Highlights
-
•
In the UAEHFS, levels of esRAGE were significantly associated with glycemic status.
-
•
In the UAEHFS, levels of sRAGE and esRAGE were significantly associated with BMI.
-
•
In the UAEHFS, sRAGE was associated with waist/hip circumference ratio.
-
•
The AGE-RAGE axis is associated with glycemia and obesity in an Arab population.
Keywords: Advanced glycation endproducts (AGEs), Receptor for AGE (RAGE), Cohort study, Diabetes mellitus, Obesity
Abstract
Aims
The transformation of the United Arab Emirates (UAE) from a semi-nomadic to a high income society has been accompanied by increasing rates of obesity and Type 2 diabetes mellitus. We examined if the AGE-RAGE (receptor for advanced glycation endproducts) axis is associated with obesity and diabetes mellitus in the pilot phase of the UAE Healthy Futures Study (UAEHFS).
Methods
517 Emirati subjects were enrolled and plasma/serum levels of AGE, carboxy methyl lysine (CML)-AGE, soluble (s)RAGE and endogenous secretory (es)RAGE were measured along with weight, height, waist and hip circumference (WC/HC), blood pressure, HbA1c, Vitamin D levels and routine chemistries. The relationship between the AGE-RAGE axis and obesity and diabetes mellitus was tested using proportional odds models and linear regression.
Results
After covariate adjustment, AGE levels were significantly associated with diabetes status. Levels of sRAGE and esRAGE were associated with BMI and levels of sRAGE were associated with WC/HC.
Conclusions
The AGE-RAGE axis is associated with diabetes status and obesity in this Arab population. Prospective serial analysis of this axis may identify predictive biomarkers of obesity and cardiometabolic dysfunction in the UAEHFS.
Introduction
The World Health Organization (WHO) reported that non-communicable diseases (NCDs) result in the death of approximately 38 million people each year. According to the WHO report [1], nearly three-quarters of the deaths from NCDs occur in countries with low- to middle income. Cardiovascular diseases (CVDs) account for the greatest proportion of these deaths; important risk factors include tobacco use, unhealthy diets, physical inactivity and harmful consumption of alcohol [1]. The consequences of obesity include insulin resistance and the development of Type 2 diabetes mellitus, which are major causes of morbidity and mortality, on account of accelerated atherosclerosis, heart attacks and strokes [2], [3].
The United Arab Emirates (UAE) has rapidly evolved from a semi-nomadic society to a flourishing high income society in which consumption of high energy foods and reduced physical activity have resulted in increasing rates of obesity and it sequelae in adults, adolescents and children [4], [5]. Hajat and colleagues reported that in the Abu Dhabi Weqaya study, which screened 50,183 adults ≥ 18 years of age, approximately 57% of the subjects were overweight or had obesity; 18% had diabetes mellitus; and 27% had pre-diabetes [6]. These considerations underscore the need to identify the underlying mediating mechanisms of cardiometabolic disease and biomarkers to track individuals particularly vulnerable to obesity and its side effects.
The receptor for advanced glycation endproducts (RAGE) transduces the signals of a unique repertoire of ligands that accumulate in NCDs, such as in obesity, hyperglycemia and aging [7]. Studies in human subjects illustrated that RAGE is highly expressed in obese adipose tissue, to a greater degree than that observed in the adipose tissue of lean subjects; and in atherosclerotic plaques, particularly in the diabetic state [8], [9]. This increased expression of RAGE and its ligands in these tissues was mechanistically linked to disease, as mice devoid of Ager (gene encoding RAGE) were protected from diet-induced obesity and from accelerated atherosclerosis in diabetes [10], [11].
RAGE ligand AGEs, such as carboxy methyl lysine (CML) AGEs, are generated to accelerated degrees in obesity and diabetes mellitus in human cardiometabolic disorders. Two forms of soluble RAGE have been detected in human serum/plasma. The first form, soluble (s)RAGE, is a cell-surface cleaved form of the receptor that results from the actions of matrix metalloproteinases (MMPs) or a disintegrin and metalloproteinase domain-containing protein 10 or ADAM10 [12], [13]. The second form, endogenous secretory or esRAGE results from alternative splicing of the human AGER mRNA leading to deletion of part of the RAGE transmembrane domain and the cytoplasmic tail [14]. Multiple published studies, largely cross-sectional in design, have reported on associations between the circulating levels of AGE, CML-AGE, sRAGE and/or esRAGE and the presence of obesity, Type 2 diabetes mellitus and its complications [7], [15], [16]. Others have shown that therapeutic intervention in cardiometabolic diseases may modulate levels of RAGE ligands and the soluble RAGEs [17].
Here, we measured levels of AGEs, CML-AGE, sRAGE and esRAGE in 517 pilot study subjects of the UAEHFS and tested their potential association with obesity, diabetes status (HbA1c) and other risk factors and biomarkers of cardiometabolic disease.
Subjects
517 Emirati subjects were enrolled into the pilot study of the UAEHFS from January 2015 to April 2015 from the Zayed Military Primary Health Care Clinic (ZMH PHCC) and the Abu Dhabi Blood Bank (ADBB), both of which are licensed for clinical research by the Health Authority of Abu Dhabi (HAAD). In each location, individuals who visited the clinic either for bi-annual medical screening (at the ZMH PHCC) or to donate blood (at ADBB) were invited to participate in the study. Inclusion criteria included: age ≥ 18 years and UAE national residents in the Abu Dhabi Emirate. Exclusion criteria included: age < 18 years, inability to give informed consent for the study, any acute medical illness (such as acute infection, chest pain or breathlessness, etc.) and pregnancy. No subjects were excluded on the basis of pre-existing chronic medical conditions, such as diabetes, hypertension or ischemic heart disease. All participants in the pilot study read and understood the information brochure and signed informed consent prior to recruitment. Subjects who agreed to participate and provided informed consent underwent a variety of physical measurements and analysis of blood samples. The percent missing data for each study variable is listed in the Table 1 and includes 6.2% missing values for HbA1c. The Institutional Review Boards (IRBs) of the Sheikh Khalifa Medical City (SKMC), Zayed Military Hospital (ZMH), Zayed University (ZU), New York University Abu Dhabi (NYUAD), NYU Langone Medical Center, New York, and United Arab Emirates University (UAEU) approved the protocols of the pilot study.
Table 1.
Descriptive table on the effect of candidate variables, including the markers of AGE-RAGE axis, obesity indices and other potential covariates, on diabetes status (HbA1c levels) for 517 subjects. Note that the data include 6.2% missing values for HbA1c.
| Continuous variables | All individuals (n = 517) |
Normal (HbA1c < 5.7) (n = 331) |
Prediabetic (5.7 ≤ HbA1c < 6.5) (n = 121) |
Diabetic (6.5 ≤ HbA1c) (n = 33) |
% of missing | †P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| CML-AGE (pmol/ml) | 6853.91 | 13618.98 | 6068.87 | 12182.11 | 8980.72 | 17477.03 | 7192.74 | 10629.61 | 7.74 | 0.095 |
| AGE (Arbitrary Units) | 1318.15 | 1085.66 | 1417.17 | 1196.84 | 1042.72 | 628 | 1222.62 | 1012.27 | 5.42 | 0.003 |
| sRAGE (pg/ml) | 1093.15 | 513.41 | 1151.6 | 544.22 | 1002.96 | 433.74 | 851.33 | 363.24 | 5.61 | <0.001 |
| esRAGE (ng/ml) | 0.22 | 0.16 | 0.23 | 0.17 | 0.21 | 0.12 | 0.17 | 0.08 | 5.61 | 0.034 |
| BMI (kg/m2) | 28.24 | 6.24 | 26.88 | 5.2 | 30.57 | 6.4 | 34.54 | 7.89 | 16.83 | <0.001 |
| WC/HC (ratio) | 0.88 | 0.1 | 0.86 | 0.09 | 0.91 | 0.1 | 0.95 | 0.09 | 21.86 | <0.001 |
| Age (years) | 31.78 | 10.48 | 29.08 | 8.45 | 36.41 | 10.5 | 43.84 | 13.03 | 9.09 | <0.001 |
| eGFR (ml/min/1.73m2) | 102.71 | 26.63 | 105.15 | 26.91 | 97.79 | 21.5 | 98.75 | 35.7 | 14.31 | 0.008 |
| Systolic blood pressure (SBP) (mm Hg) | 116.86 | 15.59 | 113.8 | 13.86 | 121.51 | 16.21 | 131.29 | 19.18 | 17.41 | <0.001 |
| Diastolic blood pressure (DBP) (mm Hg) | 77.49 | 9.94 | 76.01 | 9.02 | 79.49 | 11.23 | 85.6 | 9.44 | 17.41 | <0.001 |
| Cholesterol (mg/dL) | 196.01 | 40.8 | 192.31 | 37.19 | 208.85 | 43.11 | 188.24 | 57 | 5.42 | 0.009 |
| Triglycerides (mg/dL) | 120.58 | 88.71 | 108.98 | 74.73 | 131.55 | 83.7 | 196.85 | 167.58 | 5.42 | <0.001 |
| HDL (mg/dL) | 52.07 | 12.82 | 53.32 | 12.89 | 50.81 | 12.63 | 44.59 | 10.19 | 7.16 | 0.001 |
| HS-CRP (mg/dL) | 0.85 | 0.22 | 0.84 | 0.23 | 0.87 | 0.21 | 0.86 | 0.24 | 6 | 0.17 |
| Vitamin D (ng/ml) | 22.06 | 10.55 | 21.36 | 10.28 | 22.37 | 10.7 | 26.32 | 11.14 | 7.16 | 0.041 |
| Serum creatinine (mg/dL) | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| Categorical variables | N | % | N | % | N | % | N | % | % of missing | †P-value |
| Sex | 473 | 304 | 173 | 31 | 8.51 | 0.142 | ||||
| Male | 323 | 62.48 | 200 | 65.79 | 81 | 73.64 | 22 | 70.97 | ||
| Female | 150 | 29.01 | 104 | 34.21 | 29 | 26.36 | 9 | 29.03 | ||
Univariate analyses: The P-values were estimated based on univariate proportional odds models for the effect of each candidate covariate on diabetes status. Diabetes status was coded as 0 for normal (HbA1c < 5.7), 1 for prediabetic (5.7 ≤ HbA1c < 6.5), and 2 for diabetic (6.5 ≤ HbA1c). SD = standard deviation.
Materials and methods
Clinical measurements
Sitting and standing height was measured using a stadiometer (Seca, Hamburg Deutschland) and waist circumference (WC) and hip circumference (HC) were measured using a standard tape (Wessex non-stretchable sprung tape) [18]. Body mass was measured using the Tanita TC (Tanita Inc., Tokyo, Japan) and body mass index (BMI) was calculated according to the following formula: body weight (kg)/height2 (meters).
Brachial blood pressure (systolic and diastolic) was recorded twice on the upper left arm with appropriate cuff size with two-minute interval between readings using a semi-automated sphygmomanometer (Omron M10-IT, Omron Corporation, Kyoto, Japan).
Biological samples
Study participants provided specimens including blood (8 ml SST vacutainer and 8 ml plasma EDTA vacutainer). SST vacutainers were subjected to centrifugation (3500 rpm, 4 °C, 15 min) 30 min post-collection. All samples were refrigerated (4–8 °C) and then transported to the NYU Abu Dhabi (NYUAD) research laboratory in a temperature-controlled cooler where the SST samples were aliquoted into 1.0 ml tubes. 2 mls of whole blood were removed from the EDTA vacutainer and stored in 1 ml aliquots. The remaining sample was centrifuged at 3500 rpm at 4 °C for 15 min and plasma and red blood cells (RBCs) were aliquoted into 1.0 ml tubes. All aliquots were stored at −80 °C until further testing (see below).
Standard chemistry assays
HbA1c was measured on EDTA-derived whole blood sample and routine clinical chemistry per Table 1 was performed on SST serum. All assays were performed on the Beckman Coulter UniCel DxC 600 Synchron Clinical Systems (Beckman Coulter, USA) according to the manufacturer’s instructions. Instrument results were validated against the RIQAS external quality assessment programs for general clinical chemistry and HbA1c. HbA1c is reported in NGSP units and then converted to IFCC (mmol/mol) according to the following formula: IFCC = (10.93 × NGSP) − 23.5. Serum levels of Vitamin D were assayed using the Beckman Coulter Access 2 Immunoassay System (Beckman Coulter, USA) in accordance with the manufacturer’s instructions. eGFR was calculated according to the equation: eGFR = 175 × (Scr)−1.154 X (Age)−0.203X (0.742 if a female subject).
Research assays
Soluble RAGE, esRAGE
Soluble (s) RAGE and esRAGE levels were assayed on plasma obtained from blood in EDTA tubes on samples previously stored at −80 °C using enzyme-linked immunosorbent assay (ELISA) kits in accordance with the manufacturers’ protocol (R&D Systems Quantikine Immunoassay, Minneapolis, MN, and B-Bridge ELISA, B-Bridge International, Cupertino, CA, respectively). Reported results represent the mean of the results from two distinct wells/sample. Interassay variability for the measurement of sRAGE and esRAGE was CV 8.02 and 7.75, respectively.
Protein-bound CML
Protein-bound CML in serum was quantified using liquid chromatography (LC)-mass spectrometry (MS) as previously published [19], [20]. Agilent 6538 Accurate-Mass Quadrupole Time-of-Flight (Q-TOF) LC/MS system was used to measure CML in hydrolyzed serum samples.
AGE detection by relative fluorescence
AGE fluorescence at 440 nm (excitation at 370 nm), was determined in the acid hydrolysates of serum, as previously published [21] using a Fluorescence Microplate reader (BioTek Synergy H1 microplate reader). Relative fluorescence was determined in the hydrolyzed samples after diluting 60 μl of each sample with 2.0 ml of distilled water. A control buffer was used as a blank to subtract background fluorescence levels.
Statistical analysis
Descriptive analysis and the univariate proportional odds models on the effect of candidate variables, including the markers of AGE-RAGE, obesity indices and other potential covariates on diabetes status are reported in Table 1. The normality of each candidate variable was checked by histogram and the Shapiro-Wilk test [22] (Fig. S1). Since none of the variables passed the normality test, the non-parametric correlation method was considered in the following analyses. Pairwise Spearman correlation coefficient estimates [23] among BMI, WC/HC, eGFR, systolic blood pressure (SBP), diastolic blood pressure (DBP), cholesterol, triglycerides, high density lipoprotein (HDL), high sensitivity C-reactive protein (hs-CRP), Vitamin D and serum creatinine, as well as their p-values, are reported in 1. Since DBP is strongly correlated with SBP (correlation = 0.772, p < 0.001) and serum creatinine is strongly correlated with eGFR (correlation = −0.862, p < 0.001), we only fitted SBP and eGFR in the multiple regression models. Proportional odds models [24] were fitted to survey if CML-AGE, AGE, sRAGE, and esRAGE are associated with diabetes status, as defined by HbA1c levels, with covariate adjustments (Table 2). The diabetes status was coded for HbA1c (%) as 0 for normal (HbA1c < 5.7 (37 mmol/mol)), 1 for prediabetes (HbA1c ≥ 5.7 (37 mmol/mol) and <6.5 (48 mmol/mol)), and 2 for diabetes mellitus (HbA1c ≥ 6.5 (48 mmol/mol)) and treated as ordinal. Pairwise Spearman correlation coefficient estimates among CML-AGE, AGE, sRAGE esRAGE, BMI and WC/HC, as well as their p-values, were reported to determine if they are related to each other (Table 3). Multiple linear regression models were used to survey if CML-AGE, AGE, sRAGE, and esRAGE are associated with obesity in terms of measures of BMI (Table 4) or WC/HC (Table 5), with covariate adjustments.
Table 2.
The proportional odds models were fitted for the association between diabetes status and each of CML-AGE, AGE, sRAGE, and esRAGE. Separate models were fitted for different covariate adjustments. Diabetes status was coded as ordinal: 0 for normal stat (HbA1c < 5.7), 1 for prediabetic state (5.7 ≤ HbA1c < 6.5), and 2 for diabetic state (6.5 ≤ HbA1c). Each continuous predictor was standardized to have mean 0 and standard deviation 1.
| †Covariate adjustment | CML-AGE |
AGE |
sRAGE |
esRAGE |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value | |
| 1 | 0.099 | 0.103 | 0.334 | −0.302 | 0.144 | 0.036 | −0.287 | 0.132 | 0.029 | −0.173 | 0.118 | 0.143 |
| 2 | 0.099 | 0.103 | 0.333 | −0.31 | 0.145 | 0.033 | −0.284 | 0.132 | 0.031 | −0.17 | 0.118 | 0.15 |
| 3 | 0.073 | 0.112 | 0.515 | −0.382 | 0.165 | 0.021 | −0.063 | 0.138 | 0.649 | −0.03 | 0.125 | 0.809 |
| 4 | 0.124 | 0.127 | 0.329 | −0.308 | 0.159 | 0.053 | −0.213 | 0.141 | 0.13 | −0.126 | 0.126 | 0.318 |
| 5 | 0.061 | 0.115 | 0.597 | −0.307 | 0.155 | 0.047 | −0.235 | 0.141 | 0.094 | −0.123 | 0.124 | 0.322 |
| 6 | 0.104 | 0.105 | 0.324 | −0.273 | 0.144 | 0.058 | −0.25 | 0.134 | 0.062 | −0.15 | 0.12 | 0.212 |
| 7 | 0.09 | 0.103 | 0.378 | −0.301 | 0.145 | 0.038 | −0.181 | 0.133 | 0.173 | −0.117 | 0.12 | 0.33 |
| 8 | 0.097 | 0.103 | 0.347 | −0.367 | 0.154 | 0.017 | −0.258 | 0.132 | 0.051 | −0.159 | 0.118 | 0.178 |
| 9 | 0.098 | 0.103 | 0.343 | −0.307 | 0.145 | 0.034 | −0.284 | 0.132 | 0.031 | −0.171 | 0.118 | 0.149 |
| *10 | 0.058 | 0.117 | 0.618 | −0.404 | 0.177 | 0.023 | −0.009 | 0.145 | 0.95 | <0.001 | 0.131 | 0.998 |
| *11 | 0.095 | 0.131 | 0.465 | −0.354 | 0.172 | 0.04 | −0.087 | 0.146 | 0.549 | −0.044 | 0.13 | 0.734 |
Covariate adjustment: 1: Baseline (age + sex); 2: Baseline + eGFR; 3: Baseline + BMI; 4: Baseline + WC/HC; 5: Baseline + SBP 6: Baseline + total cholesterol + total triglycerides + HDL; 7: Baseline + hs-CRP; 8: Baseline + Vitamin D; 9: Baseline + serum creatinine; 10: Baseline + eGFR + BMI + SBP + total cholesterol + total triglycerides + HDL + hs-CRP + Vitamin D; 11: Baseline + eGFR + WC/HC + SBP + total cholesterol + total triglycerides + HDL + hs-CRP + Vitamin D.
Note: For the covariate adjustment, 10 and 11, the complete estimated models including all covariate estimates for AGE are addressed through Tables S2 and S3. SE: standard error.
Table 3.
Pairwise Spearman correlation coefficient estimates and their p-values among CML-AGE, AGE, sRAGE, esRAGE, BMI, and WC/HC. As the normality of each variable was not satisfied visually and by the Shapiro-Wilk test (Fig. S1), the non-parametric method, Spearman’s rank test, was used (Table S1).
| CML-AGE | AGE | sRAGE | esRAGE | BMI | WC/HC | |
|---|---|---|---|---|---|---|
| CML-AGE | – | −0.516, <0.001 | 0.043, 0.351 | 0.076, 0.096 | −0.036, 0.466 | 0.001, 0.986 |
| AGE | −0.516, <0.001 | – | −0.08, 0.076 | −0.037, 0.415 | 0.025, 0.609 | −0.041, 0.42 |
| sRAGE | 0.043, 0.351 | −0.08, 0.076 | – | 0.671, <0.001 | −0.322, <0.001 | −0.201, <0.001 |
| esRAGE | 0.076, 0.096 | −0.037, 0.415 | 0.671, <0.001 | – | −0.194, <0.001 | −0.093, 0.068 |
| BMI | −0.036, 0.466 | 0.025, 0.609 | −0.322, <0.001 | −0.194, <0.001 | – | 0.476, <0.001 |
| WC/HC | 0.001, 0.986 | −0.041, 0.42 | −0.201, <0.001 | −0.093, 0.068 | 0.476, <0.001 | – |
Note: In each cell, the first number in the pair is the Spearman correlation coefficient estimate and the second number is the associated p-value based on the Spearman’s rank test on whether it is significantly different from 0. For example, with respect to the correlation between sRAGE and BMI, the correlation estimate is −0.322 and its p-value is <0.001.
Table 4.
The linear regression models fitted for the association between BMI and each of CML-AGE, AGE, sRAGE, and esRAGE. Separate models were fitted for different covariate adjustments. Each continuous predictor was standardized to have mean 0 and standard deviation 1.
| †Covariate adjustment | CML-AGE |
AGE |
sRAGE |
esRAGE |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value | |
| 1 | 0.054 | 0.324 | 0.868 | 0.057 | 0.282 | 0.839 | −1.675 | 0.284 | <0.001 | −1.182 | 0.283 | <0.001 |
| 2 | 0.073 | 0.325 | 0.822 | 0.023 | 0.286 | 0.937 | −1.667 | 0.286 | <0.001 | −1.169 | 0.286 | <0.001 |
| 3 | 0.054 | 0.313 | 0.864 | −0.027 | 0.273 | 0.922 | −1.525 | 0.28 | <0.001 | −1.049 | 0.276 | <0.001 |
| 4 | 0.058 | 0.321 | 0.856 | 0.157 | 0.275 | 0.567 | −1.445 | 0.282 | <0.001 | −1.024 | 0.277 | <0.001 |
| 5 | −0.068 | 0.302 | 0.822 | 0.026 | 0.263 | 0.923 | −1.315 | 0.274 | <0.001 | −0.896 | 0.268 | 0.001 |
| 6 | 0.067 | 0.325 | 0.836 | 0.029 | 0.285 | 0.919 | −1.672 | 0.288 | <0.001 | −1.148 | 0.285 | <0.001 |
| 7 | 0.062 | 0.326 | 0.849 | 0.044 | 0.287 | 0.877 | −1.67 | 0.286 | <0.001 | −1.173 | 0.286 | <0.001 |
| 8 | −0.012 | 0.295 | 0.968 | 0.048 | 0.257 | 0.851 | −0.979 | 0.273 | <0.001 | −0.661 | 0.261 | 0.012 |
*Note: For the covariate adjustment, 8, the complete estimated model including all covariate estimates for sRAGE and esRAGE are addressed through Tables S4 and S5 respectively. SE: standard error.
Covariate adjustment: 1: Baseline (age + sex); 2: Baseline + eGFR; 3: Baseline + SBP; 4: Baseline + total cholesterol + total triglycerides + HDL; 5: Baseline + hs-CRP; 6: Baseline + Vitamin D; 7: Baseline + serum creatinine; 8: Baseline + eGFR + SBP + total cholesterol + total triglycerides + HDL + hs-CRP + Vitamin D.
Table 5.
The linear regression models fitted for the association between WC/HC and each of CML-AGE, AGE, sRAGE, and esRAGE. Separate models were fitted for different covariate adjustments. Each continuous predictor was standardized to have mean 0 and standard deviation 1.
| †Covariate adjustment | CML-AGE |
AGE |
sRAGE |
esRAGE |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value | |
| 1 | <0.001 | 0.005 | 0.993 | −0.005 | 0.004 | 0.22 | −0.016 | 0.004 | <0.001 | −0.009 | 0.004 | 0.029 |
| 2 | <0.001 | 0.005 | 0.972 | −0.004 | 0.004 | 0.282 | −0.015 | 0.004 | 0.001 | −0.008 | 0.004 | 0.051 |
| 3 | 0.001 | 0.005 | 0.874 | −0.005 | 0.004 | 0.229 | −0.015 | 0.004 | 0.001 | −0.009 | 0.004 | 0.035 |
| 4 | −0.001 | 0.005 | 0.884 | −0.004 | 0.004 | 0.308 | −0.013 | 0.004 | 0.003 | −0.008 | 0.004 | 0.068 |
| 5 | <0.001 | 0.005 | 0.975 | −0.005 | 0.004 | 0.192 | −0.014 | 0.004 | 0.001 | −0.008 | 0.004 | 0.057 |
| 6 | <0.001 | 0.005 | 0.971 | −0.005 | 0.004 | 0.213 | −0.016 | 0.004 | <0.001 | −0.009 | 0.004 | 0.029 |
| 7 | <0.001 | 0.005 | 0.985 | −0.005 | 0.004 | 0.24 | −0.015 | 0.004 | 0.001 | −0.008 | 0.004 | 0.051 |
| 8 | −0.001 | 0.005 | 0.887 | −0.003 | 0.004 | 0.486 | −0.011 | 0.004 | 0.016 | −0.006 | 0.004 | 0.189 |
*Note: For the covariate adjustment, 8, the complete estimated model including all covariate estimates for sRAGE are addressed through Table S6. SE: standard error.
Covariate adjustment: 1: Baseline (age + sex); 2: Baseline + eGFR; 3: Baseline + SBP; 4: Baseline + total cholesterol + total triglycerides + HDL; 5: Baseline + hs-CRP; 6: Baseline + Vitamin D; 7: Baseline + serum creatinine; 8: Baseline + eGFR + SBP + total cholesterol + total triglycerides + HDL + hs-CRP + Vitamin D.
Results
We determined HbA1c levels and measured BMI and WC/HC in the subjects. Based on HbA1c levels (%), the cohort was divided into three subgroups: 331 subjects had HbA1c < 5.7 (37 mmol/mol) (normal); 121 subjects had HbA1c ≥ 5.7 (37 mmol/mol) and <6.5 (48 mmol/mol) (prediabetes); and 33 subjects had HbA1c ≥ 6.5 (48 mmol/mol) (diabetes mellitus) (Table 1). Within these subgroups, the mean age ± SD was 29.08 ± 8.45 years, 36.41 ± 10.5 years and 43.84 ± 13.03 years, respectively, and the proportion of male subjects was 65.79%, 73.64% and 70.97%, respectively, and the proportion of female subjects was 34.21%, 26.36%, and 29.03%, respectively (Table 1). The mean BMI ± SD of the subjects in the three subgroups by HbA1c levels was 26.88 ± 5.2, 30.57 ± 6.4 and 34.54 ± 7.89, (in kg/m2), respectively, and the mean WC/HC ± SD in the three subgroups by HbA1c levels was 0.86 ± 0.09, 0.91 ± 0.1 and 0.95 ± 0.09, respectively. These data demonstrate a step-wise increase in BMI and WC/HC with markers predictive of diabetes status (p < 0.001) (Table 1).
Table 1 reports the univariate analysis and related summary statistics for the relationships between the measures of CML-AGE, AGE, sRAGE and esRAGE, with the first major clinical parameter of this study, diabetes status, based on the HbA1c levels. AGE, sRAGE and esRAGE were significantly associated with diabetes status, but no significant relationship was observed for CML-AGE. Other potential covariates, eGFR, SBP, DBP, total cholesterol, triglyceride, HDL, hs-CRP, Vitamin D and serum creatinine, were also surveyed and are reported in Table 1.
We next performed multivariate analyses to assess if CML-AGE, AGE, sRAGE and esRAGE are associated with diabetes status defined by HbA1c levels, after adjusting for other confounding factors. Here, we report eleven different covariate adjustments, as described in Table 2. We could not find any significant association for CML-AGE with or without covariate adjustments (Tables 1 and 2). The significant univariate association of sRAGE and esRAGE with diabetes status (Table 1) was due to other confounding factors, as their statistical significance was lost after adjusting for the covariates investigated through models 1–11 (Table 2). In contrast, the significant and negative association between AGE and HbA1c levels was retained after covariate adjustment except in Model 4 and Model 6 with p-value = 0.053 and 0.058, respectively (Table 2).
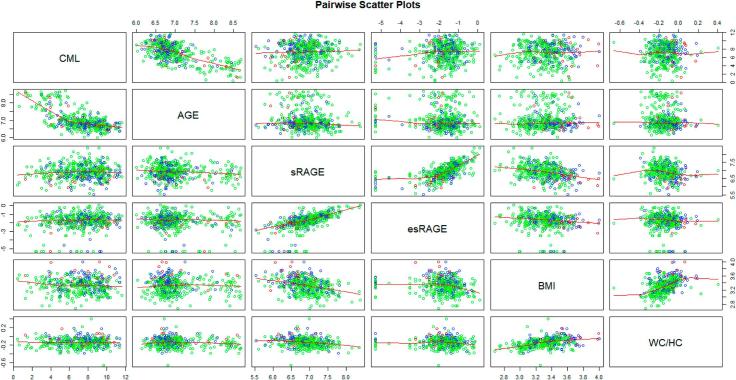
We next surveyed how the markers of the AGE/RAGE axis, CML-AGE, AGE, sRAGE and esRAGE, and BMI and WC/HC within individual subjects may be related to each other. Our analyses revealed (Table 3, Fig. 1) that 1) CML-AGE is significantly correlated with AGE, but not with sRAGE, esRAGE, BMI or WC/HC; 2) AGE is significantly correlated with CML-AGE, but not with sRAGE, esRAGE, BMI or WC/HC; 3) sRAGE is significantly correlated with esRAGE, BMI and WC/HC, but not with AGE or CML-AGE; 4) esRAGE is significantly correlated with sRAGE and BMI, but not with CML-AGE or AGE or WC/HC; (5) BMI is significantly correlated with sRAGE, esRAGE and WC/HC, but not with CML-AGE or AGE; and 6) WC/HC is significantly correlated with sRAGE and BMI, but not with CML-AGE, AGE or esRAGE. Hence, these data suggest an intra-correlation structure between the markers of the AGE-RAGE axis and BMI or WC/HC. We performed the following two analyses to test the association between them.
Fig. 1.
Pairwise scatter plots among CML-AGE, AGE, sRAGE, esRAGE, BMI, and WC/HC. Green circles are for normal individuals (HbA1c < 5.7), blue circles are for prediabetic individuals (5.7 ≤ HbA1c < 6.5), and red circles are for diabetic individuals (6.5 ≤ HbA1c). As the normality of each variable was not satisfied by the Shapiro-Wilk test, logarithm was taken to each variable. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
First, we addressed the association of CML-AGE, AGE, sRAGE and esRAGE with the second major clinical parameter of study, BMI. In the univariate analyses, only sRAGE and esRAGE were statistically significantly and negatively associated with BMI (Table 3). Here, we report eight different covariate adjustments, as described in Table 4, which revealed that the significant and negative association for sRAGE and esRAGE with BMI remained after any further covariate adjustment, but no significance was observed for CML-AGE and AGE.
Second, we addressed the association of CML-AGE, AGE, sRAGE and esRAGE with the third major clinical parameter of study, WC/HC. In the univariate analysis, only sRAGE was significantly and negatively associated with WC/HC (Table 3). Table 5, using the covariate adjustments as shown in Table 4, reports that only sRAGE is significantly and negatively associated with WC/HC after any further covariate adjustment. No statistically significant differences were observed for AGE, CML-AGE or esRAGE.
Discussion
In the United Arab Emirates, the rising rates of excessive body mass, metabolic dysfunction and Type 2 diabetes mellitus and their cardiovascular consequences threaten the health and well-being of its citizens. The UAEHFS aims to identify the causes of these common metabolic diseases in a prospective manner and to identify predictive biomarkers. In the context of RAGE, published studies suggested that in subjects with Type 2 diabetes mellitus and obesity with BMI 30-35 undergoing bariatric surgery, higher baseline levels of sRAGE predicted weight loss and remission from Type 2 diabetes mellitus at six months and three years post-surgical intervention [25], [26]. Hence, this pilot study served to test the feasibility of this approach with respect to predictors of obesity and metabolic disease in an Arab population.
Although there were no significant associations with levels of sRAGE and diabetes status levels of sRAGE remained significantly associated with BMI and WC/HC after all covariate adjustment. The general directionality observed in this study was that as diabetes status progressed from normal to diabetes and as BMI and WC/HC rose, levels of sRAGE declined. Associations between diabetes mellitus and obesity and sRAGE have been reported in other populations but the results are discordant on whether higher or lower sRAGE levels associated with overall cardiometabolic disease. Sebeková and colleagues reported in young to middle-aged subjects free of diabetes mellitus or medications that levels of sRAGE and RAGE ligands CML-AGE declined prior to the manifestation of metabolic syndrome [27]. These considerations highlight a number of important points: First, Gaens and colleagues showed that in obese individuals, lower levels of RAGE ligand CML-AGE were identified in plasma compared to lean subjects and, thus, the CML-AGEs were believed to be “trapped” in obese adipose tissue, in which higher tissue levels of RAGE were noted [9]. Second, with respect to the discordance between “high” vs. “low” levels of sRAGE and status of metabolic disease, Thomas and colleagues noted that studies of the general population cohorts revealed that lower levels of sRAGE were associated with poor health outcomes [28], [29], [30]. This point bears direct relevance to the design of the UAEHFS, a population-based cohort study. Third, genetic variations in the gene encoding AGER have been suggested to contribute to the levels of sRAGE [31], [32]. Of note, genetic factors are to be examined in the UAEHFS and the findings might provide novel insights into AGER-specific variants linked to the AGE-RAGE axis, diabetes mellitus and obesity; no such data exist at this time in this population.
The present findings indicate a number of significant associations (negative) between levels of esRAGE and BMI and WC/HC, even after covariate adjustment, but not with diabetes status. It has been shown that lower levels of esRAGE were associated with obesity and risk of metabolic syndrome [33], [34], [35]. Vazzana and colleagues showed that a weight loss program in five women with obesity resulted in increases in levels of esRAGE [33]. Comparable to findings with sRAGE, it has been reported that renal function, genetic polymorphisms in AGER, medications and the study of populations vs. known diseased subjects may affect esRAGE levels and thus account for these disparate results [36], [37], [38]. These considerations underscore the importance of measuring baseline levels and prospective repeat measures of esRAGE in the UAEHFS subjects with respect to associations with obesity and/or diabetes mellitus.
In this study, no significant associations were observed between CML-AGE and BMI, WC/HC or diabetes status, but we did observe significant associations between AGE and diabetes status, but not with BMI or WC/HC. There are multiple possible reasons for these findings, such as (1) the present pilot study was not designed to test specific hypotheses regarding the form of AGE and diabetes or obesity status and hence was insufficiently powered to detect significant differences; (2) factors relevant to local dietary patterns or exercise in the UAE might contribute to distinct effects on AGEs vs. CML-AGE in this population, which might impact the overall detectable AGE or CML-AGE circulating burden in these subjects [39]; (3) heretofore unknown polymorphisms in genes that regulate total AGE or CML-AGE burden might be present in this population, such as variations in the gene encoding glyoxalase 1 (GLO1), whose product detoxifies methylglyoxal, a pre-AGE species [40], [41], [42] or in association with variations in genes encoding AGE receptors [43]; and (4) the overall low prevalence of diabetes mellitus in this pilot study (n = 33/517 or 6.2%) suggests that frank diabetes mellitus, at least in this population, may exert the greater impact on levels of CML-AGE than prediabetes or normal states, with or without obesity. In this context, it is important to note that in obesity, as cited above, circulating AGE levels (such as CML-AGE) might be lower in obese vs. lean subjects due to tissue trapping [9]. In contrast, multiple studies have suggested higher levels of circulating AGEs in subjects with diabetes mellitus and its complications [44]. Irrespective of these caveats, it will be important to prospectively follow levels of the AGE-RAGE axis in this population to determine if levels of AGE or CML-AGE reach significance with respect to diabetes status, BMI or WC/HC, as cohort subjects age and as more subjects are expected to develop Type 2 diabetes mellitus over time.
In conclusion, the results of this pilot of the UAEHFS suggest that levels of AGE, but not CML-AGE, sRAGE or esRAGE were associated with diabetes status, and that levels of sRAGE and esRAGE, but not CML-AGE or AGE were associated with obesity status in the UAE population. Hence, prospective and serial analysis of these endpoints in the UAEHFS subjects may identify predictive biomarkers of obesity and cardiometabolic dysfunction, especially when analyzed with the results of dietary and life-style surveys, genetic analyses, microbiome factors and markers of cardiovascular disease.
Author contributions
CKI, RR and AMS designed the study, conducted data collection, analyzed data and drafted the manuscript. AA and RA designed the study, conducted data collection, analyzed data and reviewed the manuscript. AAJ, AAN, EAZ, NO, MAB, MAH, FAM, AAD, SMS, LAW, WAM, HA, FAA, AAH, MH, DG, and MJOC conducted data collection, analyzed data and reviewed the manuscript. HK and HL analyzed data and drafted the manuscript. JA, TK, SS, and RBH designed the study, conducted data collection, analyzed data and reviewed the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors gratefully acknowledge the support of the funding organization New York University Abu Dhabi. The authors are grateful to Louise Ashall, Sneha Thomas, and Nosirudeen Quadri for their assistance in the AGE and CML-AGE assays and for the expert assistance of Ms. Latoya Woods in the preparation of this manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jcte.2017.08.001.
Contributor Information
Ravichandran Ramasamy, Email: ravichandran.ramasamy@nyumc.org.
Ann Marie Schmidt, Email: annmarie.schmidt@nyumc.org.
Appendix A. Supplementary data
References
- 1.http://www.who.int/mediacentre/factsheets/fs355/en/.
- 2.Kannel W.B., McGee D.L. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2(2):120–126. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- 3.Haffner S.M., Lehto S., Ronnemaa T., Pyorala K., Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 4.Ng S.W., Zaghloul S., Ali H.I., Harrison G., Popkin B.M. The prevalence and trends of overweight, obesity and nutrition-related non-communicable diseases in the Arabian Gulf States. Obes Rev. 2011;12(1):1–13. doi: 10.1111/j.1467-789X.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- 5.Ali H.I., Ng S.W., Zaghloul S., Harrison G.G., Qazaq H.S., El Sadig M. High proportion of 6 to 18-year-old children and adolescents in the United Arab Emirates are not meeting dietary recommendations. Nutr Res. 2013;33(6):447–456. doi: 10.1016/j.nutres.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Hajat C., Harrison O., Al Siksek Z. Weqaya: a population-wide cardiovascular screening program in Abu Dhabi, United Arab Emirates. Am J Public Health. 2012;102(5):909–914. doi: 10.2105/AJPH.2011.300290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynaert N.L., Gopal P., Rutten E.P., Wouters E.F., Schalkwijk C.G. Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease. Int J Biochem Cell Biol. 2016;81(Pt B):403–418. doi: 10.1016/j.biocel.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Cipollone F., Iezzi A., Fazia M., Zucchelli M., Pini B., Cuccurullo C. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108(9):1070–1077. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 9.Gaens K.H., Goossens G.H., Niessen P.M., van Greevenbroek M.M., van der Kallen C.J., Niessen H.W. Nepsilon-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34(6):1199–1208. doi: 10.1161/ATVBAHA.113.302281. [DOI] [PubMed] [Google Scholar]
- 10.Bu D.X., Rai V., Shen X., Rosario R., Lu Y., D'Agati V. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res. 2010;106(6):1040–1051. doi: 10.1161/CIRCRESAHA.109.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song F., Hurtado del Pozo C., Rosario R., Zou Y.S., Ananthakrishnan R., Xu X. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes. 2014;63(6):1948–1965. doi: 10.2337/db13-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L., Bukulin M., Kojro E., Roth A., Metz V.V., Fahrenholz F. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem. 2008;283(51):35507–35516. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- 13.Raucci A., Cugusi S., Antonelli A., Barabino S.M., Monti L., Bierhaus A. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22(10):3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 14.Yonekura H., Yamamoto Y., Sakurai S., Petrova R.G., Abedin M.J., Li H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370(Pt 3):1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen L.J., Flyvbjerg A., Bjerre M. Soluble receptor for advanced glycation end product: a biomarker for acute coronary syndrome. Biomed Res Int. 2015;2015:815942. doi: 10.1155/2015/815942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt A.M. Soluble RAGEs – prospects for treating & tracking metabolic and inflammatory disease. Vascul Pharmacol. 2015;72:1–8. doi: 10.1016/j.vph.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santilli F., Vazzana N., Bucciarelli L.G., Davi G. Soluble forms of RAGE in human diseases: clinical and therapeutical implications. Curr Med Chem. 2009;16(8):940–952. doi: 10.2174/092986709787581888. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Noun L., Sohar E., Laor A. Neck circumference as a simple screening measure for identifying overweight and obese patients. Obes Res. 2001;9(8):470–477. doi: 10.1038/oby.2001.61. [DOI] [PubMed] [Google Scholar]
- 19.Hanssen N.M., Engelen L., Ferreira I., Scheijen J.L., Huijberts M.S., van Greevenbroek M.M. Plasma levels of advanced glycation endproducts Nepsilon-(carboxymethyl)lysine, Nepsilon-(carboxyethyl)lysine, and pentosidine are not independently associated with cardiovascular disease in individuals with or without type 2 diabetes: the Hoorn and CODAM studies. J Clin Endocrinol Metab. 2013;98(8):E1369–E1373. doi: 10.1210/jc.2013-1068. [DOI] [PubMed] [Google Scholar]
- 20.Teerlink T., Barto R., Ten Brink H.J., Schalkwijk C.G. Measurement of Nepsilon-(carboxymethyl)lysine and Nepsilon-(carboxyethyl)lysine in human plasma protein by stable-isotope-dilution tandem mass spectrometry. Clin Chem. 2004;50(7):1222–1228. doi: 10.1373/clinchem.2004.031286. [DOI] [PubMed] [Google Scholar]
- 21.Monnier V.M., Bautista O., Kenny D., Sell D.R., Fogarty J., Dahms W. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT skin collagen ancillary study group. Diabetes control and complications trial. Diabetes. 1999;48(4):870–880. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro S.S., Wilk M.B. An analysis of variance test for normality (Complete samples) Biometrika. 1965;52(3/4):591–611. [Google Scholar]
- 23.Spearman C. The proof and measurement of association between two things. Am J Psychol. 1904;15(1):72–101. [PubMed] [Google Scholar]
- 24.McCallagh P. Regression models for ordinal data. J R Stat Soc. 1980;42(2):109–142. Series B (Methodological) [Google Scholar]
- 25.Parikh M., Chung M., Sheth S., McMacken M., Zahra T., Saunders J.K. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do NOT meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg. 2014;260(4):617–622. doi: 10.1097/SLA.0000000000000919. discussion 22-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz D., Saunders J.K., Ude-Welcome A., Marie Schmidt A., Dunn V., Leon Pachter H. Three-year follow-up comparing metabolic surgery versus medical weight management in patients with type 2 diabetes and BMI 30–35. The role of sRAGE biomarker as predictor of satisfactory outcomes. Surg Obes Relat Dis. 2016;12(7):1337–1341. doi: 10.1016/j.soard.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Sebekova K., Krivosikova Z., Gajdos M. Total plasma Nepsilon-(carboxymethyl)lysine and sRAGE levels are inversely associated with a number of metabolic syndrome risk factors in non-diabetic young-to-middle-aged medication-free subjects. Clin Chem Lab Med. 2014;52(1):139–149. doi: 10.1515/cclm-2012-0879. [DOI] [PubMed] [Google Scholar]
- 28.Falcone C., Emanuele E., D’Angelo A., Buzzi M.P., Belvito C., Cuccia M. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25(5):1032–1037. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 29.Lindsey J.B., de Lemos J.A., Cipollone F., Ayers C.R., Rohatgi A., Morrow D.A. Association between circulating soluble receptor for advanced glycation end products and atherosclerosis: observations from the Dallas Heart Study. Diabetes Care. 2009;32(7):1218–1220. doi: 10.2337/dc09-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas M.C., Woodward M., Neal B., Li Q., Pickering R., Marre M. Relationship between levels of advanced glycation end products and their soluble receptor and adverse outcomes in adults with type 2 diabetes. Diabetes Care. 2015;38(10):1891–1897. doi: 10.2337/dc15-0925. [DOI] [PubMed] [Google Scholar]
- 31.Lim S.C., Dorajoo R., Zhang X., Wang L., Ang S.F., Tan C.S. Genetic variants in the receptor for advanced glycation end products (RAGE) gene were associated with circulating soluble RAGE level but not with renal function among Asians with type 2 diabetes: a genome-wide association study. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw263. [DOI] [PubMed] [Google Scholar]
- 32.Kucukhuseyin O., Ozgen T., Karagedik E.H., Cesur Y., Yilmaz Aydogan H., Yaylim I. The effects of advanced glycation end products (RAGE)-374T/A and Gly82Ser variants and soluble-RAGE levels to obesity in children. Cell Mol Biol (Noisy-le-grand) 2016;62(5):9–14. [PubMed] [Google Scholar]
- 33.Vazzana N., Guagnano M.T., Cuccurullo C., Ferrante E., Lattanzio S., Liani R. Endogenous secretory RAGE in obese women: association with platelet activation and oxidative stress. J Clin Endocrinol Metab. 2012;97(9):E1726–E1730. doi: 10.1210/jc.2012-1473. [DOI] [PubMed] [Google Scholar]
- 34.Momma H., Niu K., Kobayashi Y., Huang C., Chujo M., Otomo A. Lower serum endogenous secretory receptor for advanced glycation end product level as a risk factor of metabolic syndrome among Japanese adult men: a 2-year longitudinal study. J Clin Endocrinol Metab. 2014;99(2):587–593. doi: 10.1210/jc.2013-2936. [DOI] [PubMed] [Google Scholar]
- 35.Gurecka R., Koborova I., Csongova M., Sebek J., Sebekova K. Correlation among soluble receptors for advanced glycation end-products, soluble vascular adhesion protein-1/semicarbazide-sensitive amine oxidase (sVAP-1) and cardiometabolic risk markers in apparently healthy adolescents: a cross-sectional study. Glycoconj J. 2016;33(4):599–606. doi: 10.1007/s10719-016-9696-9. [DOI] [PubMed] [Google Scholar]
- 36.Gohda T., Tanimoto M., Moon J.Y., Gotoh H., Aoki T., Matsumoto M. Increased serum endogenous secretory receptor for advanced glycation end-product (esRAGE) levels in type 2 diabetic patients with decreased renal function. Diabetes Res Clin Pract. 2008;81(2):196–201. doi: 10.1016/j.diabres.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Peng W.H., Lu L., Wang L.J., Yan X.X., Chen Q.J., Zhang Q. RAGE gene polymorphisms are associated with circulating levels of endogenous secretory RAGE but not with coronary artery disease in Chinese patients with type 2 diabetes mellitus. Arch Med Res. 2009;40(5):393–398. doi: 10.1016/j.arcmed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Tan K.C., Chow W.S., Tso A.W., Xu A., Tse H.F., Hoo R.L. Thiazolidinedione increases serum soluble receptor for advanced glycation end-products in type 2 diabetes. Diabetologia. 2007;50(9):1819–1825. doi: 10.1007/s00125-007-0759-0. [DOI] [PubMed] [Google Scholar]
- 39.Clarke R.E., Dordevic A.L., Tan S.M., Ryan L., Coughlan M.T. Dietary advanced glycation end products and risk factors for chronic disease: a systematic review of randomised controlled trials. Nutrients. 2016;8(3):125. doi: 10.3390/nu8030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groener J.B., Reismann P., Fleming T., Kalscheuer H., Lehnhoff D., Hamann A. C332C genotype of glyoxalase 1 and its association with late diabetic complications. Exp Clin Endocrinol Diabetes. 2013;121(7):436–439. doi: 10.1055/s-0033-1345124. [DOI] [PubMed] [Google Scholar]
- 41.Tanhauserova V., Kuricova K., Pacal L., Bartakova V., Rehorova J., Svojanovsky J. Genetic variability in enzymes of metabolic pathways conferring protection against non-enzymatic glycation versus diabetes-related morbidity and mortality. Clin Chem Lab Med. 2014;52(1):77–83. doi: 10.1515/cclm-2012-0833. [DOI] [PubMed] [Google Scholar]
- 42.Peculis R., Konrade I., Skapare E., Fridmanis D., Nikitina-Zake L., Lejnieks A. Identification of glyoxalase 1 polymorphisms associated with enzyme activity. Gene. 2013;515(1):140–143. doi: 10.1016/j.gene.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Adams J.N., Raffield L.M., Martelle S.E., Freedman B.I., Langefeld C.D., Carr J.J. Genetic analysis of advanced glycation end products in the DHS MIND study. Gene. 2016;584(2):173–179. doi: 10.1016/j.gene.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nin J.W., Ferreira I., Schalkwijk C.G., Jorsal A., Prins M.H., Parving H.H. Higher plasma high-mobility group box 1 levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12 year follow-up study. Diabetologia. 2012;55(9):2489–2493. doi: 10.1007/s00125-012-2622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.