Highlights
-
•
We screened for signs of neuropathy in a diabetes population in Zanzibar.
-
•
Nerve conduction study by NC-stat DPNCheck found neuropathy in 45%.
-
•
Monofilament results suggestive of neuropathy in 61%.
-
•
Compared to nerve conduction study, monofilament had a 59% specificity.
-
•
Hyperglycaemia and hypertension are highly prevalent risk factors in this population.
Keywords: Diabetes mellitus, Sub-Saharan Africa, Diabetic polyneuropathy (DPN), Nerve conduction studies, NC-stat DPNCheck
Abstract
Aim
Scant information is available about the prevalence of diabetic polyneuropathy, as well as the applicability of screening tools in sub-Saharan Africa. We aimed to investigate these issues in Zanzibar (Tanzania).
Methods
One hundred consecutive diabetes patients were included from the diabetes clinic at Mnazi Mmoja Hospital. Clinical characteristics were recorded. Further, we investigated: a) self-reported numbness of the lower limbs, b) ten-point monofilament test, c) the Sibbald 60-s Tool and d) nerve conduction studies (NCS, using an automated handheld point-of-care device, the NC-stat DPNCheck).
Results
Mean age was 54 years, 90% had type 2 diabetes, and with 9 year average disease duration. Mean HbA1c was 8.5% (69 mmol/mol), blood pressure 155/88 mmHg. Sixty-two% reported numbness, 61% had positive monofilament and 79% positive Sibbald tool. NCS defined neuropathy in 45% of the patients. Only the monofilament showed appreciable concordance with the NCS, Cohen’s κ 0.43.
Conclusions
The patient population was characterised by poor glycaemic control and hypertension. In line with this, neuropathy was rampant. The monofilament test tended to define more cases of probable neuropathy than the NCS, however specificity was rather low. Plantar skin thickening may have led to false positives in this population. Overall concordance was, however, appreciable, and could support continued use of monofilament as a neuropathy screening tool. The NC-stat DPNCheck could be useful in cases of diagnostic uncertainty or for research purposes in a low resource setting.
Introduction
Diabetes mellitus (DM) is on the rise globally and especially in sub-Saharan Africa (SSA), with a predicted doubling the next 20 years [1], [2]. Among the causes of this rise are rural-urban migration with accompanying dietary changes, reduced physical activity and longer life expectancy [3], [4], [5]. The estimated DM prevalence in the adult population of SSA today is 14.2 million [6], [7]. Information regarding the prevalence of diabetes and diabetic complications in the region is limited, with more than three quarters of the countries in SSA lacking national data [7], [8]. In Zanzibar (Tanzania) the estimated DM prevalence is 3–7%, based on random screening of selected populations and the 2011 National survey on Non Communicable Disease [9]. In light of this, increasing diabetes complications can be expected.
Diabetic polyneuropathy (DPN) is one of the most common diabetes complications. It is characterized by progressive loss of nerve fibres resulting in clinical manifestations such as reduced sensibility, pain and eventually motor nerve dysfunction [10]. As a dismal end-result, DPN may result in foot ulcers and amputations. Seventy percent of leg amputations in Tanzania occur in patients with DM, and the mortality rates associated with foot ulcers are reported to be as high as 50% [11]. In Zanzibar the clinical impression is that DPN represents a significant disease burden, however, evidence is scarce. One multi-centre study has been performed in Tanzania, in which more than a third of DM patients were considered to have high-risk feet for DPN [12]. Loss of a limb is serious in any setting, but potentially catastrophic in a society with limited social security structures. Early detection could facilitate interventions aimed at preventing such severe endpoints [12].
Because of the insidious development of DPN, patients can be asymptomatic for years and diagnosed only after complications like foot ulcers appear. There are multiple methods for DPN evaluation, and for screening purpose a variety of tests together with clinical judgement are recommended [13]. The accepted gold standard for the diagnosis of DPN is a traditional nerve conduction study (NCS) [14], a resource demanding examination not available in Zanzibar. Simpler, commercially available, point-of-care devices have been developed. The NC-stat DPNCheck is a hand-held device that measures sural nerve conduction velocity and action potential amplitude and gives a quantitative measurement of DPN. It is validated against traditional NCS [15], [16]. To our knowledge there are no studies on the use of nerve conduction tests in SSA. Hence, the aim of this study was to compare different methods of diagnosing DPN in a sub-Saharan population, and to evaluate the NC-stat DPNCheck as a potential tool for detection of DPN in a low-resource setting.
Methods
Study design and patients
This was an open, cross-sectional study of 100 patients with previously diagnosed diabetes consecutively included from the diabetes clinic at Mnazi Mmoja Hospital (MMH), Zanzibar (Tanzania). Data collection was performed between September 28th and October 21st, 2015. MMH is one of four hospitals in Zanzibar, the local hospital for Zanzibar City, as well as the only tertiary referral hospital in the region. Between 30–60 patients attend the clinic daily, out of which the first 5–20 patients on each occasion were invited to participate. The research procedures were carried out in an air conditioned room by a local intern doctor, a local nurse and a Norwegian doctor or nurse. Participants signed a written consent form after receiving thorough oral and written information. Illiterate participants were asked to consent by fingerprint.
Anthropometric data
Weight (kg), height (cm), Body mass index (kg/m2) as well as blood pressure (mm Hg) was recorded. Weight was measured on a standing scale with light clothing. Height was measured using a wall-mounted measuring rod.
Laboratory data
Fasting capillary blood sugar was measured with an Accu-check Glucometer. Further biochemistry was analysed at the laboratory of the private TASAKHTAA Hospital Zanzibar, an associate of GLOBAL Group of Hospitals. HbA1c was measured on BS 200 Mindray Automated Chemistry Analyzer.
Neuropathy
DPN status was evaluated by four methods: (1) self-reported numbness of the lower limbs, (2) monofilament test, (3) The Sibbald 60-s Tool, a standardized foot examination tool, and (4) nerve conduction studies using the NC-stat DPN Check. Self-reported numbness was recorded as the outcome of a simple yes/no question (“Do you have numbness of the lower limbs?”). The standardized ten gram monofilament evaluation was performed at ten sites on each foot [17]: the plantar surface of all toes, the hallux, the third and fifth metatarsal heads, the base of the fifth and the heal. With eyes closed, the patients provided a yes/no response to monofilament pressure. A score ≥ four negative responses was considered as a case of reduced sensibility. The Sibbald 60-Second Tool (2012) is a standardized foot examination tool designed to identify persons with diabetes who have high risk feet, modified from the International Diabetes Federations International Working Group on the Diabetic Foot [17]. The test included ascertaining any previous history of ulcer or amputation, physical examination for deformity, foot lesions, lower limb circulation, and sensibility examined by the monofilament test (as described above). Results were recorded as ten yes/no responses. Any positive response was defined as a high-risk foot for DPN.
By use of the NC-stat DPN Check (NeuroMetrix Inc., Waltham, MA, USA) participants were categorized into four different groups (no neuropathy, mild, moderate or severe neuropathy) by combining sural nerve conduction velocity and sensory nerve action potential amplitude results. All examinations were performed according to a standard protocol by non-specialized personnel, and were done in duplicates. The instrument consists of a display, a single use biosensor pad and two metal stimulation probes placed 9.22 cm from the biosensor. The probes discharge a 100 mA current to be detected orthodromically by the biosensor. The skin overlying the anatomical position of the sural nerve, posterior to the lateral malleolus and proximally to the Achilles tendon, was first prepared using a preparation pad that sterilized and buffered the testing area. The stimulation probes were then covered in gel to promote conduction, and placed on the prepared area. The medial edge of the biosensor was placed on the lower calf, in line with the Achilles tendon. In an automated protocol the sural nerve was stimulated 4–16 times within 10–20 s, depending on the strength of the sural nerve signal detected by the biosensor. Results below 1.5 µV were automatically adjusted to zero. If a device error was observed, the test was repeated. If a second error was observed, the test was recorded as unsuccessful.
Statistics
Continuous variables were compared by independent sample T-test or Mann-Whitney U test whenever the data did not have normal distribution or when the assumption of equal variance was violated across the study groups. Chi-square test was used to compare the qualitative differences between groups. A p-value <0.05 was considered statistically significant. Results were reported as mean, standard deviation (SD) and frequencies (percentage), unless otherwise stated. Sensitivity, specificity and predictive values were calculated for the different methods and measures of agreement between methods were evaluated by Cohen’s kappa (κ).
Ethics
The study was conducted in accordance with the principles of Good Clinical Practice (CPMP/ICH/135/95) and the Declaration of Helsinki on ethical principles for medical research involving human subjects (2013 version). Prior approval was obtained by the Zanzibarian medical ethics committee (ZAMREC) and the Norwegian regional ethics committee (REK Vest).
The clinically relevant results were made available for the patients and the health care personnel at the diabetes clinic at MMH. Patients who were found to have foot ulcers were referred for surgical evaluation. Those with DPN or other risk factors were given individual counselling on how to prevent foot ulcers. Key study findings have been presented to the doctors at MMH.
Results
Clinical characteristics
Fifty-one male and forty-nine female patients with diabetes were included in the study. The characteristics of the study population are listed in Table 1. Ten patients had been clinically diagnosed with type 1 DM. These were younger (40 vs. 55 years) had longer DM duration compared to patients with clinical type 2 DM (14 vs. 8 years). Fifty-one patients were treated with oral antidiabetic medication, 44 patients with insulin, two patients with both, and two patients with diet alone. The mean DM duration was longer in the insulin vs. non-insulin treated group (11.7 vs. 6.7 years). In total, 50% were treated with metformin, and the most common dose was 500 mg twice daily. Thirty-three patients were treated with sulphonylureas (glibenclamide), the most common dose being 20 mg daily. The mean daily insulin dose was 50 IU (0.8 IU/kg). One patient reported use of lipid-lowering medication. Seventy-five percent of the patients reported use of antihypertensive medication, of which 61% used Angiotensin-Converting Enzyme (ACE) inhibitors. The majority were however still hypertensive, 60% had systolic blood pressure above 140 mmHg. A significant correlation was found between morning fasting capillary glucose and HbA1 c level, r = 0.55, p < 0.0005
Table 1.
Characteristics of the study population.
| Variables | |
|---|---|
| Number of included patients | 100 |
| Sex male/female | 51/49 |
| Age, years | 54 [29–85] |
| Body mass index, kg/m2 | 26 [17–43] |
| Type 1DM/Type 2 DM* | 10/90 |
| DM duration, years | 8.9 [0 −3 0] |
| Systolic blood pressure, mmHg | 155 [81–233] |
| Diastolic blood pressure, mmHg | 88 [51–127] |
| Previous amputation | 6 |
| HbA1c,%, (mmol/mol) | 8.5 [3.7–23] (69 [17–227]) |
| Fasting blood glucose, mmol/l | 12.4 [4.3–26] |
| Insulin treatment | 44 |
| Oral antidiabetic medication | 51 |
| Antihypertensive treatment | 75 |
| Lipid-lowering therapy | 1 |
Data are presented as number of patients or mean [range].
Clinical diagnosis, not verified.
Neuropathy
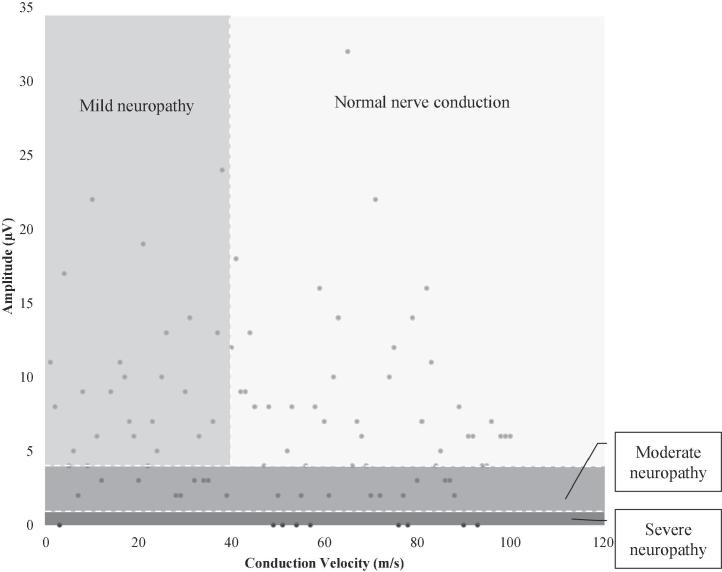
When asked about numbness of the feet, 62% answered confirmatory. The monofilament test was positive in 61%, whereas the Sibbald 60-s Tool was positive in 87%, of the patients. NCS measured by NC-stat DPNCheck, detected DPN in 45% of the patients, 15% were classified as mild, 20% as moderate and 10% as severe polyneuropathy (Fig. 1). In seven patients the NCS test results were invalid; these patients were excluded from further analysis.
Fig. 1.
Nerve conduction study results DPN grading according to NC-stat DPNCheck score.
Patients with DPN based on NC-stat DPNCheck were more likely male (62% vs. 40%, p < 0.05), of higher age (56 vs. 52 years, p < 0.05) and had more cases of present and previous foot ulcers (31 vs. 17 reported cases, p < 0.05). Out of the six participants who had undergone lower limb amputation, five had DPN based on NC-stat DPNCheck on the contralateral limb.
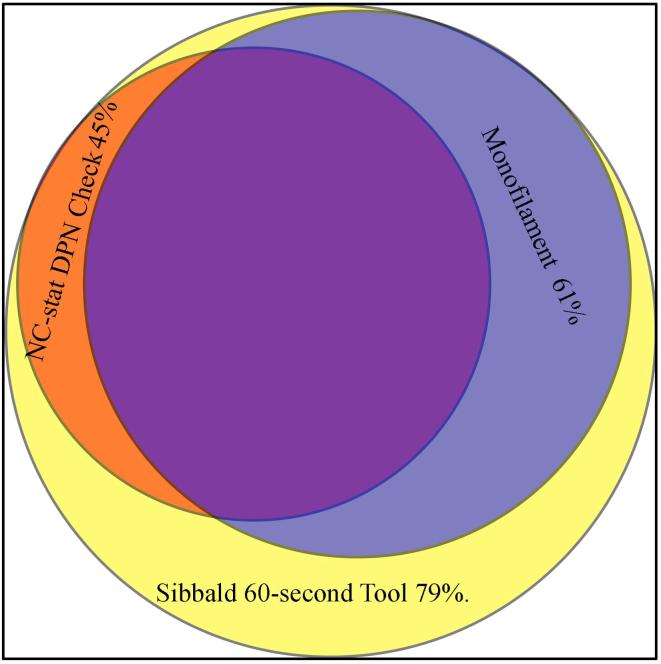
No significant differences were detected in terms of diabetes duration, BMI, blood pressure and HbA1c. The different evaluation methods for DPN were compared with NC-stat DPNCheck test results in terms of accuracy and agreement. Monofilament and the Sibbald 60-s Tool showed the highest sensitivity. Although both these tests showed a high negative predictive value, the monofilament also demonstrated the highest specificity, resulting in the highest positive predictive value and Cohen‘s kappa (κ = 0.43), suggesting better overall association with NC-stat DPNCheck (Table 2). Between-method concordances are depicted in Fig. 2.
Table 2.
Diagnostic accuracy and agreement of DPN screening methods.
| Test method | Sensitivity | Specificity | PPV | NPV | Cohen’s κ of agreement |
|---|---|---|---|---|---|
| Numbness | 74% | 49% | 54% | 69% | 0.22 |
| Monofilament | 86% | 59% | 63% | 83% | 0.43 |
| Sibbald | 98% | 47% | 54% | 94% | 0.19 |
The accuracy and degree of agreement between self-reported numbness of the lower limbs, the monofilament test and Sibbald 60-s tool, compared to the NC-stat DPNCheck. PPV = positive predictive value, NPV = negative predictive value.
Fig. 2.
Venn diagram depicting between-method concordances.
Discussion
With the help of a hand-held nerve conduction study device, DPN could be confirmed in almost half the patients attending the diabetes clinic at Mnazi Mmoja Hospital, Zanzibar This is in accordance with the estimated prevalence of DPN [13], [14], and previous investigations in of DPN in this population[12]. Compared to NC-stat DPNCheck, only the monofilament test had an acceptable level of agreement.
In this study we wanted to estimate the prevalence of DPN and to compare different screening methods for detecting DPN using nerve conduction study as our gold standard [14]. In our experience, the NC-stat DPNCheck was technically easy to use in this setting, and none of the test subjects found the test procedure any more than mildly discomforting. The NC-stat DPNCheck is dependent on the presence of an anatomically standard sural nerve. In up to 9% of adults the sural nerve can be inaccessible [18], [19], which is an obvious challenge in the use of the NC-stat DPNCheck. In line with this, we were not able to acquire test results from seven of the participants and consequently they were excluded from the between-method analysis. These were comparable with the total cohort in terms of glycaemic control, diabetes type and other neuropathy tests. Furthermore, a well-known limitation of the NC-stat DPNCheck is the possible underestimation of sural nerve amplitudes, since results <1.5 µV are not registered [15]. These cases are classified as severe neuropathy, independently of conduction velocity, as can be seen in Fig. 1.
Self-reported numbness of the lower limb is a standardized part of a diabetes anamnesis. Our findings showed low agreement with NC-stat DPNCheck (Table 2), and also with the Sibbald 60-Second Tool, suggesting this method of screening for DPN is insufficient.
The monofilament test is a recommended screening method for both DPN and identification of risk for foot ulceration [14], [20], [21]. It is universally widespread in use and available at a low cost. The monofilament test had high sensitivity (86%), and a better positive predictive value (63%) than self-reported numbness and the Sibbald 60-s Tool. The agreement with the NC-stat DPNCheck was also highest for this test (κ = 0.39), albeit still a substantial proportion (23%) of patients with positive monofilament test had normal NC-stat DPNCheck. The high sensitivity stands in contrast to previous studies that have found that monofilament used alone has low sensitivity compared to other tests [22], [23]. One can however speculate as to whether the monofilament tests estimate of DPN is too high in a population where barefoot walking is customary, resulting in thickening of palmar skin and reduced sensation.
The Sibbald 60-Second Tool was chosen for its implementability by all healthcare providers, ease of use and previous validation in an African population and other low income countries [24], [25]. As shown, the Sibbald 60-Second Tool was very sensitive (100%), more so than self-reported symptoms of numbness (74%). On the other hand, both had low specificity, resulting in a low positive predictive value when compared to NCS. Conversely, the negative predictive value for the Sibbald 60-s Tool was high, rendering it useful in many clinical settings. It is a validated screening instrument that can be implemented at low cost. Its use is limited as it is somewhat time-consuming; also our clinical experience is that it requires dedicated personnel to perform in a standardized fashion.
Surprisingly, we found a higher prevalence of DPN through screening questionnaires and clinical examination than the NC-stat DPNCheck [26], [27], [28], [29]. Several explanations may be offered. It may reflect an actual difference in neuropathy phenotype compared to studies done on different ethnic populations [30], [31]. In line with this, a formal validation of the device in specific ethnic populations could be of value. Also, it may reflect upon cultural differences in the experience and reporting of symptoms. As this study – to the best of our knowledge – is the first employing NCS in this setting, this remains to be concluded upon.
We recruited participants from the largest public out-patient diabetes clinic in Zanzibar. Patients were consecutively included, informed and investigated by their regular health care providers in their native language The cross-sectional nature of the study prohibits exploration of causal factors leading to DPN. Furthermore, we cannot determine which method of investigation predicts hard end-points such as ulcers and amputations.
The glycaemic status, as measured by HbA1c, showed an extremely wide range (3.7–23%). This could be an expression of true variation, or it could be due to technical difficulties and other confounding factors like anaemia and haemoglobinopathies known to affect HbA1c [32], [33]. Our findings suggest that there is room for improved antidiabetic treatment in the prevention of DPN, as there was little inter-patient variation in doses of both oral antidiabetic drugs and insulin doses, and especially metformin doses were consistently very low. The lack of available glucose self-monitoring severely limits the possibility of optimizing the antidiabetic treatment. Likewise, the prevalence of hypertension was very high, and the majority of patients were still hypertensive despite reporting antihypertensive treatment. Most were on ACE-inhibitors, which are known to be less effective in patients of African origin [34]. Hypertension is known to be an independent risk factor for the development of DPN, and may add to the undesirable consequences of hyperglycaemia [35]. Unfortunately, we lack information about dyslipidaemia. However, the use of statins was surprisingly low, implying a high prevalence of untreated patients.
The clinical consequence of detecting DPN is to initiate preventive interventions aimed at saving limbs and lives. Raised awareness about the condition has previously been shown to improve diabetic foot care through the Step by Step Project in Tanzania [12]. The monofilament test showed high sensitivity, with limited specificity. We would still argue for the applicability of monofilament as a screening tool for DPN, since the benefits of early identification of risk of foot ulceration, outweigh the risks associated with false positive results. Also, the interventions in terms of treating risk factors and patient education are advantageous to all DM patients. Furthermore, monofilament is inexpensive and ubiquitous. The automated point-of-care NCS device NC-stat DPNCheck could play a role as an alternative to traditional NCS in this setting.
Conclusion
We have confirmed a rampant prevalence of DPN and an unfavourable metabolic risk profile in diabetes patients in Zanzibar. Our findings suggest the utility of monofilament as a screening tool for DPN and the NC-stat DPNCheck in cases of diagnostic uncertainty or for research purposes in a low resource setting. In order to address the associated risk of ulcers and amputations, a prospective study in this diabetes population would be of great value.
Conflict of interest
The authors have no competing interest to disclose.
Funding
The project was made possible through funding from The Department of International Collaboration and The Department of Medicine Haukeland University Hospital.
Authors’ contributions
ECV contributed to study design, data acquisition, data analysis, and interpretation of the data and wrote the manuscript. ES and MØ contributed to study design, interpretation of the data and revised the manuscript. FS contributed to study design, data acquisition and revised the manuscript. BAJ contributed to the data acquisition. HBT contributed to study design and revised the manuscript. OO contributed to data acquisition and revised the manuscript. All authors approved of the final version for publication. ECV, MØ and ES are responsible for the integrity of the work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jcte.2017.09.001.
Appendix A. Supplementary data
References
- 1.World Health Organization W. Global report on diabetes. Geneva; 2016.
- 2.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey R, Julien M. Urbanisation and health. Clinical medicine (London, England). 2005;5:137–41. [DOI] [PMC free article] [PubMed]
- 4.The Lancet Diabetes E. The ageing of Africa. The lancet Diabetes & endocrinology. 2016;4:1. [DOI] [PubMed]
- 5.Chiwanga F.S., Njelekela M.A., Diamond M.B., Bajunirwe F., Guwatudde D., Nankya-Mutyoba J. Urban and rural prevalence of diabetes and pre-diabetes and risk factors associated with diabetes in Tanzania and Uganda. Global Health Action. 2016;9:31440. doi: 10.3402/gha.v9.31440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall V., Thomsen R.W., Henriksen O., Lohse N. Diabetes in Sub Saharan Africa 1999–2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11:564. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Federation ID. IDF diabetes atlas. 7th ed. 2013.
- 8.Dalal S., Beunza J.J., Volmink J., Adebamowo C., Bajunirwe F., Njelekela M. Non-communicable diseases in sub-Saharan Africa: what we know now. Int J Epidemiol. 2011;40:885–901. doi: 10.1093/ije/dyr050. [DOI] [PubMed] [Google Scholar]
- 9.Zanzibar MoH, Jorgensen JMA. NCD Survey Report: Main findings form the National Non-Communicable Disease Risk Factor Survey. Zanzibar; 2012.
- 10.Pop-Busui R., Boulton A.J., Feldman E.L., Bril V., Freeman R., Malik R.A. Diabetic neuropathy: a position statement by the american diabetes association. Diabetes Care. 2017;40:136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulam-Abbas Z., Lutale J.K., Morbach S., Archibald L.K. Clinical outcome of diabetes patients hospitalized with foot ulcers, Dar es Salaam, Tanzania. Diabetic Med: J Br Diabetic Assoc. 2002;19:575–579. doi: 10.1046/j.1464-5491.2002.00740.x. [DOI] [PubMed] [Google Scholar]
- 12.Abbas Z.G., Lutale J.K., Bakker K., Baker N., Archibald L.K. The 'Step by Step' Diabetic Foot Project in Tanzania: a model for improving patient outcomes in less-developed countries. Int Wound J. 2011;8:169–175. doi: 10.1111/j.1742-481X.2010.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesfaye S., Boulton A.J., Dyck P.J., Freeman R., Horowitz M., Kempler P. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyck P.J., Albers J.W., Andersen H., Arezzo J.C., Biessels G.J., Bril V. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes/Metab Res Rev. 2011;27:620–628. doi: 10.1002/dmrr.1226. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.A., Halpern E.M., Lovblom L.E., Yeung E., Bril V., Perkins B.A. Reliability and validity of a point-of-care sural nerve conduction device for identification of diabetic neuropathy. PLoS ONE. 2014;9:e86515. doi: 10.1371/journal.pone.0086515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins B.A., Grewal J., Ng E., Ngo M., Bril V. Validation of a novel point-of-care nerve conduction device for the detection of diabetic sensorimotor polyneuropathy. Diabetes Care. 2006;29:2023–2027. doi: 10.2337/dc08-0500. [DOI] [PubMed] [Google Scholar]
- 17.Sibbald RG, Ayello EA, Alavi A, Ostrow B, Lowe J, Botros M, et al. Screening for the high-risk diabetic foot: a 60-second tool (2012). Adv Skin Wound Care 2012;25:465–76; quiz 77–8. [DOI] [PubMed]
- 18.Burke D., Skuse N.F., Lethlean A.K. Sensory conduction of the sural nerve in polyneuropathy. J Neurol Neurosurg Psychiatry. 1974;37:647–652. doi: 10.1136/jnnp.37.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killian J.M., Foreman P.J. Clinical utility of dorsal sural nerve conduction studies. Muscle Nerve. 2001;24:817–820. doi: 10.1002/mus.1074. [DOI] [PubMed] [Google Scholar]
- 20.Perkins B.A., Orszag A., Ngo M., Ng E., New P., Bril V. Prediction of incident diabetic neuropathy using the monofilament examination: a 4-year prospective study. Diabetes Care. 2010;33:1549–1554. doi: 10.2337/dc09-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawford F, Cezard G, Chappell FM, Murray GD, Price JF, Sheikh A, et al. A systematic review and individual patient data meta-analysis of prognostic factors for foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations (PODUS). Health Technol Assess (Winchester, England). 2015;19:1–210. [DOI] [PMC free article] [PubMed]
- 22.Pambianco G., Costacou T., Strotmeyer E., Orchard T.J. The assessment of clinical distal symmetric polyneuropathy in type 1 diabetes: a comparison of methodologies from the Pittsburgh Epidemiology of Diabetes Complications Cohort. Diabetes Res Clin Pract. 2011;92:280–287. doi: 10.1016/j.diabres.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miranda-Palma B., Sosenko J.M., Bowker J.H., Mizel M.S., Boulton A.J. A comparison of the monofilament with other testing modalities for foot ulcer susceptibility. Diabetes Res Clin Pract. 2005;70:8–12. doi: 10.1016/j.diabres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Woodbury M.G., Sibbald R.G., Ostrow B., Persaud R., Lowe J.M. Tool for rapid & easy identification of high risk diabetic foot: validation & clinical pilot of the simplified 60 second diabetic foot screening Tool. PLoS ONE. 2015;10:e0125578. doi: 10.1371/journal.pone.0125578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prisca Olabisi Adejumo A.F.A., Fasanmade Adesoji A. Results of a 60 second foot screening for patients with diabetes conducted on the, World diabetes day. J Nursing Edu Pract. 2011;2013:3. [Google Scholar]
- 26.Fateh H.R., Madani S.P., Heshmat R., Larijani B. Correlation of Michigan neuropathy screening instrument, United Kingdom screening test and electrodiagnosis for early detection of diabetic peripheral neuropathy. J Diabetes Metabolic Disorders. 2015;15:8. doi: 10.1186/s40200-016-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyllienmark L., Alstrand N., Jonsson B., Ludvigsson J., Cooray G., Wahlberg-Topp J. Early electrophysiological abnormalities and clinical neuropathy: a prospective study in patients with type 1 diabetes. Diabetes Care. 2013;36:3187–3194. doi: 10.2337/dc12-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussain G., Rizvi S.A., Singhal S., Zubair M., Ahmad J. Cross sectional study to evaluate the effect of duration of type 2 diabetes mellitus on the nerve conduction velocity in diabetic peripheral neuropathy. Diabetes Metab Syndrome. 2014;8:48–52. doi: 10.1016/j.dsx.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Sharma S., Vas P.R., Rayman G. Assessment of diabetic neuropathy using a point-of-care nerve conduction device shows significant associations with the LDIFLARE method and clinical neuropathy scoring. J Diabetes Sci Technol. 2015;9:123–131. doi: 10.1177/1932296814551044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tahrani A.A., Altaf Q.A., Piya M.K., Barnett A.H. Peripheral and autonomic neuropathy in south asians and white caucasians with type 2 diabetes mellitus: possible explanations for epidemiological differences. J Diabetes Res. 2017;2017:1273789. doi: 10.1155/2017/1273789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott C.A., Malik R.A., van, Ross E.R., Kulkarni J., Boulton A.J. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34:2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziemer D.C., Kolm P., Weintraub W.S., Vaccarino V., Rhee M.K., Twombly J.G. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152:770–777. doi: 10.7326/0003-4819-152-12-201006150-00004. [DOI] [PubMed] [Google Scholar]
- 33.Vikoren T.B., Berg J.P., Berg T.J. Sources of error when using haemoglobin A1c. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke. 2014;134:417–421. doi: 10.4045/tidsskr.13.0938. [DOI] [PubMed] [Google Scholar]
- 34.Fox R. American Heart Association 2001 scientific sessions: late-breaking science-ACE inhibitor therapy in African-Americans. Circulation. 2001;104:E9052. [PubMed] [Google Scholar]
- 35.Tesfaye S., Chaturvedi N., Eaton S.E., Ward J.D., Manes C., Ionescu-Tirgoviste C. Vascular risk factors and diabetic neuropathy. New England J Med. 2005;352:341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.