Abstract
Survival outcome for elderly patients with newly diagnosed diffuse large B-cell lymphoma remains suboptimal in the rituximab era. In this systematic review, we summarize available evidence relevant to the inclusion of anthracycline in upfront chemoimmunotherapy for these elderly patients and highlight the need of prospective clinical trials. With limited prospective data, we find that pretreatment comprehensive geriatric assessment accurately predicts survival and treatment-related toxicities, suggesting its potential role in guiding overall treatment decision-making.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2235.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Alnylam, Biogen, and Pfizer Inc. Author Christopher R. Flowers served as an advisor or consultant for Spectrum Pharmaceuticals, Celgene, Optum Rx, Seattle Genetics, Gilead Sciences, and Bayer and received grants for clinical research from AbbVie, Acerta Pharma, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Janssen Pharmaceuticals, Millennium/Takeda, National Institutes of Health, Onyx Pharmaceuticals, Phamacyclics, and Spectrum Pharmaceuticals. Associate Editor David Garcia and the remaining authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Evaluate recommendations for elderly patients with diffuse large B-cell lymphoma who are considered fit or unfit for intensive therapy, based on a literature review.
Examine recommendations for assessment of elderly patients with diffuse large B-cell lymphoma to determine their level of fitness to withstand therapy, based on a literature review.
Analyze recommendations for management of elderly patients with diffuse large B-cell lymphoma and cardiac comorbidities, based on a literature review.
Release date: November 16, 2017; Expiration date: November 16, 2018
Case presentation
A 76-year-old man with hypertension, type 2 diabetes, coronary artery disease, chronic obstructive pulmonary disease, and severe osteoarthritis was diagnosed with stage IV diffuse large B-cell lymphoma (DLBCL) of activated B-cell–like subtype when he presented with generalized lymphadenopathy, night sweats, and weight loss. His International Prognostic Index was 4, and he had a performance status of 1 before diagnosis, mainly as a result of dyspnea and arthritis-related pain. Would he benefit from treatment with rituximab and anthracycline-based chemotherapy with curative intent? Are there tools available to help guide the decision-making process? This case illustrates a clinical scenario that is common in our daily practice.
Introduction
The incidence of aggressive non-Hodgkin lymphoma subtypes such as DLBCL increases with age, yet outcomes for elderly patients (defined as age older than 60 or 65 years in prospective clinical trials) are significantly and disproportionately inferior to those of younger patient populations.1,2 Although differences in tumor biology may play a partial role,3 the management of these patients is heavily influenced by their preexisting comorbidities and a variety of geriatric syndromes such as polypharmacy, risk of falling, depression, cognitive impairment, sarcopenia, and frailty.4,5 Compounding these challenges is the lack of randomized data to determine the risk-to-benefit ratio of different types of therapy and to guide treatment decision-making, because elderly patients are generally excluded from pivotal drug trials.6
The current gold standard for treating elderly patients with DLBCL was established by the Groupe d’Etude des Lymphomes de l’Adulte (GELA) non-Hodgkin lymphoma trial (GELA LNH-98-5), which showed that the addition of rituximab to 8 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy improved the long-term survival outcome for patients 60 to 80 years old.7,8 The subsequent RICOVER-60 trial confirmed the efficacy of adding rituximab to CHOP (R-CHOP) in elderly patients with DLBCL.9 Approaches that used dose-reduced CHOP with rituximab or ofatumumab also demonstrated meaningful rates of complete remission, 2-year progression-free survival, overall survival (OS), and acceptable toxicity in patients age 80 years or older.10,11 Moreover, treatment with anthracycline-based chemotherapy may be complicated by comorbidities and alterations in functional status in older adults, as illustrated in a large epidemiologic study of treatment patterns in elderly DLBCL patients in the United States.12 However, omission of anthracycline may also lead to inferior survival in these patients.13
Accumulating evidence has suggested that geriatric unfitness and frailty determined by a comprehensive geriatric assessment (CGA) are independent predictors of mortality and treatment-related toxicities in several hematologic malignancies.14-17 However, even unfit patients may still benefit substantially from curative intent rather than palliative chemotherapy as evident from a retrospective analysis of 135 elderly lymphoma patients.18 The authors found that even in unfit patients who are treated with curative intent, anthracycline-containing chemotherapy had a significantly better 1-year OS compared with patients receiving palliative measures only.18 Given the retrospective, single-institution nature of most published studies in the field and their intrinsically associated selection bias, we conducted a systematic review examining the impact on the survival and treatment-related toxicities of anthracycline-containing vs anthracycline-free chemotherapy regimens for the initial treatment of elderly patients with newly diagnosed DLBCL.
Methods
Literature search strategy and study selection criteria
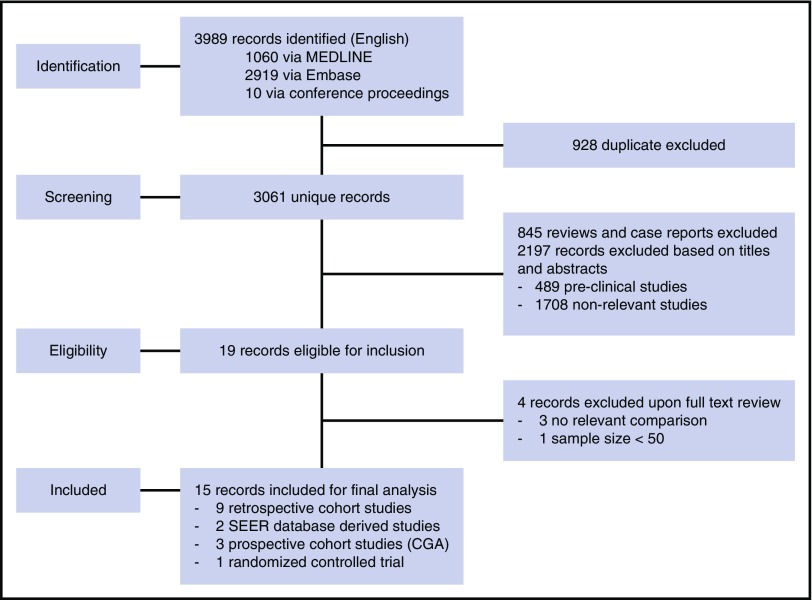
We performed a comprehensive electronic literature search using MEDLINE and Embase from 1 January 1990 to 30 December 2016 (Figure 1). Proceedings of 2 major annual conferences (58th American Society of Hematology Annual Meeting and Exposition in 2016 and the 52nd Annual Meeting of the American Society of Clinical Oncology in 2016) were also searched for abstracts whose corresponding manuscripts had not yet been published in peer-reviewed journals. In addition, the Cochrane Database of Systematic Reviews and the Clinical Trial Registry were searched for relevant systematic reviews and ongoing trials up to 30 December 2016. Two authors (R.J.L. and M.B.) independently reviewed all abstracts for eligibility assessment, and a third author (C.R.F.) mediated discordant results. The search criteria were based on a defined population, intervention, comparison, outcomes, and timing question in which the population was patients age 60 years or older with newly diagnosed DLBCL, intervention was treatment with a chemotherapy regimen containing rituximab, comparison was between regimens with or without anthracycline, the primary outcome was OS, the secondary outcomes were overall response rate (ORR) and treatment-related toxicities, and the time period for evaluation was defined as after completion of first-line therapy until relapse, death, or the last follow-up. Each study required >50 patients to be included. The medical subject heading (MeSH) search included “Lymphoma, Large B-cell, Diffuse” and “Aged” and “Anthracyclines” or “Rituximab” or “Lymphoma, Non-Hodgkin” and “Aged” and “Geriatric Assessment.” The full article was reviewed if eligibility was clearly met or if there was uncertainty regarding a priori–defined eligibility criteria based on the title and abstract. Only prospective studies using CGA were included.
Figure 1.
PRISMA article flow diagram. Step-by-step literature search using the Medical Subject Headings and subsequent record identification based on abstract or full-text review.
Quality assessment and statistical analysis
Data were abstracted by using a standard approach that was customized for the study. The data elements included the type of study (single, multicenter, or population-based cohort), method of data collection (retrospective or prospective), aim of the study, regimen used, treatment outcomes, and statistical data when analysis was performed. Quality assessment of the studies was performed with a checklist adapted from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.19 However, because this is one of the first systematic reviews on this topic, no study was excluded on the basis of the quality assessment. We also examined related previous and subsequent publications to find unreported protocol details when evaluating the quality of the study. The data extracted from the eligible studies were quantified and reported as pooled proportions. All statistical analyses were performed using STATA software (version 10).
Search results
Impacts of anthracycline-containing regimens
Fifteen studies conducted between 2010 and 2016 fulfilled the inclusion criteria, including 9 retrospective cohort studies20-28 and 2 administrative database–derived epidemiologic studies29,30 comparing chemotherapy regimens with or without anthracyclines in elderly patients who had newly diagnosed DLBCL (Table 1). No randomized control trials were conducted to specifically address our question. Five prospective cohort studies, including 1 randomized controlled study, evaluated the use of CGA to guide treatment decision making in elderly patients with newly diagnosed DLBCL, are summarized in Table 2.31-35 In total, 1732 patients were included in 9 retrospective studies. As shown in Table 1, most studies were small single-institution studies with missing data, especially data related to the comparison between anthracycline-containing or anthracycline-free regimens. The average STROBE checklist score was 15.5 (range, 0-22; higher scores indicate higher quality). After summarizing all studies with available data, it seemed that treating patients with an anthracycline-containing regimen by physician and/or patient choice was associated with a 3-year OS rate of 63% compared with 44% for anthracycline-free regimens (P < .001). Incomplete data precluded comparison of ORR and treatment-related toxicities, and no statistical difference was observed in treatment-related mortality between the 2 treatment groups (Table 1).
Table 1.
Summarized findings of retrospective studies examining the impact of chemotherapy choice on outcomes in elderly patients with DLBCL
| Reference | No. of patients | Institution/location | Age (y) | % of patients | IPI | 3-Year OS (%) | ORR (%) | Toxicities (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthracycline-containing regimen | Anthracycline-free regimen | Anthracycline-containing regimen | Anthracycline-free regimen | Anthracycline-containing regimen | Anthracycline-free regimen | ||||||
| 20 | 207 | MDACC | ≥80 | 54 | ≥3 | 63 | 25 | — | — | 10 TRM | 10 TRM |
| 21 | 141 | Mexico | ≥65 | 61 | ≥3 | 63 | 52 | 73 | 60 | 55 | 40 |
| 22 | 72 | Emory University | ≥65 | 25 | ≥4 | 59 | 38 | 84 | 63 | 24 | — |
| 23 | 128 | Switzerland | ≥60 | 49 | ≥3 | — | — | — | — | — | — |
| 24 | 378 | Portugal | ≥60 | 55 | ≥3 | — | — | — | — | — | — |
| 25 | 103 | Netherlands | ≥75 | 35 | ≥2* | — | — | — | — | — | — |
| 26 | 73 | OHSU | ≥75 | 49 | ≥3 | 68 | 54 | — | — | 9 TRM | — |
| 27 | 154 | MGH | ≥75 | — | — | — | — | — | — | — | |
| 28 | 476 | VA system | ≥80 | 49 | ≥2* | 28.1 mo (median) | 13.1 mo (median) | — | — | 15 TRM | 23 TRM |
IPI, International Prognostic Index; MDACC, MD Anderson Cancer Center; MGH, Massachusetts General Hospital; OHSU, Oregon Health & Science University; TRM, treatment-related mortality; VA, Veterans Administration.
Age-adjusted IPI.
Table 2.
Summarized findings of prospective studies that examined the impact of CGA on treatment outcomes in elderly patients with DLBCL
| Reference | No. of patients | Location | Age (y) | OS (%) | ORR (%) | Toxicities (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % of patients | IPI | Fit | Unfit/frail | Fit | Unfit/frail | Fit | Unfit/frail | ||||
| 31 | 100 | Italy | ≥70 | 22 | ≥3 | 76 (5-y) | 47 (5-y) | 85 | 76 | 31 | 51 |
| 32 | 91 | Italy | ≥65 | 30 | ≥3 | 34 (5-y) | 9 (5-y) | 83 | 73 | 28 | 57 |
| 33 | 88 | Italy | ≥65 | 60 | ≥3 | Median, NR | Median, 8.0 mo | 93 | 52 | — | — |
| 34 | 173 | Italy | ≥69 | 43 | ≥3 | 84 (2-y) | 47 (2-y) | — | — | — | — |
NR, not reached.
For 2 large analyses that used administrative databases, Williams et al29 used a Surveillance, Epidemiology, and End Results (SEER)–Medicare data set (2002-2009) to characterize treatment and survival outcomes of 1156 elderly patients (older than age 80 years) with newly diagnosed DLBCL. They found that R-CHOP compared with other anthracycline-free regimens (by physician and/or patient choice) was associated with improved OS (hazard ratio, 0.45; 95% confidence interval, 0.33-0.62) and lymphoma-related survival (hazard ratio, 0.58; 95% confidence interval, 0.38-0.88) by using a propensity score–matched multivariable Cox proportional hazards model. By using the same SEER-Medicare data set (2000-2006), Tien et al30 analyzed treatment choice and outcomes in 8262 elderly DLBCL patients. They found that among this cohort, the unadjusted 3-year OS was highest in anthracycline-containing regimens plus rituximab (65%), followed by anthracycline-containing regimens without rituximab (55%), and anthracycline-free regimens with rituximab (44%). After adjusting for clinical covariates, anthracycline-containing regimens plus rituximab (by physician and/or patient choice) demonstrated the best 3-year survival (63%). Treatment toxicity data were not reported for these 2 studies; thus, no comparison can be made between the regimens.
Impacts of CGA on treatment outcome in elderly DLBCL patients
As shown in Table 2, 5 prospective studies evaluated the use of CGA but only 1 was a prospective, randomized controlled trial.31-35 In total, 452 patients were included in the 4 prospective observation studies. CGA categorized patients into fit and unfit/frail categories. However, in all 4 studies, the choice of regimen was left to the discretion of physicians, and CGA was not used to guide treatment selection. The summarized ORR and 5-year OS were 87% and 55% for fit patients and 67% and 29% for unfit/frail patients, respectively (both P < .001). The summarized rate of grade 3 or higher toxicity was 29% for fit patients and 54% for unfit/frail patients (P = .0005). The details of regimens (anthracycline-containing vs anthracycline-free) were not available from these prospective observational studies. Merli et al35 conducted the only randomized study, which prospectively allocated 224 elderly fit (according to CGA) patients to receive either a standard dose of R-CHOP or the less intensive rituximab plus cyclophosphamide, etoposide, vincristine, and prednisone (mini-R-CEOP) with the dose of epirubicin equivalent to two-thirds that of doxorubicin. After a median follow-up of 42 months, 5-year event-free survival rates were 46% and 48% for mini-R-CEOP and R-CHOP, respectively (not significant). The 5-year OS rates were 63% and 62% for mini-R-CEOP and R-CHOP, respectively (not significant). They conclude that the less intense mini-R-CEOP may be an acceptable option for fit elderly patients with DLBCL.
We did not identify any prospective, randomized studies that specifically addressed the question of an appropriate regimen for elderly unfit DLBCL patients selected by CGA. In the unfit subgroup of patients (n = 28) from the study by Tucci et al,34 curative-intent treatment with an anthracycline-containing regimen produced a 2-year OS of 75% vs 45% (P = .32) for palliative treatment with anthracycline-free regimens, anthracycline-based regimens with less than 70% relative dose intensity (RDI), radiation treatment, rituximab monotherapy, or supportive care alone. The unfit subgroup of patients (n = 22) from the study by Olivieri et al32 all received liposomal doxorubicin. Finally, in a retrospective analysis by Yoshida et al,18 patients classified as unfit by CGA (n = 53) who received curative-intent treatment with anthracycline-containing regimens had a significantly better 1-year OS when compared with those receiving palliative measures (66.1% vs 19.0%; P < .001).
Summary and recommendations
Historically, the initial treatment strategy for elderly patients with newly diagnosed DLBCL has been based on the judgment of clinicians.36-38 Treatment-related toxicities of R-CHOP worsen with increasing comorbidity, functional disability, and advanced age.39,40 An important question facing the treating physician is to identify fit patients who can tolerate curative-intent anthracycline-containing regimens and frail patients who cannot. Whether unfit elderly patients still derive benefits from anthracycline-based regimens is unclear. Our systematic review is designed to address these questions. We found that there was a lack of data from randomized controlled studies to answer the question of whether anthracycline-based regimens would benefit elderly patients with DLBCL. However, we identified multiple retrospective cohort studies and 2 SEER database–derived analyses of a total of more than 11 000 patients that support an association between anthracycline-containing regimens and improved OS and ORR with acceptable toxicities. Moreover, available subgroup analyses of unfit patients identified prospectively by CGA also support an association between anthracycline-containing regimens and improved outcomes. Our results make it imperative to undertake a careful assessment of fitness for R-CHOP before considering less toxic and potentially less effective alternatives.
Although the retrospective nature of most of the studies we identified in this review were associated with intrinsic selection bias (eg, patient choices or physicians’ conscious or subconscious selection of patients on the basis of fitness, thus leading to treatment bias), the improved rates of OS and ORR seen with anthracycline-based therapy are consistent with historical cohorts of elderly lymphoma patients.7-9 Given the key role of anthracycline-based therapy in maintaining response rate and cure rate in elderly patients with DLBCL, dosage may be adjusted on the basis of the level of frailty while maintaining a certain degree of efficacy such as the mini-R-CHOP regimen (50% dose reduction of cyclophosphamide, doxorubicin, and vincristine) used successfully in patients older than age 80 years,10 or the mini-R-CEOP regimen used in fit elderly patients.35 Another article not selected for inclusion in our analysis included 192 elderly DLBCL patients and compared outcomes between regimens of different anthracycline and cyclophosphamide RDI; it showed that anthracycline RDI of >60% was associated with improved survival, consistent with our findings.41
For patients with severe cardiovascular dysfunction, which precluded the use of anthracyclines, several potential newer alternative regimens could be considered despite the lack of prospective randomized controlled studies. For example, substitution of an anthracycline with another drug such as gemcitabine (R-GCVP) or etoposide (R-CEOP) has produced reasonable response rates and OS for patients.42,43 Another alternative regimen, bendamustine and rituximab, produced a high response rate of 78% in a small phase II trial with elderly DLBCL patients, albeit with a median OS of only 10.2 months and progression-free survival of only 5.4 months; even the study authors counseled that this regimen should be used with caution.44 Whether these less toxic regimens can substituted for R-CHOP in elderly fit patients warrants evaluation in prospective, randomized trials.
The use of geriatric assessment tools such as CGA may help physicians identify those patients who are suitable for R-CHOP.45,46 As shown in our analysis of 4 prospective cohort studies, CGA accurately predicted rates of OS, ORR, and treatment-related toxicities. Interestingly, the only randomized trial identified in our study suggests that fit elderly patients may achieve similar outcomes with mini-R-CEOP using epirubicin, another anthracycline that is two-thirds as potent as doxorubicin; therefore, reduced doses of anthracyclines may be considered in fit patients without affecting outcomes.35 Whether CGA can help guide treatment decision making, thus improving survival and reducing treatment-related toxicity, needs prospective validation. In addition, some studies have suggested that steroid pretreatment of patients with a performance status of 3 or 4 may render them suitable for treatment with standard R-CHOP.47 Primary granulocyte colony-stimulating factor prophylaxis in patients older than age 65 years with poor performance status and nutritional status or significant comorbidity reduces the risk of febrile neutropenia and related morbidity associated with R-CHOP treatment and is recommended for elderly patients receiving initial chemoimmunotherapy.48
In summary, our systematic review confirms the lack of prospective, randomized studies to define the optimal first-line therapy for elderly DLBCL patients. We acknowledge the intrinsic limitations and selection bias of retrospective and database-derived studies, but our analysis highlights the significant association of an anthracycline-containing regimen in elderly patients who are fit enough to tolerate this treatment with improved survival. Our analysis also demonstrated that CGA can stratify treatment outcomes and should be considered as a useful tool to guide treatment decision making between standard R-CHOP treatment and alternative regimens. We recommend that the initial treatment decision for elderly patients with DLBCL should be based on assessment of fitness or frailty and comorbidities rather than age alone, ideally in the setting of a prospective clinical trial that uses CGA. We recommend that patients considered fit for intense treatment should be treated with standard-dose R-CHOP because it produces the best survival outcomes, and R-CHOP with or without dosage and/or individual drug adjustments should be considered for those unfit by CGA. Finally, for frail patients or patients with significant cardiac comorbidities, an alternative regimen that substitutes anthracyclines should be used, along with expert management of the patient’s geriatric comorbidities.
Acknowledgments
R.J.L. and C.R.F. acknowledge the American Society of Hematology Clinical Research Training Institute program and David Garcia for developing research concepts.
This work was supported in part by the Winship Research Informatics Shared Resource of the Winship Cancer Institute of Emory University (P30CA138292) and by award K24CA208132 from the National Institutes of Health, National Cancer Institute (C.R.F.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: R.J.L., M.B., and C.R.F. performed the literature search and refinements; and R.J.L., M.B., C.S.D., and C.R.F. designed the research, contributed to the search terms, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: C.R.F. received a consulting fee or honorarium from Spectrum Pharmaceuticals, Celgene, OptumRx, Seattle Genetics, Gilead Sciences, and Bayer and research funding from AbbVie, Acerta Pharma, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Janssen Pharmaceuticals, Millennium/Takeda, Spectrum Pharmaceuticals, Onyx Pharmaceuticals, Pharmacyclics, and the National Institutes of Health. The remaining authors declare no competing financial interests.
Correspondence: Richard J. Lin, Memorial Sloan Kettering Cancer Center, Box 8, 1275 York Ave, New York, NY 10065; e-mail: linr@mskcc.org; and Christopher R. Flowers, Department of Hematology and Medical Oncology, Winship Cancer Institute, Emory University, 1365 Clifton Rd NE, Room B4300, Atlanta, GA 30322; e-mail: crflowe@emory.edu.
References
- 1.Vose JM, Armitage JO, Weisenburger DD, et al. The importance of age in survival of patients treated with chemotherapy for aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 1988;6(12):1838-1844. [DOI] [PubMed] [Google Scholar]
- 2.International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987-994. [DOI] [PubMed] [Google Scholar]
- 3.Mareschal S, Lanic H, Ruminy P, Bastard C, Tilly H, Jardin F. The proportion of activated B-cell like subtype among de novo diffuse large B-cell lymphoma increases with age. Haematologica. 2011;96(12):1888-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. Blood. 2010;116(24):5103-5110. [DOI] [PubMed] [Google Scholar]
- 5.Fields PA, Linch DC. Treatment of the elderly patient with diffuse large B cell lymphoma. Br J Haematol. 2012;157(2):159-170. [DOI] [PubMed] [Google Scholar]
- 6.Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol. 2012;30(17):2036-2038. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. [DOI] [PubMed] [Google Scholar]
- 8.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23(18):4117-4126. [DOI] [PubMed] [Google Scholar]
- 9.Pfreundschuh M, Schubert J, Ziepert M, et al. ; German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105-116. [DOI] [PubMed] [Google Scholar]
- 10.Peyrade F, Jardin F, Thieblemont C, et al. ; Groupe d’Etude des Lymphomes de l’Adulte (GELA) investigators. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12(5):460-468. [DOI] [PubMed] [Google Scholar]
- 11.Peyrade F, Bologna S, Delwail V, et al. Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: an open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematol. 2017;4(1):e46-e55. [DOI] [PubMed] [Google Scholar]
- 12.Hamlin PA, Satram-Hoang S, Reyes C, Hoang KQ, Guduru SR, Skettino S. Treatment patterns and comparative effectiveness in elderly diffuse large B-cell lymphoma patients: a surveillance, epidemiology, and end results-medicare analysis. Oncologist. 2014;19(12):1249-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastion Y, Blay JY, Divine M, et al. Elderly patients with aggressive non-Hodgkin’s lymphoma: disease presentation, response to treatment, and survival--a Groupe d’Etude des Lymphomes de l’Adulte study on 453 patients older than 69 years. J Clin Oncol. 1997;15(8):2945-2953. [DOI] [PubMed] [Google Scholar]
- 14.Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy--a systematic review. Leuk Res. 2014;38(3):275-283. [DOI] [PubMed] [Google Scholar]
- 15.Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99(8):1373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida M, Nakao T, Horiuchi M, et al. Analysis of elderly patients with diffuse large B-cell lymphoma: aggressive therapy is a reasonable approach for ‘unfit’ patients classified by comprehensive geriatric assessment. Eur J Haematol. 2016;96(4):409-416. [DOI] [PubMed] [Google Scholar]
- 19.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and Elaboration. Ann Intern Med. 2007;147(8):W163-W194. [DOI] [PubMed] [Google Scholar]
- 20.Chihara D, Westin JR, Oki Y, et al. Management strategies and outcomes for very elderly patients with diffuse large B-cell lymphoma. Cancer. 2016;122(20):3145-3151. [DOI] [PubMed] [Google Scholar]
- 21.Nolasco-Medina D, Reynoso-Noveron N, Mohar-Betancourt A, Aviles-Salas A, García-Perez O, Candelaria M. Comparison of three chemotherapy regimens in elderly patients with diffuse large B cell lymphoma: experience at a single National Reference Center in Mexico. BioMed Res Int. 2016;2016:9817606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis CC, Cohen JB, Shah KS, et al. Efficacy and tolerability of anthracycline-based therapy in elderly patients with diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2015;15(5):270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diem S, Ess S, Cerny T, Früh M, Hitz F. Diffuse large B-cell lymphoma in elderly patients: a retrospective analysis. Eur J Intern Med. 2014;25(6):577-582. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez R, Esteves S, Chacim S, et al. What determines therapeutic choices for elderly patients with DLBCL? Clinical findings of a multicenter study in Portugal. Clin Lymphoma Myeloma Leuk. 2014;14(5):370-379. [DOI] [PubMed] [Google Scholar]
- 25.Boslooper K, Kibbelaar R, Storm H, et al. Treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone is beneficial but toxic in very elderly patients with diffuse large B-cell lymphoma: a population-based cohort study on treatment, toxicity and outcome. Leuk Lymphoma. 2014;55(3):526-532. [DOI] [PubMed] [Google Scholar]
- 26.Smith SD, Chen A, Spurgeon S, et al. Diffuse large B-cell lymphoma in adults aged 75 years and older: a single institution analysis of cause-specific survival and prognostic factors. Ther Adv Hematol. 2013;4(6):349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toomey CE, Muzikanksy A, Lee AI, et al. Treatment choice and outcome in diffuse large B cell lymphoma (DLBCL) patients 75 years and older [abstract]. Blood 2010;116(21). Abstract 733. [Google Scholar]
- 28.Carson KR, Riedell P, Lynch R, et al. Comparative effectiveness of anthracycline-containing chemotherapy in United States veterans age 80 and older with diffuse large B-cell lymphoma. J Geriatr Oncol. 2015;6(3):211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams JN, Rai A, Lipscomb J, Koff JL, Nastoupil LJ, Flowers CR. Disease characteristics, patterns of care, and survival in very elderly patients with diffuse large B-cell lymphoma. Cancer. 2015;121(11):1800-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tien YY, Link BK, Brooks JM, Wright K, Chrischilles E. Treatment of diffuse large B-cell lymphoma in the elderly: regimens without anthracyclines are common and not futile. Leuk Lymphoma. 2015;56(1):65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spina M, Balzarotti M, Uziel L, et al. Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist. 2012;17(6):838-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivieri A, Gini G, Bocci C, et al. Tailored therapy in an unselected population of 91 elderly patients with DLBCL prospectively evaluated using a simplified CGA. Oncologist. 2012;17(5):663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tucci A, Ferrari S, Bottelli C, Borlenghi E, Drera M, Rossi G. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer. 2009;115(19):4547-4553. [DOI] [PubMed] [Google Scholar]
- 34.Tucci A, Martelli M, Rigacci L, et al. ; Italian Lymphoma Foundation (FIL). Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma. 2015;56(4):921-926. [DOI] [PubMed] [Google Scholar]
- 35.Merli F, Luminari S, Rossi G, et al. Cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab versus epirubicin, cyclophosphamide, vinblastine, prednisone and rituximab for the initial treatment of elderly “fit” patients with diffuse large B-cell lymphoma: results from the ANZINTER3 trial of the Intergruppo Italiano Linfomi. Leuk Lymphoma. 2012;53(4):581-588. [DOI] [PubMed] [Google Scholar]
- 36.Morrison VA, Hamlin P, Soubeyran P, et al. ; International Society of Geriatric Oncology. Approach to therapy of diffuse large B-cell lymphoma in the elderly: the International Society of Geriatric Oncology (SIOG) expert position commentary. Ann Oncol. 2015;26(6):1058-1068. [DOI] [PubMed] [Google Scholar]
- 37.Morrison VA, Hamlin P, Soubeyran P, et al. Diffuse large B-cell lymphoma in the elderly: impact of prognosis, comorbidities, geriatric assessment, and supportive care on clinical practice. An International Society of Geriatric Oncology (SIOG) expert position paper. J Geriatr Oncol. 2015;6(2):141-152. [DOI] [PubMed] [Google Scholar]
- 38.Chaganti S, Illidge T, Barrington S, et al. ; British Committee for Standards in Haematology. Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol. 2016;174(1):43-56. [DOI] [PubMed] [Google Scholar]
- 39.Merli F, Luminari S, Rossi G, et al. ; Fondazione Italiana Linfomi. Outcome of frail elderly patients with diffuse large B-cell lymphoma prospectively identified by Comprehensive Geriatric Assessment: results from a study of the Fondazione Italiana Linfomi. Leuk Lymphoma. 2014;55(1):38-43. [DOI] [PubMed] [Google Scholar]
- 40.Wieringa A, Boslooper K, Hoogendoorn M, et al. Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP: a population-based cohort study. Br J Haematol. 2014;165(4):489-496. [DOI] [PubMed] [Google Scholar]
- 41.Byun JM, Lee JO, Kang B, et al. Diffuse large B-cell lymphoma in the elderly: real world outcomes of immunochemotherapy in Asian population. Clin Lymphoma Myeloma Leuk. 2016;16(9):503-510. [DOI] [PubMed] [Google Scholar]
- 42.Fields PA, Townsend W, Webb A, et al. De novo treatment of diffuse large B-cell lymphoma with rituximab, cyclophosphamide, vincristine, gemcitabine, and prednisolone in patients with cardiac comorbidity: a United Kingdom National Cancer Research Institute trial. J Clin Oncol. 2014;32(4):282-287. [DOI] [PubMed] [Google Scholar]
- 43.Rashidi A, Oak E, Carson KR, Wagner-Johnston ND, Kreisel F, Bartlett NL. Outcomes with R-CEOP for R-CHOP-ineligible patients with diffuse large B-cell lymphoma are highly dependent on cell of origin defined by Hans criteria. Leuk Lymphoma. 2016;57(5):1191-1193. [DOI] [PubMed] [Google Scholar]
- 44.Park SI, Grover NS, Olajide O, et al. A phase II trial of bendamustine in combination with rituximab in older patients with previously untreated diffuse large B-cell lymphoma. Br J Haematol. 2016;175(2):281-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfreundschuh M, Trümper L, Kloess M, et al. ; German High-Grade Non-Hodgkin’s Lymphoma Study Group. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104(3):634-641. [DOI] [PubMed] [Google Scholar]
- 48.Repetto L, Biganzoli L, Koehne CH, et al. EORTC Cancer in the Elderly Task Force guidelines for the use of colony-stimulating factors in elderly patients with cancer. Eur J Cancer. 2003;39(16):2264-2272. [DOI] [PubMed] [Google Scholar]