Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Baseline metabolic tumor volume and the presence of refractory disease predict outcome for patients with relapsed/refractory HL.
Metabolic tumor volume improves the predictive power of pretransplantation PET in relapsed/refractory HL.
Abstract
Identification of prognostic factors for patients with relapsed/refractory Hodgkin lymphoma (HL) is essential for optimizing therapy with risk-adapted approaches. In our phase 2 study of positron emission tomography (PET)–adapted salvage therapy with brentuximab vedotin (BV) and augmented ifosfamide, carboplatin, and etoposide (augICE), we assessed clinical factors, quantitative PET assessments, and cytokine and chemokine values. Transplant-eligible patients with relapsed/refractory HL received 2 (cohort 1) or 3 (cohort 2) cycles of weekly BV; PET-negative patients (Deauville score ≤2) proceeded to autologous stem cell transplantation (ASCT) whereas PET-positive patients received augICE before ASCT. Serum cytokine and chemokine levels were measured at baseline and after BV. Metabolic tumor volume (MTV) and total lesion glycolysis were measured at baseline, after BV, and after augICE. Sixty-five patients enrolled (45, cohort 1; 20, cohort 2); 49 (75%) achieved complete response and 64 proceeded to ASCT. Three-year overall survival and event-free survival (EFS) were 95% and 82%, respectively. Factors predictive for EFS by multivariable analysis were baseline MTV (bMTV) (P < .001) and refractory disease (P = .003). Low bMTV (<109.5 cm3) and relapsed disease identified a favorable group (3-year EFS, 100%). For patients who received a transplant, bMTV and pre-ASCT PET were independently prognostic; 3-year EFS for pre-ASCT PET-positive patients with low bMTV was 86%. In this phase 2 study of PET-adapted therapy with BV and augICE for relapsed/refractory HL, bMTV and refractory disease were independent prognostic factors for EFS. Furthermore, bMTV improved the predictive power of pre-ASCT PET. Future studies should optimize efficacy and tolerability of salvage therapy by stratifying patients according to risk factors such as bMTV.
Introduction
The standard treatment for patients with Hodgkin lymphoma (HL) after first-line treatment has failed is second-line chemotherapy followed by consolidation with autologous stem cell transplantation (ASCT).1,2 The commonly used second-line therapies, such as ICE (ifosfamide, carboplatin, and etoposide), DHAP (dexamethasone, cytarabine, and cisplatin), GVD (gemcitabine, vinorelbine, and liposomal doxorubicin), or IGEV (ifosfamide, gemcitabine, etoposide, and vinorelbine), are similar with respect to response rate, and therefore choice of treatment is typically dependent on the physician or institution.3-6 We and others have shown that one of the strongest predictors of outcome for patients with relapsed or refractory HL is achievement of a negative positron emission tomography (PET) scan before ASCT.7-13 The importance of pretransplantation PET was demonstrated in a study that evaluated PET-adapted sequential therapy with ICE and GVD chemotherapy. In this study, patients with relapsed or refractory disease initially received ICE-based therapy; patients who did not achieve PET negativity then received additional therapy with GVD before being considered for ASCT. Among 97 patients enrolled, 60% achieved PET normalization with ICE-base therapy alone; an additional 18% achieved PET normalization after ICE/GVD, whereas 17% remained PET positive after ICE/GVD.3 Achievement of PET normalization was associated with an event-free survival (EFS) of >80%, and there was no difference in outcome for patients who required 1 or 2 multiagent regimens to achieve PET normalization. Thus, this study reinforced the concept that the goal of second-line therapy should be to achieve PET normalization and that there is no disadvantage when 2 sequential therapies are required to achieve PET negativity.
On the basis of this model, we designed this study in which patients with relapsed or refractory HL after first-line therapy initially received brentuximab vedotin (BV) alone for 2 cycles. Patients achieving PET negativity proceeded directly to consolidation with ASCT, and those with persistent abnormalities on PET received additional chemotherapy with augmented ICE (augICE). To avoid undertreating patients, we used stringent criteria to define PET negativity (Deauville score ≤2).14 We previously reported the results for cohort 1, which had 45 patients enrolled, 27% of whom achieved PET negativity to BV alone and proceeded directly to ASCT.15 An additional 69% achieved PET normalization after ICE-based chemotherapy and all but 1 patient proceeded to ASCT. The overall PET-negative rate after PET-adapted therapy with BV and augICE was 76%, and the 2-year EFS was 80%. Pretransplant PET was significant for EFS within cohort 1; however, we were unable to identify patient characteristics that predicted for achievement of PET negativity or overall outcomes. We subsequently enrolled a second cohort, which aimed to improve the complete response (CR) rate to single-agent BV by administering 3 cycles of BV rather than 2. As we report here, the response rates and outcomes among cohort 2 patients were the same as those observed in cohort 1. We thus performed an analysis of cohorts 1 and 2 combined, which aimed at identifying predictive and prognostic factors within this larger series. Here we report the results of this combined analysis in which we evaluated the impact of quantitative PET analysis, serum cytokine and chemokine levels, and clinical characteristics on response to treatment and overall outcomes.
Methods
This was an institutional review board–approved phase 2 clinical trial for patients with biopsy-proven relapsed or refractory classical HL after 1 previous chemotherapy regimen had failed (NCT01508312). The study included 2 cohorts; methods for cohort 1 were described previously.15 In brief, patients in cohort 1 received BV 1.2 mg/kg on days 1, 8, and 15 every 28 days for 2 cycles followed by evaluation by PET. Those who achieved PET normalization (defined by Deauville score of ≤2) proceeded directly to ASCT; those with persistent abnormalities on PET received 2 cycles of augICE before being considered for ASCT. Patients with localized, nodal-based disease who had not previously received radiation were treated with involved field radiation therapy (IFRT) before conditioning for ASCT.
After enrollment on cohort 1 was completed, enrollment proceeded for cohort 2 for which the eligibility and treatment were the same except that patients received 3 cycles of weekly BV rather than 2. Reported here are updated results from cohort 1, results from cohort 2, and analysis of prognostic factors, including quantitative PET analysis and pre- and post-BV cytokine and chemokine levels.
Response assessment
All PET scans were reviewed by 1 of 2 nuclear medicine physicians at Memorial Sloan Kettering Cancer Center (H.S. or R.G.). Patients underwent assessment by PET after 2 cycles of weekly BV (cohort 1) or 3 cycles of weekly BV (cohort 2) within 1 week of the last dose of BV. PET scans were interpreted by using the five-point Deauville scale in which Deauville 5 was defined as the presence of new lesions or any lesion with uptake ≥3 × SUVmax (maximum standardized uptake value) of normal liver.14,16
Image acquisition and data analysis
PET scans from mid skull to upper thighs were obtained about 60 minutes after injection on Discovery STE or LS or Discovery 690 systems (GE Medical Systems, Milwaukee, WI). A clinical imaging protocol was used with an injection of ∼444 to 555 MBq fluorodeoxyglucose after at least 6 hours of fasting and documentation of blood glucose <200 mg/dL. Subsequently, a low-dose attenuation-corrected computed tomography scan (120-140 kV; ∼80 mA) was acquired followed by PET imaging. All images were reviewed on PET volume computer assisted reading (VCAR) software (GE Healthcare). Region of interest was set by manual adjustment in 3 planes to exclude adjacent physiologic fluorodeoxyglucose-avid structures. SUVmax was defined as the maximum voxel intensity within the volumetric region of interest. A threshold of 41% of the maximum signal intensity was used to delineate the metabolic tumor volume (MTV). Patient MTV represents the sum of every individual lesion MTV. Total lesion glycolysis (TLG) was calculated as the product of MTV and the SUV mean.17
Cytokine analysis
Interleukin-6 (IL-6), IL-10, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and cysteine-cysteine thymus and activation regulated chemokine (TARC) were measured at baseline and after 2 cycles of BV (cohort 1) or after 3 cycles of BV (cohort 2). IL-6, IL-10, TNF-α, and IFN-γ were measured by a multiplex enzyme-linked immunosorbent assay array (Meso Scale Discovery, Rockville, MD). TARC was measured by Simple Plex (ProteinSimple, San Jose, CA), an automated, microfluidic immunoassay platform. All assays were validated and performed according to standards set by the Clinical Laboratory Improvement Amendments.
Statistical analysis
The sample size calculation for cohort 1 was previously described.15 The aim in cohort 2 was to assess PET-negative rate to single-agent BV after 3 cycles of weekly treatment. In cohort 1, 12 (27%) of 45 enrolled patients achieved a PET-negative response (defined by Deauville score ≤2) to single-agent BV. An additional 8 patients in cohort 1 achieved Deauville 3 responses to BV, and we hypothesized that an additional cycle of BV may have converted the Deauville 3 responses to Deauville 2. On the basis of the Deauville 3 (or better) response rate of 44% in cohort 1, we hypothesized that the PET-negative rate by Deauville 2 after 3 cycles of BV would be about 50%. Allowing for type I and type II error rates of 0.2, and assuming a PET-negative rate of 27% after 2 cycles of BV, the number of patients required to show an improvement in PET-negative rate to 50% after 3 cycles of BV was 20.
Regarding the secondary end points, associations between patient characteristics, serum cytokine levels, and PET response were analyzed by Wilcoxon rank sum test. EFS and overall survival (OS) for all enrolled patients were estimated from the time of treatment initiation using the Kaplan-Meier method; for analyses that included only patients who had received a transplant, EFS and OS were estimated from the time of transplant. The significance of potential prognostic factors was assessed first by using univariable Cox proportional hazards regression. Factors significant by univariable analysis (P ≤ .05) were further evaluated by multivariable analysis using multivariable Cox regression models. The best cutoff value for a continuous covariate was determined by a grid search of a series of cutoff values over the range of the covariate and choosing the one that gave the smallest P value of a log-rank test comparing the 2 groups defined by the cutoff value.
Results
Patient characteristics and disposition
Forty-five patients enrolled onto cohort 1 and 20 patients enrolled onto cohort 2. Patient characteristics are provided in Table 1. In brief, the 65 patients included 34 females (52%), 34 (52%) patients with primary refractory disease, 10 (15%) with B symptoms, and 24 (37%) with extranodal disease.
Table 1.
Patient characteristics
| Characteristic | Cohort 1 (n = 45) | Cohort 2 (n = 20) | Total (n = 65) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Female sex | 20 | 44 | 14 | 70 | 34 | 52 |
| Median age, y (range) | 31 (13-65) | 35 (19-59) | 33 (13-65) | |||
| Stage at initial diagnosis | ||||||
| Early | 22 | 49 | 13 | 65 | 35 | 54 |
| Advanced | 23 | 51 | 7 | 35 | 30 | 46 |
| Stage at relapse | ||||||
| Early | 23 | 51 | 13 | 65 | 36 | 55 |
| Advanced | 22 | 49 | 7 | 35 | 29 | 45 |
| Refractory | 25 | 56 | 9 | 45 | 34 | 52 |
| B symptoms | 9 | 20 | 1 | 5 | 10 | 15 |
| Extranodal disease | 19 | 42 | 5 | 25 | 24 | 37 |
| Bulk (>5 cm) | 12 | 27 | 4 | 20 | 16 | 25 |
Efficacy
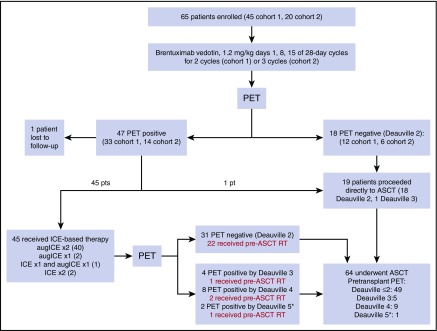
Figure 1 summarizes the treatment pathway for patients enrolled onto cohorts 1 and 2. Among the patients enrolled onto cohort 2, 16 (80%) completed all 3 cycles of weekly BV. Reasons for not completing all 3 cycles of BV included disease progression (n = 1), grade 3 rash (n = 1), grade 2 infusion-related reaction (n = 1), and patient preference (n = 1). Six (30%) of the 20 patients on cohort 2 achieved CR to BV (Deauville ≤2). Seven (6 with Deauville 2 response, 1 with Deauville 3 response) of the 20 patients proceeded directly to ASCT after receiving BV. Thirteen patients received additional treatment before ASCT; 10 received augICE for 2 cycles (as specified by the protocol) and 3 received modified treatment on the basis of treating physician preference (2 received standard ICE for 2 cycles and 1 received 1 cycle of standard ICE and 1 cycle of augICE). Among the 13 patients who received ICE-based chemotherapy after BV, 9 (69%) achieved CR. All 20 patients in cohort 2 proceeded to ASCT. There was no significant difference in response among patients in cohorts 1 and 2, and thus further analyses are based upon the 2 cohorts combined. Overall, 18 (28%) of 65 patients in cohorts 1 and 2 achieved Deauville 2 response to BV alone and 75% achieved Deauville 2 response to the entire PET-adapted treatment program with BV and ICE-based therapy. Sixty-four of 65 patients proceeded to ASCT, of whom 26 (41%) received pre-ASCT IFRT. PET statuses before pre-ASCT radiation were Deauville 2 (22), Deauville 3 (1), Deauville 4 (2), and Deauville 5 (1). PET was repeated after radiation (before ASCT) for the Deauville 4 and 5 patients and the PET scans of all 3 patients were Deauville 4 after radiation therapy. Among these 3 patients, 2 are currently event free after ASCT; 1 patient (who had a Deauville 4 response before radiation therapy) relapsed 7 months after ASCT. The Deauville 5 patient who did not receive pretransplant radiation therapy remains event free after ASCT.
Figure 1.
Treatment pathway. (*) None of the Deauville 5 patients had new SUV-avid lesions.
Survival
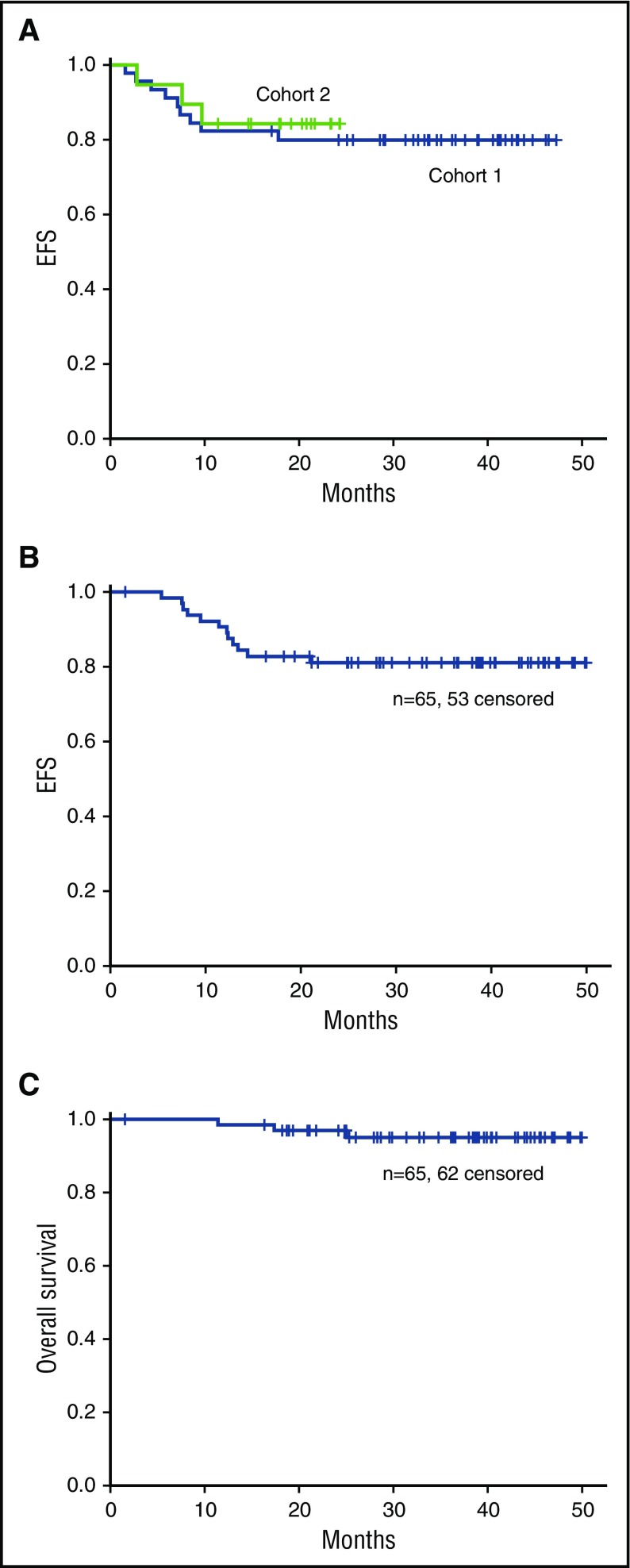
Median follow-up for survivors is 40 months and 24 months for cohorts 1 and 2, respectively. No significant difference in EFS was observed for cohorts 1 and 2 (Figure 2A). Three-year EFS and OS for all 65 patients were 82% and 95%, respectively (Figure 2B-C).
Figure 2.
Overall outcomes. (A) EFS for cohorts 1 and 2. (B) EFS and (C) OS for cohorts 1 and 2 combined.
Cytokine analysis
TARC, IL-6, IL-10, TNF-α, and IFN-γ were measured at baseline and after BV for 37 of 45 patients in cohort 1. Given the significant findings with regard to TARC in cohort 1, evaluation of TARC levels was expanded to cohort 2 and was measured for 64 patients at baseline and 57 patients after BV. Baseline and post-BV cytokine and chemokine levels are provided in Table 2. An analysis of cytokines, chemokines, and baseline clinical factors showed that patients with extranodal disease were more likely to have elevated IFN-γ (P = .003) and elevated IL-10 (P = .049) whereas patients with B symptoms were more likely to have elevated TNF-α (P = .019) and IL-10 (P = .019). Among the cytokines and chemokines measured, TARC was the only one that decreased significantly after BV treatment (median, 6852 pg/mL to 875 pg/mL; P < .0001). Furthermore, baseline TARC was predictive of EFS, and the optimal cutoff for predicting outcome in this series was determined to be 96 330 pg/mL. By using this cutoff, the 3-year EFS rates for patients presenting with low (n = 59) and high (n = 5) baseline TARC were 86% and 20%, respectively (P < .001).
Table 2.
Cytokine and chemokine levels
| Cytokine/chemokine (normal, pg/mL) | Before BV (pg/mL) | After BV (pg/mL) | ||||
|---|---|---|---|---|---|---|
| No. of patients | Median | Range | No. of patients | Median | Range | |
| IL-6 (<17.4) | 37 | 2.27 | 0.10-154 | 37 | 1.41 | 0.09-34 |
| IL-10 (<2) | 37 | 0.38 | 0.09-112 | 37 | 0.45 | 0.14-18 |
| TNF-α (<5.6) | 37 | 2.55 | 0.55-15.15 | 37 | 2.25 | 0.58-22 |
| IFN-γ (<2) | 37 | 8.66 | 1.45-1 554 | 37 | 9.01 | 2.62-113 |
| TARC (<500) | 64 | 6852 | 164-163 169 | 57 | 875 | 151-34 770 |
Quantitative PET analysis
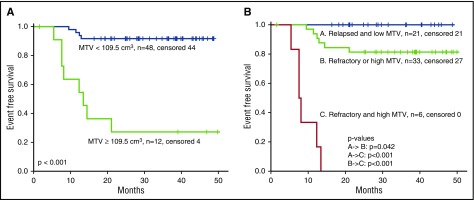
MTV and TLG values at baseline, after BV, and after ICE and/or augICE are summarized in Table 3. As expected, significant reductions in MTV and TLG were observed after BV and ICE-based chemotherapy. The optimal baseline MTV (bMTV) cutoff for predicting EFS was 109.5 cm3. Using the cutoff of 109.5 cm3 for bMTV, the 3-year EFS rates for patients with low (n = 48) and high (n = 12) MTV were 92% and 27%, respectively (P < .001) (Figure 3A).
Table 3.
Quantitative PET assessment
| PET parameter | Baseline | After BV | After ICE chemotherapy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Median | Range | No. of patients | Median | Range | No. of patients | Median | Range | |
| MTV, cm3 | 60 | 50.0 | 6.55-782 | 58 | 2.7 | 0-148 | 37 | 0 | 0-11.5 |
| TLG, g | 60 | 310 | 13.4-6472 | 58 | 6.9 | 0-572 | 37 | 0 | 0-24 |
Figure 3.
EFS and baseline risk factors. (A) EFS for low (<109.5 cm3) or high (≥109.5 cm3) bMTV. (B) EFS with respect to bMTV and primary refractory disease. Outcomes for patients with 0, 1, or 2 risk factors are shown.
Prognostic factors
Baseline factors found to be predictive for EFS included age older than 45 years (P = .016), refractory disease (lack of achievement of CR after first-line therapy) (P = .033), B symptoms (P = .032), advanced stage at relapse (P = .011), and bMTV, TARC, and TLG (all P < .001). Factors that remained prognostic by multivariable analysis were MTV (hazard ratio, 1.022; 95% confidence interval, 1.011-1.034; P < .001) and refractory disease (hazard ratio, 24.2; 95% confidence interval, 3.0-198.1; P = .003) (Table 4). Figure 3B shows the EFS with respect to bMTV and refractory disease. Three-year EFS rates for patients with 0, 1, or 2 risk factors were 100%, 81%, and 0%, respectively. There were no factors that predicted for likelihood of achieving PET negativity after BV or ICE.
Table 4.
Univariable and multivariable analysis
| Characteristic | Univariable | Multivariable | ||
|---|---|---|---|---|
| P | HR | 95% CI | P | |
| Age >45 y | .016 | |||
| Male sex | .224 | |||
| Refractory | .033 | 24.2 | 3.0-198.1 | .003 |
| Advanced stage | .011 | |||
| Extranodal | .061 | |||
| B symptoms | .032 | |||
| bMTV | <.001 | 1.022 | 1.011-1.034 | <.001 |
| TLG | <.001 | |||
| TARC | <.001 | |||
CI, confidence interval; HR, hazard ratio.
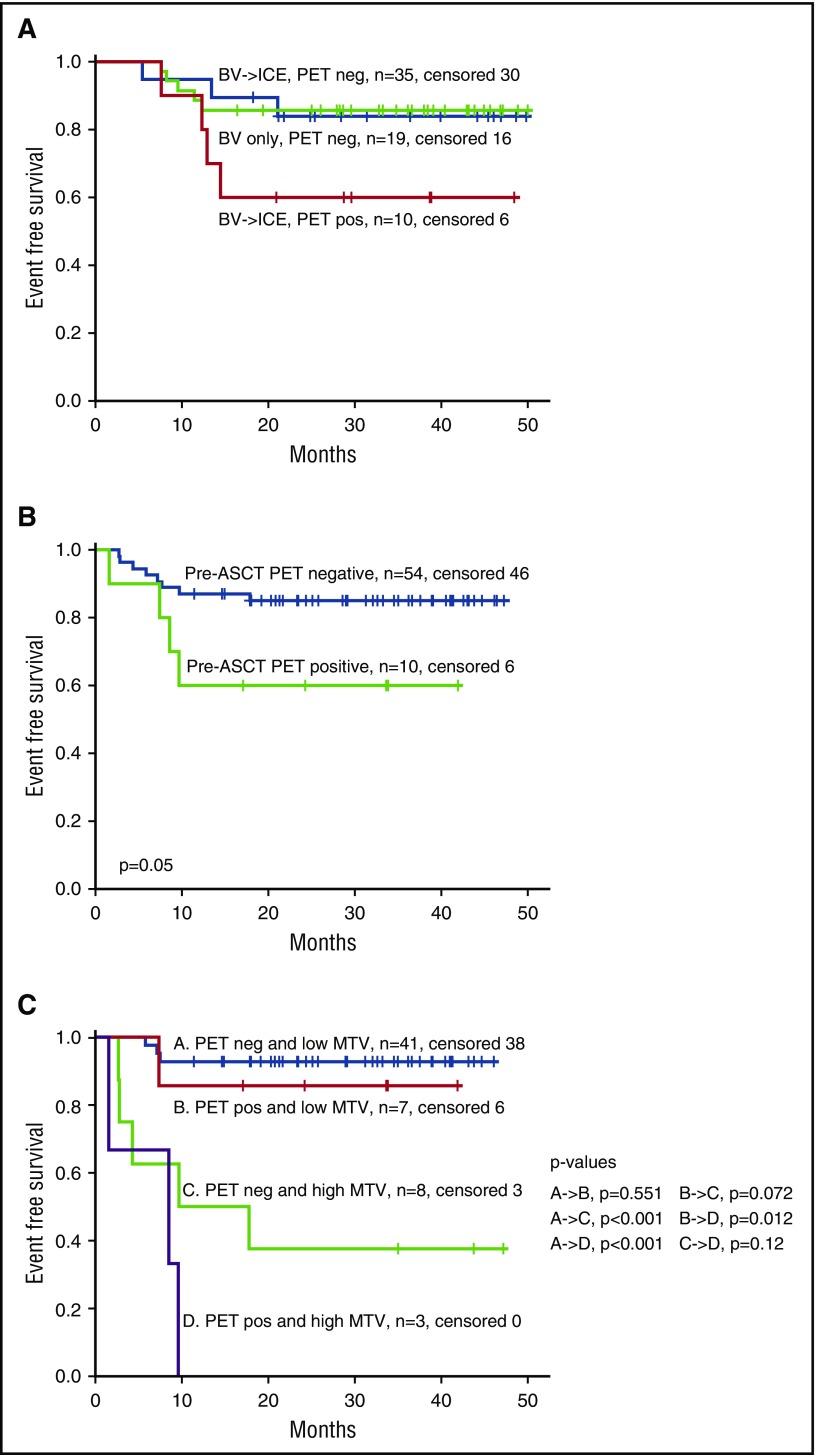
For patients who received a transplant, EFS for those who were PET negative was the same regardless of whether they achieved PET-negative status with BV alone or whether they required BV followed by ICE-based therapy (Figure 4A). Pretransplant PET was prognostic among patients who received a transplant when we defined PET negativity as Deauville ≤3 (P = .05) (Figure 4B) but only marginally significant using Deauville ≤2 (P = .06); thus, in analyses involving patients who received a transplant, we used Deauville ≤3 to define PET negativity. In multivariable analysis, bMTV and pretransplant PET remained independently significant for EFS. Three-year EFS rates for patients with low MTV/negative pretransplant PET, low MTV/positive pretransplant PET, high MTV/negative pretransplant PET, and high MTV/positive pretransplant PET were 93%, 86%, 38%, and 0%, respectively (Figure 4C).
Figure 4.
Impact of pre-ASCT PET and bMTV on EFS. (A) EFS for patients who received a transplant according to treatment (BV alone or BV followed by ICE) and pretransplant PET (PET positive defined as Deauville ≥4). (B) EFS according to pretransplant PET (PET positive defined as Deauville ≥4). (C) EFS according to bMTV and pretransplant PET (PET positive defined as Deauville ≥4). neg, negative; pos, positive.
Discussion
There is no single standard second-line therapy for relapsed or refractory HL, but there is general agreement that the goal of second-line therapy should be to achieve a negative PET.7-13 In this study, we aimed to improve both PET-negative rate and tolerability of second-line therapy by incorporating BV, which is one of the most active single agents for HL. By using Deauville ≤2 to define complete PET response, the CR rate to PET-adapted therapy with BV and ICE-based therapy was 75%. Furthermore, 28% of patients achieved a Deauville ≤2 response after BV alone and had excellent outcomes after ASCT despite never receiving more intense salvage therapy such as ICE. As expected for a salvage regimen associated with a high CR rate in relapsed or refractory HL, the post-ASCT outcomes were excellent with 3-year EFS of 82%. Of note, a considerable portion of patients in this study (55%) had early-stage disease, which potentially contributed to the favorable outcomes we observed; however, refractory disease, which is consistently associated with poor prognosis, was well represented (52% of all patients and 55% of the early-stage patients) and similar in frequency to that in other studies; thus, the results are likely not influenced significantly by the proportion of early-stage patients enrolled.18-21 Nevertheless, these data confirm the notion that the goal of second-line therapy should be to achieve a PET-negative response and that patients are not at a disadvantage by receiving less intense therapy (such as BV alone) or 2-step sequential therapy (BV followed by ICE). The CR rate of 75% is higher than previously reported with ICE-based therapy alone (60%) and sets the bar for other novel second-line therapies undergoing evaluation in relapsed or refractory HL.3
We used stringent criteria (Deauville 1 or 2) for PET negativity in this study because of the concern for treatment failure (as a result of de-escalation and avoidance of ICE) among patients achieving PET negativity after BV alone. When we analyzed the prognostic impact of pretransplant PET for the entire study, it was similar when using a definition of Deauville ≤3 rather than Deauville ≤2 to define PET negativity (P = .05 vs P = .06, respectively). Thus, achieving PET response with Deauville score ≤3 is likely sufficient in the pretransplant setting and is generally accepted as the appropriate cutoff to define interim PET negativity.22 By using the Deauville ≤3 criteria, the PET-negative rates to BV alone and after the entire PET-adapted treatment program were 42% and 83%, respectively. Similar CR rates have been reported for recently studied newer generation salvage therapies, such as bendamustine plus BV (CR, 74%) and bendamustine, gemcitabine, and vinorelbine (CR, 73%).21,23
By expanding our study to include a second cohort, we aimed to determine whether a higher CR rate would be observed after 3 vs after 2 cycles of single-agent BV. We did not observe a significant difference in CR rate with more cycles of BV and, in fact, 20% of patients could not complete all 3 cycles of BV, mostly because of poor tolerability. The lack of improved response with more cycles of BV is consistent with that observed by Chen et al24 in a similar phase 2 study that evaluated second-line BV. In that study, patients who relapsed after first-line therapy received 4 cycles of standard-dose BV and were evaluated for response after 2 and 4 cycles. All observed CRs occurred after 2 cycles, and no partial responses converted to CRs between cycles 2 and 4. Thus, maximal response to BV in HL is expected to be early and unlikely to improve with additional cycles of therapy. Accordingly, in the pretransplant setting in which the goal of treatment is to achieve a PET-negative response, if a CR is not achieved after 2 cycles of either weekly or standard-dose BV, switching to a different salvage regimen that is not cross-resistant is warranted.
When we initially analyzed results from cohort 1, the only factor that was prognostic for outcome was pretransplant PET. We were not able to identify other factors in cohort 1 that predicted for response to treatment or outcome. Given that we observed similar response rates and outcomes among patients enrolled onto cohorts 1 and 2, we combined these cohorts to evaluate for predictive or prognostic factors among the entire group. In the combined analysis of cohorts 1 and 2, the 2 baseline factors that remained independently predictive for EFS by multivariable analysis were refractory disease and bMTV. Primary refractory disease has been identified as a negative prognostic factor in HL in many other studies; thus, identification of its importance in our series was not unexpected.18-20 Likewise, MTV has more recently been identified as an important prognostic factor, particularly in the first-line setting for HL.25,26 The bMTV value that best stratified patients enrolled on our study was 109.5 cm3; however, additional studies are needed to confirm the optimal bMTV cutoff in relapsed or refractory HL. Metabolic tumor volume was recently found to influence rituximab exposure and outcome in patients with diffuse large B-cell lymphoma.27 This raises the possibility that bMTV impacted outcome in our study by affecting exposure to BV. Future studies should explore the association between MTV and BV pharmacokinetics. Furthermore, there are several methods for measuring MTV, but there is no one agreed upon approach. We chose to use a previously described method in which a 41% threshold of SUVmax was used to calculate lesion volumes. We did not assess alternative methods in our study; however, it will ultimately be important to compare different methods for MTV measurement to determine the optimal approach.28
Given the prognostic significance of bMTV and refractory disease, these factors may aid in stratifying patients for future risk-adapted treatment strategies. For example, among the patients with the most favorable prognosis in our series, those with low bMTV and relapsed (rather than refractory) disease, 100% are alive and in remission after this treatment program. It is possible that less intense therapy, potentially without transplant, may be appropriate for this group. Similarly, the patients with the most unfavorable prognosis, those with both refractory disease and high MTV, did poorly with this treatment program (none are event free at 2 years), and thus clinical trials that evaluate novel treatment approaches should be considered for these patients.
For the patients who received a transplant, bMTV and pretransplant PET were independently significant for EFS by multivariable analysis. Accordingly, bMTV improved the predictive value of pretransplant PET and thus may aid in treatment decisions in the pretransplant setting. In our series, pretransplant positive PET was associated with 3-year EFS of 60%; however, the 3-year EFS for patients with low MTV and positive pretransplant PET was much better at 86%, indicating that it may be reasonable for PET-positive patients to proceed to transplant provided they have a low bMTV. Similarly, the 3-year EFS for pretransplant PET-negative patients was 85%; however, it was considerably lower (38%) for patients with high MTV and negative pretransplant PET; thus, more novel approaches and/or maintenance strategies may be appropriate for this group.
TARC, a chemokine that is normally produced by antigen-presenting cells, is highly produced by Hodgkin Reed-Sternberg cells and is likely responsible for attracting type 2 T-helper cells to the HL microenvironment.29 Serum TARC levels have previously been reported to predict response to first-line therapy and to predict relapse after therapy in HL.30-33 Similarly, in our study, serum TARC was found to be predictive for EFS; however, MTV, which is essentially another measure of tumor burden, was a stronger predictor for outcome because TARC did not remain prognostic in multivariable analysis. Given the ease of assessing TARC, future studies should continue to assess levels throughout treatment to clarify its role in managing HL.
It is our standard practice to include pre-ASCT IFRT in treating radiation-naïve patients with localized, nodal-based relapsed or refractory HL. As a result, several patients in this study (41%) received pre-ASCT radiation therapy. The majority of these patients (22 of 26) were PET negative (Deauville 2) before radiation therapy. For the 3 patients who were PET positive (Deauville 4 or 5), PET scans were repeated after radiation therapy, before proceeding to ASCT. Although all 3 PET scans showed stability or improvement, they remained positive by Deauville 4, potentially because of the short time interval between completion of radiation therapy and the repeat scans. Because of the small number of PET-positive patients who received radiation therapy, it is not possible to determine whether radiation therapy improved their outcomes; however, 2 of the 3 Deauville 4/5 patients who received radiation therapy remain event free; therefore, it is feasible that radiation therapy contributed to better-than-expected outcomes for the PET-positive group.
In conclusion, this study shows that PET-adapted sequential therapy with BV and augICE is a highly effective treatment approach for relapsed or refractory HL associated with a CR rate of 75% (Deauville 2). With a median follow-up of 40 months and of 24 months for cohorts 1 and 2, the outcomes after this treatment program are excellent, with 82% of patients being event free at 3 years. bMTV and refractory disease were the strongest prognostic factors and should be incorporated into the design of future risk-adapted trials. Furthermore, bMTV improved the predictive value of pre-ASCT PET and could help inform treatment decisions for PET-negative and PET-positive patients in the pre-ASCT setting. The ideal salvage regimen for relapsed or refractory HL combines high efficacy with tolerability. Future studies should continue to aim to improve both efficacy and tolerability by tailoring treatment to baseline risk factors and interim response.
Acknowledgments
This work was supported by Seattle Genetics; a Core grant from the National Institutes of Health, National Cancer Institute (P30 CA 008748); and the Adam R. Spector Hodgkin Lymphoma Fund.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.J.M. and C.H.M. designed the study; and A.J.M., H.S., S.G., K.L.T., M.F., J.Y., S.J.M., B.R.C., S.Y.F., J.G., R.G., P.A.H., S.M.H., A.K., M.M., A. Ni, A. Noy, M.L.P., M.-A.P., C.S.P., C.S., D.S., A.Y., A.D.Z., and C.H.M. performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: A.J.M. received research funding and honoraria from Seattle Genetics and honoraria from Takeda. S.M.H. received research support and consulting fees from Seattle Genetics and Takeda. A.K. received research support from Seattle Genetics. M.-A.P. received consulting fees from Seattle Genetics. A.Y. received honoraria from Takeda. C.H.M. received research funding and honoraria from Seattle Genetics. The remaining authors declare no competing financial interests.
Correspondence: Alison J. Moskowitz, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY, 10065; e-mail: moskowia@mskcc.org.
References
- 1.Schmitz N, Pfistner B, Sextro M, et al. ; German Hodgkin’s Lymphoma Study Group; Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haematopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065-2071. [DOI] [PubMed] [Google Scholar]
- 2.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051-1054. [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7):1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josting A, Rudolph C, Reiser M, et al. ; Participating Centers. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin’s disease. Ann Oncol. 2002;13(10):1628-1635. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett NL, Niedzwiecki D, Johnson JL, et al. ; Cancer Leukemia Group B. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Ann Oncol. 2007;18(6):1071-1079. [DOI] [PubMed] [Google Scholar]
- 6.Santoro A, Magagnoli M, Spina M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin’s lymphoma. Haematologica. 2007;92(1):35-41. [DOI] [PubMed] [Google Scholar]
- 7.Gentzler RD, Evens AM, Rademaker AW, et al. F-18 FDG-PET predicts outcomes for patients receiving total lymphoid irradiation and autologous blood stem-cell transplantation for relapsed and refractory Hodgkin lymphoma. Br J Haematol. 2014;165(6):793-800. [DOI] [PubMed] [Google Scholar]
- 8.Akhtar M, Kyme A, Zhou V, Fulton R, Meikle S. An investigation of the challenges in reconstructing PET images of a freely moving animal. Australas Phys Eng Sci Med. 2013;36(4):405-415. [DOI] [PubMed] [Google Scholar]
- 9.Devillier R, Coso D, Castagna L, et al. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin’s lymphoma responding to prior salvage therapy. Haematologica. 2012;97(7):1073-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smeltzer JP, Cashen AF, Zhang Q, et al. Prognostic significance of FDG-PET in relapsed or refractory classical Hodgkin lymphoma treated with standard salvage chemotherapy and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1646-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mocikova H, Pytlik R, Markova J, et al. Pre-transplant positron emission tomography in patients with relapsed Hodgkin lymphoma. Leuk Lymphoma. 2011;52(9):1668-1674. [DOI] [PubMed] [Google Scholar]
- 12.Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116(23):4934-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbour E, Hosing C, Ayers G, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109(12):2481-2489. [DOI] [PubMed] [Google Scholar]
- 14.Gallamini A, Barrington SF, Biggi A, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica. 2014;99(6):1107-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskowitz AJ, Schöder H, Yahalom J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosfamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin’s lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284-292. [DOI] [PubMed] [Google Scholar]
- 16.Barrington SF, Kirkwood AA, Franceschetto A, et al. PET-CT for staging and early response: results from the Response-Adapted Therapy in Advanced Hodgkin Lymphoma study. Blood. 2016;127(12):1531-1538. [DOI] [PubMed] [Google Scholar]
- 17.Meignan M, Sasanelli M, Casasnovas RO, et al. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. Eur J Nucl Med Mol Imaging. 2014;41(6):1113-1122. [DOI] [PubMed] [Google Scholar]
- 18.Bröckelmann PJ, Müller H, Casasnovas O, et al. Risk factors and a prognostic score for survival after autologous stem-cell transplantation for relapsed or refractory Hodgkin lymphoma. Ann Oncol. 2017;28(6):1352-1358. [DOI] [PubMed] [Google Scholar]
- 19.Constans M, Sureda A, Terol MJ, et al. ; GEL/TAMO Cooperative Group. Autologous stem cell transplantation for primary refractory Hodgkin’s disease: results and clinical variables affecting outcome. Ann Oncol. 2003;14(5):745-751. [DOI] [PubMed] [Google Scholar]
- 20.Shah N, Rauenzahn S, Veltri L, et al. Long-term outcomes after thiotepa-based high-dose therapy (HDT) and autologous hematopoietic cell transplantation (auto-HCT) in non-Hodgkin lymphoma (NHL). Bone Marrow Transplant. 2017;52(2):321-322. [DOI] [PubMed] [Google Scholar]
- 21.Santoro A, Mazza R, Pulsoni A, et al. Bendamustine in combination with gemcitabine and vinorelbine is an effective regimen as induction chemotherapy before autologous stem-cell transplantation for relapsed or refractory Hodgkin lymphoma: final results of a multicenter phase II study. J Clin Oncol. 2016;34(27):3293-3299. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Fisher RI, Barrington SF, et al. ; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin’s Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaCasce AS, Bociek G, Sawas A, et al. Brentuximab vedotin plus bendamustine: a highly active salvage treatment regimen for patients with relapsed or refractory Hodgkin lymphoma [abstract]. Blood. 2015;126(23). Abstract 3982. [Google Scholar]
- 24.Chen R, Palmer JM, Martin P, et al. Results of a multicenter phase II trial of brentuximab vedotin as second-line therapy before autologous transplantation in relapsed/refractory hodgkin lymphoma. Biol Blood Marrow Transplant. 2015;21(12):2136-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song MK, Chung JS, Lee JJ, et al. Metabolic tumor volume by positron emission tomography/computed tomography as a clinical parameter to determine therapeutic modality for early stage Hodgkin’s lymphoma. Cancer Sci. 2013;104(12):1656-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanoun S, Rossi C, Berriolo-Riedinger A, et al. Baseline metabolic tumour volume is an independent prognostic factor in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2014;41(9):1735-1743. [DOI] [PubMed] [Google Scholar]
- 27.Tout M, Casasnovas O, Meignan M, et al. Rituximab exposure is influenced by baseline metabolic tumor volume and predicts outcome of DLBCL patients: a Lymphoma Study Association report. Blood. 2017;129(19):2616-2623. [DOI] [PubMed] [Google Scholar]
- 28.Schöder H, Moskowitz C. Metabolic tumor volume in lymphoma: hype or hope? J Clin Oncol. 2016;34(30):3592-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol. 2011;29(14):1812-1826. [DOI] [PubMed] [Google Scholar]
- 30.Sauer M, Plütschow A, Jachimowicz RD, et al. Baseline serum TARC levels predict therapy outcome in patients with Hodgkin lymphoma. Am J Hematol. 2013;88(2):113-115. [DOI] [PubMed] [Google Scholar]
- 31.Jones K, Vari F, Keane C, et al. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res. 2013;19(3):731-742. [DOI] [PubMed] [Google Scholar]
- 32.Harrison SJ, Hsu AK, Neeson P, et al. Early thymus and activation-regulated chemokine (TARC) reduction and response following panobinostat treatment in patients with relapsed/refractory Hodgkin lymphoma following autologous stem cell transplant. Leuk Lymphoma. 2014;55(5):1053-1060. [DOI] [PubMed] [Google Scholar]
- 33.Farina L, Rezzonico F, Spina F, et al. Serum thymus and activation-regulated chemokine level monitoring may predict disease relapse detected by PET scan after reduced-intensity allogeneic stem cell transplantation in patients with Hodgkin lymphoma. Biol Blood Marrow Transplant. 2014;20(12):1982-1988. [DOI] [PubMed] [Google Scholar]