Abstract
Ingestion of coffee (which is a mixture of over 1000 hydrosoluble substances) is known to protect from type-2 diabetes mellitus and its complications, and other chronic disorders associated with increased oxidative damage in blood and tissues. This protection is generally attributed to polyphenols and melanoidins. Very few studies were conducted on the amelioration of classic blood markers of oxidative stress induced after a few days of caffeine administration, but results vary.
To assess whether caffeine per se could account for antioxidant properties of coffee in the short-term, we tested the ability of pure caffeine ingestion (5 mg/kg body weight/day in two daily doses for seven consecutive days) to improve plasma levels of six biochemical indices in healthy male volunteers (n = 15). These indices were total antioxidant capacity (TAC), glutathione (GSH), oxidized glutathione (GSSG), GSH to GSSG ratio, lipid hydroperoxides (LOOH) and malondialdehyde (MDA).
We found that all indices changed significantly (P < .05 or < .01) in a favourable manner, ranging from −41% for GSSG to −70% for LHP levels, and +106% for GSH levels to +249% for the GSG/GSSG ratio. Changes of any given index were uniform across subjects, with no outliers.
We conclude that caffeine has unequivocal, consistent antioxidant properties.
Keyword: Oxidative stress, Coffee, Caffeine, Lipid peroxidation, Gluthathione, Malondialdehyde
Introduction
Oxidative stress is involved in ageing [1], [2], [3], [4], [5], [6], [7] and in various diseases, including diabetes mellitus [8], [9], [10], atherosclerosis [11], [12], rheumatoid arthritis [13], [14], [15], [16], Alzheimer’s disease [17], [18], [19], Parkinson’s disease [20], [21], [22] and cancer [23], [24], [25], [26], [27], [28], [29], [30], [31]. Coffee has an antioxidant power three to five-fold greater than that red wine and tea [32], [33]. Accordingly, coffee consumption is associated with a decrease in incidence of the above disease, a beneficial effect that is generally attributed to polyphenols and melanoidins [34], [35].
Concerning endocrine and metabolic disorders, coffee exerts a protective effect on type-2 diabetes mellitus [36], decreasing the prevalence of newly detected hyperglycemia [37]. The antioxidants contained in coffee also protect from lipid peroxidation [38], [39]. Studies in rats showed that green tea and coffee both inhibited intestinal cholesterol absorption due to their content in epigallocatechin gallate and caffeine [40]. Coffee has recently aroused interest also because supplementation studies have shown that the consumption of coffee increased the concentration of plasma total homocysteine (tHcy) in humans [39], [41]. Elevated plasma tHcy concentrations have been associated with increased lipid peroxidation [42] and it is also suggested to be an independent risk factor for cardiovascular disease [43].
Tools for obesity management including caffeine and green tea have been proposed as strategies for weight loss and weight maintenance. A green tea–caffeine mixture improves weight maintenance, through thermogenesis, fat oxidation, and sparing fat free mass. [41]. Coffee is a complex mixture of potential ‘‘nutraceuticals.’’ Indeed, coffee contains about 1500 different substances, approximately half of which are soluble [36]. In order of abundance, typical values for the water-soluble constituents are phenolic polymers (pulp) 8%, polysaccharides 6%, chlorogenic acids 4%, minerals 3%, caffeine 1%, organic acids 0.5%, sugars 0.3%, lipids 0.2%, and aroma 0.1%. The water-soluble constituents of coffee impair the intestinal absorption of l-thyroxine, most likely as a result of physical sequestration of the hormone [36].
The aim of the study is to assess in human volunteers whether the short term administration of caffeine would be beneficial on lipid peroxidation and a number of indices of oxidative stress.
Materials and methods
Study group
Male volunteers had to meet the following criteria in addition to signing the consent form: being of age 18–25 years, nonsmokers, nondrinkers, having normal body mass index (BMI), having a diet that met the dietary reference values indicated by the Società Italiana di Nutrizione Umana (Italian Society for Human Nutrition) [46]. Fifteen volunteers, regular coffee drinkers, were recruited.
The water solution of caffeine given to these volunteers was a galenic formulation prepared by a local pharmacy. This caffeine solution was administered orally, at room temperature, at the dose of 5 mg/kg body weight/day in two daily doses (2.5 mg/kg in the morning and 2.5 mg/kg after lunch) for seven days. The daily dose was equivalent to five cups of coffee. We evaluated the biochemical oxidative markers specified below. Oxidative stress markers were analyzed in plasma before and after the intake of caffeine. Blood for the two time points (baseline and end of the study) was drawn in the morning, with the baseline sample taken prior to the first dose of caffeine and the final sample taken on the morning on day 8.
The markers of oxidative stress measured were (i) total antioxidant capacity (TAC); (ii) Glutathione (GSH); (iii) oxidized glutathione (GSSG); (iv) GSH to GSSG ratio; (v) lipid hydroperoxides (LOOH); (vi) malondialdehyde (MDA). As well known, decreased oxidative stress is associated with an increase in TAC, GSH, GSH to GSSG ratio, and a decrease in the remaining three indices.
Assays
Lipid peroxidation, was quantified by assessing the oxidative state of the plasma through determination of the levels of lipid hydroperoxides (LOOH, µmol/l) by means of spectrophotometric technique analysis, and malondialdehyde (MDA) levels by high-performance liquid chromatography (HPLC). For LOOH, we used the Oxis Bioxytech® LPO-560™ Assay (Oxis International, Inc., Portland, OR, USA). This assay is based on the oxidation of ferrous ions (Fe2+) to ferric ions (Fe3+) by hydroperoxides under acidic conditions. Ferric ions then bind with the indicator dye, xylenol orange, and form a colored complex. The absorbance of the complex was measured at 560 nm. For MDA measurement, 250 μl serum was added to 50 μl NaOH 6 M and then incubated at 60 °C in water bath for 30 min. Afterwards, proteins were precipitated with 125 μl 35% perchloric acid (v/v), with subsequent centrifugation and the mixture was centrifuged at 2800 rpm for 10 min. Next, 250 μl of the supernatant were transferred into an Eppendorf tube and mixed with 25 μl DNPH, which had been prepared as 5 mM solution in 2 M hydrochloric acid. This mixture was incubated for 30 min at room temperature in the dark and 50 μl were analyzed by HPLC [47].
The total antioxidant power (TAC, μmol/l) was determined by a colorimetric technique, using a commercial kit (DIACRON (Grosseto, Italy).
The modulation of antioxidant defenses was determined by analyzing plasma levels of reduced glutathione (GSH, μmol/ml), oxidized glutathione (GSSH, μmol/ml) and GSH/GSSH ratio. GSH and GSSH were measured by means of HPLC. This extraction procedure requires that blood samples are collected in vacutainer tubes containing K3-EDTA. After collection, 100 μl fresh blood were mixed with 12 μl phosphate buffer 10 mmol/l, pH 7.2 (for free GSH), or 12 μl phosphate buffer 10 mmol/l, pH 7.2, containing 10 mM N-ethylmaleimide (for oxidized GSH). One hundred μl of this mixture were hemolyzed by adding 900 μl distilled water and immediately deproteinized by adding 200 μl sulfosalicylic acid (12% volume). The content of GSH was assessed in the acid-soluble fraction [48].
Statistics
For each group, the arithmetic mean of the values found and the relative standard deviation (SD) were calculated. The significance of differences between groups was evaluated by the analysis of variance (ANOVA); P values < .05 were considered statistically significant.
Results
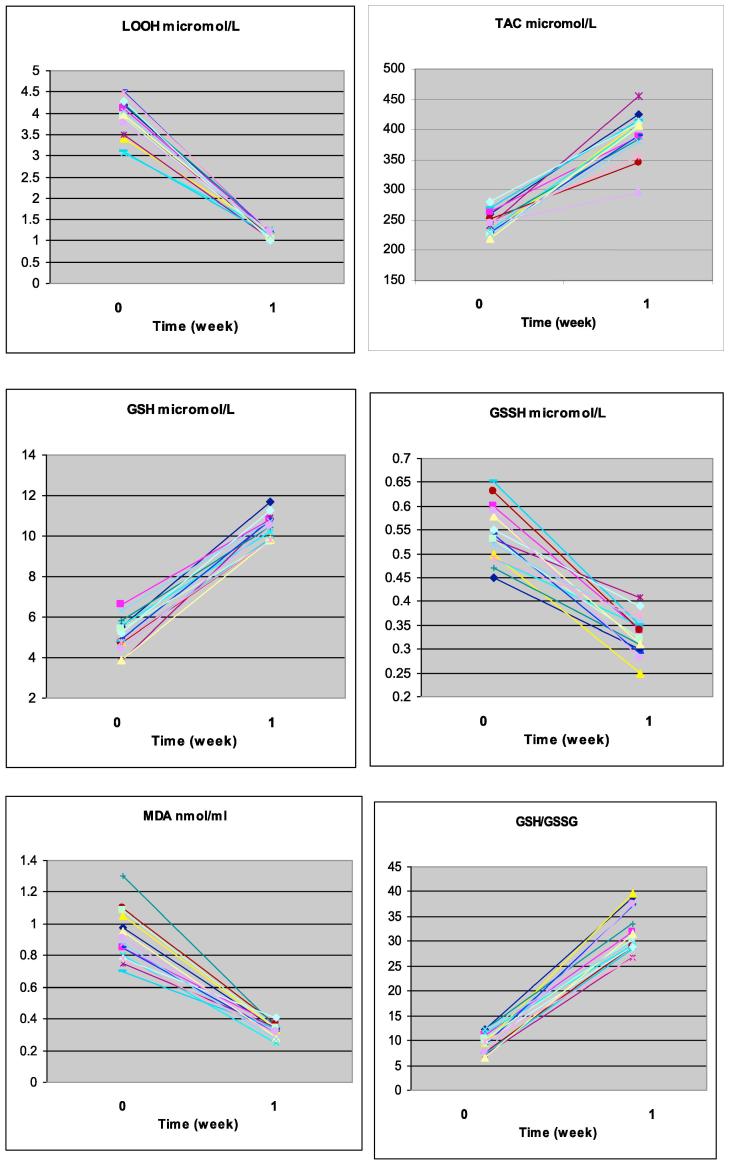
Data are illustrated individually in Fig. 1, and summarized in Table 1.
Fig. 1.
Individual data in the 15 volunteers for each of the six indices measured. Abbreviations are: GSH = glutathione; GSSG = oxidized glutathione; LOOH = lipid hydroperoxides; MDA = malondialdehyde; TAC = Total antioxidant capacity.
Table 1.
Changes in the indicated indices of oxidative stress observed in 15 healthy male volunteers after one-week administration of 5 mg/kg body weight/day in two daily doses.
| Index | Caffeine administration |
Statistics (P) | |
|---|---|---|---|
| Before | After | ||
| Lipid hydroperoxides (LOOH), µmol/L | 3.88 ± 1.85 | 1.16 ± 0.35 (−70%) | <.05 |
| Malondialdehyde (MDA), nmol/ml | 0.9 ± 0.3 | 0.3 ± 0.1 (−67%) | <.01 |
| Oxidized glutathione (GSSG), µmol/L | 0.56 ± 0.3 | 0.33 ± 0.4 (−41%) | <.01 |
| Glutathione (GSH), µmol/L | 5.1 ± 1.5 | 10.5 ± 2.7 (+106%) | <.01 |
| GSH to GSSG ratio | 9.11 ± 2.8 | 31.8 ± 3.4 (+249%) | <.01 |
| Total antioxidant capacity (TAC), µmol/L | 244.5 ± 40.3 | 398.2 ± 37.0 (+163%) | <.05 |
*The beneficial outcome after caffeine administration is a decrease for the first three indices and an increase for the last three indices.
Data are mean ± SD. Differences between means ± SD by ANOVA.
All indices changed in a favourable manner, ranging from −41% for GSSG to −70% for LOOH levels, and +106% for GSH levels to +249% for the GSG/GSSG ratio. We did not have any side effects, except for a slight, non-statistically significant, increase in heart rate.
Fig. 1 shows that changes of any given index were uniform across subjects, with no outliers.
Discussion
As summarized in Table 2, the indices of oxidative stress we have studied in the present paper are of relevance, including the diabetes mellitus setting. Concerning the object of our study, viz. caffeine, data from the literature show beneficial effects on TAC and lipid peroxidation [44], [45], [49], [50] with important additional actions of DNA protection from on oxidative breakage by hydroxyl radicals [51] and of decreased platelet aggregation [52], [53].
Table 2.
Summary of indices of oxidative stress and diabetes mellitus.
| Index | General | Pertinence for diabetes |
|---|---|---|
| Lipid hydroperoxides (LOOH) | Peroxidation of lipids produces highly reactive aldehydes, including MDA, acrolein, 4-hydroxynonenal, 4-oxononenal, and isolevuglandins [68]. It has been reported that peroxyl radicals can remove hydrogen from lipids, producing hydroperoxides that further propagate the free-radical pathway [69] | Increased lipid peroxidation occurs in both type 1 and type 2 diabetes mellitus [38] LOOH increase particularly in patients with vascular complications [70]. Lipid peroxidation in diabetes induces many secondary chronic complications including atherosclerosis and neural disorders [71], [72] |
| Malondialdehyde (MDA) | MDA is a three carbon, low molecular weight aldehyde representing the main product of polyunsaturated fatty acid peroxidation. It is characterized by a high toxicity due to its ability to react with other molecules like DNA and protein [54], [55], [56], [57], [58], [28], [59], [30], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75] MDA is documented as a primary biomarker of free radical mediated lipid damage and oxidative stress [74] |
Increased MDA level in plasma and many tissues was reported in diabetic patients [76], [77] Increased levels of MDA in diabetics suggests that peroxidative injury may be involved in the development of diabetic complications |
| Glutathione (GSH) | GSH is the most abundant nonprotein thiol that defends against oxidative stress [76]. GSH is an efficient antioxidant present in almost all living cells and is also considered as a biomarker of redox imbalance at cellular level [78], [79] | Reduced levels of GSH are found in diabetes [79]. Decreased GSH level may be one of the factors in the oxidative DNA damage in type 2 diabetics |
| Oxidized glutathione (GSSG) | GSSG is reduced back to GSH by the nicotinamide adenine dinucleotide phosphate (NADPH)-dependent catalysis of the flavoenzyme GSH reductase | GSSG levels in plasma from diabetic subjects were higher than those from controls |
| GSH to GSSG ratio | This ratio is used to evaluate oxidative stress status in biological systems | Plasma GSH/GSSG showed a significant decrease in type 2 diabetes as compared to normal. Hyperlipidemia, inflammation, and altered antioxidant profiles are the usual complications in diabetes mellitus as a result of decreased GSH/GSSG ratio |
| Total antioxidant capacity (TAC) | TAC is the primary measure and marker to evaluate the status and potential of oxidative stress in the body | TAC is significantly lower in diabetic subjects with poor glycaemic control than healthy subjects, while patients with good glycaemic control had plasma antioxidative values similar to controls [66]. Decrease in TAC of plasma is associated with increased complications of diabetes, which include cardiovascular disease, nerve damage, blindness, and nephropathy TAC is markedly reduced in sciatic nerve homogenates of diabetic animals [61] |
As recently reviewed [54] prior to us others [39], [43], [55], [56], [57] have evaluated the short-term effects of drinking caffeine on the oxidative stress. While in 4/5 such studies, the number of subjects is lower than ours, only a few have evaluated all the six markers of blood oxidative stress we did. Effects on DNA protection are demonstrable as early as two hours after coffee ingestion [43], confirming previous intervention studies that provided evidence for long-term coffee consumption correlating with reduced DNA background damage in healthy volunteers. Continued coffee intake was associated with further decrements in background DNA damage within the 8 h intervention. Mean tail intensities (TIs%) decreased from 0.33 TI% (baseline, 0 h) to 0.22 TI% (within 8-h coffee consumption). The authors concluded that repeated coffee consumption was associated with reduced background DNA strand breakage, clearly measurable as early as 2 h after first intake resulting in a cumulative overall reduction by about one-third of the baseline value [43].
As reviewed elsewhere, the total antioxidant capacity of plasma is the primary measure and marker to evaluate the status and potential of oxidative stress in the body [58]. Lipid hydroperoxides and MDA have been documented as a primary biomarker of free radical mediated lipid damage and oxidative stress [58]. GSH, the most abundant nonprotein thiol that defends against oxidative stress, is considered as a biomarker of redox imbalance at cellular level [58]. In contrast, GSSG is unable to perform antioxidant functions. GSSG can be reduced back to GSH (and the GSH:GSSG ratio maintained high) by glutathione reductase and associated oxidation of NADPH to NAD+, unless such enzymatic activity is overwhelmed by excessive amounts of reactive oxygen species (59).
The effect of coffee consumption on the modulation of plasma antioxidant capacity was evaluated in 10 studies [54]. Eight studies (seven chronic interventions and one acute trial) also investigated the role of coffee in the modulation of blood GSH levels as a substrate of GPx [glutathione peroxidase] and GST [glutathione S-transferases] enzymes. Four out of seven chronic intervention studies documented an increase in GSH levels [42], [58], [28], while two long-term studies [12], [22] and one study performing both an acute and a chronic intervention [55] did not show any significant effect. Coffee ineffectiveness was attributed to the degradation and metabolic conversion of different coffee constituents in the body or to the short duration of the intervention [55].
The effect of coffee consumption on markers of lipid oxidation has been investigated [11], [12], [18], [19], [21], [22], [23], [31], [56]. Five out of 12 studies investigated only the acute effect of coffee consumption [16], [19], [21], [23], [56], [61], five were chronic intervention studies [12], [18], [19], [21], [22], [61], [62], while two studies investigated both acute and chronic effects [23], [64]. In these studies, isoprostanes (IsoPs) and malondialdehyde (MDA) were the most frequently considered markers of lipid damage. Besides 8-IsoPGF2 and MDA, further markers of lipid damage and/or protection considered in the present review were oxidized LDL, resistance to LDL oxidation, serum LDL-conjugated dienes and hydroxyl fatty acids. The analysis of the main findings revealed that most of the interventions failed to demonstrate a significant decrease in markers of lipid damage with exception of results found by Ochiai et al. [57] and Sirota et al. [65]. The former reported a significantly reduced urinary 8-epiPGF2 following consumption of a coffee beverage (providing 600 mg of CGAs) when compared with placebo in healthy men. Results showed that consumption of 200 mL Turkish roasted coffee during a meal based on red-meat cutlets resulted in a significant inhibition of postprandial plasma MDA. No effect between treatments and control/placebo were instead found by other authors [16], [23], [61]. The investigation by Leelarungrayub et al. [56] deserves a special mention, because it reports a significant higher level of MDA in men consuming caffeinated coffee, when compared to decaffeinated coffee or control, followed by a submaximal exercise test. Authors reported that, similarly to what observed in previous investigations, results demonstrated an increased intramuscular fat oxidation following consumption of caffeine-rich foods. For what concerns the other markers of lipid damage, only Yukawa et al. [66] found a modest reduction of LDL oxidation susceptibility and a decrease of MDA levels following consumption of 3 coffees/day for 1 week. No significant effect was instead found by Mursu et al. [39] on serum LDL-conjugated dienes and plasma hydroxyl fatty acids, or by Teekachunhatean et al. [55] on MDA levels and by Hoelzl et al. [67] on both MDA and oxidized LDL.
In their article [54], Martini et al. conclude that, despite the high inter-study heterogeneity, data suggest that consumption of coffee may increase glutathione levels and reduce the levels of DNA damage. These effects are more evident in chronic interventions than in acute studies.
In summary, we have demonstrated that 7-day administration of pure caffeine induces unequivocally beneficial changes in a number of oxidative-stress biochemical indices, the magnitude of these changes being the greatest for the GSH to GSSG ratio.
The authors declare no conflict of interest.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Arranz L., Fernández C., Rodríguez A., Ribera J.M., De la Fuente M. The glutathione precursor N-acetylcysteine improves immune function in postmenopausal women. Free Radical Biol Med. 2008;45:1252–1262. doi: 10.1016/j.freeradbiomed.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto K., Takasaki W., Yamoto T., Manabe S., Sato I., Tsuda S. Effect of glutathione (GSH) depletion on DNA damage and blood chemistry in aged and young rats. J Toxicol Sci. 2008;33:421–429. doi: 10.2131/jts.33.421. [DOI] [PubMed] [Google Scholar]
- 3.Christon R., Haloui R.B., Durand G. Dietary polyunsaturated fatty acids and aging modulate glutathione-related antioxidants in rat liver. J Nutr. 1995;125:3062–3070. doi: 10.1093/jn/125.12.3062. [DOI] [PubMed] [Google Scholar]
- 4.Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4:288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Rebrin I., Bayne A.C., Mockett R.J., Orr W.C., Sohal R.S. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebrin I., Sohal R.S. Pro-oxidant shift in glutathione redox state during aging. Adv Drug Delivery Rev. 2008;60:1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samiec P.S., Drews-Botsch C., Flagg E.W., Kurtz J.C., Sternberg P., Jr, Reed R.L. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radical Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 8.Cerielo A., Motz E., Cavarape A., Lizzio S., Russo A., Quatraro A. Hyperglycemia counterbalances the antihypertensive effect of glutathione in diabetic patients: evidence linking hypertension and glycemia through the oxidative stress in diabetes mellitus. J Diabetes Complications. 1997;11:250–255. doi: 10.1016/s1056-8727(97)00021-4. [DOI] [PubMed] [Google Scholar]
- 9.Dincer Y., Akcay T., Alademir Z., Ilkova H. Effect of oxidative stress on glutathione pathway in red blood cells from patients with insulin-dependent diabetes mellitus. Metabolism. 2002;51:1360–1362. doi: 10.1053/meta.2002.35192. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida K., Hirokawa J., Tagami S., Kawakami Y., Urata Y., Kondo T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia. 1995;38:201–210. doi: 10.1007/BF00400095. [DOI] [PubMed] [Google Scholar]
- 11.Margutti P., Matarrese P., Conti F., Colasanti T., Delunardo F., Capozzi A. Autoantibodies to the C-terminal subunit of RLIP76 induce oxidative stress and endothelial cell apoptosis in immune-mediated vascular diseases and atherosclerosis. Blood. 2008;111:4559–4570. doi: 10.1182/blood-2007-05-092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Signorelli S.S., Neri S., Di Pino L., Costa M.P., Pennisi G., Digrandi D. Oxidative stress and endothelial damage in patients with asymptomatic carotid atherosclerosis. Clin Exp Med. 2001;1:9–12. doi: 10.1007/s10238-001-8002-7. [DOI] [PubMed] [Google Scholar]
- 13.Hassan M.Q., Hadi R.A., Al-Rawi Z.S., Padron V.A., Stohs S.J. The glutathione defense system in the pathogenesis of rheumatoid arthritis. J Appl Toxicol. 2001;21:69–73. doi: 10.1002/jat.736. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen-Lane J.H., Zurier R.B., Lawrence D.A. Analysis of the thiol status of peripheral blood leukocytes in rheumatoid arthritis patients. J Leukoc Biol. 2007;81:934–941. doi: 10.1189/jlb.0806533. [DOI] [PubMed] [Google Scholar]
- 15.Seven A., Guzel S., Aslan M., Hamuryudan V. Lipid, protein, DNA oxidation and antioxidant status in rheumatoid arthritis. Clin Biochem. 2008;41:538–543. doi: 10.1016/j.clinbiochem.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Karelson E, Mahlapuu R, Zilmer M, Soomets U, Bogdanovic N, Langel U. Possible signaling by glutathione and its novel analogue through potent stimulation of frontocortical G proteins in normal aging and in Alzheimer’s disease. In: Diederich M, editor. Cell Signaling, Transcription, and Translation as Therapeutic Targets. New York Academy of Sciences; New York; 2002. 973: 537–40. [DOI] [PubMed]
- 17.Liu HL, Wang H, Shenvi S, Hagen TM, Liu RM. Glutathione metabolism during aging and in Alzheimer disease. In: De Grey ADN, editor. Strategies for Engineered Negligible Senescence: Why Genuine Control of Aging May Be Foreseeable. New York Academy of Sciences; 2004. 1019:346–9. [DOI] [PubMed]
- 18.Resende R., Moreira P.I., Proenca T., Deshpande A., Busciglio J., Pereira C. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radical Biol Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Lang A.E. The progression of Parkinson disease: a hypothesis. Neurology. 2007;68:948–952. doi: 10.1212/01.wnl.0000257110.91041.5d. [DOI] [PubMed] [Google Scholar]
- 20.Spina M.B., Cohen G. Dopamine turnover and glutathione oxidation: implications for Parkinson disease. Proc Natl Acad Sci USA. 1989;86:1398–1400. doi: 10.1073/pnas.86.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto N, Sawada H, Izumi Y, Kume T, Katsuki H, Shimohama S, et al. Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress: relevance to Parkinson disease. J Biol Chem; 282:4364–72. [DOI] [PubMed]
- 22.Barranco S.C., Perry R.R., Durm M.E., Quraishi M., Werner A.L., Gregorcyk S.G. Relationship between colorectal cancer glutathione levels and patient survival: early results. Dis Colon Rectum. 2000;43:1133–1140. doi: 10.1007/BF02236562. [DOI] [PubMed] [Google Scholar]
- 23.Kigawa J., Minagawa Y., Kanamori Y., Itamochi H., Cheng X., Okada M. Glutathione concentration may be a useful predictor of response to second-line chemotherapy in patients with ovarian cancer. Cancer. 1998;82:697–702. doi: 10.1002/(sici)1097-0142(19980215)82:4<697::aid-cncr12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A., Sharma S., Pundir C.S., Sharma A. Decreased plasma glutathione in cancer of the uterine cervix. Cancer Lett. 1995;94:107–111. doi: 10.1016/0304-3835(95)03832-h. [DOI] [PubMed] [Google Scholar]
- 25.Wong D.Y., Hsiao Y.L., Poon C.K., Kwan P.C., Chao S.Y., Chou S.T. Glutathione concentration in oral cancer tissues. Cancer Lett. 1994;81:111–116. doi: 10.1016/0304-3835(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 26.Yeh C.C., Hou M.F., Wu S.H., Tsai S.M., Lin S.K., Hou L.A. A study of glutathione status in the blood and tissues of patients with breast cancer. Cell Biochem Funct. 2006;24:555–559. doi: 10.1002/cbf.1275. [DOI] [PubMed] [Google Scholar]
- 27.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 28.Hayes J.D., Pulford D.J. The glutathione S-Transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 29.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Hayes J.D., McLellan L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radical Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 31.Dalle-Donne I., Rossi R., Colombo R., Giustarini D., Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 32.Richelle M., Tavazzi I., Offord E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J Agric Food Chem. 2001;49(7):3438–3442. doi: 10.1021/jf0101410. [DOI] [PubMed] [Google Scholar]
- 33.Metro D., Muraca U., Manasseri L. Role of green tea in oxidative stress prevention. Clin Ter. 2006;157(6):507–510. [PubMed] [Google Scholar]
- 34.Borrelli R.C., Visconti A., Mennella C., Anese M., Fogliano V. Chemical characterization and antioxidant properties of coffee melanoidins. J Agric Food Chem. 2002;50(22):6527–6533. doi: 10.1021/jf025686o. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Gonzales I., Jimenez-Escrig A., Saura-Calixto F. In vitro antioxidant activity of coffees brewed using different procedures (Italian, espresso and filter) Food Chem. 2005;90:133–139. [Google Scholar]
- 36.Benvenga S., Bartolone L., Pappalardo M.A., Russo A., Lapa D., Giorgianni G. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid. 2008;18:293–301. doi: 10.1089/thy.2007.0222. [DOI] [PubMed] [Google Scholar]
- 37.Van Dam R.M., Hu F.B. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 38.Davì G., Falco A., Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signaling. 2005;7:256–268. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- 39.Mursu J., Voutilainen S., Nurmi T., Alfthan G., Virtanen J.K., Rissanen T.H. The effects of coffee consumption on lipid peroxidation and plasma total homocysteine concentrations: a clinical trial. Free Radical Biol Med. 2005;38(4):527–534. doi: 10.1016/j.freeradbiomed.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 40.Wang S., Noh S.K., Koo S.I. Epigallocatechin gallate and caffeine differentially inhibit the intestinal absorption of cholesterol and fat in ovariectomized rats. J Nutr. 2006;136:2791–2796. doi: 10.1093/jn/136.11.2791. [DOI] [PubMed] [Google Scholar]
- 41.Westerterp-Plantenga M.S. Green tea catechins, caffeine and body-weight regulation. Physiol Behav. 2010;100:42–46. doi: 10.1016/j.physbeh.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Tiwari B.K., Pandey K.B., Abidi A.B., Rizvi S.I. Markers of Oxidative Stress during Diabetes Mellitus. J Biomarkers. 2013;2013:378790. doi: 10.1155/2013/378790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakuradze T., Lang R., Hofmann T., Schipp D., Galan J., Eisenbrand G. Coffee consumption rapidly reduces background DNA strand breaks in healthy humans: Results of a short-term repeated uptake intervention study. Mol Nutr Food Res. 2016;60:682–686. doi: 10.1002/mnfr.201500668. [DOI] [PubMed] [Google Scholar]
- 44.Devasagayam T.P., Kamat J.P., Mohan H., Kesavan P.C. Caffeine as an antioxidant: inhibition of lipid peroxidation induced by reactive oxygen species. Biochim Biophys Acta. 1996;1282(1):63–70. doi: 10.1016/0005-2736(96)00040-5. [DOI] [PubMed] [Google Scholar]
- 45.Lee C. Antioxidant ability of caffeine and its metabolities based on the study of oxygen radical absorbing capacity and inhibition of LDL peroxidation. Clin Chim Acta. May 2000;295(1–2):141–154. doi: 10.1016/s0009-8981(00)00201-1. [DOI] [PubMed] [Google Scholar]
- 46.LARN – Livelli di assunzione di riferimento di nutrienti ed energia per la popolazione italiana. Revisione 2012 – SINU (Società Italiana di Nutrizione Umana).
- 47.Mateos R., Lecumberri E., Ramos S., Goya L., Bravo L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827(1):76–82. doi: 10.1016/j.jchromb.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 48.Pastore A., Piemonte F., Locatelli M., Lo Russo A., Gaeta L.M., Tozzi G. Determination of blood total, reduced, and and oxidized glutathione in pediatric subjects. Clin Chem. 2001;47(8):1467–1469. [PubMed] [Google Scholar]
- 49.Natella F., Nardini M., Giannetti I., Dattilo C., Scaccini C. Coffee drinking influences plasma antioxidant capacity in humans. J Agric Food Chem. 2002;50(21):6211–6216. doi: 10.1021/jf025768c. [DOI] [PubMed] [Google Scholar]
- 50.Bydlowski S.P., Yunker R.L., Rymaszewski Z., Subbiah M.T. Coffee extracts inhibit platelet aggregation in vivo and in vitro. Int J Vitam Nutr Res. 1987;57(2):217–223. [PubMed] [Google Scholar]
- 51.Azam S, Hadi N, Khan NU, Hadi SM. Antioxidant and prooxidant properties of caffeine, theobromineand xanthine. Med Sci Monit 2003. 9(9):BR325–30. [PubMed]
- 52.Choi J.W. Influence of caffeine on the responsiveness of human plateled to agonists. Thromb Res. 2003;110(4):209–212. doi: 10.1016/s0049-3848(03)00348-7. [DOI] [PubMed] [Google Scholar]
- 53.Varani K., Portaluppi F., Gessi S., Merighi S., Ongini E., Belardinelli L. Dose and time effects of caffeine intake on human plateled adenosine A(2A) receptors: functional and biochemical aspects. Circulation. 2000;102(3):285–289. doi: 10.1161/01.cir.102.3.285. [DOI] [PubMed] [Google Scholar]
- 54.Martini D., Del Bo' C., Tassotti M., Riso P., Del Rio D., Brighenti F. Coffee consumption and oxidative stress: A review of human intervention studies. Molecules. 2016;21(8) doi: 10.3390/molecules21080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teekachunhatean S., Tosri N., Sangdee C., Wongpoomchai R., Ruangyuttikarn W., Puaninta C. Antioxidant effects after coffee enema or oral coffeeconsumption in healthy Thai male volunteers. Hum. Exp. Toxicol. 2012;31:643–651. doi: 10.1177/0960327111432499. [DOI] [PubMed] [Google Scholar]
- 56.Leelarungrayub D., Sallepan M., Charoenwattana S. Effects of acute caffeinated coffee consumption on energy utilization related to glucose and lipid oxidation from short submaximal Treadmill exercise in sedentary Men. Nutr Metab Insights. 2011;4:65–72. doi: 10.4137/NMI.S8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ochiai R., Sugiura Y., Otsuka K., Katsuragi Y., Hashiguchi T. Coffee bean polyphenols ameliorate postprandial endothelial dysfunction in healthy male adults. Int J Food Sci Nutr. 2015;66:350–354. doi: 10.3109/09637486.2015.1007453. [DOI] [PubMed] [Google Scholar]
- 58.Droge W. Free radicals in the physiological control of cell function. Physiol. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 59.Mišík M., Hoelzl C., Wagner K.H., Cavin C., Moser B., Kundi M. Impact of paper filtered coffee on oxidative DNA-damage: results of a clinical trial. Mutat Res. 2010;692(1–2):42–48. doi: 10.1016/j.mrfmmm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Catanzaro O., Capponi J.A., Michieli J., Labal E., Di Martino I., Sirois P. Bradykinin B1 antagonism inhibits oxidative stress and restores Na+K+ ATPase activity in diabetic rat peripheral nervous system. Peptides. 2013;44:100–104. doi: 10.1016/j.peptides.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 61.Korkmaz G.G., Konukoglu D., Kurtulus E.M., Irmak H., Bolayirli M., Uzun H. Total antioxidant status and markers of oxidative stress in subjects with normal orimpaired glucose regulation (IFG, IGT) in diabetic patients. Scand J Clin Lab Invest. 2013;3(8):641–649. doi: 10.3109/00365513.2013.846477. [DOI] [PubMed] [Google Scholar]
- 62.Rein D., Paglieroni T.G., Pearson D.A., Wun T., Schmitz H.H., Gosselin R. Cocoa and wine polyphenols modulate platelet activation and function. J Nutr. 2000;130(8S Suppl):2120S–2126S. doi: 10.1093/jn/130.8.2120S. [DOI] [PubMed] [Google Scholar]
- 63.Schiffrin E.L. Antioxidants in hypertension and cardiovascular disease. Mol Interv. 2010;10(6):354–362. doi: 10.1124/mi.10.6.4. [DOI] [PubMed] [Google Scholar]
- 64.Sirota R., Gorelik S., Harris R., Kohen R., Kanner J. Coffee polyphenols protect human plasma from postprandial carbonyl modifications. Mol Nutr Food Res. 2013;57(5):916–919. doi: 10.1002/mnfr.201200557. [DOI] [PubMed] [Google Scholar]
- 65.Yukawa G.S., Mune M., Otani H., Tone Y., Liang X.M., Iwahashi H. Effects of coffee consumption on oxidative susceptibility of low-density lipoproteins and serum lipid levels in humans. Biochemistry (Mosc) 2004;69(1):70–74. doi: 10.1023/b:biry.0000016354.05438.0f. [DOI] [PubMed] [Google Scholar]
- 66.Hoelzl C., Knasmüller S., Wagner K.H., Elbling L., Huber W., Kager N. Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol Nutr Food Res. 2010;54(12):1722–1733. doi: 10.1002/mnfr.201000048. [DOI] [PubMed] [Google Scholar]
- 67.Guo L, Chen Z, Amarnath V, Davies SS. Identification of novel bioactive aldehyde-modified phosphatidylethanolamines formed by lipid peroxidation. Free Radical Biol Med 2012. 53(6)6:1226–38. [DOI] [PMC free article] [PubMed]
- 68.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fowler M.J. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77–82. [Google Scholar]
- 69.Baynes J.W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 70.Ramesh B., Karuna R., Sreenivasa R.S., Haritha K., Sai M.D., Sasi B.R. Effect of Commiphora mukul gum resin on hepatic marker enzymes, lipid peroxidation and antioxidants status in pancreas and heart of streptozotocin induced diabetic rats. Asian Pac J Trop Biomed. 2012;2(11):895–900. doi: 10.1016/S2221-1691(12)60249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Del Rio D., Stewart A.J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 72.Shodehinde S.A., Oboh G. Antioxidant properties of aqueous extracts of unripe Musa paradisiaca on sodium nitroprusside induced lipid peroxidation in rat pancreas in vitro. Asian Pac J Trop Biomed. 2013;3(6):449–457. doi: 10.1016/S2221-1691(13)60095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moussa S.A. Oxidative stress in diabetes mellitus. Romanian J Biophys. 2008;18:225–236. [Google Scholar]
- 74.Bandeira Sde M., Guedes Gda S., da Fonseca L.J., Pires A.S., Gelain D.P., Moreira J.C. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and SOD activity. Oxid Med Cell Longevity. 2012:819310. doi: 10.1155/2012/819310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu S.C. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chakravarty S., Rizvi S.I. Day and night GSH and MDA levels in healthy adults and effects of different doses of melatonin on these parameters. Int J Cell Biol. 2011;2011:404591. doi: 10.1155/2011/404591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rahigude A., Bhutada P., Kaulaskar S., Aswar M., Otari K. Participation of antioxidant and cholinergic system in protective effect of naringenin against type-2 diabetes-induced memory dysfunction in rats. Neuroscience. 2012;226:62–72. doi: 10.1016/j.neuroscience.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 78.Calabrese V., Cornelius C., Leso V., Trovato-Salinaro A., Ventimiglia B., Cavallaro M. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim Biophys Acta. 2012;1822(5):729–736. doi: 10.1016/j.bbadis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 79.Dinçer Y., Akçay T., Alademir Z., Ilkova H. Assessment of DNA base oxidation and glutathione level in patients with type 2 diabetes. Mutat Res. 2002;505(1–2):75–81. doi: 10.1016/s0027-5107(02)00143-4. [DOI] [PubMed] [Google Scholar]