Abstract
Background:
CD8+ T cells and natural killer (NK) cells are cytotoxic cells that use granzyme B (GrB) and perforin. Defective cytotoxic function is known to play a role in dysregulated immune response as seen in chronic sinusitis, also referred to as chronic rhinosinusitis (CRS). However, to our knowledge, in the United States, neither GrB or perforin expression has been reported in patients with CRS.
Objective:
The aim of this study was to investigate sinonasal cytotoxic cells, their mediators, and cell-specific distribution of these mediators in patients with CRS with nasal polyp (CRSwNP) and in patients with CRS without nasal polyp (CRSsNP).
Methods:
Blood and sinus tissue samples were taken from patients with CRSsNP (n = 8) and CRSwNP (n = 8) at the time of surgery. Control subjects (n = 8) underwent surgery for cerebrospinal fluid leak repair or to remove non–hormone-secreting pituitary tumors. The cells were analyzed via flow cytometry by using CD8 expression to identify cytotoxic T cells and CD56 expression to identify NK cells. Intracellular GrB and perforin expression were analyzed with flow cytometry.
Results:
We observed no significant differences in plasma or peripheral blood immune cell numbers or specific levels of GrB or perforin among the groups. In the sinonasal mucosa of the patients with CRSsNP and the patients with CRSwNP, there was a significant decrease in GrB and perforin levels (p < 0.05) despite similar or increased numbers of cytotoxic cells when compared with the controls. The overall decrease in GrB and perforin in the sinonasal mucosa of the patients with CRSsNP and the patients with CRSwNP was due to decreased T cell production. There was no difference in total NK cell count or expression of perforin or GrB among all the groups.
Conclusion:
Total levels of sinonasal GrB and perforin were decreased in the sinonasal mucosa of both the patients with CRSwNP and the patients with CRSsNP compared with the controls, whereas sinonasal CD8+ T cells, (but not NK cells,), intracellular stores of GrB and perforin were reduced in the patients with CRSwNP compared with the controls.
Keywords: Sinusitis, T-cell, NK cell, granzyme B, perforin, nasal polyp, chronic rhinosinusitis, cytotoxic, sinonasal, rhinosinusitis
Chronic rhinosinusitis (CRS) is a highly prevalent inflammatory disease of the upper airway that affects 5–11% of the world's adult population.1 CRS is characterized by chronic sinonasal symptoms that result from extensive infiltration of inflammatory cells into the sinonasal mucosa. However, more research is needed because the complex immunologic processes and dysregulation that underlie CRS onset and progression are not fully understood. Granzyme B (GrB) is a serine protease that regulates inflammation, apoptosis, and antiviral immunity.2,3 As such, GrB has also been shown to be a marker of a cell's cytotoxic capacity.4 The entry of GrB into a cell is facilitated by the pore-forming protein, perforin. GrB and perforin work synergistically to maintain homeostasis and regulate inflammation. The two main cytotoxic effector cells that use these mediators are CD8+ T cells and CD56+ natural killer (NK) cells. Results of studies indicate that dysregulation in the release of GrB and perforin can contribute to inflammation associated with allergic rhinitis.5,6 Furthermore, loss of GrB can lead to impaired killing of viral infected cells.7
Results of recent studies indicate that there may be dysregulations in CD8+ T cell and NK cell functions in patients with CRS. For example, a number of studies found that CD8+ T cells accumulate in the sinonasal tissues of patients with CRS.5,8–10 Other studies that investigated NK cells found that, in patients with CRS and other allergic airway diseases, NK cells have an impaired ability to produce cytokines and to degranulate, which indicates a dysregulation in effector functions.11–13 In addition, a previous study that analyzed a CRS murine model found that the depletion of NK cells further aggravated eosinophilic inflammation.14 In a recent study from China that examined differences in GrB expression in controls and individuals with eosinophilic or noneosinophilic CRS with nasal polyps (CRSwNP), no differences were found in comparisons of GrB expression in peripheral blood or sinonasal tissue between the groups.8 Given the widely reported immunologic differences in Western versus Asian patients with CRSwNP,15 herein we investigated NK cell and CD8+ T cell expression of GrB and perforin in patients with CRSwNP or in patients with CRS without nasal polyps (CRSsNP) in a population of patients from the southeastern United States.
METHODS
Patients
The institutional review board at the Medical University of South Carolina approved these studies, and written informed consent was obtained from all the patients. We enrolled patients with CRSsNP (n = 8) and patients with CRSwNP (n = 8) who fulfilled European Position Paper on Rhinosinusitis and Nasal Polyps 2012 criteria.16 Obviously inflamed ethmoid sinus tissue was collected from patients with CRSsNP and those with CRSwNP. Control tissue was obtained from the sinuses of patients who were undergoing surgery for nonsecreting pituitary tumors or cerebrospinal fluid leaks (n = 8). Based on radiographic and endoscopic examinations, these patients were free from the presence of sinonasal inflammation. Exclusion criteria were the use of oral steroids or immunomodulatory agents within the preceding 30 days, other immunologic disorders, cystic fibrosis, or aspirin exacerbated respiratory disease.
Tissue Procurement and Processing
Sinus tissue explants and blood samples were returned immediately to the laboratory and processed as previously described.17–19 Blood samples were processed as previously described to obtain peripheral blood mononuclear cells, which were cryogenically preserved for later use.20
Immunostaining and Flow Cytometric Analysis
T cell and NK cell expression of intracellular GrB and perforin was conducted similar to our previously described methods.19–22 Peripheral blood mononuclear cells or single-cell suspensions of sinonasal tissue were thawed, rinsed twice, then stained with antibodies (BD Bioscience, Franklin Lakes, NJ) to identify extracellular markers. CD8 was used to identify cytotoxic T cells, whereas CD56 was used for the identification of NK cells The cells were permeabilized by using Cytofix/Perm reagent (BD Bioscience, Franklin Lakes, NJ) and then stained for intracellular expression of GrB and perforin. Isoform-matched isotypes were used as controls for extra- and intracellular staining. The cells were immediately analyzed by using a Guava 8HT flow cytometer (EMD Millipore, Billerica, MA) and FCS Express 5.0 software (De Novo Software, Glendale, CA). Dead cells (7-Aminoactinomycin D positive) were excluded from the final data analysis. Quantification of GrB and perforin was done by using arithmetic mean fluorescent intensity.
Statistics
At the time that these studies were conducted, to our knowledge, no data were available in the literature on GrB or perforin levels in sinonasal tissue to use for sample size calculations. In an interim power analysis that examined the differences in GrB expression (mean ± standard deviation) in the first four control (5.95 ± 2.6) and four CRSwNP (1.7 ± 0.41) samples examined, when assuming α = 0.05 and a power = 0.80, it was determined that we would require a sample size of four per group. For additional statistical power, we decided to pursue a sample size of eight per group.
Statistical analysis was conducted by using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA). A D'Agostino and Pearson omnibus test was used to determine if the data sets were normally distributed. A χ2 test was used to determine if there were statistically significant differences in population compositions with regard to gender and race. A one-way analysis of variance was used to determine if there were differences in age among the groups. For data in which all the groups were normally distributed, a one-way analysis of variance with a post hoc Tukey multiple comparisons test was used to determine statistical significance. For data in which all the groups were not normally distributed, a Kruskal-Wallis test, followed by a post hoc Dunn multiple comparisons test was used when appropriate.
RESULTS
Circulating GrB and Perforin Levels Were Not Different Between Controls and Patients with CRsNP or CRSwNP
Herein, we sought to investigate sinonasal GrB and perforin levels from CD8+ T cells and NK cells in a control group and patients with CRSsNP and patients with CRSwNP. There were 24 patients enrolled in this study who were divided evenly into three groups: control, CRSsNP, and CRSwNP. Each of the three groups consisted of eight patients. No significant differences were found among the three groups for demographic factors (Table 1). Asthma status was determined by patient history. Given that allergy testing was not a part of standard of care for control subjects or patients with CRSsNP, atopic status was only available for those with CRSwNP. Initially, we focused our investigation on circulating levels of GrB and perforin in plasma as well as intracellular levels in circulating peripheral blood immune cells. However, we observed no significant differences in plasma levels of GrB or perforin between the controls or either subset of CRS (data not shown). Similarly, there was no difference in the total or the cell-specific expression of GrB or perforin (data not shown). As such, we shifted our investigation to focus on sinonasal levels of these mediators.
Table 1.
Patient demographics

CRSsNP = Chronic rhinosinusitis without nasal polyp; CRSwNP = chronic rhinosinusitis with nasal polyp; SD = standard deviation.
T Cells and, to a Lesser Degree, NK Cells Account for the Majority of GrB and Perforin in the Sinonasal Mucosa
First, we examined the cell-specific distribution of GrB and perforin in the sinonasal mucosa. In doing so, we found that, in all the groups, T cells accounted for the majority of GrB and perforin, with NK cells being the next most abundant source of these mediators (Fig. 1). Interestingly, in the control patients, T cells were responsible for producing the highest percentage of GrB and perforin (Fig. 1 A and B, respectively). NK cells were the next most abundant cell type for producing GrB and perforin. In addition, we observed that 5–10% of cells positive for GrB were neither CD8+ T cells nor NK cells.
Figure 1.
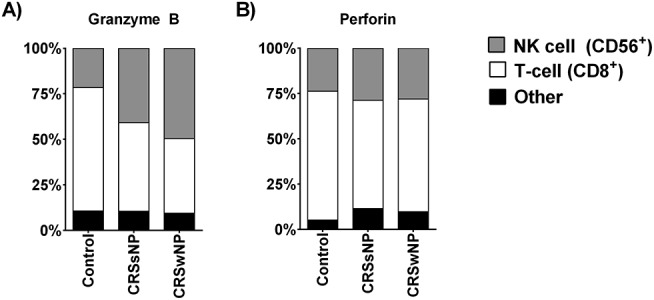
Cell-specific distribution of granzyme B (GrB) (A) and perforin (B) in sinonasal tissue. CD8+ T cells are the predominate source of GrB (except in patients with chronic rhinosinusitis with nasal polyp [CRSwNP]) and perforin in all the groups. Natural killer (NK) cells are the next most abundant source of GrB and perforin. Another unknown cell type accounts for 5–10% of sinonasal GrB and perforin in all the groups.
Total Levels of GrB and Perforin Were Reduced in the Sinonasal Mucosa
Next, we examined the difference in the total percentage of cells that produce GrB and perforin. Compared with the control patients, we observed a significant decrease in the percentage of total viable cells that stained positively for GrB and perforin (Fig. 2 A and B, respectively) in patients with CRSsNP or CRSwNP compared with the controls (p < 0.5). We next quantified the total amount of GrB and perforin (Fig. 2 C and D, respectively) present by using mean fluorescent intensity of staining. Similarly, a significant difference was seen for both total GrB and perforin expressions in patients with CRSsNP or CRSwNP compared with the controls (p < 0.5, for both comparisons). There was no significant difference between CRSsNP and CRSwNP percentages of cells that positively stained for either mediator or the amount of staining for either mediator.
Figure 2.
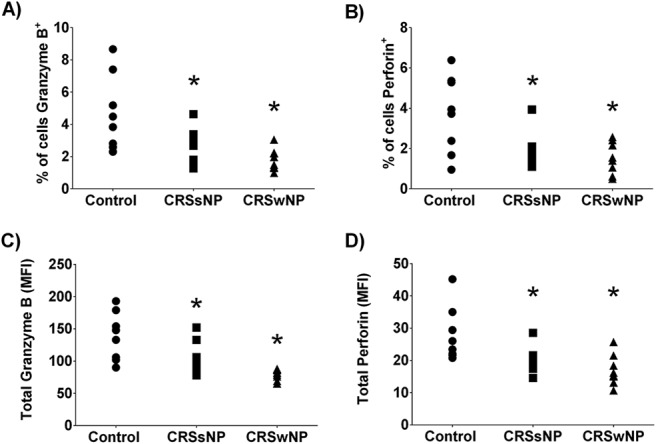
The percentage of cells positive for granzyme B (GrB) (A) and perforin (B), and quantified levels of GrB (C) and perforin (D) in the sinonasal mucosa. Note that the total percentage of cells and the amount of GrB and perforin present, as quantified mean fluorescent intensity (MFI), was reduced in both chronic rhinosinusitis (CRS) groups. *p < 0.05 versus control.
The Percentage of Sinonasal CD8+ T Cell Levels, But Not NK Cell Levels, Was Increased in Patients with CRSwNP Compared with Controls
We next examined if a reduced sinonasal presence of CD8+ T cells or NK cells was responsible for the decreased sinonasal levels of GrB and perforin. We observed that patients with CRSwNP, but not those with CRSsNP, had a significantly higher percentage of sinonasal CD8+ T cells than the control patients (Fig. 3 A). Interestingly, we did not find any significant difference in the percentage of sinonasal CD56+ NK cells across the groups (Fig. 3 B). Taken together, analysis of these data indicated that the decreases in GrB and perforin were not the result of reduced numbers of sinonasal CD8+ T cells or NK cells.
Figure 3.
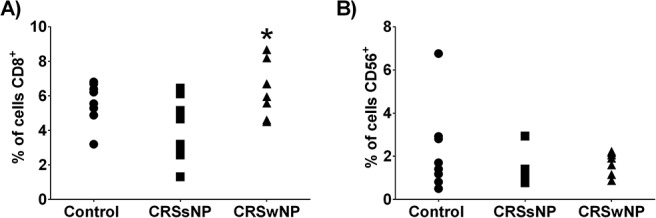
The percentage of CD8+ T cells and CD56+ natural killer (NK) cells present in the sinonasal mucosa. (A) Patients with chronic rhinosinusitis with nasal polyp (CRSwNP) had increased numbers of sinonasal CD8+ T cells compared with the controls. (B) There was no significant difference in the number of sinonasal NK cells between the groups. *p < 0.05 versus control.
The Amount of GrB and Perforin Was Reduced in Sinonasal CD8+ T Cells But Not in NK Cells in CRS
Next, we examined the cell-specific production of each cytotoxic mediator. We did so by evaluating the percentage of cells that stained positively for both CD8+ and GrB+, that stained positively for both CD8+ and perforin, that stained positively for both CD56+ and GrB+, and that stained positively for both CD56+ and perforin. We found significant reductions in the percentage of all sinonasal cells that were positively stained for both CD8+ and GrB+, and that were stained positively for both CD8+ and perforin in patients with CRSsNP and in patients with CRSwNP compared with the controls (Fig. 4 A and B). To gain greater insight into cell-specific production of each mediator, we next examined the percentage of total CD8+ T cells that stained positively for both GrB and perforin. We observed that the percentages of total CD8+ T cells positive for GrB and perforin were significantly reduced in patients with CRSwNP, not in patients with CRSsNP, compared with the controls, which indicated that, although there were more T cells, fewer of them were producing GrB and perforin (Fig. 4 C and D). We did not see any significant difference among the groups when we analyzed the NK cell-specific production of GrB or perforin (Fig. 5 A–D). Together, analysis of these data indicated that, in patients with CRSwNP, although there was an increase in sinonasal CD8+ T cells, they had reduced intracellular stores of GrB and perforin.
Figure 4.
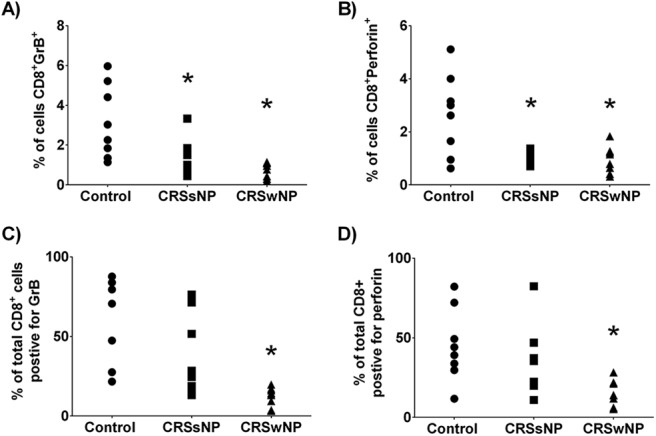
T-cell production of granzyme B (GrB) and perforin was reduced in patients with chronic rhinosinusitis without nasal polyp (CRSsNP) and patients with chronic rhinosinusitis with nasal polyp (CRSwNP). The percentage of sinonasal cells that stained positively for both CD8 and GrB (A) or perforin (B). To correct for the difference in cell infiltrate, GrB (C) and perforin (D) staining was examined as a percentage of total CD8+ T cells present. *p < 0.05 versus control.
Figure 5.
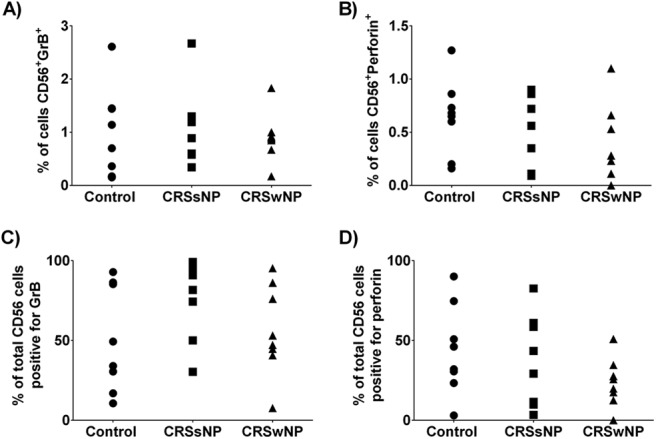
Natural killer (NK) cell production of granzyme B (GrB) and perforin was not altered in patients with chronic rhinosinusitis without nasal polyp (CRSsNP) or chronic rhinosinusitis with nasal polyp (CRSwNP). The percentage of sinonasal cells that stained positively for both the NK cell marker CD56 and GrB (A) or perforin (B). To correct for differences in cell infiltrate, GrB (C), and perforin (D) staining was examined as a percentage of total CD56+ NK cells present. *p < 0.05 versus control.
DISCUSSION
GrB and perforin are two critical mediators used by cytotoxic T cells and NK cells to modulate inflammation and to facilitate apoptosis, such as in cells that are virally infected. There is increasing evidence that abnormalities in cytotoxic effector cells and their mediators play an important role in inflammatory airway diseases. GrB expression has previously been examined by histology and flow cytometry in Chinese patients with eosinophilic CRSwNP and those with noneosinophilic CRSwNP. However, to our knowledge, in the United States, GrB or perforin expression in patients with CRSsNP or patients with CRSwNP has not be reported, and, therefore, was the focus of our studies.
A number of studies report that significant increases in CD8+ T cells are seen in the sinonasal mucosa of patients with CRS compared with controls.5,8–10,23 Analysis of our data supported these previous findings in that we observed a significant increase in sinonasal CD8+ T cells in patients with CRSwNP. One hypothesis to explain these abnormal levels is that upregulated levels of CD8+ T cells could be the result of decreased perforin levels. Low levels of perforin production have been proven in both human and murine models to result in the increased expansion and decreased death of CD8+ T cells.6,24–26 In addition, our results indicated that there was a significant decrease in perforin levels in both subsets of patients with CRS, which supported the notion that significantly lower levels of total perforin could play a role in abnormal regulation and thereby the drastically increased CD8+ T cell levels seen in patients with CRSwNP.
Interestingly, we only found significantly different levels of perforin expression in CD8+ T cells but not in NK cells. As previously discussed, NK cells have been found to regulate CD8+ T cell levels by using perforin and have also been observed to have lost their effector functions in patients with CRS.11,12,27 Although we did not analyze degranulation markers or the efficacy of NK cell function, our results showed no significant difference in the total NK cell count or expression of perforin or GrB. Given that GrB has also been shown to be a marker of a cell's cytotoxic capacity,4 it may be possible that the CD8+ T cells in the sinonasal mucosa of patients with CRSwNP could have reduced the cytotoxic potential.
Ma et al.,8 reported that CD8+ T cells in sinonasal mucosa have reduced effector functions via loss of cytotoxicity and reduced GrB compared with cells from the peripheral blood. With regard to comparisons between controls and patients with CRSwNP, similar to Ma et al.,8 we did not find any difference in peripheral blood GrB levels between the groups. However, unlike that study, we did find reductions in sinonasal CD8+ T cell production of GrB and perforin in sinonasal mucosa. These differences may be due, in part, to differences in the cytokines present in each of these populations. For example, patients with CRSwNP who live in countries such as China, Korea, and Japan often present with type 1 and type 3 inflammation. This varies from Western populations (Europe and North America) in which CRSwNP is characterized by a predominately type 2 microenvironment.15 Consistent with this hypothesis is that the type 2 cytokine, interleukin 4, has been shown to be a suppressor of cytotoxic functions.28 However, no studies have yet to report an exact reason why there is an observed loss of GrB and perforin expression in sinonasal CD8+ T cells from patients with CRS, and this could be the focus of future studies.
Ultimately, analysis of the current and previous findings indicated that CD8+ T cells and NK cells may not function as classic cytotoxic cells in the sinonasal mucosa of patients with CRS.8,11–13 Although most of the data presented herein focused on sinonasal levels of the major mediators involved in cytotoxic effector functions, it is important to note that the circulating levels of these factors were, in addition, examined. In agreement with previous studies, we did not observe any difference in these mediators systemically.8,9,23 Our results, however, did differ from studies that evaluated peripheral levels in patients with asthma and chronic obstructive pulmonary disease.29–33 This further supported the notion that there may be changes in the T cell profile specific to the localized region of the sinonasal mucosa in CRS that results in a loss of cytotoxicity.
To date, to our knowledge, there have been no published findings with regard to the cell-specific distribution of cytotoxic mediators in the sinonasal mucosa of patients with CRS. Interestingly, we found that there was a significant reduction in GrB and perforin levels in sinonasal CD8+ T cells of the patients with CRS compared with the controls but no significant difference in the GrB and perforin levels in sinonasal NK cells of the patients with CRS compared with the controls. In addition, it is important to note that 10% of both the GrB and perforin distributions in the controls, patients with CRSsNP, and patients with CRSwNP was not within NK cells or CD8+ T cells. Although CD8+ and NK cells are most commonly known to produce GrB and perforin, they can also be produced by mast cells, neutrophils, T-regulatory cells, dendritic cells, macrophages, or basophils.34–39 Further investigation is required to determine the exact cell type that is responsible for the production of the remaining 10% of the cytotoxic mediators.
There were several limitations to the current study. Here we did not determine the role of these mediators in cytotoxic function nor did we investigate the relative contribution of cytotoxic effector mechanisms to each cell type. In addition, there was a limitation in the relatively small sample size in that it was underpowered to determine the possible role of atopic status. It is also worth mentioning that the control cohort was predominately female, which had the potential to introduce a gender bias to these studies. However, in our CRSwNP cohort, which was composed equally of male and female patients, we observed no difference in GrB or perforin expression between the genders.
CONCLUSION
Compared with the control subjects, patients with CRSsNP and patients with CRSwNP had reduced sinonasal levels of the cytotoxic mediators GrB and perforin. Furthermore, although patients with CRSwNP had an increase in their number of sinonasal CD8+ T cells present, these cells had reduced intracellular stores of GrB and perforin. Future studies may examine how diminished levels of these mediators may impact antiviral immunity and inflammation in patients with CRS.
Footnotes
These studies received support in funding by grants from the Flight Attendants Medical Research Institute (092401 [J.K.M.] and 140030 [Z.M.S.]). J.K. Mulligan is supported by the South Carolina Clinical and Translational Research Institute, with an academic home at the Medical University of South Carolina, National Institute of Health/National Center for Advancing Translational Sciences grants KL2TR001452 and UL1TR001450
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Patel ZM, Thamboo A, Rudmik L, et al. Surgical therapy vs continued medical therapy for medically refractory chronic rhinosinusitis: A systematic review and meta-analysis. Int Forum Allergy Rhinol 7:119–127, 2017. [DOI] [PubMed] [Google Scholar]
- 2. Salti SM, Hammelev EM, Grewal JL, et al. Granzyme B regulates antiviral CD8+ T cell responses. J Immunol 187:6301–6309, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: Function, dysfunction and human pathology. Nat Rev Immunol 15:388–400, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Waterhouse NJ, Sedelies KA, Clarke CJ. Granzyme B; the chalk-mark of a cytotoxic lymphocyte. J Transl Med 2:36, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qiu S, Du Y, Duan X, et al. Cytotoxic T lymphocytes mediate chronic inflammation of the nasal mucosa of patients with atypical allergic rhinitis. N Am J Med Sci 3:378–383, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 290:1354–1358, 2000. [DOI] [PubMed] [Google Scholar]
- 7. Salti SM, Hammelev EM, Grewal JL, et al. Granzyme B regulates antiviral CD8+ T cell responses. J Immunol 187:6301–6309, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma J, Shi LL, Deng YK, et al. CD8(+) T cells with distinct cytokine-producing features and low cytotoxic activity in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Clin Exp Allergy 46:1162–1175, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Pant H, Hughes A, Miljkovic D, et al. Accumulation of effector memory CD8+ T cells in nasal polyps. Am J Rhinol Allergy 2013;27: e117–e126, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Cho KS, Kim CS, Lee HS, et al. Role of interferon-gamma-producing T cells in the pathogenesis of chronic rhinosinusitis with nasal polyps associated with staphylococcal superantigen. J Otolaryngol Head Neck Surg 39:600–605, 2010. [PubMed] [Google Scholar]
- 11. Kim JH, Kim GE, Cho GS, et al. Natural killer cells from patients with chronic rhinosinusitis have impaired effector functions. PLoS One 2013;8: e77177, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim JH, Choi GE, Lee BJ, et al. Natural killer cells regulate eosinophilic inflammation in chronic rhinosinusitis. Sci Rep 6:27615, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scordamaglia F, Balsamo M, Scordamaglia A, et al. Perturbations of natural killer cell regulatory functions in respiratory allergic diseases. J Allergy Clin Immunol 121:479–485, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Kim JH, Gong CH, Choi GE, et al. Natural killer cell deficits aggravate allergic rhinosinusitis in a murine model. ORL J Otorhinolaryngol Relat Spec 78:199–207, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy 45:328–346, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50:1–12, 2012. [DOI] [PubMed] [Google Scholar]
- 17. Oyer SL, Nagel W, Mulligan JK. Differential expression of adhesion molecules by sinonasal fibroblasts among control and chronic rhinosinusitis patients. Am J Rhinol Allergy 27:381–386, 2013. [DOI] [PubMed] [Google Scholar]
- 18. Mulligan JK, Nagel W, O'Connell BP, et al. Cigarette smoke exposure is associated with vitamin D3 deficiencies in patients with chronic rhinosinusitis. J Allergy Clin Immunol 134:342–349, 2014. [DOI] [PubMed] [Google Scholar]
- 19. Psaltis AJ, Schlosser RJ, Yawn JR, et al. Characterization of B-cell subpopulations in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 3:621–629, 2013. [DOI] [PubMed] [Google Scholar]
- 20. O'Connell BP, Schlosser RJ, Wentzel JL, et al. Systemic monocyte-derived dendritic cells and associated Th2 skewing in chronic rhinosinusitis. Otolaryngol Head Neck Surg 150:312–320, 2014. [DOI] [PubMed] [Google Scholar]
- 21. Schlosser RJ, Carroll WW, Soler ZM, et al. Reduced sinonasal levels of 1alpha-hydroxylase are associated with worse quality of life in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol 6:58–65, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Mulligan JK, White DR, Wang EW, et al. Vitamin D3 deficiency increases sinus mucosa dendritic cells in pediatric chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg 147:773–781, 2012. [DOI] [PubMed] [Google Scholar]
- 23. Pant H, Hughes A, Schembri M, et al. CD4(+) and CD8(+) regulatory T cells in chronic rhinosinusitis mucosa. Am J Rhinol Allergy 28:e83–e89, 2014. [DOI] [PubMed] [Google Scholar]
- 24. Stepp SE, Dufourcq-Lagelouse R, Kumar V. Pillars article: Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 1999. 286: 1957–1959. J Immunol 194:5044–5046, 2015. [PubMed] [Google Scholar]
- 25. Chen M, Felix K, Wang J. Critical role for perforin and Fas-dependent killing of dendritic cells in the control of inflammation. Blood 119:127–136, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou S, Ou R, Huang L, Moskophidis D. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J Virol 76:829–840, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zecher D, Li Q, Oberbarnscheidt MH, et al. NK cells delay allograft rejection in lymphopenic hosts by downregulating the homeostatic proliferation of CD8+ T cells. J Immunol 184:6649–6657, 2010. [DOI] [PubMed] [Google Scholar]
- 28. Gardiner CM, Reen DJ. Differential cytokine regulation of natural killer cell-mediated necrotic and apoptotic cytotoxicity. Immunology 93:511–517, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vernooy JH, Moller GM, van Suylen RJ, et al. Increased granzyme A expression in type II pneumocytes of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175:464–472, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Hodge S, Hodge G, Nairn J, et al. Increased airway granzyme b and perforin in current and ex-smoking COPD subjects. COPD 3:179–187, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Vesterinen E, Timonen T. Natural killer cell activity in specific and non-specific bronchial challenge. Ann Allergy 60:247–249, 1988. [PubMed] [Google Scholar]
- 32. Lin SJ, Chang LY, Yan DC, et al. Decreased intercellular adhesion molecule-1 (CD54) and L-selectin (CD62L) expression on peripheral blood natural killer cells in asthmatic children with acute exacerbation. Allergy 58:67–71, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Jira M, Antosova E, Vondra V, et al. Natural killer and interleukin-2 induced cytotoxicity in asthmatics. I. Effect of acute antigen-specific challenge. Allergy 43:294–298, 1988. [DOI] [PubMed] [Google Scholar]
- 34. Strik MC, de Koning PJ, Kleijmeer MJ, et al. Human mast cells produce and release the cytotoxic lymphocyte associated protease granzyme B upon activation. Mol Immunol 44:3462–3472, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Wagner C, Iking-Konert C, Denefleh B, et al. Granzyme B and perforin: Constitutive expression in human polymorphonuclear neutrophils. Blood 103:1099–1104, 2004. [DOI] [PubMed] [Google Scholar]
- 36. Grossman WJ, Verbsky JW, Tollefsen BL, et al. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 104:2840–2848, 2004. [DOI] [PubMed] [Google Scholar]
- 37. Rissoan MC, Duhen T, Bridon JM, et al. Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, 3 novel transcripts in human plasmacytoid dendritic cells. Blood 100:3295–3303, 2002. [DOI] [PubMed] [Google Scholar]
- 38. Choy JC, McDonald PC, Suarez AC, et al. Granzyme B in atherosclerosis and transplant vascular disease: Association with cell death and atherosclerotic disease severity. Mod Pathol 16:460–470, 2003. [DOI] [PubMed] [Google Scholar]
- 39. Tschopp CM, Spiegl N, Didichenko S, et al. Granzyme B, a novel mediator of allergic inflammation: Its induction and release in blood basophils and human asthma. Blood 108:2290–2299, 2006. [DOI] [PubMed] [Google Scholar]


