Abstract
The see-through medaka is a vertebrate model with a transparent body in the adult stage, as well as during the embryonic stages, that was generated from a small laboratory fish, medaka (Oryzias latipes). In this fish model, most of the pigments are genetically removed from the entire body by a combination of recessive alleles at four loci. The main internal organs, namely, heart, spleen, blood vessels, liver, gut, gonads, kidney, brain, spinal cord, lens, air bladder, and gills, in living adult fish are visible to the naked eye or with a simple stereoscopic microscope. This fish is healthy and fertile. A transgenic see-through medaka was produced by using the green fluorescent protein (GFP) gene fused to the regulatory regions of the medaka vasa gene, in which germ cell-specific expression of GFP was visualized. The fluorescent tag also efficiently improved visibility of gonadal tissues. The process of oocyte maturation in the ovary was monitored by repeated observations from the outside of the body during one spawning cycle in the same living females of the transgenic see-through stock. The see-through medaka will provide an opportunity for noninvasive studies of morphological and molecular events that occur in internal organs in the later stages of life.
The bodies of most vertebrates are opaque, and internal organs are not visible from the outside. This makes noninvasive studies of internal organs difficult in vertebrate models. Small laboratory fish, such as the medaka (Oryzias latipes) (1, 2) and the zebrafish (Brachydanio rerio) (3), are recognized as excellent vehicles for studies of the embryonic development of vertebrates, because of the transparency of their bodies. However, this advantage is lost after the hatching stage because of the development of pigment cells in the skin, peritoneum, and some other tissues.
Pigment cells in some fish species, including medaka, are classified into four main types, namely, melanophores, iridophores, leucophores, and xanthophores, based on their color (4, 5). Melanophores contain melanin in melanosomes, the melanin-containing organelles, and show black or brown color. Iridophores exhibit various structural colors and iridescence through the reflection of light from the surface of orderly distributed organelles, reflecting platelets. The organelles contain guanine as the main component, although it is not a true pigment. Leucophores are considered as an extreme type of iridophore and show the structural white color. Xanthophores are pale yellow to bright red cells containing pteridines and carotenoids in pterinosomes and carotenoid vesicles, respectively, in the cytoplasm.
About 50 natural color mutants of medaka are maintained in the Laboratory of Freshwater Fish Stocks, Bioscience Center, Nagoya University (Nagoya, Japan) (6, 7). Some of these color mutants show deficiency in pigmentation. By crossing selected mutants, we genetically removed the pigments from the entire body of the fish, and thereby generated a transparent fish, the see-through medaka. In this fish stock, the main internal organs are visible through the body wall of the living adults.
The green fluorescent protein (GFP) gene fused to the regulatory regions of the medaka vasa gene (8, 9) was introduced into the genome of the see-through medaka to study gene expression in the gonadal tissues and to examine the efficiency of the fluorescent tag in this fish model. Continuous observation of oocyte maturation was carried out in the same living females during one spawning cycle.
Materials and Methods
Fish Breeding.
Fish were bred in 16-liter tanks with a water-circulating system (MH; Meito-suien, Nagoya, Japan) at 26°C under a 14-h light and 10-h dark cycle. They were fed Artemia larvae and commercially available feeds, such as powdered TetraMin flakes (TetraWerke, Melle, Germany) and Otohime β1 (Nisshin Feed, Tokyo), twice a day.
Parental Strains Used for Crossings.
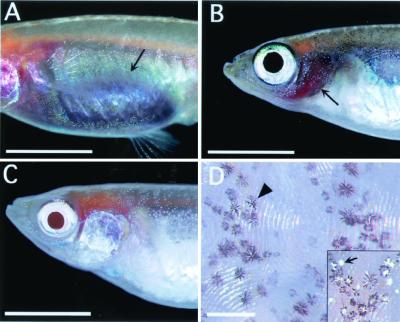
Four color mutants were used as parents for crossings to produce progeny that are deficient in pigments. These mutants were collected by Tomita (6, 7) from natural and commercially available populations. They are guanineless (gu; Fig. 1A), iridophoreless-1 (il-1; Fig. 1B), albino-3 (i-3; Fig. 1C), and leucophore-free (lf; Fig. 1D). These stocks are homozygous recessive at the nonlinked gu, il-1, i-3, and lf loci, respectively. All of the stocks are healthy and fertile.
Figure 1.
Four medaka color mutants used for crossing. (A) gu fish. The abdomen (arrow) appears dark because the melanophore layer inside the peritoneum is visible through the transparent iridophore layer, which lacks iridescence. (B) il-1 fish. The operculum is transparent because of lack of iridescent reflection by iridophores in the skin. The red color of the gills (arrow) is seen through the operculum. (C) i-3 fish. An albino with pink eyes and without melanin pigment in all parts of the body. (D) The skin of lf fish. Leucophores (arrow) are observed in the skin of the wild-type fish (Inset), but not in that of lf fish. Dark dendritic cells (arrowhead) are melanophores. (Bars represent 5 mm in A–C and 300 μm in D.)
In the wild-type medaka, iridophores are found in the skin, mainly on the ventral side, in the eyes, and on the peritoneum. On the outside of the peritoneal membrane, a reflecting iridophore layer normally obscures the underlying melanophore layer. In the gu/gu mutant, the intensity of iridescent color exhibited by iridophores is reduced, and the belly appears dark because the melanophore layer is visible through the transparent iridophore layer in the peritoneum. The il-1 mutation also reduces the degree of iridescence exhibited by iridophores, but in this case the skin is more strongly affected than the peritoneum and eyes. The red color of the gills is visible through the operculum because of lack of reflection in the skin. The i-3 stock appears to display typical albinism with pink eyes and absence of melanin pigment from all parts of the body. The gu, il-1, and i-3 loci are autosomal. The lf locus is sex-linked and is carried on both X and Y chromosomes (10). Fish of the lf stock show no visible leucophores throughout life (11).
The olvas-GFP Transgenic Medaka.
The olvas-GFP transgenic medaka of the orange-red variety (9) is heterozygous for a transgene, polvas-GFP, consisting of the coding region of GFP gene fused to the regulatory regions of the medaka homologue of the Drosophila vasa gene, olvas. The olvas gene is known to show germ-line-specific expression during the embryonic development of medaka (8). The transgenic medaka expresses GFP specifically in the germ line (9). This fish was used as the parent to introduce the transgene into the genome of the see-through fish.
Observation of Internal Organs in the See-Through Medaka.
Internal organs in the see-through medaka were externally observed in swimming fish in transparent plastic tanks by the naked eye or in anesthetized specimens under a stereoscopic microscope (MZAPO; Leica, Heerbrugg, Switzerland). For anesthetization, fish were treated with 0.1% 3-aminobenzoic acid ethyl ester (MS-222,Sigma) and kept in water in a 9-cm dish at 10°C on a cooling plate (MATS–500SW; Tokai Hit, Sizuoka, Japan) on the stage of the microscope.
Observation and Imaging of GFP Fluorescence.
Fish were anesthetized with the method described above. GFP expression in the fish was detected by using a fluorescence stereoscopic microscope (MZFLIII; Leica) equipped with a filter set, GFP2, composed of a 480/40-nm excitation filter and a 510-nm barrier filter. Fluorescent images were recorded by using a color digital cooled charge-coupled device camera (C4742–95; Hamamatsu Photonics, Hamamatsu, Japan) mounted on the microscope and analyzed by using IPLAB SPECTRUM 3.5 software (Scanalytics, Fairfax, VA).
Results
Generation of the See-Through Medaka.
To generate the see-through medaka, a mass mating technique was used, with more than three individuals for each crossing. First, the gu and i-3 fish were mated to produce the wild-type F1 offspring of the genotype gu/+ i-3/+ (step 1, Table 1). Among the F2 offspring were double-mutant fish with the phenotype characteristic of gu/gu i-3/i-3 (step 2, Table 1), and this phenotype bred true in the F3 generation (step 3, Table 1) and beyond. This stock was named STI (see-through medaka no. 1) and maintained as a closed colony. STI fish are fairly transparent because they lack black pigmentation in all locations of the body and are deficient in iridescence, particularly in the peritoneum. Through the transparent skin and peritoneum, the main internal organs are externally discernible by the naked eye.
Table 1.
Crossing of parent stocks to produce the see-through medaka
| Step no. of crossing | Parent stocks
|
Offspring
|
Name of the see-through medaka | ||
|---|---|---|---|---|---|
| Males | Females | Generation | Genotype used for the next step of crossing | ||
| 1 | i-3 (or gu) | gu (or i-3) | F1 | gu/+, i-3/+ | |
| 2 | F1 | F1 | F2 | gu/gu, i-3/i-3 | |
| 3 | F2 | F2 | F3 | gu/gu, i-3/i-3 | STI |
| 4 | lf | STI | F1 | gu/+, i-3/+, Ylf/X+ (Xlf/X+) | |
| 5 | F1 | F1 | F2 | gu/gu, i-3/i-3, Ylf/Xlf | |
| 6 | F2 | F1 | bc* | gu/gu, i-3/i-3, Ylf/Xlf (Xlf/Xlf) | |
| 7 | bc | bc | Intercross | gu/gu, i-3/i-3, Ylf/Xlf (Xlf/Xlf) | STII |
| 8 A | STII | il-1 | F1(A) | gu/+, i-3/+, Ylf/X+ (Xlf/X+), il-1/+ | |
| 8 B | il-1 | STII | F1(B) | gu/+, i-3/+, Y+/Xlf (Xlf/X+), il-1/+ | |
| 9 A | F1(A) | F1(A) | F2(A) | gu/gu, i-3/i-3, Ylf/Xlf, il-1/il-1 | |
| 9 B | F1(B) | F1(B) | F2(B) | gu/gu, i-3/i-3, Xlf/Xlf, il-1/il-1 | |
| 10 | F2(A) | F2(B) | F3 | gu/gu, i-3/i-3, Ylf/Xlf (Xlf/Xlf), il-1/il-1 | STIII |
bc, Backcross.
However, it was hypothesized that the transparency of the body could be improved by the introduction of the lf mutation into the STI stock to reduce the interference of leucophores in the transmission of light through the body of the fish. This improvement was accomplished by using Mendelian crosses similar to those described above to produce a new stock of the genotype gu/gu i-3/i-3 lf/lf (steps 4 and 5, Table 1). Because the lf locus is sex-linked, most of the triply homozygous offspring obtained in the F2 generation were male (step 5, Table 1). Therefore, triply homozygous males from the F2 generation were mated with females of the F1 generation that were heterozygous for the three loci. Triply homozygous males and females were then obtained at a normal sex ratio (step 6, Table 1). Their offspring bred true (step 7, Table 1) and were maintained as a closed colony. The new stock was named STII (see-through medaka no. 2). These STII fish were indeed more transparent than the STI. The main internal organs were more externally visible than those in the STI.
The gu/gu genotype of STII fish reduced the intensity of iridescent color exhibited by iridophores of the peritoneum. However, the skin of the fish retained some iridescent reflectance that interfered with transparency, particularly under the microscope. Therefore, to further reduce the degree of reflectance of the skin, a fourth recessive mutation, il-1, was introduced into the STII stock to generate the see-through medaka of the genotype gu/gu i-3/i-3 lf/lf il-1/il-1. Considering the sex linkage of the lf locus, two series of crosses, A and B, were used to obtain male (steps 8A and 9A, Table 1) and female (steps 8B and 9B, Table 1) quadruple mutants, respectively. Because the il-1 phenotype becomes apparent during the course of growth after hatching, all embryos of F2 (A) (step 9A, Table 1) and F2 (B) (step 9B, Table 1), which showed triple phenotypes of gu/gu i-3/i-3 lf/lf mutations, were first reared to the adult stage, and then individuals with the il-1/il-1 phenotype were selected for breeding. When male quadruple mutants were mated with female quadruple mutants, their offspring bred true (step 10, Table 1). They were named STIII (see-through medaka no.3) (Fig. 2) and maintained as a closed colony. These fish have the same degree of transparency to the naked eye as the STII, except for the operculum, which is more transparent in the STIII. Under the stereoscopic microscope, the STIII appear more transparent than the STII because of the lack of reflection of light in the skin.
Figure 2.
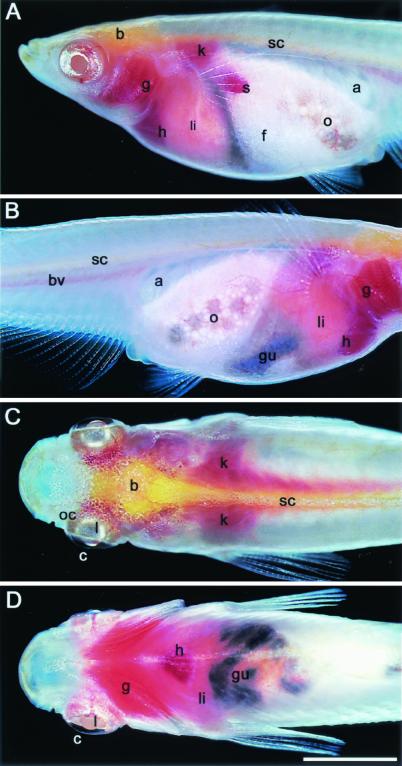
Adult STIII fish. The left (A) and right (B) sides of the body of a female. The dorsal (C) and ventral (D) views of a male. a, Air bladder; b, brain; bv, blood vessels; c, conjunctiva; f, fat tissue; g, gill; gu, gut; h, heart; k, kidney; l, lens; li, liver; o, ovary; oc, optic cup; s, spleen; sc, spinal cord. The dark color of the gut comes from ingested feed. (The bar represents 4 mm.)
These three stocks of the see-through medaka, STI, STII, and STIII, have been bred for several generations over 1–2 years after their establishment. All of the stocks are healthy and fertile and reach the adult stage in two months after hatching, as do many other stocks of medaka. The females spawn 20 to 30 eggs, a normal brood size, every day under laboratory conditions. Almost 100% of the embryos develop normally and hatch.
Observation of Internal Organs in the See-Through Medaka.
In the STIII stock, all organ systems were visible in the living, intact organism. From the lateral view, the fine comb-like structure of the branchial arches and their associated primary lamellae could be seen through the transparent operculum (Fig. 2 A and B). From the lateral and ventral views, the pulsating red heart was discernible by the naked eye immediately caudal to the branchial chamber and slightly to the right of the ventral midline (Fig. 2 A, B, and D). From the left lateral view, the dark red spleen was clearly seen (Fig. 2A). The liver was pink and occupied the anterior one-third to one-fourth of the abdominal cavity (Fig. 2 A, B, and D). The gallbladder was visible in some individuals because of its characteristic greenish-blue color and position at the caudal margin of the liver on the right ventral side (data not shown). Portions of the gut could be visualized through the lateral or ventral body walls (Fig. 2 B and D). It was easier to visualize when it contained ingested food, giving it a reddish or dark coloration depending on the diet. The gut could be seen to change its position from time to time in the same individuals. Gonads were seen anteroventral to the transparent air bladder (Fig. 2 A and B). In adult females, the ovary often occupied more than half of the abdominal cavity. Fat tissues often intervened between the left lateral aspect of the ovary and the body wall (Fig. 2A). This intervention apparently led to improved clarity of ovarian structures seen from the right (Fig. 2B) versus the left side. From the right, young oocytes at various stages of maturation were discernible in the ovary by the naked eye. The testis was also more visible from the right than the left side, for the same reason, in males (data not shown). From the dorsal view (Fig. 2C), the yellowish brain and spinal cord occupied the midline. Bilaterally symmetrical kidneys, on either side of the vertebral column, extended from the bases of the pectoral fins, where they reached their greatest area and width, to the caudal reaches of the abdominal cavity. The round clear lenses of the eyes were observed between the thin conjunctivae and the optic cups on the dorsum of the head. A fine red line ventral to the vertebral column and easily seen in lateral views was the site of the aorta and associated veins (Fig. 2B). In addition, many smaller blood vessels could be observed throughout the body under the stereoscopic microscope.
Observations of Gonads in the olvas-GFP Transgenic See-Through Medaka.
In the olvas-GFP transgenic medaka of the orange-red variety, GFP fluorescence in the gonad becomes no longer visible after the embryo hatches because the skin and the peritoneum of these fish become covered with pigment cells. Therefore, the olvas-GFP-transgenic see-through medaka was produced by mating the transgenic orange-red fish with the STII stock and backcrossing the offspring to the same stock with selection to maintain the transgene.
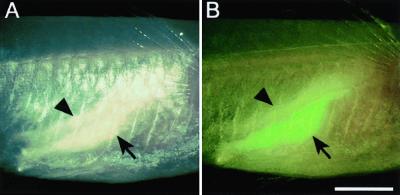
In the olvas-GFP-transgenic see-through fish, GFP expression was found exclusively in germ-line cells in adult fish as well as embryos and fry. The testis in the male was often difficult to distinguish from the surrounding fat tissues under white light (Fig. 3A), but it could be observed clearly under excitation light for GFP (Fig. 3B).
Figure 3.
The testis of an adult male of the transgenic see-through stock expressing the olvas-GFP gene. The photographs were taken from the right side of the body through the abdominal wall under weak white light. The fish is oriented with its head at the right side of each picture. The testis (arrow) and fat tissues (arrowhead) are observed without excitation light (A) and with excitation light (B). (The bar represents 2 mm.)
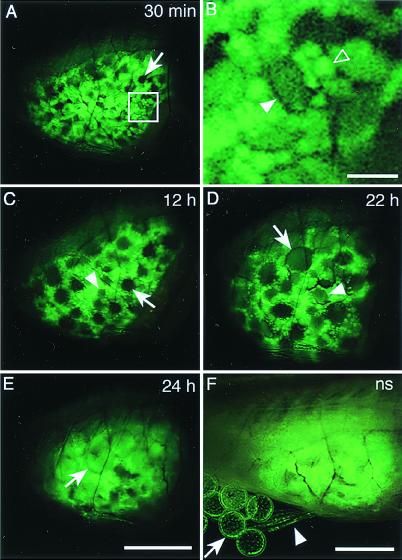
In females of the transgenic see-through fish, ovarian tissues were more clearly visible under excitation light than white light. To monitor the process of oocyte maturation, we repeatedly observed ovarian tissues in the same living individuals during one spawning cycle (24 h) under excitation light. Immediately after spawning, the ovary was filled with oocytes differing in the size and intensity of GFP fluorescence (Fig. 4A). At least three types of oocytes were discernible. The small oocytes (smaller than 100 μm in diameter) exhibiting strong fluorescence predominated in the tissues (Fig. 4B). This type of oocytes could be identified only under excitation light, but not under white light. Dozens of medium oocytes (more than 100 μm in diameter) exhibited weaker fluorescence. A dozen or so large oocytes (more than 200 μm in diameter) appeared dark in the photograph, but exhibited faint GFP fluorescence (Fig. 4A). The sizes of these medium and large oocytes could not be determined accurately because they were embedded in the ovarian tissues filled with small oocytes exhibiting strong fluorescence, and only a part of their cell body might have appeared on the surface. An increase in diameter was observed in the large oocytes and observed in at least a part of the medium oocytes during the following 1-day period (Fig. 4 A–D). In some cases, growth of oocytes could be traced individually by inferring their location in the ovarian tissues. An increase in the volume of the ovary was also observed. Rhythmical contractile movement was sometimes observed in the tissues. During the period after ovulation and before spawning, the ovarian tissues were covered with ovulated eggs in the ovarian cavity and were somewhat obscured (Fig. 4E). The females spawned when they were returned to the tanks with males (Fig. 4F). After spawning, the ovary was clearly visible again. The size and fluorescent image of the ovary were similar to those seen at the beginning of the repeated observations.
Figure 4.
Continuous observation of oocyte maturation in the ovary from the same living adult female of the transgenic see-through stock expressing olvas-GFP. Photographs were taken from the right side of the body through the abdominal wall. The fish is oriented with its head at the right side of each picture. The image under white light is similar to that of the ovary in Fig. 2B. (A) Thirty minutes after spawning. The ovary is filled with oocytes that differ in size and intensity of fluorescence. Small oocytes with strong GFP fluorescence predominate. The arrow shows a dark large oocyte. (B) Enlargement of the outlined area in A. Small (open arrowhead) and medium (solid arrowhead) oocytes are clearly seen. (C) Twelve hours after spawning. Apparent growth is observed in the medium (arrowhead) and large (arrow) oocytes. (D) Twenty-two hours after spawning. Growth in medium (arrowhead) and large dark (arrow) oocytes progressed. An increase in the volume of the ovary is also apparent. (E) Twenty-four hours after spawning. Mature eggs (arrow) had ovulated into the ovarian cavity. The ovarian tissues were somewhat obscured by being covered with the ovulated eggs. (F) The female spawned again when she was put back into the tank with a male after observation of the ovary shown in E. The ovarian tissues became clearly visible again. The cluster of the spawned eggs with weak fluorescence (arrow) is attached to the belly. Medaka females carry eggs in this fashion for several hours after spawning. The fish body was visualized by weak white light. The arrowhead shows the ventral fin. (The magnification is the same in A, and C–E, and the bar in E represents 2 mm. Bars in B and F represent 200 μm and 2 mm, respectively.) ns, Next spawning.
Discussion
A Fish Model That Is Transparent in the Adult Stage.
The medaka has been used as an ideal fish model in various fields of biology (1, 2). It has a small body size of 3 cm in total length and a short generation time of 2 months, and is easily maintained in the laboratory. Females spawn 20 to 30 eggs every day of the year under controlled conditions of temperature and light. Embryos develop externally and are transparent. The small genome size of the medaka, which is about one-fourth of that of mammalian species, is advantageous to the molecular analyses in this species.
In the present study, we successfully generated an improved fish model, the see-through medaka, that is transparent in the adult stage as well as embryonic stages, by genetically removing pigments from the skin and the peritoneum by the crossing of color mutants. In this fish, the main organ systems are visible from the outside of the body of living fish to the naked eye or with a simple stereoscopic microscope. No other vertebrate model with such transparency in the adult stage is available thus far. The potential of the medaka as an experimental model is greatly improved by the generation of the see-through medaka.
The see-through medaka has advantages in research applications to noninvasive studies of normal and pathological alterations of the internal structures at the later stage of life, such as organogenesis in postembryonic stages, growth and aging, carcinogenesis and genesis of other diseases, and inflammation or other alterations caused by exposure to chemical substances including endocrine disrupters. These alterations may be rapidly screened with ease in this fish. Continuous observations of internal organs are possible in the same individual throughout life, as shown in observations of oocyte maturation of the transgenic olvas-GFP see-through fish in the present study. This fish is also a useful alternative model to decrease the number of animals used for experiments, because many types of experiments can be conducted without having to kill the animals.
By using large-scale mutagenesis, great success has been achieved in screening mutations that affect early developmental processes in the zebrafish (12). Recently, similar screening processes have been started in the medaka (13). The transparency of the embryos in these fish makes the screening of an enormous number of embryos possible. The use of see-through medaka opens up the possibility of large-scale mutagenesis to screen mutations affecting postembryonic or late-onset biomedical phenomena because of the transparency in the adult stage.
Furthermore, the see-through medaka will also be an attractive viable model for classroom lessons in anatomy, physiology, and other biological subjects, because vital and dynamic activities of internal organs can be visualized easily in living fish.
In Vivo Visualization of Gene Expression in the Transgenic See-Through Medaka.
In vivo visualization of gene expression has been successful in transgenic medaka when GFP is used as a reporter. For example, a transgenic medaka carrying a GFP transgene that is driven by the promoter of the medaka translation elongation factor 1α-A gene (pEF-1α-A/GFP) expresses GFP in many of the tissues, except muscle from the embryonic, through the adult stage (14). The olvas-GFP-transgenic medaka of the orange-red variety, which is used as the parental stock to introduce the transgene into the see-through stock in the present study, exhibits germ-line-specific expression of the transgene (9). In these examples, transgene expression is highly visible in living organisms in their embryonic stages, but is difficult to see in the internal organs in the adult stage without killing the fish.
In the present study, we showed that in vivo visualization of gene expression was possible, even in the adult stage, in the see-through medaka. In the transgenic olvas-GFP see-through medaka, the testis was discernible from the surrounding fat tissues by GFP fluorescence. Ovarian tissues were also visualized clearly, and the process of oocyte maturation was monitored by repeated observations in the same viable females during one spawning cycle. Three types of oocytes differing in the size and intensity of GFP fluorescence were identified in the ovarian tissues, that is, small, medium, and large oocytes exhibiting strong, weaker, and faint GFP fluorescence, respectively. They appear to be at the previtellogenic, vitellogenic, and postvitellogenic phases of oocyte maturation, respectively, by referring to data obtained from dissected materials (15). Molecular mechanisms for the different intensities of GFP fluorescence in the three types of oocytes are unknown. These results demonstrated that fluorescence imaging of the transgenic see-through medaka is useful not only for monitoring the molecular processes of gene expression but also for improving the visibility of internal organs or tissues in living specimens. This method may be applied to any other gene constructs.
Recently, in vivo imaging of gene expression, progression of infection, and growth and metastases of tumors has been attempted in experimental animals with opaque bodies by using newer technologies such as MRI (16, 17), positron emission tomography (18), and bioluminescence technology (19, 20). The see-through medaka can now be used for these types of experiments to greatly simplify the experimental protocols and thereby enhance the validity of results.
Stability of the See-Through Medaka as Fish Stocks.
The see-through medaka, even the STIII, retains some pigmentation, that is, the orange-red coloration caused by xanthophores in the skin and the reflection by iridophores in the skin, peritoneum, and eyes. Introduction of other mutations in the pigmentation of the medaka may further improve the transparency of the body of this fish. However, one of the most obvious functions of pigments or pigment cells is to protect animals from the deleterious effects of sunlight. Melanin effectively absorbs light rays over a wide wavelength range, including harmful UV light, and acts as a sunscreen (21). Body color plays other important roles in the survival of lower vertebrates in their habitats (4, 5). The long-term effects of the transparent body of the see-through medaka over many generations on its growth, reproduction, and lifespan are unknown, although it is healthy and fertile under the present laboratory conditions. The effects of a combination of recessive mutations on this fish are also unknown, although the parental mutant strains of the see-through fish have been healthy and fertile since they were established 20 to 30 years ago. These problems are left for future studies.
Acknowledgments
We thank M. L. Lamoreux of Texas A&M University and D. Hinton of Duke University for reading the manuscript, and C. Inoue, H. Torihara, Y. Ishiguro, and J. Ishikawa for their technical assistance. This study was supported by grants from the Ministry of the Environment; Research for Millennium Project by the Ministry of Education, Culture, Sports, Science and Technology; and The Research for the Future Project of the Japan Society for the Promotion of Science.
Abbreviations
- GFP
green fluorescent protein
- gu
guanineless
- il-1
iridophoreless-1
- i-3
albino-3
- lf
leucophore-free
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ozato K, Wakamatsu Y. Dev Growth Differ. 1994;36:437–443. doi: 10.1111/j.1440-169X.1994.00437.x. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa Y. BioEssays. 2000;22:487–495. doi: 10.1002/(SICI)1521-1878(200005)22:5<487::AID-BIES11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Fishman M C, Stainier D Y, Breitbart R E, Westerfield M. Methods Cell Biol. 1997;52:67–82. doi: 10.1016/s0091-679x(08)60374-x. [DOI] [PubMed] [Google Scholar]
- 4.Fujii R. Int Rev Cytol. 1993;143:191–255. [Google Scholar]
- 5.Bagnara J T. In: The Pigmentary System: Physiology and Pathophysiology. Nordlund J J, Boissy R E, Hearing V J, King R A, Ortonne J-P, editors. Oxford: Oxford Univ. Press; 1998. pp. 9–40. [Google Scholar]
- 6.Tomita H. In: Medaka (Killifish): Biology and Strains. Yamamoto T, editor. Tokyo: Keigaku; 1975. pp. 251–272. [Google Scholar]
- 7.Tomita H. Fish Biol J Medaka. 1992;4:45–47. [Google Scholar]
- 8.Shinomiya A, Tanaka M, Kobayashi T, Nagahama Y, Hamaguchi S. Dev Growth Differ. 2000;42:317–326. doi: 10.1046/j.1440-169x.2000.00521.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Kinoshita M, Kobayashi D, Nagahama Y. Proc Natl Acad Sci USA. 2001;98:2544–2549. doi: 10.1073/pnas.041315498. . (First Published February 20, 2001; 10.1073/pnas.041315498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada H, Shimada A, Fukamachi S, Naruse K, Shima A. Zool Sci. 1998;15:123–126. doi: 10.2108/zsj.15.123. [DOI] [PubMed] [Google Scholar]
- 11.Tomita H. Fish Biol J Medaka. 1992;4:37–39. [Google Scholar]
- 12.Mullins M C, Hammerschmidt M, Haffter P, Nüsslein-Volhard C. Curr Biol. 1994;4:189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 13.Loosli F, Koster R W, Carl M, Kuhnlein R, Henrich T, Mucke M, Krone A, Wittbrodt J. Mech Dev. 2000;97:133–139. doi: 10.1016/s0925-4773(00)00406-8. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita M, Kani S, Ozato K, Wakamatsu Y. Dev Growth Differ. 2000;42:469–478. doi: 10.1046/j.1440-169x.2000.00530.x. [DOI] [PubMed] [Google Scholar]
- 15.Iwamatsu T, Ohta T, Oshima E, Sakai N. Zool Sci. 1988;5:353–373. [Google Scholar]
- 16.Louie A Y, Hüber M M, Ahrens E T, Rothbacher U, Moats R, Jacobs R E, Fraser S E, Meade T J. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 17.Chen X J, Hedlund L W, Möller H E, Chawla M S, Maronpot R R, Johnson G A. Proc Natl Acad Sci USA. 2000;97:11478–11481. doi: 10.1073/pnas.97.21.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Annala A J, Barrio J R, Toyokuni T, Satyamurthy N, Namavari M, Cherry S R, Phelps M E, Herschman H R, Gambhir S S. Nat Med. 2000;6:933–937. doi: 10.1038/78704. [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Baranov E, Jiang P, Sun F-X, Li X-M, Li L, Hasegawa S, Bouvet M, Al-Tuwaijri M, Chishima T, et al. Proc Natl Acad Sci USA. 2000;97:1206–1211. doi: 10.1073/pnas.97.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocchetta H L, Boylan C J, Foley J W, Iversen P W, LeTourneau D L, McMillian C L, Contag P R, Jenkins D E, Parr T R., Jr Antimicrob Agents Chemother. 2001;45:129–137. doi: 10.1128/AAC.45.1.129-137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordlund J J, Ortonne J-P. In: The Pigmentary System: Physiology and Pathophysiology. Nordlund J J, Boissy R E, Hearing V J, King R A, Ortonne J-P, editors. Oxford: Oxford Univ. Press; 1998. pp. 475–487. [Google Scholar]