Abstract
Accumulating evidence suggests a key role of the gut–microbiota–brain axis in the antidepressant actions of certain compounds. Ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist, showed rapid and sustained antidepressant effects in treatment-resistant depressed patients. In contrast, another NMDAR antagonist, lanicemine, did not exhibit antidepressant effects in such patients. (R)-ketamine, the (R)-enantiomer of ketamine, has rapid-acting and long-lasting antidepressant effects in rodent models of depression. Here we compared the effects of (R)-ketamine and lanicemine on depression-like phenotype and the composition of the gut microbiota in susceptible mice after chronic social defeat stress (CSDS). In behavioral tests, (R)-ketamine showed antidepressant effects in the susceptible mice, whereas lanicemine did not. The 16S ribosomal RNA gene sequencing of feces demonstrated that (R)-ketamine, but not lanicemine, significantly attenuated the altered levels of Bacteroidales, Clostridiales and Ruminococcaceae in the susceptible mice after CSDS. At the genus level, (R)-ketamine significantly attenuated the marked increase of Clostridium in the susceptible mice. In contrast, the effects of lanicemine were less potent than those of (R)-ketamine. This study suggests that the antidepressant effects of (R)-ketamine might be partly mediated by the restoration of altered compositions of the gut microbiota in a CSDS model.
Introduction
Ketamine is an N-methyl-D-aspartate receptor (NMDAR) antagonist. Since the first report of its antidepressant effects in depressed patients by Berman et al.1, a number of clinical studies have replicated ketamine’s antidepressant effects in treatment-resistant unipolar2,3 and bipolar4,5 depression. Recent meta-analysis demonstrated that ketamine exhibits rapid and sustained antidepressant effects in treatment-resistant depressed patients6,7. Interestingly, ketamine demonstrated a rapid reduction of suicidal ideation in treatment-resistant depressed patients8,9. In contrast, it is well recognized that ketamine produces acute transient psychotomimetic side effects after single or repeated infusions1–5,10,11. Taken together, ketamine is the most prominent antidepressant for treatment-resistant depression, although the psychotomimetic side effects and abuse potential of ketamine should not be ignored12–19. Ketamine’s antidepressant actions are accompanied by the stimulation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), the mammalian target of rapamycin (mTOR), brain-derived neurotrophic factor (BDNF)-TrkB signaling, and increased synaptogenesis12,20–23. However, the mechanisms of action through which ketamine exerts its rapid-acting and sustained antidepressant effects are not fully understood.
Ketamine (Ki = 0.53 μM for NMDAR) is a racemic mixture comprising equal parts of (R)-ketamine and (S)-ketamine. (S)-ketamine (Ki = 0.30 μM for NMDAR) exhibits an approximately 3–4-fold greater binding affinity for NMDAR than (R)-ketamine (Ki = 1.40 μM for NMDAR), which pharmacologically explains the fact that (S)-ketamine has an approximately 4-fold greater anesthetic potency and greater undesirable psychotomimetic side effects than (R)-ketamine13–15,24. We demonstrated that (R)-ketamine has more potent and longer lasting antidepressant effects than (S)-ketamine in the animal models of depression25–29. Unlike (S)-ketamine, (R)-ketamine appears to lack psychotomimetic side effects and abuse potential30,31.
Lanicemine (AZD6765: (1 S)-1-phenyl-2-pyridin-2-ylethaneamine) (Ki = 0.56–1.5 μM for NMDAR) is a low-trapping NMDAR channel blocker sharing many pharmacological effects of ketamine at the NMDAR32,33. A single intravenous dose of lanicemine showed rapid but short-lived antidepressant effects in treatment-resistant depressed patients (n = 22)32. A subsequent phase IIb study reported that lanicemine (100 or 150 mg, three intravenous infusions per week for 3 weeks, as an adjunct to one antidepressant) was associated with a significant improvement of depressive symptoms in treatment-resistant depressed patients (n = 152)33. However, a recent phase IIb study using a larger sample size reported that lanicemine (50 or 100 mg, three intravenous infusions per week for 12 weeks, as an adjunct to one ongoing antidepressant) did not improve depressive symptoms in treatment-resistant depressed patients (n = 302)34. These data suggest no evidence to support the efficacy of lanicemine augmentation over placebo in treating treatment-resistant depressive symptoms. Collectively, it is unclear how NMDAR inhibition plays a role in the antidepressant properties of ketamine14,15.
Accumulating evidence suggests that the gut microbiota might play a role in the pathogenesis of depression and the antidepressant actions of antidepressants35–44. The present study was therefore conducted to examine whether the gut microbiota plays a role in the mechanisms underlying the antidepressant actions of (R)-ketamine and lanicemine in a chronic social defeat stress (CSDS) model.
Results
(R)-ketamine, but not lanicemine, shows rapid and sustained antidepressant effects in susceptible mice after CSDS
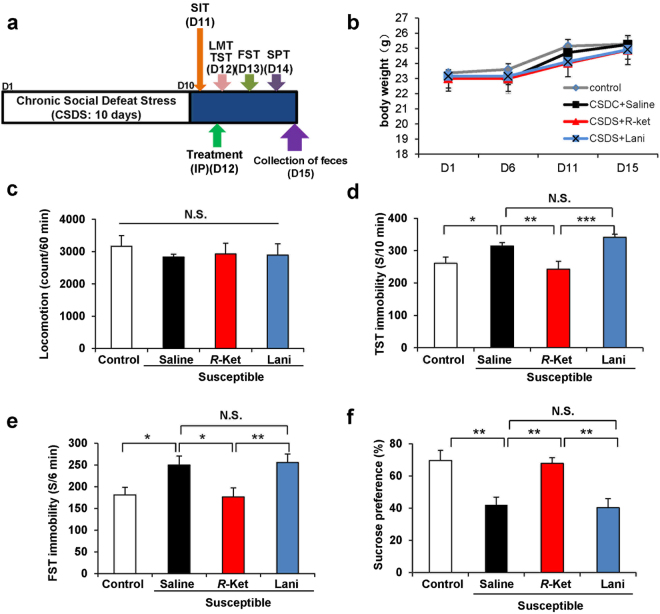
The social interaction test (SIT) after CSDS was performed on day 11 (Fig. 1a). In the SIT (no target), the social interaction time of two groups was the same (Fig. S1). In the SIT (target), we divided the susceptible or resilient mice by the evaluation of the time of mice in the interaction area (Fig. S1). The antidepressant effects of (R)-ketamine (10 mg/kg) and lanicemine (10 mg/kg) in susceptible mice after CSDS were examined (Fig. 1a). There were no changes in the body weight among the four groups (Fig. 1b). There were also no significant differences in the locomotion among the four groups (Fig. 1c). In the tail suspension test (TST) and forced swimming test (FST), (R)-ketamine, but not lanicemine, significantly decreased the increased immobility time in the susceptible mice (Fig. 1d and e). In the sucrose preference test (SPT), (R)-ketamine exerted potent anti-anhedonia effects two days after a single dose (Fig. 1f). These data indicate that (R)-ketamine exerts potent antidepressant and anti-anhedonia effects in a CSDS model, consistent with our previous reports26,28,29. In contrast, lanicemine did not show antidepressant effects in the CSDS model, consistent with the recent clinical results34.
Figure 1.
Effects of (R)-ketamine and lanicemine in the depression-like phenotype susceptible mice after CSDS. (a) The schedule of CSDS model, treatment, behavioral tests and feces collection. CSDS was performed from day 1 to day 10, and social interaction test (SIT) was performed on day 11. Saline (10 ml/kg), (R)-ketamine (10 mg/kg), or lanicemine (10 mg/kg) were administered i.p. into CSDS susceptible mice on day 12. Behavioral tests and SPT were performed form day 12 to day 14. On day 15, feces were collected. (b) Body weight (two-way ANOVA, time: F3,15 = 20.99, P < 0.001, treatment: F3,15 = 1.688, P = 0.176, interaction: F9,15 = 0.337, P = 0.960). (c–e) Behavioral tests including LMT (one-way ANOVA, F3,20 = 0.241, P = 0.867), TST (F3,20 = 7.025, P = 0.002) and FST (F3,20 = 4.722, P = 0.012) were performed. (f) SPT was performed 2 days after a single dose (F3,20 = 9.555, P < 0.001). Data are shown as mean ± S.E.M. (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001. NS: not significant.
The Principal Coordinate Analysis (PCoA) of the gut bacterium data
The PCoA analysis plots of Bray-Curtis dissimilarity among the four groups showed that the dots of (R)-ketamine treated group (c1-c6) were different from the dots of saline treated group (b1-b6) whereas the dots of lanicemine treated group (d1-d6) were similar to the dots of saline treated group (b1-b6) (Fig. 2a). Thus, it is likely that (R)-ketamine has more potency to improve the altered composition of gut microbiota after CSDS than lanicemine.
Figure 2.
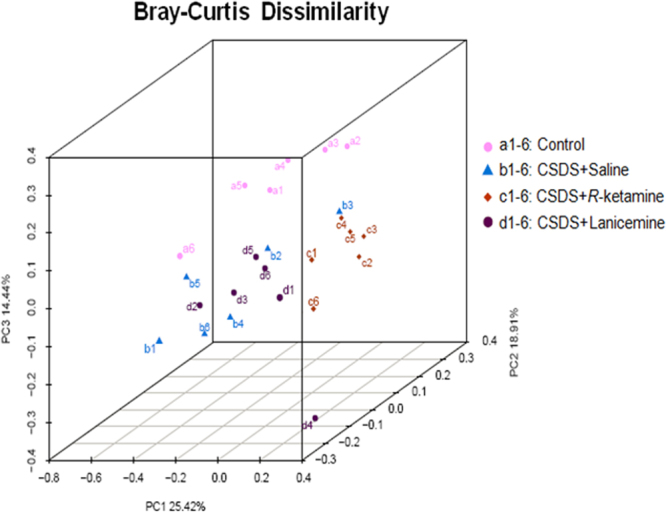
The PCoA of the gut bacterium data. The PCoA analysis plots of Bray-Curtis dissimilarity among the four groups (n = 6).
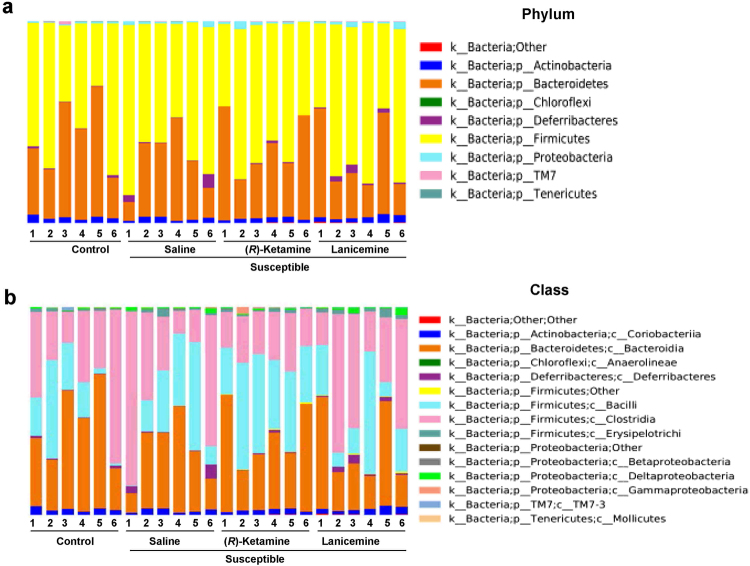
Composition in the gut bacteria at the levels of phylum and class
The compositions of gut bacterium of feces from all groups 3 days after a single dose of saline, (R)-ketamine or lanicemine were determined using 16S ribosomal RNA gene sequencing. At the level of phylum, the relative abundances of Bacteroidetes and Firmicutes were shown in the all groups (Fig. 3a). At the level of class, the relative abundances of Bacteroidia, Bacilli, and Clostridia were shown in all groups (Fig. 3b). At both levels, there were no significant differences among the four groups (Fig. 3a and b).
Figure 3.
Composition in the gut bacterium at the levels of phylum and class. (a) The relative abundances of phylum in fecal samples of the four groups 3 days after a single dose of saline, (R)-ketamine or lanicemine. The relative abundances of Bacteroidetes and Firmicutes were shown in the all groups. (b) The relative abundances of class in fecal samples of the four groups 3 days after a single dose of saline, (R)-ketamine or lanicemine. The relative abundances of Bacteroidia, Bacilli, and Clostridia were shown in the all groups. Data of each mouse are shown (n = 6).
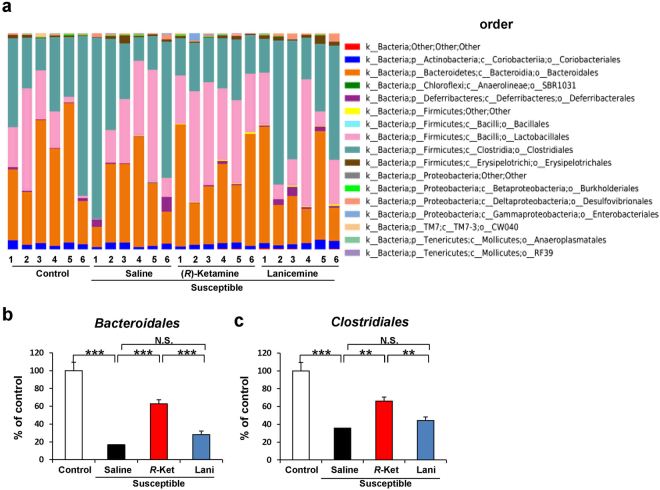
Altered composition in the gut bacteria at the order level
The order level of gut bacterium 3 days after a single dose of saline, (R)-ketamine or lanicemine are shown (Fig. 4a). At the order level, Bacteroidales and Clostridiales were significantly lower in the CSDS susceptible mice than in the control mice. Furthermore, (R)-ketamine significantly improved the reduced levels of Bacteroidales and Clostridiales in the CSDS susceptible mice (Fig. 4b and c). In contrast, lanicemine did not alter the levels of these two bacteria (Fig. 4b and c).
Figure 4.
Altered composition in the gut bacteria at the order level. (a) The relative abundances of order in fecal samples of the four groups 3 days after a single dose of saline, (R)-ketamine or lanicemine. (b)The order levels of Bacteroidales were significantly altered (one-way ANOVA: F3,20 = 45.166, P < 0.001). (c) The order level of Clostridiales were significantly altered (one-way ANOVA: F3,20 = 30.221, P < 0.001). Data are shown as mean ± S.E.M. (n = 6). **P < 0.01, ***P < 0.001. NS: not significant.
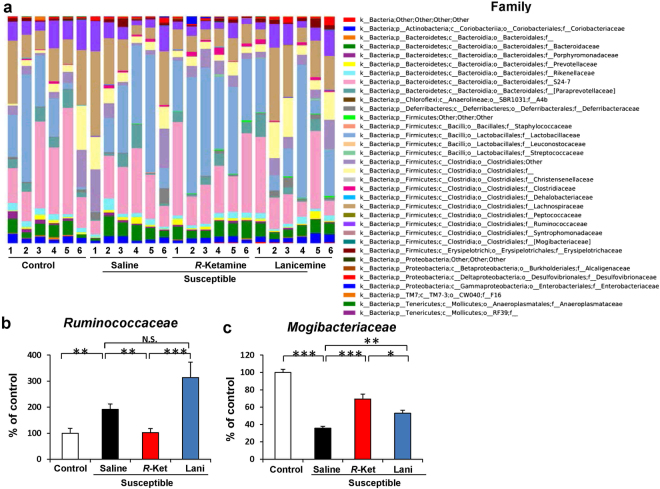
Altered composition in the gut bacteria at the family level
The family levels of gut bacterium 3 days after a single dose of saline, (R)-ketamine or lanicemine are shown (Fig. 5a). At the family level, levels of Ruminococcaceae were significantly increased in the susceptible mice after CSDS. Furthermore, (R)-ketamine, but not lanicemine, significantly attenuated the increased levels of Ruminococcaceae in the susceptible mice (Fig. 5b). In contrast, levels of Mogibacteriaceae were significantly decreased in the susceptible mice. Furthermore, both (R)-ketamine and lanicemine significantly attenuated the reduced levels of Mogibacteriaceae in the susceptible mice. Notably, (R)-ketamine was more potent than lanicemine at increasing the levels of Mogibacteriaceae in the susceptible mice (Fig. 5c).
Figure 5.
Altered composition in the gut bacteria at the family level. (a) The relative abundances of family in fecal samples of the four groups 3 days after a single dose of saline, (R)-ketamine or lanicemine. (b) The family levels of Ruminococaceae were significantly altered (one-way ANOVA: F3,20 = 32.341, P < 0.001). (c) The family levels of Mogibacteriaceae were significantly altered (one-way ANOVA: F3,20 = 49.097, P < 0.001). Data are shown as mean ± S.E.M. (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001. NS: not significant.
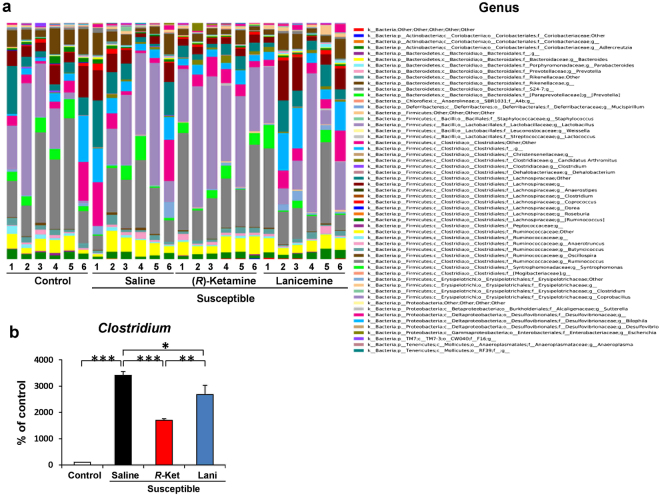
Altered composition in the gut bacteria at the genus level
The genus levels of gut bacterium 3 days after a single dose of saline, (R)-ketamine or lanicemine are shown (Fig. 6a). At the genus level, levels of Clostridium were dramatically increased in the susceptible mice after CSDS. Furthermore, both (R)-ketamine and lanicemine significantly attenuated the increased levels of Clostridium in the susceptible mice. Notably, (R)-ketamine was more potent than lanicemine in reducing the levels of Clostridium in the susceptible mice (Fig. 6b).
Figure 6.
Altered composition in the gut bacteria at the genus level. (a) The relative abundances of genus in fecal samples of the four groups 3 days after a single dose of saline, (R)-ketamine or lanicemine. (b) The genus levels of Clostridium were significantly altered (one-way ANOVA: F3,20 = 55.538, P < 0.001). Data are shown as mean ± S.E.M. (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The major findings of the present study are as follows: First, (R)-ketamine showed rapid and sustained antidepressant effects in a CSDS model of depression. In contrast, lanicemine did not show antidepressant effects in the same model. A previous study showed that inhibitory effects on in vivo [3H]MK-801 binding in the mouse brain by ketamine (10 mg/kg) and lanicemine (10 mg/kg) were the same35. It is, therefore, unlikely that NMDAR inhibition may play a role in the differential effects of (R)-ketamine and lanicemine although a further study is needed. Second, at the level of order, Bacteroidales and Clostridiales were significantly decreased in the CSDS susceptible mice. Interestingly, (R)-ketamine, but not lanicemine, significantly attenuated the reduced levels of Bacteroidales and Clostridiales in the susceptible mice. Third, at the family level, Ruminococcaceae and Mogibacteriaceae were significantly altered in the CSDS susceptible mice. Interestingly, (R)-ketamine, but not lanicemine, significantly attenuated the increased levels of Ruminococcaceae in the susceptible mice. Furthermore, both (R)-ketamine and lanicemine significantly attenuated the reduced levels of Mogibacteriaceae in the susceptible mice although the effects of lanicemine were less potent than (R)-ketamine. Finally, (R)-ketamine significantly attenuated the increased levels of Clostridium, the genus of bacteria, in the susceptible mice although the effects of lanicemine were less potent than (R)-ketamine. These findings suggest that altered composition of gut microbiota in the CSDS susceptible mice might play a role in the depression-like phenotype, and that the improvement of the altered composition of gut microbiota in the susceptible mice by (R)-ketamine might play, in part, a role in its rapid antidepressant effect.
At the order level, we found marked reductions of Bacteroidales and Clostridiales levels in the susceptible mice after CSDS. There was a report showing altered levels of Bacteroidales in patients with depression46. In addition, low levels of Bacteroidales are associated with obesity; which is related with depression through inflammation47,48. There was a significant increase in Bacteroidales in high-fat fed rats compared with low-fat fed rats49. Furthermore, levels of Bacteroidales were correlated significantly to better memory performance in the mice on a high-fat diet50, suggesting a possible link between food-related behaviors and gut microbiota. Collectively, it seems that reduced levels of Bacteroidales might play a role in the pathogenesis of depression.
The order Clostridiales include the polysaccharolytic and gram-positive bacteria that contribute strongly to the production of short chain fatty acids (e.g., butyrate) in the gut51. A randomized, double-blind, placebo-controlled study showed that probiotic intake markedly redistributed the microbial taxa of Clostridiales 52. In this study, we found that (R)-ketamine, but not lanicemine, significantly improved the reduced levels of Bacteroidales and Clostridiales in a CSDS model, consistent with the beneficial antidepressant effects of (R)-ketamine. Therefore, it is likely that the restoration of Bacteroidales and Clostridiales by (R)-ketamine may partly explain its robust antidepressant actions. Nonetheless, further studies underlying the role of Bacteroidales and Clostridiales in the antidepressant actions of (R)-ketamine are needed.
Ruminococcaceae is the family of the class Clostridia which are anaerobic, Gram-positive microbes. Ruminococcaceae is also known to be cellulolytic, as well as active in acetate, formate, and hydrogen production53. In healthy Japanese adults, the scores for bowel movement frequency were significantly correlated with the abundances of Mogibacteriaceae in the fecal microbiota54. Although the exact physiological functions of Ruminococcaceae and Mogibacteriaceae are not fully understood, it is likely that the altered composition of these microbiota in the gut may contribute to the pathogenesis of depression. Interestingly, we found that (R)-ketamine, but not lanicemine, significantly attenuated the increased levels of Ruminococcaceae in CSDS susceptible mice. We also found that both (R)-ketamine and lanicemine significantly attenuated the reduced levels of Mogibacteriaceae in CSDS susceptible mice. Interestingly, the increase in the levels of Mogibacteriaceae induced by (R)-ketamine was more potent than that by lanicemine. Although the precise physiological implications of Ruminococcaceae and Mogibacteriaceae in depression are unknown, it is likely that the restoration in the level of these bacteria induced by (R)-ketamine partially mediates its antidepressant action. Nonetheless, further studies on the relationship between (R)-ketamine’s antidepressant effects and these bacteria are needed.
Clostridium is a genus of Gram-positive bacteria, which includes several significant human pathogens, including the causative agent of botulism and an important cause of diarrhea Clostridium difficile. A study showed that adults with depression seem to be more likely to develop Clostridium difficile infection55. In addition, patients with depressive symptoms exhibit greater and more prolonged inflammatory responses after antigen challenge than subjects without depressive symptoms, suggesting that depression may result in immune dysregulation56. A recent study reported increased levels of Clostridium in actively-depressed patients compared to healthy subjects37. Furthermore, there was a negative correlation between Clostridium levels and serum levels of brain-derived neurotrophic factor in depressed patients37. In this study, we found marked increases of Clostridium in CSDS susceptible mice. We also found that (R)-ketamine significantly attenuated the increased levels of Clostridium in the susceptible mice. Interestingly, a decrease in the levels of Clostridium induced by (R)-ketamine was more potent than that by lanicemine. Taken together, it is likely that the restoration of Clostridium by (R)-ketamine may partly explain its robust antidepressant actions. Nonetheless, further studies on the role of the order Clostridiales, including the genus Clostridium, in depression and antidepressant actions of (R)-ketamine are needed.
The present data do not provide direct evidence of the effect of gut microbiota on the antidepressant actions of (R)-ketamine because behavioral experiments using germ-free mice were not performed. However, it is well known that the anti-microbial effects of currently available antidepressants are important for the correction of the intestinal dysbiosis observed in depressed patients45. Therefore, the gut microbiota–brain axis possibly plays a role in the antidepressant actions of (R)-ketamine. Nonetheless, additional studies elucidating the relationship between the gut microbiota axis and the antidepressant actions of (R)-ketamine are needed.
In conclusion, the present study suggests that the gut microbiota–brain axis might be associated with the antidepressant actions of (R)-ketamine, namely via (R)-ketamine-induced changes in the levels of the order Bacteroidales, Clostridiales; the family Ruminococcaceae, Mogibacteriaceae; and the genus Clostridium.
Methods and Materials
Animals
Male adult C57BL/6 mice, aged 8 weeks (body weight 20–25 g, Japan SLC, Inc., Hamamatsu, Japan) and male adult CD1 (ICR) mice, aged 13–15 weeks (body weight > 40 g, Japan SLC, Inc., Hamamatsu, Japan) were used. Animals were housed under controlled temperatures and 12 hour light/dark cycles (lights on between 07:00–19:00 h), with ad libitum food (CE-2; CLEA Japan, Inc., Tokyo, Japan) and water. The protocol was approved by the Chiba University Institutional Animal Care and Use Committee. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA.
Materials
(R)-ketamine hydrochloride was prepared by recrystallization of (R,S)-ketamine (Ketalar®, ketamine hydrochloride, Daiichi Sankyo Pharmaceutical Ltd., Tokyo, Japan) and D-(−)-tartaric acid, as described previously25. The purity of (R)-ketamine was determined by a high-performance liquid chromatography (CHIRALPAK® IA, Column size: 250 × 4.6 mm, Mobile phase: n-hexane/dichloromethane/diethylamine (75/25/0.1), Daicel Corporation, Tokyo, Japan). The dose (10 mg/kg as ketamine hydrochloride) of (R)-ketamine and lanicemine (AZD6765; Sigma-Aldrich Co. Ltd, St Louis, MO, USA) was used as previously reported26–30,57.
Chronic social defeat stress (CSDS) model
The procedure of CSDS was performed as previously reported26,28,29,57–59. Every day the C57BL/6 mice were exposed to a different CD1 aggressor mouse for 10 min, total for 10 days. When the social defeat session ended, the resident CD1 mouse and the intruder mouse were housed in one half of the cage separated by a perforated Plexiglas divider to allow visual, olfactory, and auditory contact for the remainder of the 24-h period. At 24 h after the last session, all mice were housed individually. On day 11, a social interaction test (SIT) was performed to identify subgroups of mice that were susceptible and unsusceptible (resilient) to social defeat stress. This was accomplished by placing mice in an interaction test box (42 × 42 cm) with an empty wire-mesh cage (10 × 4.5 cm) located at one end. The movement of the mice was tracked for 2.5 min, followed by 2.5 min in the presence of an unfamiliar aggressor confined in the wire-mesh cage. The duration of the subject’s presence in the “interaction zone” (defined as the 8-cm-wide area surrounding the wiremesh cage) was recorded by a stopwatch. The interaction ratio was calculated as time spent in an interaction zone with an aggressor/time spent in an interaction zone without an aggressor. An interaction ratio of 1 was set as the cutoff: mice with scores <1 were defined as “susceptible” to social defeat stress and those with scores ≥1 were defined as “unsusceptible”. Approximately 70–80% of mice were susceptible after CSDS (Fig. S1). Susceptible mice were randomly divided in the subsequent experiments. Control mice without social defeat stress were housed in the same cage before the behavioral tests.
Treatment and behavioral tests
Saline (10 ml/kg), (R)-ketamine (10 mg/kg) or lanicemine (10 mg/kg) was administered intraperitoneally (i.p.) into the susceptible mice after CSDS. Saline (10 ml/kg) was also administered i.p. into control mice (Fig. 1a). Behavioral tests, including locomotion test (LMT), tail suspension test (TST), forced swimming test (FST) and 1% sucrose preference test (SPT), were performed as reported previously26,28,29,57–59. Behavioral test were also performed by two observers who were blinded to the group assignment of mice. Each treatment group was equally represented in each experimental cohort.
Locomotion
The locomotor activity was measured by an animal movement analysis system SCANETMV-40 (MELQUEST Co., Ltd., Toyama, Japan). The mice were placed in experimental cages (length × width × height: 560 × 560 × 330 mm). The cumulative exercise was recorded for 60 minutes. Cages were cleaned between testing session.
TST
A small piece of adhesive tape placed approximately 2 cm from the tip of the tail for mouse. A single hole was punched in the tape and mice were hung individually, on a hook. The immobility time was recorded for 10 minutes. Mice were considered immobile only when they hung passively and completely motionless.
FST
The FST was tested by an automated forced-swim apparatus SCANETMV-40 (MELQUEST Co., Ltd., Toyama, Japan). The mice were placed individually in a cylinder (diameter: 23 cm; height: 31 cm) containing 15 cm of water, maintained at 23 ± 1 °C. Immobility time from activity time as (total) – (active) time was calculated by the apparatus analysis software. The immobility time for mouse was recorded for 6 minutes.
SPT
Mice were exposed to water and 1% sucrose solution for 48 h, followed by 4 hours of water and food deprivation and 1 hour exposure to two identical bottles, one is water, and another is 1% sucrose solution. The bottles containing water and sucrose were weighed before and at the end of this period. The sucrose preference was calculated as a percentage of sucrose solution consumption to the total liquid consumption.
16S rRNA analysis of fecal samples
The fecal samples were collected 3 days after a single dose of saline (10 ml/kg), (R)-ketamine (10 mg/kg) or lanicemine (10 mg/kg). They placed in 1.5 ml tubes, snap-frozen on dry ice and stored at −80 °C. The 16S rRNA analysis of fecal samples was performed at Takara Bio. Inc. (Shiga, Japan). The DNA extraction was performed using the MoBio Powerlyzer Powersoil DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA). The V4 hypervariable region of the bacterial 16S rRNA gene was amplified from the fecal DNA extracts using modified universal bacterial primer pairs 515 F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGCCAGCMGCCGCGGTAA-3′) and 806 R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT-3′) with Illumina adaptor overhang sequences. Amplicons were generated, cleaned, indexed and sequenced according to the Illumina MiSeq 16S Metagenomic Sequencing Library Preparation protocol (http://support.illumina.com/ downloads/16s_metagenomic_sequencing_library_preparation.html) with certain modifications. Sequencing data were combined and sample identification assigned to multiplexed reads using the MOTHUR software environment42,60,61. The data were denoised; low quality sequences, pyrosequencing errors, and chimeras were removed, and then sequences were clustered into operational taxonomic units (OTUs) at 97% identity using the CD-HITOTU pipeline (available from http://eeizhong-lab.ucsd.edu/cd-hit-otu)43,61,62. OTUs containing fewer than four reads per individual diet/animal combination were excluded due to the likelihood of there being a sequencing artifact. The samples were normalized by randomly resampling sequences used to the lowest number of sequences per sample (each diet/animal combination) using Daisychopper (http://www.festinalente.me/bioinf/). Taxonomic classification of OTUs was conducted using the Ribosomal Database Project Classifier43,61,63.
Statistical analysis
The data show as the mean ± standard error of the mean (S.E.M.). Analysis was performed using PASW Statistics 20 (formerly SPSS Statistics; SPSS, Tokyo, Japan). The data were analyzed using the one-way analysis of variance (ANOVA) or two-way ANOVA, followed by post-hoc Tukey test. Furthermore, Principal Coordinate Analysis (PCoA) was performed to visualize similarities or dissimilarities of the data of four groups. The P-values of less than 0.05 were considered statistically significant.
Electronic supplementary material
Acknowledgements
This study was supported by the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and Development, AMED (to K.H.) and National Natural Science Foundation of China (to C.Y., No.: 81703482). Drs Chun Yang and Qian Ren were supported by Research Fellowship of the Japan Society for the Promotion of Science (JSPS) (Tokyo, Japan). Ms. Min Ma was supported by the Nurture of Creative Research Leaders in Immune System Regulation and Innovative Therapeutics Program of Chiba University. Dr. Chao Dong was supported by the Uehara Memorial Foundation (Tokyo, Japan).
Author Contributions
K.H. designed the study and wrote the protocol. Y.Q., C.Y., Q.R., M.M., and C.D. performed all the pharmacological experiments. Y.Q. and C.Y. undertook the statistical analysis. Y.Q. and K.H. wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Competing Interests
Dr. Hashimoto is an inventor on a filed patent application on “The use of R-ketamine in the treatment of psychiatric diseases” by Chiba University. Dr. Hashimoto has received research support from Dainippon Sumitomo, Mochida, Otsuka, and Taisho. Other authors declare no conflict of interest.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16060-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 2.Zarate CA, Jr, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 3.Murrough JW, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diazgranados N, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarate CA, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newport DJ, et al. APA Council of Research Task Force on Novel Biomarkers and Treatments (2015): Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172:950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto T, et al. Single-dose infusion ketamine and non-ketamine N-methyl-D-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46:1459–1472. doi: 10.1017/S0033291716000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diazgranados N, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price RB, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014;31:335–343. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–241. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 11.Singh JB, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant repression. Am J Psychiatry. 2016;173:816–826. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 12.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without risk of brain toxicity. Psychol Med. 2016;46:2449–2451. doi: 10.1017/S0033291716000969. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto K. Ketamine’s antidepressant action: beyond NMDA receptor inhibition. Expert Opin Ther Targets. 2016;173:1389–1392. doi: 10.1080/14728222.2016.1238899. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto K. Chapter 4. Rapid antidepressant activity of ketamine beyond NMDA receptor. In: Hashimoto K, editor. The NMDA Receptors, Humana Press, New York. pp.69–81 (2017).
- 16.Newport DJ, Schatzberg AF, Nemeroff CB. Whither ketamine as an antidepressant: panacea or toxin? Depress Anxiety. 2016;33:685–688. doi: 10.1002/da.22535. [DOI] [PubMed] [Google Scholar]
- 17.Yang C, Hashimoto K. Rapid antidepressant effects and abuse liability of ketamine. Psychopharmacology (Berl) 2014;231:2041–2042. doi: 10.1007/s00213-014-3543-0. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto K. Detrimental side effects of repeated ketamine infusions in the brain. Am J Psychiatry. 2016;173:1044–1045. doi: 10.1176/appi.ajp.2016.16040411. [DOI] [PubMed] [Google Scholar]
- 19.Sanacora G, et al. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry. 2017;74:399–405. doi: 10.1001/jamapsychiatry.2017.0080. [DOI] [PubMed] [Google Scholar]
- 20.Maeng S, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2012;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113:678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 25.Zhang JC, Li SX, Hashimoto K. R(−)-ketamine shows greater potency and longer lasting antidepressant effects than S(+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Yang C, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukumoto K, et al. Antidepressant potential of (R)-ketamine in rodent models: Comparison with (S)-ketamine. J Pharmacol Exp Ther. 2017;361:9–16. doi: 10.1124/jpet.116.239228. [DOI] [PubMed] [Google Scholar]
- 28.Yang C, et al. (R)-ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry. 2017;82:e43–e44. doi: 10.1016/j.biopsych.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Yang C et al. Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry, 10.1016/j.biopsych.2017.05.016. [DOI] [PubMed]
- 30.Yang C, Han M, Zhang JC, Ren Q, Hashimoto K. Loss of parvalbumin-immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R-ketamine. Psychiatry Res. 2016;239:281–283. doi: 10.1016/j.psychres.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto K, Kakiuchi T, Ohba H, Nishiyama S, Tsukada H. Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci. 2017;267:173–176. doi: 10.1007/s00406-016-0692-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarate CA, Jr., et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanacora G, et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. 2014;19:978–985. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanacora G, et al. Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology. 2017;42:844–853. doi: 10.1038/npp.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes A, et al. Inhibition of in vivo [3H]MK-801 binding by NMDA receptor open channel blockers and GluN2B antagonists in rats and mice. Eur J Pharmacol. 2015;766:1–8. doi: 10.1016/j.ejphar.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 36.Kelly JR, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbiota axis: challenges for translation in psychiatry. Ann Epidemiol. 2016;26:366–372. doi: 10.1016/j.annepidem.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Wong ML, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21:797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng P, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 40.Dinan TG, Cryan JF. Mood by microbe: towards clinical translation. Genome Med. 2016;6:36. doi: 10.1186/s13073-016-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers GB, et al. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang C, et al. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci Rep. 2017;7:45942. doi: 10.1038/srep45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang JC, et al. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl Psychiatry. 2017;7:e1138. doi: 10.1038/tp.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burokas A et al. Biol Psychiatry82, 472–487 (2017). [DOI] [PubMed]
- 45.Macedo D, et al. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord. 2017;208:22–32. doi: 10.1016/j.jad.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Naseribafrouei A, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 47.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;44:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 48.de La Serre CB, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pyndt Jørgensen B, et al. A possible link between food and mood: dietary impact on gut microbiota and behavior in BALB/c mice. PLoS One. 2014;9:e103398. doi: 10.1371/journal.pone.0103398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry. 2003;54:330–337. doi: 10.1016/S0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- 51.Chinda D, et al. The fermentation of different dietary fibers is associated with fecal clostridia levels in men. J Nutr. 2004;134:1881–1886. doi: 10.1093/jn/134.8.1881. [DOI] [PubMed] [Google Scholar]
- 52.Ferrario C, et al. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr. 2014;144:1187–1796. doi: 10.3945/jn.114.197723. [DOI] [PubMed] [Google Scholar]
- 53.Biddle AS, Black SJ, Blanchard JL. An in vitro model of the horse gut microbiome enables identification of lactate-utilizing bacteria that differentially respond to starch induction. PLoS One. 2013;8:e77599. doi: 10.1371/journal.pone.0077599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oki K, et al. Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol. 2016;16:284. doi: 10.1186/s12866-016-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers MA, et al. Depression, antidepressant medications, and risk of Clostridium difficile infection. BMC Med. 2013;11:121. doi: 10.1186/1741-7015-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 57.Zhang JC, et al. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2015;232:4325–4335. doi: 10.1007/s00213-015-4062-3. [DOI] [PubMed] [Google Scholar]
- 58.Yang B, et al. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2016;233:3647–3657. doi: 10.1007/s00213-016-4399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren Q, et al. Gene deciency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci USA. 2016;113:E1944–1952. doi: 10.1073/pnas.1601532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schloss PD, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang C et al. Possible role of gut-microbiota in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry in press (2017). [DOI] [PMC free article] [PubMed]
- 62.Li W, Fu L, Niu B, Wu S, Wooley J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief Bioinform. 2012;13:656–668. doi: 10.1093/bib/bbs035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.