to be event-related if both the response onset and peak occurred between 1 and 6 sec after stimulus onset, with an onset to peak amplitude of at least 0.01 μS.
In line with the common finding that a substantial proportion of healthy adults are considered “stabile” electro-dermal responders (i.e., they produce relatively few if any SCRs; Schell, Dawson, & Filion, 1988), we excluded 8 of the 39 participants (20.5%), because they generated event-related SCRs to fewer than 5% of the IAPS images.
For each participant, we calculated the average number of event-related SCRs per image across all images that evoked high-arousal experiences (of both positive and negative valence based on published norms; Lang et al., 2008). To control for the electrodermal orienting response that can occur for any type of image, we subtracted from this average event-related SCRs to normatively high-arousal images the average event-related SCRs to normatively neutral images, creating a high-arousal SCR difference score (referred to hereafter as SCR Reactivity). This measure was our autonomic physiological variable of interest. Similarly, for each participant, we obtained a high-arousal subjective rating difference score (referred to hereafter as Subjective Arousal) by calculating the average arousal rating for all normatively high-arousal images and subtracting from this the average arousal rating for all normatively neutral images.
MRI Data Acquisition and Processing Procedures
Participants underwent brain imaging between 1 and 11 days (mean = 3.87, SD = 2.85) after completing the affective image task. Imaging data were collected on a 3T Magnetom Tim Trio system (Siemens Medical Systems, Iselin, NJ) at Massachusetts General Hospital, equipped for EPI with a 12-channel phased-array head coil. Head motion was minimized using head restraints, including a pillow and foam padding. Noise was attenuated with earplugs. Structural MRI data were acquired using a T1-weighted 3D MPRAGE sequence (repetition time/echo time/flip angle = 2530 msec/3.48 msec/7°, resolution = 1.0 mm isotropic). Whole-brain resting-state fMRI data were acquired with an echo-planar sequence (repetition time = 5000 msec; echo time = 30 msec; flip angle = 90°, 2.0 mm isotropic voxels, 55 slices; 76 time points per 384 sec [6:40 min] run). During the resting-state fMRI run, participants were directed to keep their eyes open without fixating and to remain as still as possible. As this study was part of a larger project on age-related differences in affect and memory, participants also completed a memory task in the scanner following the resting-state fMRI scan.
Preprocessing of the resting-state fMRI data involved a series of previously established resting-state functional connectivity MRI procedures (Van Dijk et al., 2010; Vincent et al., 2007; Biswal, Yetkin, Haughton, & Hyde, 1995). After removing the first four functional volumes, the following steps were completed: correction for slice-dependent time shifts (SPM2, Wellcome Department of Cognitive Neurology, London, United Kingdom), correction for head motion with rigid-body transformation in three translations and three rotations (FMRIB, Oxford, UK), spatial normalization to Montreal Neurological Institute (MNI) atlas space, resampling to 2-mm isotropic voxels, spatial smoothing using a 6-mm FWHM Gaussian kernel, and temporal band-pass filtering to remove frequencies >0.08 Hz. We then removed sources of spurious variance and their temporal derivatives from the data through linear regression (six parameters derived from the rigid body head motion correction, the signal averaged over a region within the deep white matter, and the signal averaged over the ventricles). In addition to these factors, we regressed out the signal averaged over the whole brain, as it has been suggested that the use of global signal regression in brain systems expected to show positive correlations between regions within a circuit (such as the salience network; Touroutoglou et al., 2012; Seeley et al., 2007) reduces certain elements of noise related to false positives (Fox, Zhang, Snyder, & Raichle, 2009; Weissenbacher et al., 2009). The residual BOLD time course was retained for functional connectivity analysis.
Task-free fMRI Analysis
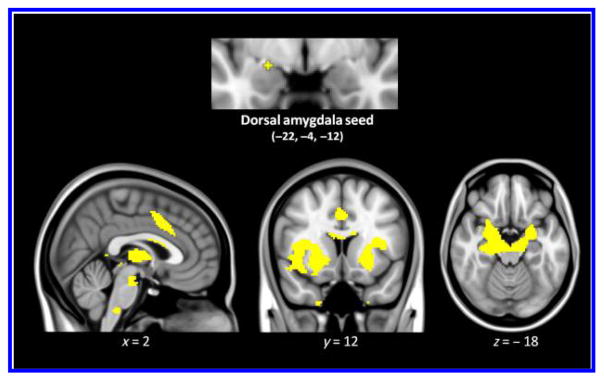
Seeley and colleagues first identified the low-frequency spontaneous BOLD fluctuations bilaterally in the salience network using a seed-based approach (Seeley et al., 2007). The dorsal amygdala has been connected with the salience network (Seeley et al., 2007) and has been associated with arousal feelings during tasks (Wilson-Mendenhall et al., 2013; Touroutoglou et al., 2012; Gerdes, 2010; Barrett, Bliss-Moreau, Duncan, Rauch, & Wright, 2007; Phan et al., 2004). In this study, we used a hypothesis-driven seed-based resting-state functional connectivity MRI analysis and created a spherical ROI (2-mm radius) around the dorsal amygdala (MNI coordinates: −22, −4, −12), as previously identified in Touroutoglou et al. (2014) and Bickart, Hollenbeck, Barrett, and Dickerson (2012). This seed ROI was used to generate a whole-brain map of Fisher’s r-to-z correlation coefficient values or connectivity strength expressed as z(r). Using the large-scale dorsal amygdala-anchored salience network mask (see Figure 1), as previously defined in an independent sample (Bickart et al., 2012), we calculated the salience network connectivity strength for each participant (referred to hereafter as Salience Network Connectivity), as the strength of task-free connectivity between the mean BOLD signal time course in the dorsal amygdala seed ROI and the mean BOLD signal time course in all of the voxels in the rest of the large-scale salience network. The averaged pairwise connectivity measure of z(r) values of the dorsal amygdala within the salience network was used for the brain–SCR–behavioral analyses. We removed data for one additional participant whose Salience Network Connectivity was an outlier (3 SDs below the group mean). We conducted subsequent analyses with the remaining 30 participants.
Figure 1.
The dorsal amygdala map, previously defined in Bickart et al. (2012), was used as the salience network mask binarized at z < 0.10. This map is overlaid on T1 MNI152 1.0 mm template brain in radiological convention to demonstrate the cortical and limbic structures within the salience network.
Relationships between Subjective Arousal, SCR Reactivity, and Salience Network Connectivity
To examine whether SCR Reactivity and Salience Network Connectivity are additive, have a mediational relationship, or interact with each other in predicting Subjective Arousal, we first performed bivariate correlations between Salience Network Connectivity, SCR Reactivity, and Subjective Arousal. We then performed a multiple regression analysis using both SCR Reactivity and Salience Network Connectivity as predictor variables and Subjective Arousal as the outcome variable: Salience Network Connectivity and SCR Reactivity were entered as predictors in the first step of a hierarchical regression analysis, and Subjective Arousal was entered as the dependent variable; the interaction term (Salience Network Connectivity × SCR Reactivity) was then added at the second step of the regression analysis. A simple slopes analysis was subsequently conducted to further explain the interaction effect. Statistical analyses were conducted using IBM SPSS Statistics Version 21. Results were considered statistically significant at p < .05.
RESULTS
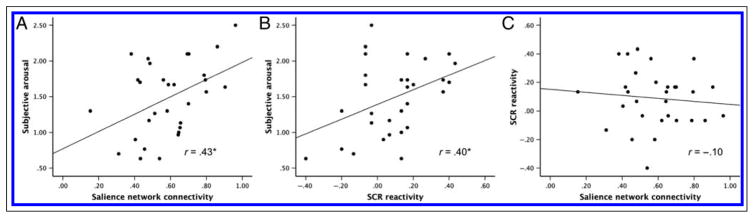
As predicted, individuals with stronger Salience Network Connectivity reported more intense experiences of Subjective Arousal while they viewed images known to normatively evoke both positive and negative high-arousal experience (r = 0.43, p < .02), replicating our prior findings in an independent sample (Touroutoglou et al., 2012, 2014). However, unlike our prior study, where resting-state fMRI was recorded just moments before participants viewed evocative images, in this study the measurement of Subjective Arousal and Salience Network Connectivity were measured several days apart, demonstrating the durability of that relationship. Individuals with greater SCR Reactivity also reported more intense Subjective Arousal (r = 0.40, p < .03). Salience Network Connectivity and SCR Reactivity were not correlated (r = −0.10, p < .61), which effectively ruled out any mediational relationship between these two predictors. Figure 2 depicts the pairwise relationships between Salience Network Connectivity, SCR Reactivity, and Subjective Arousal.
Figure 2.
A, B, and C illustrate the relationships between Salience Network Connectivity, SCR Reactivity, and Subjective Arousal. Each dot represents one individual participant. More intense Subjective Arousal is predicted by both stronger Salience Network Connectivity (A) and higher SCR Reactivity (B), whereas Salience Network Connectivity and SCR Reactivity were not related to each other (C). Negative values for SCR reactivity indicate that the participant generated, on average, more SCRs for neutral than highly arousing images. *p < .05.
To confirm that both Salience Network Connectivity and SCR Reactivity independently explained a comparable amount of the variance in ratings of Subjective Arousal, both predictors were centered and included together at the first step of a hierarchical regression analysis with Subjective Arousal as the dependent variable. Individuals with stronger Salience Network Connectivity (b = 1.332, SEb = 0.422, β = 0.478, p < .004) and greater SCR Reactivity (b = 1.146, SEb = 0.389, β = 0.446, p < .007) reported more intense experiences of Subjective Arousal. Together, the two predictors accounted for 39% of the variance in Subjective Arousal (F(2,27) = 8.5, p < .001; R2 = .39). To test whether Salience Network Connectivity moderated the extent to which SCR Reactivity predicted Subjective Arousal, the interaction term (Salience Network Connectivity × SCR Reactivity) was computed on the centered predictor variables and added at the second step of the regression analysis. The interaction significantly predicted the experience of Subjective Arousal, (b = −9.307, SEb = 2.218, β = −0.524, p < .001), explaining an additional 25% of the variance, with the overall model explaining 63% of the variance in Subjective Arousal [F(3, 26) = 15.01, p < .001, ΔR2 = .25, p < .001; Total R2 = .63)] (Table 2).
Table 2.
Full Regression Model Showing Interaction between Salience Network Connectivity and SCR Reactivity Predicts Subjective Arousal
| β | F(3, 26) | Total R2 | |
|---|---|---|---|
| Salience Network Connectivity | 0.46** | ||
| SCR Reactivity | 0.28* | 15.01** | .63 |
| Salience Network Connectivity × SCR Reactivity | −0.52** |
Standardized regression coefficients (β) of Salience Network Connectivity, SCR Reactivity, and their interaction entered as predictors in a hierarchical multiple linear regression analysis, where Subjective Arousal is the outcome.
p < .05.
p < .001.
This statistically significant interaction indicates that the contribution of SCR Reactivity to the prediction of the intensity of Subjective Arousal depends on the level of Salience Network Connectivity. To clarify this interaction, we conducted a simple slopes analysis probing the relationship between SCR Reactivity and Subjective Arousal in individuals with weak (−1 SD below the mean), moderate (mean), or strong (+1 SD above the mean) Salience Network Connectivity (Figure 3). In those with weak Salience Network Connectivity, greater SCR Reactivity strongly predicted more intense Subjective Arousal (b = 2.431, SEb = 0.433, β = 0.946, p < .001). In those with moderate Salience Network Connectivity, greater SCR Reactivity still significantly predicted more intense Subjective Arousal (b = 0.723, SEb = 0.322, β = 0.282, p < .034), but to a weaker extent. In contrast, in those with strong Salience Network Connectivity, SCR Reactivity was no longer related to Subjective Arousal (b = −0.984, SEb = 0.593, β = −0.383, p < .109).
Figure 3.

Higher SCR Reactivity predicts more intense Subjective Arousal for individuals with weak Salience Network Connectivity. This association is reduced at moderate Salience Network Connectivity and is absent at strong Salience Network Connectivity. Lines represent regression equations for the relationship between SCR Reactivity and Subjective Arousal at different levels of Salience Network Connectivity. Weak, moderate, and strong Salience Network Connectivity represent 1 SD below the mean, mean, and 1 SD above the mean values, respectively. Low, average, and high SCR Reactivity represent 1 SD below the mean, mean, and 1 SD above the mean values, respectively. *p < .05, ***p < .001.
DISCUSSION
Arousal is a fundamental property of consciousness that is a ubiquitous element of cognition, emotion, and perception; it is considered to be a core element of brain function in some constructs of the major brain–behavior domains (Cuthbert & Insel, 2013). Disturbed arousal experience has been linked to various mental illnesses such as anxiety disorders, depression, and bipolar disorders (for a review, see Posner, Russell, & Peterson, 2005), as well as medical disorders including coronary heart disease (Gianaros & Wager, 2015; Blascovich & Katkin, 1993; Matthews et al., 1986) and immunosuppression (Manuck et al., 1991; Kennedy, Glaser, & Kiecolt-Glaser, 1990). Given its centrality to our inner life, as well as mental and physical health and disease, it is important to understand the neurophysiological basis of arousal experience. Here, we found that, although sympathetic autonomic nervous system activity (SCR reactivity) did not correlate with CNS function (salience network resting-state functional connectivity), they nevertheless interacted in how they shape individual differences in arousal experience, such that across individuals, SCR reactivity contributes more to arousal experience when salience network connectivity is weak.
The lack of a simple correlation between salience network connectivity and SCR reactivity may in part reflect the multifaceted relationship between salience network and EDA. Although many lesion (Damasio, 1996; Tranel & Damasio, 1994) and functional imaging studies (Zhang et al., 2014; Fan et al., 2012; Critchley et al., 2000; Fredrikson et al., 1998; for a meta-analysis, see Beissner et al., 2013) in humans have shown that the generation and regulation of EDA is subserved by a large network of regions, which include key nodes of the salience network such as anterior cingulate, anterior insula, and amygdala, these regions may not uniformly influence EDA in the same manner. For example, activity in the cingulate cortex is positively correlated with EDA, whereas that in the insula is negatively correlated (Fredrikson et al., 1998). Differences in laterality may also be relevant, whereby damage to right-sided neural structures including the cingulate has a greater effect on EDA (Zahn et al., 1999). Therefore, the salience network and EDA relate to each other in myriad ways such that it precludes a simple correlative relationship between them and increases the likelihood that these two measures provide distinct information about subjective arousal.
The difference in how sympathetic and parasympathetic activity of the autonomic nervous system is reflected in salience network connectivity and SCR reactivity may also explain the independent relationship between the two measures. Although most regions forming the core of the salience network such as the amygdala, insula, and cingulate cortices are more closely associated with sympathetic function, some, such as the left amygdala and a small subregion in the right anterior insula, have been found to serve dual sympathetic and parasympathetic functions (Beissner et al., 2013). In addition, lesion studies in stroke and dementia patients as well as electrical stimulation studies in postsurgical epilepsy patients have argued for the right-sided regions of the salience network being predominantly involved in sympathetic function and the left-sided regions in parasympathetic function (Guo et al., 2016; Oppenheimer, Kedem, & Martin, 1996; Oppenheimer, Gelb, Girvin, & Hachinski, 1992). In contrast, SCR is a relatively pure measure of sympathetic nervous system activity (Critchley, 2002). Therefore, given that the salience network connectivity measure in our study likely reflects a combination of sympathetic and parasympathetic activity of individuals’ autonomic nervous system activity whereas that of SCR reactivity reflects predominantly the sympathetic branch, it is not surprising that the two did not correlate with each other.
The difference in timing between salience network connectivity and SCR reactivity likely also led to these two measures providing distinct contributions in shaping subjective arousal. Although SCR reactivity may reflect individuals’ baseline propensities to generate SCRs (Schell et al., 1988), the stimulus-induced EDA influenced by momentary exposure to emotional stimuli used in this study, as similar to others (Bradley, Codispoti, Cuthbert, Lang, & Bruce, 2001; Lang et al., 1993, 1998), more likely reflect a moment-to-moment or state measure of affective reactivity. In contrast, strength of connectivity of certain large-scale networks has been shown to be stable across different time points (Touroutoglou, Andreano, Barrett, & Dickerson, 2015; Shehzad et al., 2009); therefore, it seems reasonable to speculate that intrinsic network connectivity is a stable marker of trait-level differences between individuals. Although intrinsic connectivity has also been found to be malleable by recent experiences (Stevens, Buckner, & Schacter, 2010; Tambini, Ketz, & Davachi, 2010) and thus hypothesized as a state measure of affective reactivity, our findings showing that salience network connectivity predicts arousal even though the two were measured several days apart seem more consistent with the former position. Therefore, salience network connectivity and SCR reactivity, dispositional and momentary measures of affective reactivity, respectively, likely contributed distinct information in shaping arousal experience. In addition, a previous study from our laboratory using a similar paradigm showed that task-evoked fMRI amygdala activation and resting-state salience network connectivity were not correlated with each other, and independently predicted arousal experience, further argues that two measures of affective reactivity with distinct temporal properties will contribute independently to arousal experience (Touroutoglou et al., 2014).
Beyond contributing distinct information about arousal experience, SCR reactivity and salience network connectivity interacted with each other such that the relationship between SCR reactivity and arousal experience varies between individuals as a function of salience network connectivity: In individuals with weak salience network connectivity, a strong relationship exists between SCR reactivity and arousal—individuals generating more SCRs to normatively arousing stimuli report higher arousal; no such relationship exists in individuals with strong salience network connectivity—they report high arousal regardless of the number of SCRs generated. We and others have reported that some of the substantial differences between individuals in their arousal experience are attributable to differences in the strength of their salience network connectivity (Touroutoglou et al., 2012, 2014; Seeley et al., 2007). Our current findings demonstrate that a peripherally measured bodily signal indexing sympathetic autonomic nervous system activity—SCR reactivity—enables us to refine our understanding of the relationship between salience network connectivity and individual differences in subjectively reported arousal. SCR reactivity has been linked to arousal experience in psychophysiological studies (e.g., Mauss & Robinson, 2009; Lang et al., 1993, 1998; Greenwald et al., 1989), but the relationship of SCR reactivity to self-reported arousal has not yet been examined in conjunction with a measure of CNS activity. We found that the relative contribution of these two neurophysiological measures differed between individuals, an example of degeneracy—a phenomenon in which different processes within a larger biological system can lead to the same functional outcome (Edelman & Gally, 2001). This study is a first step toward understanding degeneracy in the neural mechanisms driving experiences of arousal across different individuals.
This interaction between salience network connectivity and SCR reactivity in predicting arousal experience may stem from the role the salience network plays in the afferent neural pathways of EDA. In addition to being implicated in eliciting and generating EDA through efferent pathways, regions of the salience network such as the in-sula, cingulate, and amygdala have also been postulated to represent afferent signals concerning peripheral autonomic states (Cechetto & Saper, 1990) via interoception, a process through which sensory signals (including those conveyed through autonomic afferents) that collectively describe the physiological state of the body’s internal milieu are integrated and perceived consciously (Critchley & Harrison, 2013; Craig, 2002). We may speculate that this representation of afferent signals from EDA is carried out by specific subregions or subsystems of the salience network. In our study, high salience network connectivity in certain individuals may therefore in part reflect an increased ability to accurately represent moment-to-moment peripheral physiological manifestations such as SCR and the variance measured in the SCR itself makes little to no additional contributions to explain differences in arousal experience. In contrast, lower resting salience network connectivity may reflect a poorer ability to represent afferent signals from peripheral physiological responses, and therefore, the signals themselves including SCR will play a greater role in explaining variances in arousal experience.
Future research should focus on clarifying mechanisms underlying this interaction between salience network connectivity and SCR reactivity, as well as how they relate to other neuroimaging and psychophysiological markers in predicting arousal experience. Furthermore, future studies should examine whether subregions of the salience network, as well as brain regions outside the salience network, interact differently with SCR reactivity in predicting subjective arousal and whether these differences relate to sympathetic versus parasympathetic function. For example, the Embodied Predictive Interoception Coding model recently proposed by Barrett and Simmons (2015), which posits that different layers and subdivisions of the limbic and paralimbic regions are involved in issuing efferent and representing afferent autonomic signals as well as resolving the discrepancy between the two, could serve as a foundation to test hypotheses regarding the interaction between salience network and SCRs in relation to arousal experience. We also need to better understand how salience network connectivity and SCR reactivity fit more broadly with contributions from other neurophysiological markers related to arousal, such as task-evoked activation of regions within the salience network (e.g., amygdala; Touroutoglou et al., 2014) or the structural integrity of regions within the salience network (e.g., amygdala and medial pFC; Holmes et al., 2012; Kim & Whalen, 2009). Beyond the salience network and SCR reactivity, measures from other neural regions and psycho-physiological markers may also further illuminate neural mechanisms of arousal experience. For example, the ventromedial pFC—usually classified as part of the default mode network—has visceromotor projection (Carrive & Morgan, 2012) and activity in this region has also been associated with EDA (Carrive & Morgan, 2012; Nagai et al., 2004; Patterson, Ungerleider, & Bandettini, 2002). Ultimately, a more thorough account of the neural systems physiology that shapes individual differences in arousal experience requires multimodal investigation of markers of both central and peripheral autonomic function under both task-evoked and resting-state conditions.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health (AG030311) to L. F. B. and B. C. D. C. X.’s effort was supported by a fellowship award from the Fonds de Recherche du Québec-Santé, and K. S. Q.’s effort was supported by the Department of Veterans Affairs (I01CX001053, PPO 14-144-2), the National Science Foundation (BCS-1422327), and the Army Research Institute (W5J9CQ-11-R-0017).
References
- Barrett LF. Solving the emotion paradox: Categorization and the experience of emotion. Personality and Social Psychology Review. 2006;10:20–46. doi: 10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E. Chapter 4. Affect as a psychological primitive. Advances in Experimental Social Psychology. 2009;41:167–218. doi: 10.1016/S0065-2601(08)00404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E, Duncan SL, Rauch SL, Wright CI. The amygdala and the experience of affect. Social Cognitive and Affective Neuroscience. 2007;2:73–83. doi: 10.1093/scan/nsl042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nature Reviews Neuroscience. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. Journal of Neuroscience. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. Journal of Neuroscience. 2012;32:14729–14741. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Katkin ES. Cardiovascular reactivity to psychological stress & disease. Washington, DC: American Psychological Association; 1993. Cardiovascular reactivity to psychological stress and disease: Conclusions; pp. 225–237. [Google Scholar]
- Boucsein W. Electrodermal activity. New York: Plenum; 1992. [Google Scholar]
- Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME, et al. Publication recommendations for electrodermal measurements. Psychophysiology. 2012;49:1017–1034. doi: 10.1111/j.1469-8986.2012.01384.x. [DOI] [PubMed] [Google Scholar]
- Bradley M, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ, Bruce N. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Carrive P, Morgan M. Periaqueductal gray. In: Mai JK, Paxinos G, editors. The human nervous system. 3. San Diego, CA: Academic Press; 2012. pp. 367–400. [Google Scholar]
- Cechetto D, Saper C. Role of the cerebral cortex in autonomic function. In: Loewy A, Spyer K, editors. Ventral regulation of autonomic functions. Oxford, UK: Oxford University Press; 1990. pp. 208–223. [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Electrodermal responses: What happens in the brain. Neuroscientist. 2002;8:132–142. doi: 10.1177/107385840200800209. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: A functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77:624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine. 2013;11:126–133. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Dawson M, Schell A, Filion D. The eletrodermal system. In: Cacioppo J, Tassinary L, Berntson G, editors. Handbook of psychophysiology. Cambridge, MA: Cambridge University Press; 2000. pp. 200–223. [Google Scholar]
- Edelman GM, Gally JA. Degeneracy and complexity in biological systems. Proceedings of the National Academy of Sciences, USA. 2001;98:13763–13768. doi: 10.1073/pnas.231499798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM, Tononi G. A universe of consciousness: How matter becomes imagination. New York: Basic Books; 2000. [Google Scholar]
- Fan J, Xu P, Van Dam NT, Eilam-Stock T, Gu X, Luo Y, et al. Spontaneous brain activity relates to autonomic arousal. Journal of Neuroscience. 2012;32:11176–11186. doi: 10.1523/JNEUROSCI.1172-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson M, Furmark T, Olsson MT, Fischer H, Andersson J, Långström B. Functional neuroanatomical correlates of electrodermal activity: A positron emission tomographic study. Psychophysiology. 1998;35:179–185. [PubMed] [Google Scholar]
- Gerdes A. Brain activations to emotional pictures are differentially associated with valence and arousal ratings. Frontiers in Human Neuroscience. 2010;4:175. doi: 10.3389/fnhum.2010.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD. Brain-body pathways linking psychological stress and physical health. Current Directions in Psychological Science. 2015;24:313–321. doi: 10.1177/0963721415581476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Cook EW, Lang PJ. Affective judgment and psychophysiological response: Dimensional covariation in the evaluation of pictorial stimuli. Journal of Psychophysiology. 1989;3:51–64. [Google Scholar]
- Guo CC, Sturm VE, Zhou J, Gennatas ED, Trujillo AJ, Hua AY, et al. Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proceedings of the National Academy of Sciences, USA. 2016;113:E2430–2439. doi: 10.1073/pnas.1509184113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJF, Ossewaarde L, Henckens MJAG, Qin S, van Kesteren MTR, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, et al. Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. Journal of Neuroscience. 2012;32:18087–18100. doi: 10.1523/JNEUROSCI.2531-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S, Glaser R, Kiecolt-Glaser J. Psychoneuroimmunology. In: Cacioppo JT, Tassinary LG, editors. Principles of psychophysiology: Physical, social and inferential elements. New York: Cambridge; 1990. pp. 177–190. [Google Scholar]
- Kensinger EA, Schacter DL. Memory and emotion. In: Lewis M, Haviland-Jones J, Barrett L, editors. Handbook of emotions. 2008. New York: Guilford Press; 2008. pp. 601–617. [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. Gainesville FL: NIMH, Center for the Study of Emotion & Attention; 2008. [Google Scholar]
- Lang PJ, Greenwald MC, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cerebral Cortex (New York, NY: 1991) 2007;17:742–748. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangina CA, Beuzeron-Mangina JH. Direct electrical stimulation of specific human brain structures and bilateral electrodermal activity. International Journal of Psychophysiology. 1996;22:1–8. doi: 10.1016/0167-8760(96)00022-0. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Cohen S, Rabin BS, Muldoon MF, Bachen EA, Manuck SB, et al. Individual differences in cellular immune response to stress. Psychological Science. 1991;2:111–115. [Google Scholar]
- Matthews KA, Weiss SM, Detre X, Dembroski TM, Falkner B, Manuck SB, et al. Handbook of stress, reactivity, and cardiovascular disease. New York: Wiley; 1986. [Google Scholar]
- Mauss IB, Robinson MD. Measures of emotion: A review. Cognition & Emotion. 2009;23:209–237. doi: 10.1080/02699930802204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich M, Dautoff R, Dickerson BC, Wright CI, et al. Differential hemodynamic response in affective circuitry with aging: An fMRI study of novelty, valence, and arousal. Journal of Cognitive Neuroscience. 2011;23:1027–1041. doi: 10.1162/jocn.2010.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: A physiological account of a “default mode” of brain function. Neuroimage. 2004;22:243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Nielen MMA, Heslenfeld DJ, Heinen K, Van Strien JW, Witter MP, Jonker C, et al. Distinct brain systems underlie the processing of valence and arousal of affective pictures. Brain and Cognition. 2009;71:387–396. doi: 10.1016/j.bandc.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clinical Autonomic Research. 1996;6:131–140. doi: 10.1007/BF02281899. [DOI] [PubMed] [Google Scholar]
- Patterson J, Ungerleider L, Bandettini P. Task-independent functional brain activity correlation with skin conductance changes: An fMRI study. Neuroimage. 2002;17:1797–1806. doi: 10.1006/nimg.2002.1306. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: A trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science. 2006;17:292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Russell JA, Peterson BS. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Development and Psychopathology. 2005;17:715–734. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. doi: 10.1037//0022-3514.79.2.286. [DOI] [PubMed] [Google Scholar]
- Russell JA. Culture and the categorization of emotions. Psychological Bulletin. 1991;110:426–450. doi: 10.1037/0033-2909.110.3.426. [DOI] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychological Review. 2003;110:145–172. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: Dissecting the elephant. Journal of Personality and Social Psychology. 1999;76:805–819. doi: 10.1037//0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- Schell AM, Dawson ME, Filion DL. Psychophysiological correlates of electrodermal lability. Psychophysiology. 1988;25:619–632. doi: 10.1111/j.1469-8986.1988.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, et al. The resting brain: Unconstrained yet reliable. Cerebral Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cerebral Cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Andreano JM, Barrett LF, Dickerson BC. Brain network connectivity-behavioral relationships exhibit trait-like properties: Evidence from hippocampal connectivity and memory. Hippocampus. 2015;25:1591–1598. doi: 10.1002/hipo.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Bickart KC, Barrett LF, Dickerson BC. Amygdala task-evoked activity and task-free connectivity independently contribute to feelings of arousal. Human Brain Mapping. 2014;35:5316–5327. doi: 10.1002/hbm.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D. Electrodermal activity in cognitive neuroscience: Neuroanatomical and neuropsychological correlates. In: Lane R, Nadel L, editors. Cognitive neuroscience of emotion. New York: Oxford University Press; 2000. pp. 192–224. [Google Scholar]
- Tranel D, Damasio H. Neuroanatomical correlates of electrodermal skin conductance responses. Psychophysiology. 1994;31:427–438. doi: 10.1111/j.1469-8986.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. Journal of Neurophysiology. 2010;2138:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables P, Christie M. Electrodermal activity. In: Martin I, Venables P, editors. Techniques in psychophysiology. New York: Wiley; 1980. pp. 3–67. [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF. Novelty as a dimension in the affective brain. Neuroimage. 2010;49:2871–2878. doi: 10.1016/j.neuroimage.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, et al. Arousal dissociates amygdala and hippocampal fear responses: Evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14:1070–1079. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- Wilson-Mendenhall CD, Barrett LF, Barsalou LW. Neural evidence that human emotions share core affective properties. Psychological Science. 2013;24:947–956. doi: 10.1177/0956797612464242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wundt W. Outlines of psychology (1897) In: Gentile BF, Miller BO, editors. Foundations of psychological thought: A history of psychology. New York: Sage; 2009. pp. 36–44. [Google Scholar]
- Zahn TP, Grafman J, Tranel D. Frontal lobe lesions and electrodermal activity: Effects of significance. Neuropsychologia. 1999;37:1227–1241. doi: 10.1016/s0028-3932(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Ide JS, Luo X, Farr OM, et al. Ventromedial prefrontal cortex and the regulation of physiological arousal. SCAN. 2014;9:900–908. doi: 10.1093/scan/nst064. [DOI] [PMC free article] [PubMed] [Google Scholar]