Abstract
Understanding human mating patterns, which can affect population genetic structure, is important for correctly modeling populations and performing genetic association studies. Prior studies of assortative mating in humans focused on trait similarity among spouses and relatives via phenotypic correlations. Limited research has quantified the genetic consequences of assortative mating. The degree to which the non-random mating influences genetic architecture remains unclear. Here, we studied genetic variants associated with human height to assess the degree of height-related assortative mating in European-American and African-American populations. We compared the inbreeding coefficient estimated using known height associated variants with that calculated from frequency matched sets of random variants. We observed significantly higher inbreeding coefficients for the height associated variants than from frequency matched random variants (P < 0.05), demonstrating height-related assortative mating in both populations.
Introduction
Human mate choice is relevant to a wide range of scientific disciplines, including biology, sociology, population genetics, evolutionary biology, and psychology1–5. Physical location, race, religion, ancestry, socioeconomic status (SES) and physical characteristics all influence mate choice3,6–9. Assortative mating, a phenomenon in which people choose mates with similar phenotypes to theirs in terms of physical traits and/or socio-cultural factors, is the most common deviation from random mating in Western societies6,10,11. Assortative mating studies have examined a wide array of factors for diverse purposes11. In general, age, education, race, religion and ethnic background show the strongest degree of assortative mating3,11–19. In addition to underlying biological traits, patterns of mate selection is often affected by the distribution of wealth and socioeconomic status, and taken together can impact on genetic structures of traits in a population if they are associated with genetic variation11,16,20,21.
From the population genetics perspective, assortative mating can affect heritability estimates, create correlations among traits that were initially unrelated and affect trait variance within and between families22. A key outcome of assortative mating is that it increases homozygosity of variants associated with traits that affect mate choice and causes an increase in genetic variance in a population and the corresponding trait variance, but does not change the allele frequencies unless the genetic variants are under differential selection5. When a trait forms a basis on which to select mates, it will inflate the estimated heritability for this trait based on parent-offspring studies23,24. If parental traits are correlated, then the offspring will have a higher probability of having the same alleles that affect the trait compared to their genomic backgrounds. In contrast, when estimating the heritability from twin studies, it is assumed that monozygotic (MZ) twins are genetically identical and share 100% of their genetic patterns, and dizygotic (DZ) twins share half of their genomes. Therefore, assortative mating does not affect trait correlation between MZ twins because MZ twins are genetically identical, but increases the correlation between DZ twins. As a result, assortative mating reduces the difference between MZ and DZ correlations, and which may lead to an underestimated heritability, if mating patterns are ignored6,25,26.
Assortative mating can create correlations between previously uncorrelated traits when these traits are involved in the mating selection preference11,16. Without accounting for assortative mating in genetic association studies, spurious associations may be observed for loci involved in the assortative mating process, and thus lead to an inflated false positive rate27,28. Another important aspect of assortative mating is that it increases the correlations between relatives for traits involved in mate choice, thereby increasing between-family variance11. Without properly modeling assortative mating, parameter estimates in association studies could be biased. Lastly, variants involved in assortative mating may be incorrectly eliminated from analyses because they violate Hardy-Weinberg equilibrium.
Among the traits that affect mate choice, e.g., education, SES, skin color, height is one that has been shown to be highly heritable, has a polygenic architecture, and is well studied genetically5,10. And because height has been associated with a range of health problems, such as cancers29, heart disease30, stroke31 and Alzheimer’s disease32, understanding how mate choice affects genotypes associating loci may help us to interpret results for these other traits as well. The estimated heritability of height is approximately 0.80 based on full-sib pair analysis33, but may be overestimated due to shared common environmental factors. Large GWAS studies identified common variants that together explain 50% to 60% of the heritability of adult height34–36. Genome wide association studies have identified about 700 variants associated with human height in individuals of European-ancestry34,37. These variants cumulatively explain approximately one fifth of the phenotypic variation in height and provide the most complete description of the genetic bases of a polygenic effect in humans. Although numerous height loci have been identified by GWA studies in Europeans, fewer have been reported in African-American populations, possibly because of smaller sample sizes and small estimated effect sizes of individual variants38,39. There is some debate whether spouse similarity for height can be explained by ancestry assortative mating7,40. Sebro et al.3 noted ancestry assortative mating in European Americans reflects a North-South European cline, which correlates with height. A recent study by the same group indicated that the height-related assortative mating is smaller than that for assortative mating by ancestry41. Thus, it is unclear whether assortative mating for height can be separated from the assortative mating for ancestry. Since assortative mating for height will only affect loci that contribute to height variation (and those in linkage disequilibrium with them), the genotype distributions of the identified height associated variants can be used to evaluate the evidence of assortative mating. In this study, we sought to quantify the genetic bases of height-related assortative mating by estimating the inbreeding coefficients of the height associated variants as compared to expectations for non-height associated loci. Simply, we tested the hypothesis that height associated variants have larger inbreeding coefficients than those for other loci in the genome. Results consistent with this hypothesis can provide complementary evidence that these variants are in fact height associated as it has previously been shown that deviations from Hardy Weinberg Equilibrium can provide independent evidence for association42–45.
Results
Spouse correlations of heights in CFS
The Cleveland Family Study (CFS) is an epidemiologic longitudinal study of participants who reside in Cleveland, Ohio. CFS recruited 645 European-Americans from 139 families and 652 African-Americans from 147 families46. We first calculated the height correlations between spouses. Table 1 shows the interclass spouse correlations in European-American and African-American cohorts in CFS. As expected, both European-Americans and African-Americans have a high height spouse correlation: r = 0.4 (P < 0.001) for European Americans and r = 0.24 (P = 0.14) for African Americans. The correlation in the African-American cohort was not significant, which was likely due to the smaller number of spouse-pairs (n = 39). Since ages of spouses may contribute to the height spouse correlation, we also calculated height residuals after adjusting for age in CFS founders. The height residual correlations between spouses are similar to those without adjusting for age (Table 1). The spouse height correlations provide support for height-related assortative mating in the European American cohort and modest support in the African American cohort.
Table 1.
Spousal Correlations of height in the CFS cohorts.
| Cohort | Correlation before adjusting for age | Correlation after adjusting for age | Number of spouse pairs | ||||
|---|---|---|---|---|---|---|---|
| Correlation | 95% CI | P-value | Correlation | 95% CI | P-value | ||
| CFS European | 0.40 | (0.21,0.57) | 1.3 × 10−04 | 0.38 | (0.19,0.55) | 2.8 × 10−04 | 85 |
| CFS African | 0.24 | (−0.08,0.52) | 0.14 | 0.14 | (−0.19,0.44) | 0.40 | 39 |
CFS–Cleveland Family Study.
CI–Confidence Interval.
Genetic impact of height-related assortative mating
We estimated the inbreeding coefficients of height associating SNPs in the two European-American cohorts and five African-American cohorts by maximizing the likelihood in equations (2) and (3) (See Analytical Methods). For European-American populations, we obtained the 697 independent height associated SNPs from the European GWAS of the Genetic Investigation of Anthropometric Traits (GIANT) Consortium34. These 697 independent variants are located in 432 loci, and their corresponding genes are enriched in biological pathways for human skeletal growth. Among the 697 height-associated SNPs, 196 and 270 SNPs were directly genotyped in ARIC and CFS cohorts, respectively. An additional 315 and 325 SNPs could be replaced by proxy SNPs based on LD (r 2 > 0.9) derived using the 1000 G reference panel, which provides 511 and 595 height-associated SNPs for the two European-American cohorts, respectively (Table 2). Since height is a polygenic trait, we further selected the 2,500 and 5,000 independent SNPs with smallest P-values from the GWAS of the GIANT consortium34, respectively. We calculated the inbreeding coefficients using the 2,500 and 5,000 independent SNPs and compared these to frequency matched random SNPs in ARIC European cohort.
Table 2.
Comparison of inbreeding coefficient estimated from height associated variants with randomly sampled frequency matched variants: single locus analysis.
| Populations | Height-associated SNPs | Randomly sampled Frequency matched SNPs | P-value | Sample size | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (sd) | # snp | Mean (sd) | # snp available for resampling | KS-test* | T-test | |||
| European American | ARIC | −1.137 × 10−03 (1.7 × 10−02) | 521 | −3.296 × 10–03 (1.5 × 10–02) | 68,423 | 4.18 × 10−01 | 6.14 × 10−01 | 6,787 |
| 6.02 × 10−04 (1.4 × 10−02) | 2,500 | −2.125 × 10−03 (1.47 × 10−02) | 1.65 × 10−05 | 3.74 × 10−09 | ||||
| 6.5 × 10−04 (1.4 × 10−02) | 5,000 | −1.801 × 10−03 (1.46 × 10−02) | 8.08 × 10−08 | 3.41 × 10−15 | ||||
| CFS | 4.173 × 10–03 (7.6 × 10–02) | 595 | −4.917 × 10–03 (7.6 × 10–02) | 64,749 | 1.23 × 10–09 | 3.83 × 10–03 | 171 | |
| African American | CARDIA | 9.764 × 10–03 (6.6 × 10–02) | 158 | −2.21 × 10–03 (4.0 × 10–02) | 139,703 | 1.17 × 10–02 | 2.31 × 10–02 | 828 |
| MESA | 1.276 × 10–02 (9.4 × 10–02) | 168 | 2.445 × 10–04 (3.1 × 10–02) | 141,317 | 1.43 × 10–02 | 2.96 × 10–02 | 1,147 | |
| JHS | 1.22 × 10–02 (6.7 × 10–02) | 165 | −6.388 × 10–04 (3.4 × 10–02) | 141,484 | 1.80 × 10–03 | 1.48 × 10–02 | 941 | |
| CFS | 2.414 × 10–02 (1.16 × 10–02) | 166 | −9.103 × 10–03 (9.0 × 10–02) | 119,600 | 7.51 × 10–07 | 2.97 × 10–04 | 121 | |
| ARIC | 8.4687 × 10–03 (6.1 × 10–02) | 159 | −1.796 × 10–03 (2.9 × 10–02) | 139,239 | 3.56 × 10–01 | 3.60 × 10–02 | 1,504 | |
*Kolmogorov–Smirnov test.
sd–standard deviation.
ARIC–Atherosclerosis Risk in Communities; CFS - Cleveland Family Study; CARDIA - Coronary Artery Risk Development in Young Adults; JHS - Jackson Heart Study.
For African-American cohorts, we included the top 169 SNPs (P < 5 × 10−5) identified from the GWAS of the Women’s Health Initiative (WHI)39 for the height-related assortative mating analysis. The number of SNPs genotyped in African-American cohorts range from 158 to 168 (Table 2).
Assortative mating analysis at a single locus
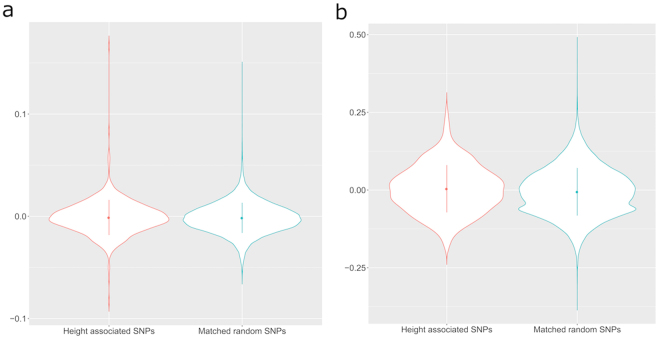
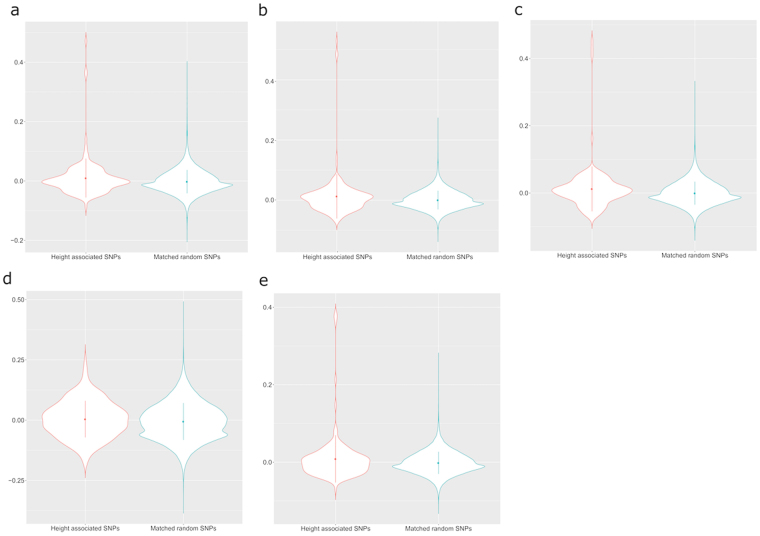
Average inbreeding coefficients in the two European-American and five African-American cohorts, using the height associated SNPs, were calculated and compared to frequency matched randomly selected SNPs from the same cohorts, as well as to the whole genome. (Table 2 and Supplementary Table S2) (equation (2) in Analytical Methods). In the two European-American cohorts, the average inbreeding coefficients for height associated SNPs are −1.137 × 10−3 and 4.173 × 10−3 for ARIC and CFS, respectively. The average of single inbreeding coefficients for height associated SNPs ranges from 8.4687 × 10−3 to 2.414 × 10−2 in five African-American cohorts. We randomly selected the same number of independent SNPs with minor allele frequencies matched to the height associated SNPs for each cohort and estimated their corresponding inbreeding coefficients. We observed significant differences for the inbreeding coefficients between the height associated SNPs and the random set of SNPs in all the cohorts except the ARIC European cohort (P-value < 0.05 for all cohorts except for ARIC European cohort, Table 2), with the height associated SNPs always having higher inbreeding coefficients. Although not statistically significant, the trend in the ARIC European cohort was the same as for the other cohorts. The violin plots also show the distribution difference between inbreeding coefficients estimated using height associated variants and randomly matched variants across the genome except ARIC European cohort (Figs 1 and 2). Thus, the genetic results provide evidence of assortative mating for height associated SNPs in all cohorts except for the ARIC European one.
Figure 1.
The violin plots of the inbreeding coefficient at single locus level for European-American cohorts. Red represents height associated loci and teal represents frequency matched random SNPs. (a) ARIC; (b) CFS.
Figure 2.
The violin plots of the inbreeding coefficient at single locus level for African-American cohorts. Red represents height associated loci and teal represents frequency matched random SNPs. (a) CARDIA; (b) MESA; (c) JHS; (d) CFS; (e) ARIC.
We observed negative average inbreeding coefficients for randomly selected SNPs in most of our studied cohorts (Table 2), although average inbreeding coefficients were close to 0. We also observed a negative average inbreeding coefficient for height associated SNPs in the ARIC European cohort. Since height is a polygenic trait, we selected the independent 2,500 and 5,000 SNPs with the smallest P-values in the height GWAS of the GIANT consortium34, respectively. We repeated the analysis using these 2,500 and 5,000 SNPs in the ARIC European cohort. We observed that the average inbreeding coefficients became more positive as more top height-associated SNPs were included, with the average inbreeding coefficients changing to 6.02 × 10−4 and 6.5 × 10−4 for the top 2,500 and 5,000 SNPs, respectively, among ARIC European Americans (Table 2), as compared to a negative value for the GWAS significant SNPs only. The difference became more significant when comparing with frequency matched random SNPs (P < 2 × 10−5 for the 2,500 SNPs and P < 9 × 10−8 for the 5,000 SNPs for all conducted tests). We calculated the correlation between effect size and inbreeding coefficient using the 521 genome wide significant SNPs and their corresponding inbreeding coefficients. We did not observe a significant correlation (r = −0.02, p = 0.545). Our result indicates that inbreeding coefficient is independent of the effect size of height associated variants, and the estimated average inbreeding coefficient is likely underestimated when only top of height associated markers are used for analysis.
As population structure will impact inbreeding coefficient estimates, we examined the population structure in the ARIC European cohort using principal component (PC) analysis9,47,48. The North-South European admixture can be clearly observed (Fig. 3). We then excluded the outliers identified using the first two PCs (Fig. 3) and calculated inbreeding coefficients again. The estimated inbreeding coefficients are consistent with those obtained from all samples, which ranges from −9.24 × 10−4 to 6.63 × 10−4 using a variable number of variants. Again we observed a significant shift of inbreeding coefficients using height associated variants as compared to randomly selected frequency matched variants (P < 0.05 for top 2,500 SNPs and 5,000 SNPs) (Supplementary Table S1).
Figure 3.
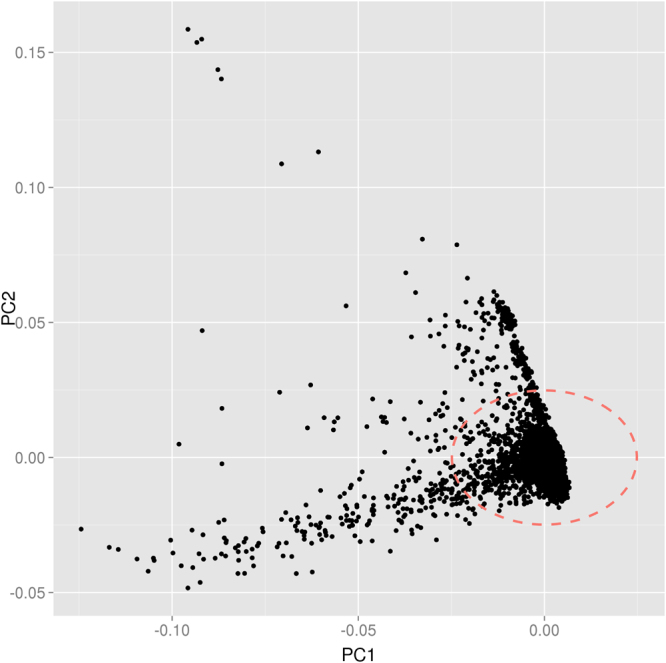
Plot of the first two principal components for 6,787 unrelated ARIC European subjects.
Assortative mating analysis with multiple loci
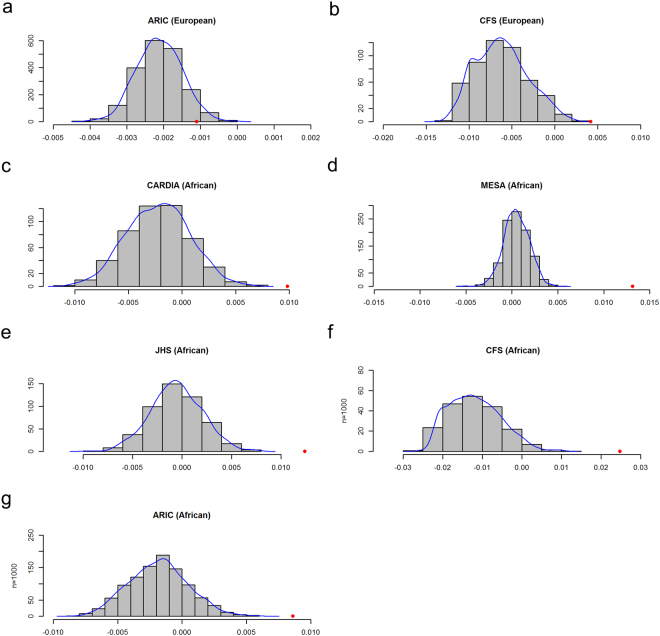
We further calculated the inbreeding coefficient using all of the height associated variants using equation (3) in Analytical Methods. Table 3 lists the inbreeding coefficients estimated from all height-associated variants in each cohort. The estimated inbreeding coefficients are −1.1 × 10−3 and 4.2 × 10−3 for ARIC European and CFS European, respectively. For the five African-American cohorts, the estimated inbreeding coefficients range from 8.62 × 10−3 to 2.477 × 10−2. The estimated inbreeding coefficients using all height-associated loci are approximately equivalent to the average of inbreeding coefficient for the single locus analysis, as expected. We observed that the inbreeding coefficients estimated using height associated variants fall in the right tails of the inbreeding coefficient distributions calculated using randomly sampled allele frequency matched SNPs (see Analytical Methods) for all the cohorts, and they are all statistically significant (Fig. 4, Table 3, ). Thus, our results are consistent with assortative mating by height driving increased homozygosity of SNPs associated with height in both European-American and African-American cohorts. As expected, when including more of the most associated SNPs in the ARIC European cohort, the inbreeding coefficients become positive and remain statistically significant (Table 3, ), supporting the polygenic basis of human height.
Table 3.
Comparison of inbreeding coefficient estimated from height associated variants and lipids associated variants with randomly sampled frequency matched variants: multiple loci analysis.
| Trait | Population | (sd) | average of frequency matched variants (sd) | P-value* | # of SNPs analyzed | |
|---|---|---|---|---|---|---|
| Height | European American | ARIC | −1.10 × 10–03 (5.32 × 10–04) | −2.19 × 10–03 (6.16 × 10–04) | 0.027 | 521 |
| 6.164 × 10–04 (2.561 × 10–04) | −2.15 × 10–03 (3.21 × 10–04) | 0.001 | 2,500 | |||
| 6.476 × 10–04 (1.889 × 10–04) | −2.09 × 10–03 (6.16 × 10–04) | 0.001 | 5,000 | |||
| CFS | 4.20 × 10–03 (3.16 × 10–03) | −6.19 × 10–03 (3.01 × 10–03) | 0.025 | 595 | ||
| African American | CARDIA | 9.847 × 10–03 (2.83 × 10–03) | −2.211 × 10–03 (2.94 × 10–03) | 0.001 | 158 | |
| MESA | 1.313 × 10–02 (2.36 × 10–03) | 4.749 × 10–04 (2.48 × 10–03) | 0.001 | 168 | ||
| JHS | 1.242 × 10–02 (2.62 × 10–03) | −5.618 × 10–04 (2.60 × 10–03) | 0.001 | 165 | ||
| CFS | 2.477 × 10–02 (7.38 × 10–03) | −7.483 × 10–03 (4.56 × 10–03) | 0.001 | 166 | ||
| ARIC | 8.622 × 10–03 (2.10 × 10–03) | −1.825 × 10–03 (2.29 × 10–03) | 0.001 | 159 | ||
| Lipids | European American | ARIC | −1.167 × 10–03 (9.87 × 10–04) | −2.21 × 10–03 (1.15 × 10–03) | 0.29 | 152 |
| CFS | 3.992 × 10–03 (8.806 × 10–03) | −5.28 × 10–03 (8.40 × 10–03) | 0.152 | 157 | ||
*P-value is comparing using height variants and randomly sampled frequency matched variants.
sd–standard deviation.
ARIC–Atherosclerosis Risk in Communities; CFS - Cleveland Family Study; CARDIA - Coronary Artery Risk Development in Young Adults; JHS–Jackson Heart Study.
Figure 4.
The histogram of the inbreeding coefficient using multiple loci for both European-American and African-American cohorts. Distributions were based on 1,000 resampling. The observed inbreeding coefficient for height associated SNPs are marked in red points. (a) ARIC (European); (b) CFS (European); (c) CARDIA (African); (d) MESA (African); (e) JHS(African); (f) CFS (African); (g) ARIC (African).
To test whether any trait associated SNPs will be affected by assortative mating, we repeated the analyses using blood lipids associated variants obtained from the Global Lipids Genetics Consortium49 in European populations. The estimated inbreeding coefficients for lipids associated SNPs were not statistically significant for all analyses (Table 3), indicating that there is no or much weaker assortative mating for blood lipids than for height.
Linkage Disequilibrium Analysis
We further assessed assortative mating for height by regressing pairwise linkage disequilibrium (LD) score on the products of the first two PC loadings and the product of effect sizes of height associated variants in the ARIC European cohort, a method demonstrated to be robust with respect to population structure5,22. We calculated the unstandardized LD parameter D16,50 for height associated SNPs located on different chromosomes and their corresponding PC loadings for PC1 and 2 in the ARIC European cohort. Using linear regression, we obtained the effect sizes for these height variants. We then regressed the D values for a pair of height variants on the products of height effect sizes and the products of PC-loadings for each pair of SNPs41. We observed significance for both height effect size products (P = 9.62 × 10−12) and PC-loading products (P = 6.33 × 10−56 for PC1 and P = 5.06 × 10−41 for PC2) (Table 4), providing further evidence for strong assortative mating by height that was independent of ancestry and population structure.
Table 4.
Regression analysis of linkage disequilibrium parameter D on the product of height effect sizes and PC-loadings for unlinked SNPs in ARIC European cohort.
| Estimate (sd) | T-value | P-value | |
|---|---|---|---|
| Product of height effect sizes | 1.186 × 10−03 (1.741 × 10−04) | 6.813 | 9.62 × 10−12 |
| Product of PC1-loading | 1.069 × 10−04 (6.782 × 10−06) | 15.766 | 6.33 × 10−56 |
| Product of PC2-loading | 8.777 × 10−05 (6.540 × 10−06) | 13.420 | 5.06 × 10−41 |
sd–standard deviation.
Discussion
In this study, we examined assortative mating for height, using both phenotype and genotype data. Estimates of assortative mating based on spousal correlations was consistent with the literature6,8,11,20,51, with estimates of correlation between spouse-pairs ranging from 0.24 to 0.4. We observed that the estimated inbreeding coefficients for height associated variants were consistently larger than that for frequency matched random markers using either single or multiple locus analyses in both European Americans and African Americans. Since assortative mating can be affected by socio-demographic factors, Laurent et al.4 suggested to use the genome wide distribution as a control. We estimated the inbreeding coefficients across the genome in the studied cohorts (Supplementary Table S2 and Supplementary Figs S1–S3); the estimated inbreeding coefficients for height associated variants were consistently larger than that based on genome wide estimates. Assortative mating for height was also independent of ancestry as determined by regressing pairwise linkage disequilibrium (LD) score on the products of the first two PC loadings and the product of effect sizes of height associated variants in the ARIC European cohort (Table 4). Thus, our results show that genetic variants associated with height exhibit significant inbreeding coefficients as predicted by our hypothesis. These results clearly demonstrate the genetic effects of phenotype-based mating in humans.
Although assortative mating for height has been reported10,11,18,25, it was not clear whether assortative for height could be explained by ancestry assortative mating or population structure.7,40 Nor did prior studies estimate how strong the height-related assortative mating was after controlling population structure41. Since population structure should impact genotype distributions equally across the genome as long as the assessed variants are not under selection, our results show trait specific effects of mating behavior by comparing the inbreeding coefficients estimated using height associated variants with a frequency matched random variants. Since most genetic variants are neutral or nearly neutral our comparison should be representative of random mating across the genome52. Additionally, genetic variants with large fitness are generally rare or low frequency and we removed all the variants with MAF <0.01 to reduce the potential bias due to selection pressure. Finally, selection may also cause departure from HWE and such variants were also excluded. Therefore, our observations of larger inbreeding coefficients of height associated variants than that of random frequency matched variants most likely reflects assortative mating for height. The result is also consistent with that from regression analysis of pairwise linkage disequilibrium (LD) score on the products of the first two PC loadings and the product of effect sizes of height associated variants in the ARIC European cohort. We observed significant association between LD and height effect size after adjusting for the PC loadings of the first two PCs (Table 4). Sebro et al.41 using the same analysis in Framingham Heart Study only observed strong assortative mating for ancestry, but not height, possibly due to relatively small sample size and small number of height associated markers used in their analyses.
Another possible cause of increased inbreeding coefficients in our analyses, is that GWAS significant SNPs may have different characteristics than random SNPs from across the genome. If this is the case, our evidence for assortative mating for height may reflect a general characteristic for GWAS significant SNPs in general. To assess this possibility, we performed the same analysis with the GWAS significant SNPs associated with blood lipids, and no significant inbreeding coefficient inflation was observed, although SNPs associated with blood lipids did show a trend towards assortative mating (Table 3). We are not clear what causes this tendency. However, it is possible that the tendency may reflect the correlation between growth in height and blood lipids53. This result indicates that GWAS associating SNPs, in general, do not inflate inbreeding coefficients, further supporting our main conclusions.
The inbreeding coefficient for height associated SNPs was negative in the multiple locus analysis in the ARIC European cohort, although the results demonstrated significantly larger inbreeding coefficients as compared to the randomly selected SNPs (Table 3 and Fig. 4). This was an unexpected observation. However, multiple reasons can lead to negative inbreeding coefficient estimates. (1) When sample size is finite, population genetics theory indicates that the heterozygote frequencies are increased by 1/(2N-1), where N is population effective size under random mating (Crow and Kimura, Introduction to Population Genetics Theory5, page 55), and this may result in negative average inbreeding coefficient estimates. (2) In F1 populations, the homozygote frequency will decrease by an amount of the variance of frequency among subpopulations (Crow and Kimura, Introduction to Population Genetics Theory5, page 54). In admixed populations, there can be many subjects whose parents are from different ancestries, even if defined as European. For example, the ARIC cohort probably has numerous samples where one parent was from Northern Europe and the other from Southern Europe (Fig. 3). When we assessed only individuals with less admixture as identified with the first two PCs (Fig. 3), the estimated inbreeding coefficients shifted to being less negative, although the differences were small (Supplementary Table S1). Similar population admixture occurs in the other cohorts (Supplementary Fig. S4). Hence, as predicted population admixture leads to lower inbreeding coefficients via increased heterozygosity across all loci, whether they have a phenotypic impact or not. (3) We estimated the pairwise kinship coefficient among individuals and excluded one individual of each pair with an estimated kinship coefficient >0.025, which will bias average inbreeding coefficient estimates in a negative direction.
To further investigate the negative inbreeding coefficients, we analyzed the ~2,500 and ~5,000 most significant height associated SNPs from the GIANT height genome wide association study. The estimated inbreeding coefficients became more positive on average with an increasing number of height-associated SNPs. Increasing the number of marginally significant height SNPs in the estimates of inbreeding coefficients increased the difference with respect to the random SNPs (P < 2 × 10−5 for top 2,500 SNPs and P < 9 × 10−8 for top 5,000 SNPs), further providing evidence of height-based assortative mating in the ARIC European cohort (Tables 2 and 3). As height is a highly heritable trait with an estimated heritability of 80% and a very large number of genetic variants (as many as 100,000 variants54) that may contribute to its variation33, it is possible that some of our randomly selected SNPs are actually associated with height. If this is the case, then our resampling analyses are conservative in testing for assortative mating. Nonetheless, we found evidence for height related assortative mating in all studied cohorts. It should be noted that our method cannot differentiate active assortative mating from passive assortative mating, i.e., that related to social or geographical homogamy.
We noted that the inbreeding coefficients estimated from either single variant or multiple variants are small and may not have substantial effect to HWE estimates. One reason is that we eliminated all variants with substantial evidence of the departure from HWE via QCs. The second reason is that there are a large number of height variants. When assortative mating involves a large number of variants, it will be less likely to affect HW deviations37,55. However, we still observed consistent larger inbreeding coefficients for the height associated variants than for a random set of variants.
We observed that the minor allele frequencies after LD pruning have a U-shape distribution with an excess of variants with intermediary frequencies56,57 (Supplementary Figs S5 and S6). The enrichment of higher minor allele frequency SNPs was caused by the LD pruning procedure as implemented in PLINK that keeps the SNPs with higher minor allele frequencies when performing LD pruning58. However, the inbreeding coefficient does not depend on allele frequency. To examine whether the allele frequency spectrums affect our result, we redid the LD pruning by selecting the retained SNPs at random. The inbreeding coefficients from height-associated SNPs compared to the randomly selected frequency matched SNPs from the LD pruning was not affected by MAF. We observed the same assortative mating signature for height. (Supplementary Table S3 and Supplementary Figs S5, S6). Our results suggested that the LD pruning process did not affect our conclusions.
It is possible that the estimation of inbreeding coefficient may be biased if a disease is associated with height and study cohorts were disease oriented. However, our study cohorts are population based samples. We only included adults and adult height is less impacted by disease. Therefore, our conclusion of assortative mating for height should not be affected even if our study cohorts include some unhealthy subjects.
In summary, our results confirmed previous reports of assortative mating by height in both European-American and African-American populations, but in contrast to studies of just assessing phenotypic correlations, we were able to demonstrate measurable genetic effects of this mating behavior. Our results indicate that mate choice with respect to height affects genotypes at loci associating with height, providing independent evidence of the veracity of these variants as associating with height. However, it is still not clear how much impact non-random mating has on genetic association studies that typically assume random mating. Our results indicate that care will need to be taken when assessing variants for association with respect to assumptions of random mating and levels of heritability as previous work has shown that heritability estimates will be inflated when the phenotypic correlation reflects genotypic correlation59. Statistical approaches considering non-random mating may be helpful in genetic association analysis, heritability estimation or interpretation of results.
Materials and Methods
The study used existing datasets, including CFS phenotype and genotype data and CARe genotype data. The CFS phenotype data were analyzed anonymously at Case Western Reserve University. The CFS study was approved by Partners Human Research Committee with the proposal number 2011D001860. Our study has been approved by Case Western Reserve University Institutional Review Board (IRB-2013-525). The genotype data from the Candidate Gene Association Resource (CARe) consortium were downloaded from the dbGaP.
Cohort description
The European cohorts included Cleveland Family Study (CFS) and Atherosclerosis Risk in Communities (ARIC). The CFS is a family-based longitudinal study starting in 1990 comprised of index cases with laboratory diagnosed sleep apnea, their family members, and neighborhood control families60,61. Four examinations over 16 years included measurements of sleep apnea, anthropometry, and other related phenotypes, as detailed previously60,61. The CFS (dbGaP phs000284.v1.p1) includes 645 European Americans in 139 families who were genotyped on the OmniChip 2.5 M array. The ARIC data were downloaded from dbGaP database (dbGaP phs000090.v1.p1). The ARIC study, sponsored by the National Heart, Lung and Blood Institute (NHLBI), is a prospective epidemiologic study designed to investigate the etiology and natural history of atherosclerosis, the etiology of clinical atherosclerotic diseases, and variation in cardiovascular risk factors, medical care and disease by race, gender, location, and date. It includes 9,707 independent subjects genotyped by Affymetrix 6.0 array.
The African-American samples are from the Candidate Gene Association Resource (CARe) consortium62. CARe has assembled samples from 9 community-based cohorts representing four ethnic groups: European-American, African-American, Hispanic, or Chinese-American, as described in detail62. The African-American samples for our assortative mating analysis were obtained from five CARe cohorts: Atherosclerosis Risk in Communities (ARIC: dbGaP phs000280.v1.p1), Coronary Artery Risk Development in Young Adults (CARDIA: dbGaP phs000285.v2.p2), Cleveland Family Study (CFS: dbGaP phs000284.v1.p1), Jackson Heart Study (JHS: dbGaP phs000286.v1.p1), Multi-Ethnic Study of Atherosclerosis (MESA: dbGaP phs000283.v1.p1), a detailed description of each cohort can be found in63. Genotyping for those cohorts was performed with Affymetrix 6.0 array.
Quality Controls
All data quality controls (QCs) were performed for each cohort separately, and only autosomal loci were used. We selected the height associated variants from the most recent GWAS34,39 in both European-American and African-American populations to determine the degree of height-based assortative mating. The remaining SNPs were considered for use in a comparison group. For the set of non-height associated loci, we excluded SNPs in each individual dataset that had either a call rate (CR) < 0.95, a minor allele frequency (MAF) < 0.01 or from a Hardy-Weinberg equilibrium test, using software PLINK58. Individuals with a missing genotype rate > 0.1 were also removed. After QCs, ~600,000 markers remained in European-American cohorts for analysis. For the five African-American cohorts, ~800,000 markers passed QCs. Since our analysis assumed all markers are independent, we pruned SNPs using PLINK58 (r2 < 0.1). After pruning, the number of SNPs in analysis were between 68,453 and 65,069 for ARIC and CFS European-American cohorts, and between 119,725 and 189,966 SNPs for African-American cohorts, respectively. The minor allele frequency distributions for height associated variants and all variants across the genome are shown in Supplementary Figs S5 and S6.
To ensure the estimated inbreeding coefficients were not confounded by the related family members, we selected unrelated founders for the family-based cohorts (CFS and JHS). To avoid cryptic relatedness, we estimated the pairwise kinship coefficient among individuals using genome wide SNPs in each cohort by software GCTA64 and excluded one individual of each pair with an estimated kinship coefficient >0.025. The final sample sizes were presented in Table 2. For admixed populations, it may be more accurate to use REAP65 that requires allele frequency distributions in ancestral populations, which were not available for our European American cohorts. Since the estimated kinship coefficients from GCTA and REAP are highly correlated and we only estimated kinship coefficients, it should have little effect for the inbreeding coefficient estimates. Therefore, the difference in method should not affect our conclusions.
Analytical Methods
Assume that a marker with two alleles A and a, and the corresponding three genotypes are aa, Aa, or AA, with allele frequency and subject to the constraint . If a population displays random mating, the expected genotype frequencies follow the Hardy-Weinberg law with the genotype frequencies , and for AA homozygotes, Aa heterozygotes and aa homozygotes, respectively. The Hardy-Weinberg principle describes a panmictic population with no mutation, migrations or selection. Either inbreeding or assortative mating will lead to Hardy-Weinberg disequilibrium, although inbreeding will affect all genetic variants while assortative mating will only involve loci related to traits associated with phenotypes affecting mate selection5. In either case, the genotype frequencies can be written as:
| 1 |
where is the inbreeding coefficient5. Both inbreeding and assortative mating will increase homozygote and decrease heterozygote frequencies. An inbreeding coefficient ranges between and 1. In the extreme case of self-fertilization, the inbreeding coefficient is 1. When the frequency of heterozygotes equals the HW expectation then the inbreeding coefficient is 0.
Assortative mating at a single locus
Assuming and are the observed number of homozygotes, the observed number of heterozygotes. To estimate the inbreeding coefficient at a single locus, we applied the maximum likelihood method66 which maximizing the following log likelihood (logl):
| 2 |
Note that the allele frequency is unaffected by inbreeding and assortative mating. Thus, we can maximize the inbreeding coefficient using an estimated allele frequency and .
To test whether assortative mating exists in each cohort, we calculated the inbreeding coefficients when estimated using resampled frequency matched variants across the genome after excluding the height associated loci. Since these resampled SNPs are less likely to be height associated, the distribution of estimated inbreeding coefficient from resampling should reflect the distribution without assortative mating on this trait. We further performed a two sample T-test as well as a Kolmogorov–Smirnov test (KS-test) to compare the height-associated SNPs with the randomly selected frequency matched SNPs.
Assortative mating with multiple loci
We extended the maximum likelihood method to estimate the inbreeding coefficient at a set of height-associated loci. Consider a set of independent SNPs, the inbreeding coefficient at multiple loci is denoted by . For the SNP, the minor allele frequency is assumed to be , and and denote the observed number of homozygotes, denote the observed number of heterozygotes. Then the likelihood function for the independent SNPs is
| 3 |
Here we assume that the inbreeding coefficient is the same for the independent SNPs, and therefore, the estimated inbreeding coefficient can be interpreted as the common inbreeding coefficient for the independent SNPs. Using the same considerations as for a single variant, the allele frequency for each SNP does not change for either inbreeding or assortative mating and can be estimated independently. The inbreeding coefficient can then be estimated using computational optimizations.
When a set of SNPs contributes to trait variation involved in assortative mating, the estimated inbreeding coefficient from equation (3) will be affected by both inbreeding (genome wide effects) and assortative mating (locus specific). Population substructure is also a confounder for estimating the inbreeding coefficient, but should affect all loci similarly. We estimate the empirical distribution of under the null hypothesis that there is no height associated assortative mating, but possibly population structure or cryptic relatedness. To obtain a distribution of under the null of no assortative mating, we applied a resampling procedure. In each resampling, we randomly sample the same number, , of independent SNPs with matched allele frequencies from the genome and calculate the inbreeding coefficient . This resampling procedure was repeated 1,000 times to obtain a null distribution of . Since most of genome wide variants either do not contribute to the height variation or have effect sizes that are small, the estimated is the approximate distribution under the null hypothesis of absence of assortative mating. The test for height-related assortative mating can be obtained by comparing this empirical distribution to the distribution for height associated variants. Since there are many height associated variants across the genome, this resampling procedure may bias to the null hypothesis, which can be conservative. A similar resampling procedure was used as we previously described.
CARe
The authors wish to acknowledge the support of the National Heart, Lung, and Blood Institute and the contributions of the research institutions, study investigators, field staff and study participants in creating this resource for biomedical research. The following nine parent studies have contributed parent study data, ancillary study data, and DNA samples through the Broad Institute (N01-HC-65226) to create this genotype/phenotype data base for wide dissemination to the biomedical research community:
Atherosclerotic Risk in Communities (ARIC)
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021 and N01-HC-55022, and grants R01HL087641, R01HL59367, R37HL051021, R01HL086694 and U10HL054512; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research; Cleveland Family Study (CFS): Case Western Reserve University (RO1 HL46380-01-16); Coronary Artery Risk in Young Adults (CARDIA): University of Alabama at Birmingham (N01-HC-48047), University of Minnesota (N01-HC-48048), Northwestern University (N01-HC-48049), Kaiser Foundation Research Institute (N01-HC-48050), University of Alabama at Birmingham (N01-HC-95095), Tufts-New England Medical Center (N01-HC-45204), Wake Forest University (N01-HC-45205), Harbor-UCLA Research and Education Institute (N01-HC-05187), University of California, Irvine (N01-HC-45134, N01-HC-95100); Jackson Heart Study (JHS): Jackson State University (N01-HC-95170), University of Mississippi (N01-HC-95171), Tougaloo College (N01-HC-95172); Multi-Ethnic Study of Atherosclerosis (MESA): University of Washington (N01-HC-95159), Regents of the University of California (N01-HC-95160), Columbia University (N01-HC-95161), Johns Hopkins University (N01-HC-95162), University of Minnesota (N01-HC-95163), Northwestern University (N01-HC-95164), Wake Forest University (N01-HC-95165), University of Vermont (N01-HC-95166), New England Medical Center (N01-HC-95167), Johns Hopkins University (N01-HC-95168),Harbor-UCLA Research and Education Institute (N01-HC-95169).
Electronic supplementary material
Acknowledgements
The work was supported by the National Institutes of Health grants HG003054 from the National Human Genome Research Institute, HL113338 and HL046380 from the National Heart, Lung, Blood Institute, National Institutes of Health grants LM10098, and the National Science Foundation CAREER award.
Author Contributions
X.Z. designed the study. X.L. performed the experiments and analyzed the data. X.Z. and X.L. prepared the manuscript. S.R., X.Z. and S.M.W. gave conceptual advice and edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15864-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kocsor F, Rezneki R, Juhasz S, Bereczkei T. Preference for Facial Self-Resemblance and Attractiveness in Human Mate Choice. Arch Sex Behav. 2011;40:1263–1270. doi: 10.1007/s10508-010-9723-z. [DOI] [PubMed] [Google Scholar]
- 2.Geary DC, Vigil J, Byrd-Craven J. Evolution of human mate choice. J Sex Res. 2004;41:27–42. doi: 10.1080/00224490409552211. [DOI] [PubMed] [Google Scholar]
- 3.Sebro R, Hoffman TJ, Lange C, Rogus JJ, Risch NJ. Testing for non-random mating: evidence for ancestry-related assortative mating in the Framingham heart study. Genetic epidemiology. 2010;34:674–679. doi: 10.1002/gepi.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurent R, Toupance B, Chaix R. Non-random mate choice in humans: insights from a genome scan. Mol Ecol. 2012;21:587–596. doi: 10.1111/j.1365-294X.2011.05376.x. [DOI] [PubMed] [Google Scholar]
- 5.Crow, J. F. & Kimura, M. An introduction to population genetics theory. (Harper & Row, 1970).
- 6.Conley D, et al. Assortative mating and differential fertility by phenotype and genotype across the 20th century. P Natl Acad Sci USA. 2016;113:6647–6652. doi: 10.1073/pnas.1523592113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domingue BW, Fletcher J, Conley D, Boardman JD. Genetic and educational assortative mating among US adults. P Natl Acad Sci USA. 2014;111:7996–8000. doi: 10.1073/pnas.1321426111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spuhler JN. Assortative Mating with Respect To Physical Characteristics. Eugen Quart. 1968;15:128–140. doi: 10.1080/19485565.1968.9987763. [DOI] [PubMed] [Google Scholar]
- 9.Campbell, C. D. et al. Demonstrating stratification in a European American population. Nature genetics37, 868-872, doi:10.1038/ng1607 (2005). [DOI] [PubMed]
- 10.Courtiol A, Raymond M, Godelle B, Ferdy JB. Mate Choice And Human Stature: Homogamy as a Unified Framework for Understanding Mating Preferences. Evolution. 2010;64:2189–2203. doi: 10.1111/j.1558-5646.2010.00985.x. [DOI] [PubMed] [Google Scholar]
- 11.Buss, D. M. Human mate selection. Am Sci73 (1985).
- 12.Mare RD. Five Decades of Educational Assortative Mating. American Sociological Review. 1991;56:15–32. doi: 10.2307/2095670. [DOI] [Google Scholar]
- 13.Rele JR. Trends and Differentials in the American Age at Marriage. The Milbank Memorial Fund Quarterly. 1965;43:219–234. doi: 10.2307/3349031. [DOI] [PubMed] [Google Scholar]
- 14.McClendon D. Religion, Marriage Markets, and Assortative Mating in the United States. Journal of Marriage and Family. 2016;78:1399–1421. doi: 10.1111/jomf.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glenn ND. Interreligious Marriage in the United States: Patterns and Recent Trends. Journal of Marriage and Family. 1982;44:555–566. doi: 10.2307/351579. [DOI] [Google Scholar]
- 16.Risch N, et al. Ancestry-related assortative mating in Latino populations. Genome Biology. 2009;10:1–16. doi: 10.1186/gb-2009-10-11-r132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt HD, Glavce C, Hartog J. Influences on assortative mating. Anthropol Anz. 1987;45:261–267. [PubMed] [Google Scholar]
- 18.Hur YM. Assortative mating for personality traits, educational level, religious affiliation, height, weight, and body mass index in parents of a Korean twin sample. Twin Research. 2003;6:467–470. doi: 10.1375/136905203322686446. [DOI] [PubMed] [Google Scholar]
- 19.Cavalli-Sforza, L. L., Menozzi, P. & Piazza, A. The history and geography of human genes. (Princeton University Press, 1994).
- 20.Greenwood J, Guner N, Kocharkov G, Santos C. Marry Your Like: Assortative Mating and Income Inequality. Am Econ Rev. 2014;104:348–353. doi: 10.1257/aer.104.5.348. [DOI] [Google Scholar]
- 21.Dribe M, Lundh C. Status homogamy in the preindustrial marriage market: partner selection according to age, social origin, and place of birth in nineteenth-century rural Sweden. J Fam Hist. 2009;34:387–406. doi: 10.1177/0363199009344708. [DOI] [PubMed] [Google Scholar]
- 22.Crow JF, Felsenstein J. The effect of assortative mating on the genetic composition of a population. Eugen Q. 1968;15:85–97. doi: 10.1080/19485565.1968.9987760. [DOI] [PubMed] [Google Scholar]
- 23.Plomin, R., DeFries, J. C., Knopik, V. S. & Neiderhiser, J. Behavioral genetics. (Worth Publishers, 2013).
- 24.Sebro R, Risch NJ. A brief note on the resemblance between relatives in the presence of population stratification. Heredity. 2012;108:563–568. doi: 10.1038/hdy.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silventoinen K, Kaprio J, Lahelma E, Viken RJ, Rose RJ. Assortative mating by body height and BMI: Finnish twins and their spouses. Am J Hum Biol. 2003;15:620–627. doi: 10.1002/ajhb.10183. [DOI] [PubMed] [Google Scholar]
- 26.Zietsch BP, Verweij KJH, Heath AC, Martin NG. Variation in Human Mate Choice: Simultaneously Investigating Heritability, Parental Influence, Sexual Imprinting, and Assortative Mating. Am Nat. 2011;177:605–616. doi: 10.1086/659629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redden DT, Allison DB. The Effect of Assortative Mating upon Genetic Association Studies: Spurious Associations and Population Substructure in the Absence of Admixture. Behavior Genetics. 2006;36:678–686. doi: 10.1007/s10519-006-9060-0. [DOI] [PubMed] [Google Scholar]
- 28.Dawson PS. The Use of Assortative Mating for Heritability Estimation. Genetics. 1964;49:991–994. doi: 10.1093/genetics/49.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collaborative Group on Epidemiological Studies of Ovarian, C. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS medicine9, e1001200, 10.1371/journal.pmed.1001200 (2012). [DOI] [PMC free article] [PubMed]
- 30.Paajanen TA, Oksala NKJ, Kuukasjarvi P, Karhunen PJ. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur Heart J. 2010;31:1802–1809. doi: 10.1093/eurheartj/ehq155. [DOI] [PubMed] [Google Scholar]
- 31.Goldbourt U, Tanne D. Body height is associated with decreased long-term stroke but not coronary heart disease mortality? Stroke. 2002;33:743–748. doi: 10.1161/hs0302.103814. [DOI] [PubMed] [Google Scholar]
- 32.Petot GJ, et al. Height and Alzheimer’s disease: findings from a case-control study. Journal of Alzheimer’s disease: JAD. 2007;11:337–341. doi: 10.3233/JAD-2007-11310. [DOI] [PubMed] [Google Scholar]
- 33.Visscher PM, et al. Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. Plos Genetics. 2006;2:316–325. doi: 10.1371/journal.pgen.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood AR, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nature genetics. 2014;46:1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang, J. et al. Common SNPs explain a large proportion of the heritability for human height. Nature genetics42, 565–569, http://www.nature.com/ng/journal/v42/n7/suppinfo/ng.608_S1.html (2010). [DOI] [PMC free article] [PubMed]
- 36.Yang, J. et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nature genetics43, 519–525, http://www.nature.com/ng/journal/v43/n6/abs/ng.823.html#supplementary-information (2011). [DOI] [PMC free article] [PubMed]
- 37.Lango Allen H, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang SJ, et al. Genome-wide association of anthropometric traits in African- and African-derived populations. Human molecular genetics. 2010;19:2725–2738. doi: 10.1093/hmg/ddq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carty CL, et al. Genome-wide association study of body height in African Americans: the Women’s Health Initiative SNP Health Association Resource (SHARe) Human molecular genetics. 2012;21:711–720. doi: 10.1093/hmg/ddr489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdellaoui A, Verweij KJH, Zietsch BP. No evidence for genetic assortative mating beyond that due to population stratification. P Natl Acad Sci USA. 2014;111:E4137–E4137. doi: 10.1073/pnas.1410781111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sebro R, Peloso GM, Dupuis J, Risch NJ. Structured mating: Patterns and implications. PLoS Genet. 2017;13:e1006655. doi: 10.1371/journal.pgen.1006655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryckman, K. & Williams, S. M. In Current Protocols in Human Genetics (John Wiley & Sons, Inc., 2001).
- 43.Feder JN, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nature genetics. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 44.Ryckman KK, et al. A prevalence-based association test for case-control studies. Genetic epidemiology. 2008;32:600–605. doi: 10.1002/gepi.20342. [DOI] [PubMed] [Google Scholar]
- 45.Wittke-Thompson, J. K., Pluzhnikov, A. & Cox, N. J. Rational inferences about departures from Hardy-Weinberg equilibrium. Am J Hum Genet76, 967–986, doi:10.1086/430507 (2005). [DOI] [PMC free article] [PubMed]
- 46.Liang JJ, et al. Comparison of Heritability Estimation and Linkage Analysis for Multiple Traits Using Principal Component Analyses. Genetic epidemiology. 2016;40:222–232. doi: 10.1002/gepi.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu XF, Zhang SL, Zhao HY, Cooper RS. Association mapping, using a mixture model for complex traits. Genetic epidemiology. 2002;23:181–196. doi: 10.1002/gepi.210. [DOI] [PubMed] [Google Scholar]
- 48.Zhu X, Li S, Cooper RS, Elston RC. A unified association analysis approach for family and unrelated samples correcting for stratification. Am J Hum Genet. 2008;82:352–365. doi: 10.1016/j.ajhg.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Global Lipids Genetics C, et al. Discovery and refinement of loci associated with lipid levels. Nature genetics. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devlin B, Risch N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics. 1995;29:311–322. doi: 10.1006/geno.1995.9003. [DOI] [PubMed] [Google Scholar]
- 51.Assortative mating in man. A cooperative study. Biometrika2, 481-498 (1902).
- 52.Hartl, D. L. & Clark, A. G. Principles of Population Genetics. (Sinauer, 2007).
- 53.Kouda K, Nakamura H, Fan W, Takeuchi H. Negative relationships between growth in height and levels of cholesterol in puberty: a 3-year follow-up study. Int J Epidemiol. 2003;32:1105–1110. doi: 10.1093/ije/dyg207. [DOI] [PubMed] [Google Scholar]
- 54.Boyle EA, Li YI, Pritchard JK. An Expanded View of Complex Traits: From Polygenic to Omnigenic. Cell. 2017;169:1177–1186. doi: 10.1016/j.cell.2017.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lynch, M. & Walsh, B. Genetics and Analysis of Quantitative Traits. (Sinauer, 1998).
- 56.Marth GT, Czabarka E, Murvai J, Sherry ST. The allele frequency spectrum in genome-wide human variation data reveals signals of differential demographic history in three large world populations. Genetics. 2004;166:351–372. doi: 10.1534/genetics.166.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keinan A, Mullikin JC, Patterson N, Reich D. Measurement of the human allele frequency spectrum demonstrates greater genetic drift in East Asians than in Europeans. Nature genetics. 2007;39:1251–1255. doi: 10.1038/ng2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Purcell, S. et al. a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet, 81 (2007). [DOI] [PMC free article] [PubMed]
- 59.Robinson, M. R. et al. Genetic evidence of assortative mating in humans. Nature Human Behaviour1, 0016, 10.1038/s41562-016-0016http://www.nature.com/articles/s41562-016-0016#supplementary-information (2017).
- 60.Larkin EK, et al. A Study of the Relationship between the Interleukin-6 Gene and Obstructive Sleep Apnea. Clinical and translational science. 2010;3:337–339. doi: 10.1111/j.1752-8062.2010.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: The relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–2237. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 62.Musunuru K, et al. Candidate gene association resource (CARe): design, methods, and proof of concept. Circulation. Cardiovascular genetics. 2010;3:267–275. doi: 10.1161/CIRCGENETICS.109.882696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu X, et al. Combined admixture mapping and association analysis identifies a novel blood pressure genetic locus on 5p13: contributions from the CARe consortium. Human molecular genetics. 2011;20:2285–2295. doi: 10.1093/hmg/ddr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J, Lee S, Goddard M, Visscher P. GCTA: A Tool for Genome-wide Complex Trait Analysis. The American Journal of Human Genetics. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thornton T, et al. Estimating kinship in admixed populations. Am J Hum Genet. 2012;91:122–138. doi: 10.1016/j.ajhg.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curie-Cohen M. Estimates of inbreeding in a natural population: a comparison of sampling properties. Genetics. 1982;100:339–358. doi: 10.1093/genetics/100.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.