Abstract
The adherens junction protein p120-catenin (p120ctn) shuttles between E-cadherin–bound and cytoplasmic pools to regulate E-cadherin/catenin complex stability and cell migration, respectively. When released from the adherens junction, p120ctn promotes cell migration through modulation of the Rho GTPases Rac1, Cdc42, and RhoA. Accordingly, the down-regulation and cytoplasmic mislocalization of p120ctn has been reported in all subtypes of lung cancers and is associated with grave prognosis. Previously, we reported that cigarette smoke induced cytoplasmic translocation of p120ctn and cell migration, but the underlying mechanism was unclear. Using primary human bronchial epithelial cells exposed to smoke-concentrated medium (Smk), we observed the translocation of Rac1 and Cdc42, but not RhoA, to the leading edge of polarized and migrating human bronchial epithelial cells. Rac1 and Cdc42 were robustly activated by smoke, whereas RhoA was inhibited. Accordingly, siRNA knockdown of Rac1 or Cdc42 completely abolished Smk-induced cell migration, whereas knockdown of RhoA had no effect. p120ctn/Rac1 double knockdown completely abolished Smk-induced cell migration, whereas p120ctn/Cdc42 double knockdown did not. These data suggested that Rac1 and Cdc42 coactivation was essential to smoke-promoted cell migration in the presence of p120ctn, whereas migration proceeded via Rac1 alone in the absence of p120ctn. Thus, Rac1 may provide an omnipotent therapeutic target in reversing cell migration during the early (intact p120ctn) and late (loss of p120ctn) stages of lung carcinogenesis.
Cigarette smoke contains >4000 active constituents, ≥60 of which are established carcinogens and/or mutagens.1 With a 20-fold greater risk of lung cancer and accounting for 87% of lung cancer–related deaths,2 smoking continues to represent the single most important carcinogenic exposure. Because treatment of lung cancer is largely ineffective, recent research has been focused on efforts to identify and reverse early events leading to the initiation of lung cancer by smoke.3 Emerging evidence suggests that smoke mediates epithelial-mesenchymal transition (EMT) and pretumor cell migration by disrupting cell-cell adhesion in polarized mucosal epithelia.4, 5 During EMT, cells switch from a polarized immobile epithelial phenotype to a highly motile fibroblast phenotype.6 Unregulated EMT confers epithelial cells with stem cell–like properties capable of self-renewal, metastasis, and resistance to apoptosis.6, 7 Little is known about how smoke mediates EMT during the early stages of lung cancer.
E-cadherin (E-cad)–based adherens junctions (AJs) interact with catenins to modulate cell-cell adhesion.8 Structural analysis by X-ray crystallography revealed that p120-catenin (p120ctn) binds to the juxtamembrane domain of E-cad, where it regulates stability and turnover of E-cad by concealing the juxtamembrane domain residues implicated in endocytosis and ubiquitination of E-cad.9, 10 The disruption of p120ctn leads to E-cad degradation, a major hallmark of EMT and malignancy.8 Accumulating evidence suggests that p120ctn shuttles between E-cad–bound and cytoplasmic pools. When bound to E-cad, p120ctn stabilizes the AJ and acts as a tumor and/or metastasis suppressor.11 When released from the AJ, p120ctn can promote EMT and cell migration through the degradation of E-cad and the modulation of Rho GTPase activity, respectively.8, 11, 12, 13, 14, 15, 16, 17 Accordingly, membrane loss, down-regulation, and cytoplasmic mislocalization of E-cad and p120ctn have been reported in most epithelial cancers, including all subtypes of lung cancers, and are frequently associated with a grave prognosis.18, 19
In lung cancer, ectopic cytoplasmic expression of p120ctn and E-cad has been associated with elevated expression of Rho GTPases.19 Rac1, Cdc42, and RhoA shuttle between their inactive GDP– and active GTP–bound forms to regulate the dynamics of the actin cytoskeleton, cell motility, cadherin-dependent adhesion, and cell proliferation.20, 21, 22 Lamellipodia, filopodia, and stress fibers are regarded as typical phenotypes of activated Rac1, Cdc42, and RhoA, respectively.23 Active Rac1 and Cdc42 drive protrusion formation at the leading edge of a migrating leukocyte, whereas active RhoA aggregates at the rear and sides of the cell, preventing protrusion formation.21 p120ctn can act as a guanine nucleotide dissociation inhibitor to inhibit RhoA through preferential interaction and sequestration of RhoA in its GDP-bound form.12 Alternatively, p120ctn indirectly activates Rac1 and Cdc42 through its interaction with Vav2, a guanine nucleotide exchange factor that promotes the exchange of GDP with GTP.13, 14
We sought to investigate the role of p120ctn in regulating Rho GTPase activity in the initiating stages of cigarette smoke–induced cell migration. Given the opposing roles of membrane versus cytoplasmic p120ctn in carcinogenesis, this study was performed in primary human bronchial epithelial (HBE) cells with intact AJs. Realizing that cancer is a multistep process requiring numerous chemically mediated insults, we mimicked the exposure of airway epithelial cells to smoke using an established model of smoke-conditioned medium (Smk).24, 25 Primary HBE cells exposed to Smk medium underwent malignant transformation in 8 days, demonstrating rapid proliferation, anchorage-independent growth, and tumorigenesis in nude mice.26 With this approach, we discovered p120ctn-dependent and p120ctn-independent pathways mediating cell migration provoked by cigarette smoke. In the presence of p120ctn, coactivation of Rac1 and Cdc42 was essential to promote cell migration, whereas in the absence of p120ctn, activation of Rac1 alone induced migration. These data reveal new details regarding the molecular events promoting cell migration in the earliest stages of cigarette smoke–induced tumorigenesis and open the way for novel approaches to the prevention of lung cancer in smokers.
Materials and Methods
Culture of Primary HBE Cells
Human tissue was handled according to the Declaration of Helsinki and was approved by the University of California Committee for Human Research. Informed consent was obtained from patients undergoing lung transplantation. Surface epithelial cells were isolated from the first through fourth bronchial generations obtained from human lungs that were removed at the time of lung transplantation. Primary cultures were established using previously published methods.27 Briefly, human tracheal tissue strips were washed in PBS. The bronchial epithelium was separated from underlying stroma using enzymatic digestion followed by vigorous agitation to dislodge the epithelial sheets. Single cells were isolated from epithelial sheets after a short incubation in 0.25% trypsin/EDTA. Primary HBE cells were plated 1 × 105 cells/cm2 onto 0.4-μm-pore Transwell polycarbonate membrane (Corning Inc., Palo Alto, CA) or cell culture plates precoated with 15 mg/cm2 of human placental collagen (Sigma-Aldrich, St. Louis, MO). The cells seeded on Transwell membrane were grown in defined air-liquid interface medium for 2 to 3 weeks to produce differentiated, polarized HBE cultures resembling natural airway epithelium as described elsewhere.27 The cells seeded on cell culture plates were grown in bronchial epithelial cell growth medium (BEGM BulletKit; Lonza, Basel, Switzerland) according to the manufacturer's instructions.
Preparation and Treatment of Cigarette Smoke–Conditioned Medium
To closely mimic the volatile and particulate-phase constituents inhaled by smokers, we used Smk medium prepared by Dr. Kent Pinkerton (University of California, Davis, CA) following the established method.28 In brief, smoke was generated by burning Kentucky 3R4F reference cigarettes with standardized 35-mL puffs for 2 seconds, once per minute, for a total of 8 puffs per cigarette. Smoke was then drawn into a conditioning chamber between puffs, mixed, and diluted with fresh filtered air to the appropriate concentration before entering an exposure chamber. The chamber atmosphere was monitored for 291 to 319 ppm of carbon monoxide, 8 mg/m3 of nicotine, and 100 mg/m3 of total suspended particulate matter at a relative humidity level of 53% to 60%. Cell culture medium (50 to 100 mL) was exposed to smoke for 6 continuous hours in an open Petri dish placed in the exposure chamber. This smoke closely mimicked cigarette smoke extracts used routinely by other investigators.24, 25 Aliquots of the smoke condensate (93 mg/m3 of total suspended particulate matter) were stored at −80°C until use. The cells were starved in basal medium without growth factors for 1 hour, followed by incubation in smoke-free medium (Ctrl) or Smk 48 mg/m3 of total suspended particulate matter) for the time points indicated in the text.
p120ctn, Rac1, Cdc42, and RhoA Knockdown and Rac1 Q61L and Rac1 T17N Overexpression
The p120ctn siRNA pool was composed of p120ctn siRNA19 and p120ctn siRNA2.29 Sequences of Rac1 siRNA, Cdc42 siRNA, and RhoA siRNA were obtained from a previous publication.30 p120ctn siRNA1, p120ctn siRNA2, Rac1 siRNA, Cdc42 siRNA, and RhoA siRNA were synthesized by Sigma-Aldrich. Plasmids pRK5-myc-Rac1-Q61L (#12983) and pRK5-myc-Rac1-T17N (#12984) were obtained from Addgene (Cambridge, MA). Primary HBE cells were transfected with the previously mentioned siRNAs and plasmids using the Amaxa Nucleofector normal HBE kit (Lonza) according to the manufacturer's protocol. Experiments were generally performed 48 hours after transfection.
Immunofluorescence Staining
Cell culture inserts were fixed with 4% paraformaldehyde and embedded in paraffin following a standard protocol. Sections (5 μm) were cut using a rotary microtome and were subjected to antigen retrieval by incubation in 10 mmol/L Tris, 1 mmol/L EDTA, and 0.05% Tween 20, pH 9.0, for 20 minutes at 85°C. Cells cultured on 24-well plastic plates were fixed with 4% paraformaldehyde. The cells were permeabilized with 0.2% Triton X-100 (Roche Diagnostics GmbH, Mannheim, Germany), blocked with 5% goat serum for 30 minutes, and incubated with primary antibodies overnight at 4°C. Mouse p120ctn (dilution 1:200), Rac1 (dilution 1:100), and RhoA (dilution 1:200) were obtained from BD Transduction Laboratories (San Jose, CA). Rabbit Rac1 (dilution 1:200), Cdc42 (dilution 1:200), and RhoA (dilution 1:200) were obtained from Cell Signaling Technology Inc. (Danvers, MA) and Santa Cruz Biotechnology (Santa Cruz, CA). Subsequently, the sections were stained with appropriate Alexa Fluor 488– or 594 (Invitrogen, Grand Island, NY)–conjugated secondary antibodies for 1 hour at room temperature. Actin was stained with Alexa Fluor 488–conjugated phalloidin (Invitrogen). Cell nuclei were stained with DAPI. Images were obtained using an Eclipse Ti fluorescence microscope and NIS-elements AR software version 3.07 (Nikon Instruments, Melville, NY).
Active Rac1, Cdc42, and RhoA GTPase Assay
Active Rac1 and Cdc42 in the cell lysates were pulled down by glutathione S-transferase (GST) conjugated with the p21-binding domain of p21-activated protein kinase-1 (GST-Pak1) using an active Rac1 or active Cdc42 pulldown and detection kit (Thermo Scientific Pierce, Rockford, IL) following the manufacturer's instructions. Active RhoA was pulled down by the rhotekin-binding domain of rhotekin using an active Rho pulldown and detection kit (Thermo Scientific Pierce). GTP-bound (active) and total Rac1, Cdc42, and RhoA were detected by Western blot analysis using specific Rac1, Cdc42, and RhoA antibodies included in the kits.
Immunoprecipitation
Protein concentrations of cell lysates were determined by a bicinchoninic acid protein assay kit (Thermo Scientific Pierce). Cell lysates containing 200 μg per sample were incubated with 2 μg of p120ctn antibody (BD Transduction Laboratories) for 1 hour at 4°C under rotary agitation. Subsequently, 50 μL of protein agarose A/G beads was added to each sample. The lysate-beads mixture was incubated overnight at 4°C under rotary agitation, washed in lysis buffer four times, and eluted in 2x SDS sample buffer.
Western Blot Analysis
Equal amounts of protein were separated by SDS-PAGE on 4% to 12% Bis-Tris gels (Invitrogen) and transferred to nitrocellulose membrane. The blots were blocked and incubated with primary antibodies overnight at 4°C. p120ctn (dilution 1:1000), Rac1 (dilution 1:1000), and RhoA (dilution 1:1000) antibodies were purchased from BD Transduction Laboratories. Cdc42 (dilution 1:1000), phosphorylated Ser3 cofilin (Cof-P; dilution 1:1000), and glyceraldehyde-3-phosphate dehydrogenase (dilution 1:3000) were obtained from Cell Signaling Technology Inc. After washing four times in Tris-buffered saline and Tween 20, blots were incubated with appropriate horseradish peroxidase conjugated secondary antibodies and were visualized using the ECL chemiluminescence detection system (GE Healthcare Life Sciences, Pittsburgh, PA). Densitometric quantification of bands was conducted using ImageJ software version 2.0 (NIH, Bethesda, MD).
Cell Migration Assay
HBE cell migration was measured using 24-well Boyden chambers containing Transwell filters with 8-μm pores (BD Biosciences, San Jose, CA). p120ctn siRNA, Rac1 siRNA, Cdc42 siRNA, RhoA siRNA, scrambled siRNA, and Rac1 Q61L– or Rac1 T17N–transfected cells were trypsinized to single-cell suspension in growth factor–free medium 48 hours after transfection. Subsequently, 2 × 104 cells were loaded onto the filter. Ctrl or Smk was applied to the lower chambers. After 4 hours of incubation at 37°C, the nonmigratory cells on the upper surface of the filters were removed using a cotton swab. Cells that migrated to the bottom of filters were fixed, stained with 0.01% crystal violet, then washed in 200 μL of 5% acetic acid and 5% methanol as described elsewhere.16 The number of migratory cells was estimated using colorimetric readings taken at OD 595 nm.
Statistical Analysis
Three to four independent repeats were conducted in all the experiments. Data are presented as means ± SEM. A Student’s t-test was used, and P < 0.05 was considered statistically significant.
Results
Smoke Activates Rac1 and Cdc42, but Not RhoA, in Primary HBE Cells
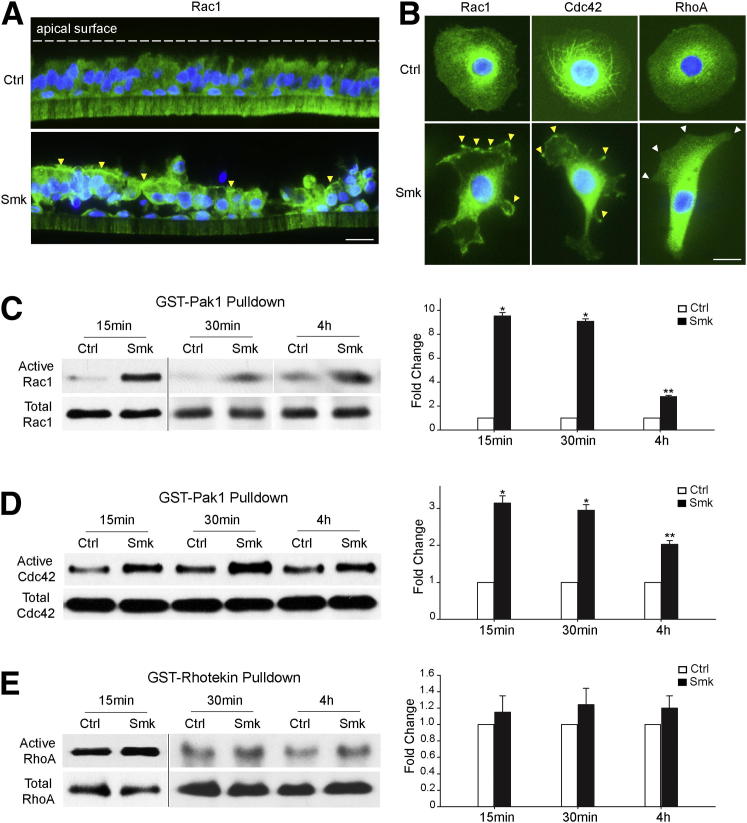
Rac1, Cdc42, and RhoA are small GTPases that regulate cell migration by shuttling between the inactive GDP– and the active GTP–bound forms.21 Using primary HBE cells cultured at an air-liquid interface to reconstitute a polarized and differentiated epithelium that mimicked the in vivo airway, we observed the translocation of cytoplasmic Rac1 (green) to the apical surface of polarized HBE cells (Figure 1A). Although cytoplasmic Cdc42 was also translocated to the apical cell surface in response to smoke, the localization of RhoA remained unchanged (data not shown). At the single-cell level, we observed the aggregation of cytoplasmic Rac1 and Cdc42 (both green) at lamellipodia or filopodia of migrating HBE cells after 30 minutes of Smk exposure, whereas cytoplasmic RhoA (green) was distributed in the cytoplasm away from lamellipodia (Figure 1B). Rac1, Cdc42, and RhoA activities were measured in primary HBE cells treated with Ctrl or Smk at time points ranging from 15 minutes to 4 hours. The GST-Pak1 pulldown assay revealed a robust increase in active Rac1 after Smk treatment, with a 9.6-fold increase after 15 minutes, a 9.1-fold increase after 30 minutes, and a 2.8-fold increase after 4 hours (Figure 1C). Similarly, we noted a 3.1-fold increase in active Cdc42 as early as 15 minutes after smoke exposure that was largely maintained through 4 hours (Figure 1D). Conversely, the GST-rhotekin pulldown assay revealed a nonsignificant increase in active RhoA after Smk exposure (Figure 1E). These data fit the current view of Rac1-RhoA31, 32 and Cdc42-RhoA33 antagonism in coordinating cell migration with cell-cell adhesion and point to Rac1 and Cdc42 as major players promoting cell migration in response to smoke.
Figure 1.
Smoke activated Rac1 and Cdc42, but not RhoA, in migratory HBE cells. A: Rac1 (green) in pseudostratified HBE cells was stained by immunofluorescence after exposure to Ctrl and Smk for 4 hours. Nuclei were visualized with DAPI (blue). Yellow arrowheads point to apical areas where Rac1 accumulated in response to Smk exposure. B: Immunofluorescence staining of Rac1, Cdc42, and RhoA (all green) in HBE cells treated with Ctrl or Smk for 30 minutes. Nuclei were counterstained with DAPI (blue). Yellow arrowheads indicate localization of Rac1 and Cdc42 at the lamellipodia or filopodia of Smk-stimulated cells. White arrowheads indicate the absence of RhoA at the lamellipodia of Smk-treated cells. C–E: HBE cells were treated with Ctrl or Smk for the indicated times. Active Rac1 and Cdc42 were pulled down by a GST-Pak1 fusion protein and were analyzed by Western blot probed with anti-Rac1 (C) or anti-Cdc42 (D). Active RhoA was pulled down by a GST-rhotekin fusion protein and was detected by Western blot analysis with anti-RhoA (E). Total Rac1, Cdc42, and RhoA were used as loading controls. Relative fold change in active Rac1 (C), Cdc42 (D), or RhoA (E) after Smk treatment was normalized to untreated Ctrl (designated onefold), reported as means ± SEM fold change and graphed next to the representative blots. ∗P < 0.01, ∗∗P < 0.05, Smk-treated cells versus Ctrl. Scale bars: 50 μm.
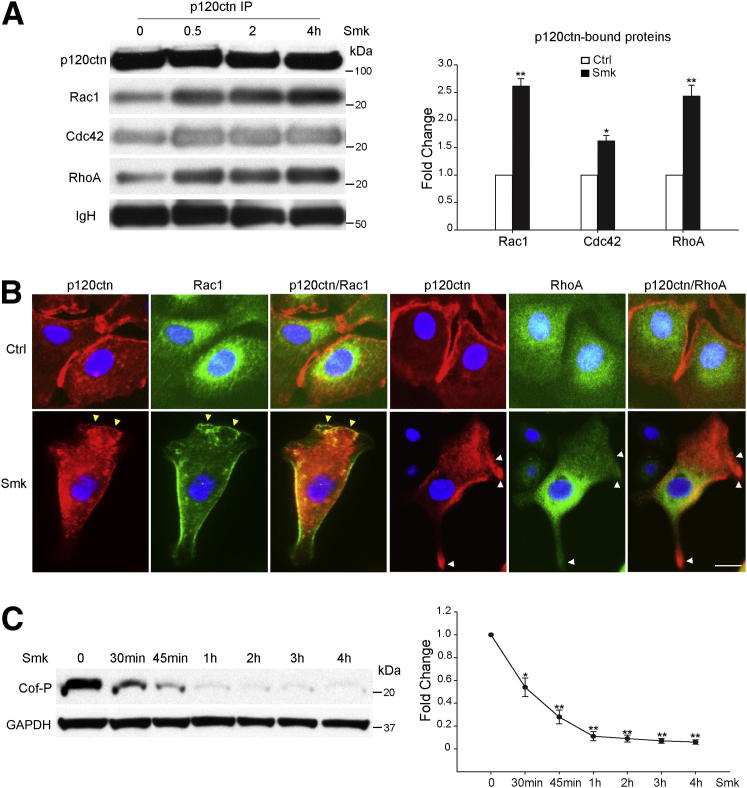
p120ctn/Rac1, p120ctn/Cdc42, and p120ctn/RhoA Complex Formation in Response to Smoke Occurs in Conjunction with Dephosphorylation of Cofilin
We recently identified an epidermal growth factor receptor (EGFR)–dependent signaling pathway linking cigarette smoke to the dissociation and degradation of AJs.15, 16, 17 Loss of AJs was accompanied by the accumulation of p120ctn in the cytoplasm,16, 17 where it has been shown to interact with and inhibit RhoA activity.12 To explore the potential role of p120ctn in regulating Rho GTPase activity during exposure to smoke, we examined its interaction with Rac1, Cdc42, and RhoA. Immunoprecipitates of p120ctn were obtained from HBE cell lysates treated with Ctrl or Smk for 0, 0.5, 2, and 4 hours. Western blot analysis and densitometric quantitation revealed a 2.6-fold increase in p120ctn-bound Rac1, a 1.6-fold increase in p120ctn-bound Cdc42, and a 2.4-fold increase in p120ctn-bound RhoA after Smk exposure (Figure 2A). The interaction between p120ctn and Rho GTPase peaked at 0.5 hours and was sustained through 4 hours of smoke treatment (Figure 2A). Colocalization of p120ctn with Rac1 or RhoA was visualized in Smk-stimulated HBE cells by immunofluorescence (Figure 2B). In control cells, cytoplasmic Rac1 and RhoA (both green) were spatially segregated from junctional/membranous p120ctn (red) (Figure 2B). After 30 minutes of exposure to Smk, junctional p120ctn translocated to the cytoplasm and the leading-edge membrane of migrating HBE cells, where it colocalized with Rac1 (Figure 2B). Conversely, RhoA colocalized with p120ctn in the cytoplasm but away from the leading edge of migratory HBE cells (Figure 2B). Together, these data demonstrated that the smoke-induced interaction between p120ctn/Rac1 and not p120ctn/RhoA occurs at the leading edge of migrating HBE cells.
Figure 2.
Smoke-mediated p120ctn/Rac1 and p120ctn/RhoA complex formation occurred in conjunction with smoke-induced dephosphorylation of cofilin. A: HBE cells were incubated with Ctrl or Smk for 0, 0.5, 2, and 4 hours. p120ctn immunoprecipitates (IPs) were immunoblotted for p120ctn, Rac1, Cdc42, and RhoA. Equal loading was revealed by immunoglobulin heavy chain (IgH). Densitometric quantitation of Rac1, Cdc42, and RhoA immunoprecipitated by p120ctn after 0.5 hours of Smk treatment was normalized to untreated Ctrl (designated onefold) and reported as means ± SEM fold change. B: HBE cells were incubated with Ctrl or Smk for 0.5 hours. Immunofluorescence staining of p120ctn (red), Rac1 (green), and RhoA (green) in HBE cells. Cell nuclei were counterstained with DAPI (blue). Yellow arrowheads indicate colocalization of p120ctn and Rac1 (merged yellow signals) at the leading edge of Smk-treated cells, and white arrowheads indicate the lack of p120ctn/RhoA colocalization in the lamellipodia and the leading edge of Smk-exposed cells. Scale bar = 50 μm. C: Cell lysates obtained from HBE cells treated with Ctrl or Smk for the indicated times were analyzed by Western blot with Cof-P. Equal loading was confirmed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative fold change in Cof-P (reported as means ± SEM) induced by smoke was normalized to Ctrl (designated onefold) and graphed as a dose-response curve. ∗P < 0.05, ∗∗P < 0.01.
The actin binding protein cofilin mediates the depolymerization of actin filaments,34 promotes lamellipodia formation, and controls the polarity of migrating cells.35 When phosphorylated on Ser,3 cofilin is unable to bind actin, and, thereby, its actin-severing activity is abolished.36 Cof-P can be regulated by Rac1, Cdc42, and RhoA through their downstream effector Lim kinase-1 or Lim kinase-2.37, 38 In conjunction with smoke-mediated coactivation of Rac1 and Cdc42 (Figure 1, C and D), smoke promoted a time-dependent dephosphorylation (activation) of cofilin at Ser3 (Cof-P), reaching a maximum 90% decrease by 1 hour that was maintained through 4 hours (Figure 2C).
Rac1 Knockdown Abolished Smoke-Induced HBE Cell Migration in the Presence and Absence of p120ctn, Whereas Cdc42 Knockdown Abrogated Smoke’s Action Only in the Presence of p120ctn
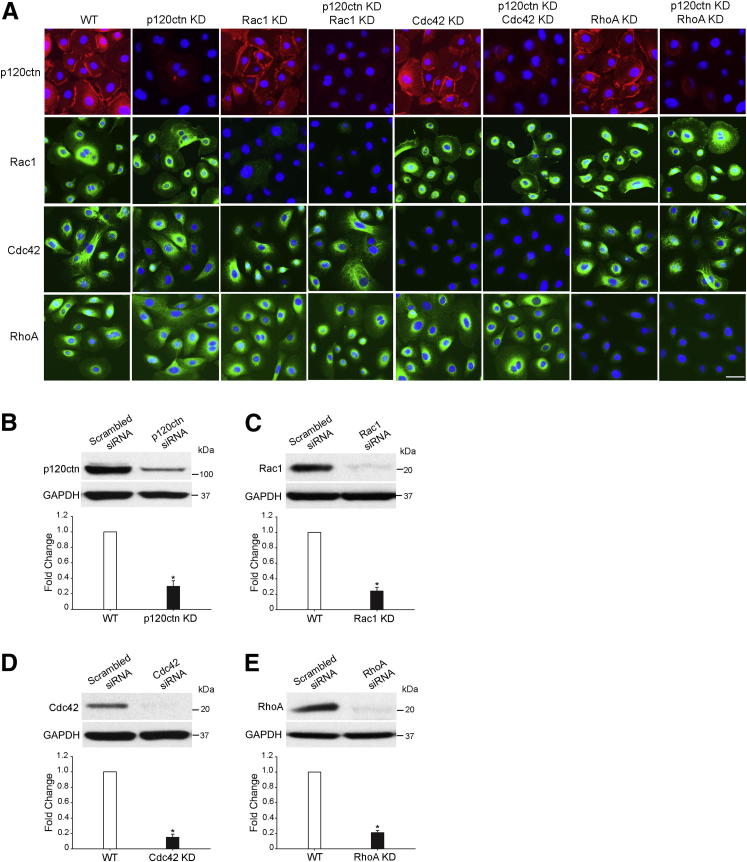
To determine whether Rho GTPase activation was essential to the regulation of smoke-induced cell migration in the presence and/or absence of p120ctn, we used siRNA silencing to knock down (KD) Rac1, Cdc42, RhoA, and p120ctn expression in primary HBE cells. HBE cells were transfected with scrambled siRNA [wild type (WT)], p120ctn siRNA, Rac1 siRNA, p120ctn + Rac1 siRNA, Cdc42 siRNA, p120ctn + Cdc42 siRNA, RhoA siRNA, and p120ctn + RhoA siRNA. Immunostaining 48 hours after transfection revealed p120ctn knockdown in 70% of p120ctn KD cells, Rac1 knockdown in 80% of Rac1 KD cells, Cdc42 knockdown in 80% of Cdc42 KD cells, and RhoA knockdown in 80% of RhoA KD cells (Figure 3A). Western blot analysis 48 hours after transfection confirmed 70% inhibition of p120ctn, 76% inhibition of Rac1, 85% suppression of Cdc42, and 79% suppression of RhoA expression by their respective siRNA compared with WT cells (Figure 3, B–E). These data demonstrated the feasibility of depleting endogenous p120ctn, Rac1, Cdc42, and RhoA from primary HBE cells.
Figure 3.
siRNA knockdown of p120ctn, Rac1, Cdc42, and RhoA in primary HBE cells. HBE cells were transfected with scrambled siRNA (WT), p120ctn siRNA (p120ctn KD), Rac1 siRNA (Rac1 KD), p120ctn and Rac1 siRNA (p120ctn KD, Rac1 KD), Cdc42 siRNA (Cdc42 KD), p120ctn and Cdc42 siRNA (p120ctn KD, Cdc42 KD), RhoA siRNA (RhoA KD), and p120ctn and RhoA siRNA (p120ctn KD, RhoA KD), respectively. A: Cells were fixed 48 hours after transfection and were analyzed by immunofluorescence staining with p120ctn (red), Rac1 (green), Cdc42 (green), and RhoA (green). Cell nuclei were visualized with DAPI (blue). Scale bar = 50 μm. B–E: Cell lysates were obtained 48 hours after transfection and were analyzed by Western blot probed with p120ctn (B), Rac1 (C), Cdc42 (D), and RhoA (E). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control. Densitometric quantitation of protein levels in WT and KD cells 48 hours after transfection is graphed beneath the corresponding blot. The levels of proteins in KD cells were normalized to those in WT cells (designated onefold) and reported as means ± SEM fold change. ∗P < 0.01, KD cells versus WT cells.
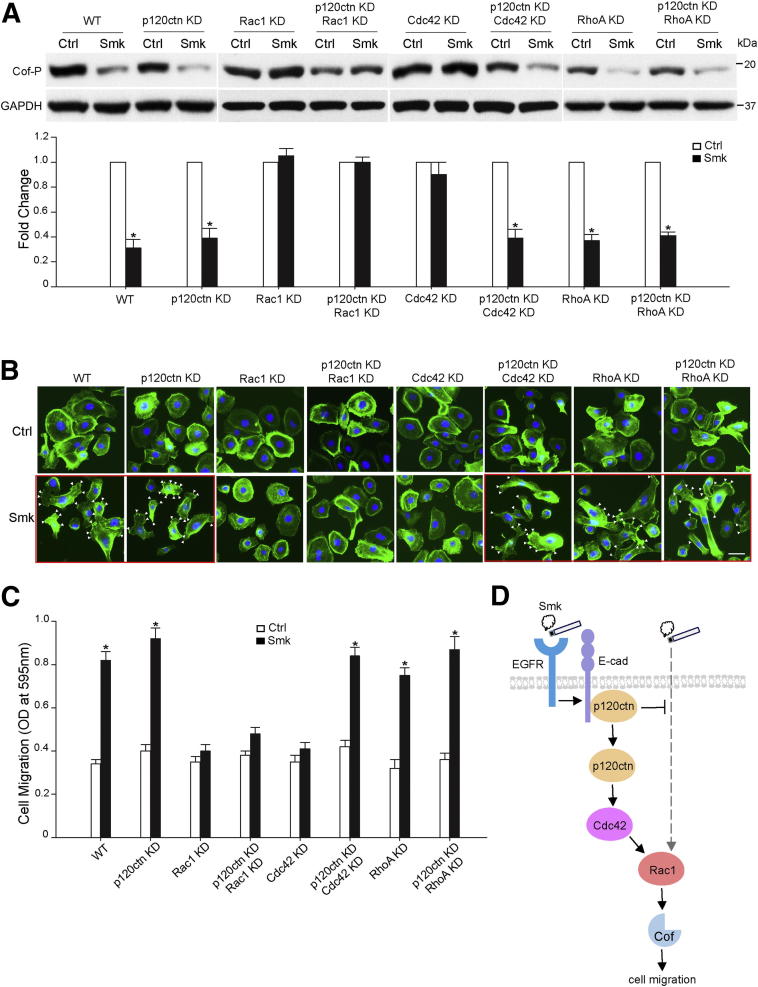
Next, we compared smoke-induced dephosphorylation of cofilin in WT and p120ctn KD cells exposed to Ctrl or Smk for 4 hours. Similar to the data shown in Figure 2C, Cof-P was decreased 60% to 70% by Smk in WT and p120ctn KD cells (Figure 4A). Dephosphorylation of Cof-P in p120ctn KD cells exposed to smoke was consistent with the continued induction of lamellipodia formation and Transwell cell migration measured by a Boyden chamber assay in smoke-exposed p120ctn KD cells (Figure 4, B and C). These data indicated the presence of p120ctn-dependent and p120ctn-independent signaling pathways linking smoke to cell migration.
Figure 4.
Rac1 mediated smoke-induced HBE cell migration in the presence and absence of p120ctn, whereas Cdc42 functioned only in the presence of p120ctn. A–C: HBE cells were transfected with scrambled siRNA (WT), p120ctn siRNA (p120ctn KD), Rac1 siRNA (Rac1 KD), p120ctn and Rac1 siRNA (p120ctn KD, Rac1 KD), Cdc42 siRNA (Cdc42 KD), p120ctn and Cdc42 siRNA (p120ctn KD, Cdc42 KD), RhoA siRNA (RhoA KD), and p120ctn and RhoA siRNA (p120ctn KD, RhoA KD), respectively. A and B: Forty-eight hours after transfection, HBE cells were treated with Ctrl and Smk for 4 hours. A: Cell lysates were obtained and analyzed by Western blot with Cof-P. Equal loading was confirmed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative fold change of Cof-P in Smk-exposed cells was normalized to unexposed Ctrl (designated onefold) and reported as means ± SEM fold change. B: HBE cells were fixed and stained with fluorescein isothiocyanate–labeled phalloidin to reveal actin (green). Cell nuclei were counterstained with DAPI (blue). Frames with the red outline indicate treatment conditions under which HBE cell migration was preserved. White arrowheads indicate the formation of lamellipodia in Smk-stimulated cells. Scale bar = 50 μm. C: Forty-eight hours after transfection, siRNA-transfected cells were loaded on Boyden chambers and incubated with Ctrl or Smk for 4 hours. Migrating cells on the bottom side of the membrane were quantified by measuring the OD at 595 nm and plotted as the means ± SD of three independent chambers. D: Differential role of Rac1 and Cdc42 in regulating smoke-promoted HBE cell migration. In the normal human airway, the AJ proteins E-cad and p120ctn mediate cell-cell adhesion. Smoke disrupts AJs and provokes the cytoplasmic translocation of p120ctn through EGFR signaling.15, 16, 17 In the early stages of lung cancer, when intracellular p120ctn is abundant, smoke promotes the activation (dephosphorylation) of actin-severing protein, Cof, to promote cell migration.34 Rac1 and Cdc42 are implicated in this p120ctn-dependent pathway because knocking down either Rac1 or Cdc42 abrogated smoke-induced cell migration in the presence of p120ctn. In the advanced stages of lung cancer, when p120ctn is lost, Cof dephosphorylation is mediated through Rac1. The alternative Rac1 pathway seems to be suppressed by membrane p120ctn and only unveiled after the loss of tumor-suppressing p120ctn. The Rac1 pathway differs from the p120ctn/Rac1 pathway in that its action does not require Cdc42. Thereby, knockdown of Rac1 alone succeeds in abolishing smoke-induced cell migration in the absence of p120ctn. Because Rac1 activation is essential for the p120ctn-dependent and p120ctn-independent cell migratory pathways induced by smoke, Rac1 likely acts at the convergent point of the two pathways and, thus, downstream of Cdc42. The present data support the potential of Rac1 as an omnipotent therapeutic target in treating the early and advanced stages of lung cancer. P < 0.01, Smk-treated cells versus Ctrl.
We next investigated the essential mediators of smoke-promoted cell migration under two conditions: intact p120ctn and loss of p120ctn. Using dephosphorylation of Cof-P, lamellipodia formation, and Transwell cell migration as readouts, smoke-induced HBE cell migration was completely abolished by introducing either Rac1 or Cdc42 siRNA, but not RhoA siRNA, into cells expressing p120ctn (Figure 4, A–C). In the absence of p120ctn, smoke-induced HBE cell migration using the same readouts was abrogated only by the suppression of Rac1 expression (Figure 4, A–C). Knocking down Cdc42 was able to block smoke-induced cell migration in p120ctn WT cells but failed to do so in p120ctn KD cells (Figure 4, A–C). To confirm that Rac1 plays an essential role in smoke-induced cell migration, constitutively active Rac1 Q61L and dominant-negative Rac1 T17N were overexpressed in Rac1 KD and Rac1/p120ctn double knockdown cells. Rac1 Q61L, but not Rac1 T17N, salvaged smoke-provoked cofilin dephosphorylation (Supplemental Figure S1A) and Transwell HBE cell migration (Supplemental Figure S1B) in the presence and absence of p120ctn. Thus, Rac1 and Cdc42 seemed to coordinately mediate smoke-induced cell migration through a p120ctn-dependent pathway, and Rac1 was essential to promote cell migration in the absence of p120ctn. A proposed model of smoke-modulated cell migration through p120ctn-dependent and p120ctn-independent pathways is presented in Figure 4D.
Discussion
Using primary HBE cells as an experimental model, we discovered that cell migration induced by cigarette smoke can occur in the presence and absence of the AJ protein p120ctn. In the presence of p120ctn, smoke-induced cell migration seemed to require the sequential activation of p120ctn, Cdc42, and Rac1. Alternatively, in the absence of p120ctn, direct activation of Rac1 seemed to be sufficient to provoke cell migration, independent of Cdc42 (Figure 4D).
Activation of promigration pathways in cells undergoing EMT is reported to depend on the presence of cytoplasmic p120ctn and the downstream activation of Rho GTPases.39 Recently, we reported a smoke-activated, EGFR-dependent signaling pathway that led to the disruption of E-cad/catenin complexes at the AJ, degradation of E-cad, and cytoplasmic translocation of membrane p120ctn.15, 16, 17 Herein, we showed Rho GTPases acting downstream of this EGFR/p120ctn-dependent pathway (Figure 4D) to promote migration via the coactivation and translocation of Rac1 and Cdc42 to lamellipodia or filopodia (Figure 1, A–D). Rac1 and Cdc42 seemed to be downstream effectors of the EGFR/p120ctn pathway (Figure 4D) because siRNA knockdown of either Rac1 or Cdc42 completely abolished smoke-induced dephosphorylation of cofilin, lamellipodia or filopodia formation, and Transwell cell migration (Figure 4, A–C). Conversely, smoke inhibited RhoA activity, which seemed to remain largely in the cytoplasm and away from the leading edge of migrating cells (Figure 1, B and E). Accordingly, suppression of RhoA by siRNA had no effect on smoke-induced cell migration using the same readouts (Figure 4, A–C). In line with the results obtained with Smk, certain constituents of cigarette smoke have been shown to mediate cell migration through Rac1- and/or Cdc42-implicated pathways. Nicotine-induced cell migration was abolished by Cdc42 knockdown in breast cancer cells.40 Benzo[α]pyrene, a potent procarcinogen found in smoke, was reported to enhance Rac1 and Cdc42 activities in 293T and HeLa cells.41
The interaction between p120ctn and Rho GTPase (Rac1, Cdc42, and RhoA) unanimously peaked 0.5 hours after exposure of migratory HBE cells to smoke (Figure 2A), approximately 0.5 hours earlier than cofilin reaching its maximal level of dephosphorylation (activation) (Figure 2C). It seems that these interactions played a functional role in regulating cell migration. Previous reports demonstrating the indirect activation of Rac1 and Cdc42 by p120ctn via the guanine nucleotide exchange factor Vav213, 14 provide evidence to support this functional role, as does the ability of p120ctn to directly inhibit RhoA by functioning as a guanine dissociation inhibitor.12 Rac1 and Cdc42 can inhibit RhoA by recruiting p190RhoGAP to the p120ctn/RhoA complex, where it provokes the intrinsic GTP-hydrolytic activity of RhoA.31 Biochemical evidence has also demonstrated the cross regulation of Rho GTPases: Cdc42 has been shown to activate Rac1,23 and Rac1 has been reported to be mutually inhibitory with RhoA.32 Thus, we hypothesize that in response to smoke, Rac1 and RhoA are incorporated into cytoplasmic and/or leading-edge p120ctn/Cdc42 complexes, where Rac1 is activated and RhoA is suppressed.
Although cytoplasmic p120ctn is known to mediate Rho GTPase activity,39 p120ctn knockdown alone did not affect smoke-induced cell migration (Figure 4, A–C). In the absence of p120ctn, cell migration continued to occur through an alternative, Rac1-dependent pathway (Figure 4D). With Rac1 knockdown completely abrogating HBE cell migration in the presence and absence of p120ctn (Figure 4, A–C), we propose that Rac1 acts at the point of intersection between p120ctn-dependent and p120ctn-independent pathways (Figure 4D). Constitutively active Rac1 Q61L, but not dominant-negative Rac1 T17N, salvaged smoke-induced cell migration in Rac1 KD and Rac1 KD/p120ctn KD cells (Supplemental Figure S1), confirming the crucial role of Rac1 in the mediation of both pathways. Alternatively, cell migration induced by smoke via the activation of Cdc42 seemed to occur upstream of this intersection point but downstream of p120ctn (Figure 4, A–C), thus, blocking Cdc42 activation had no effect on Rac1-mediated cell migration in the absence of p120ctn (Figure 4D). Consistent with the data obtained in primary HBE cells treated with smoke, this EGFR/p120ctn/Cdc42/Rac1 pathway seems to be operated in cancer cells in response to growth factors. Concurrent activation of Rac1 and Cdc42 at lamellipodia and membrane ruffles by EGF has been reported in lung cancer cells,42, 43 which was probably achieved by phosphorylation and activation of Asef, a Rac1-Cdc42 guanine nucleotide exchange factor functioning downstream of EGFR.43 These data highlight the potential significance of Rac1 in advanced disease as tumor-suppressing p120ctn is lost from cells. Activation of Rac1 via an EGFR/p120ctn-independent pathway is feasible because its activation is complex with multiple signaling inputs that include EGFR-independent receptor tyrosine kinases, integrins, and cadherins.31, 44
Previously, we described an EGFR/p120ctn-independent pathway linking cigarette smoke to cell migration.16 EGFR activation by smoke was essential to promote cell migration in the presence of p120ctn, but as p120ctn was lost from cells, chemotherapeutics directed against EGFR were no longer effective at blocking smoke-induced cell migration.16 This study provided insight regarding a potential mechanism of chemotherapeutic resistance in advanced disease when tumor-suppressing p120ctn is lost from smoke-exposed cells. Although the specific identity of signaling molecules that promote cell migration in the absence of p120ctn is unknown, we believe that Rac1 is an essential component. These data suggest that EGFR activation in response to smoke mediates membrane-to-cytoplasmic translocation of p120ctn16, 17 to facilitate Cdc42/Rac1 activation and cell migration. In contrast, we believe that Rac1 activation via an alternative, EGFR/p120ctn-independent pathway is normally suppressed by membrane p120ctn but disinhibited after its loss with tumor progression (Figure 4D).8, 16 Both pathways may occur in former smokers whose lung cancer risk persists beyond 30 years of smoking cessation.45 Lung cancer in nonsmokers and in patients with lung adenocarcinoma that is unrelated to active or second-hand smoke are associated with a higher frequency of EGFR mutation,46, 47 whereas lung cancer in former and/or current smokers demonstrates more frequent KRAS and p53 mutations.48 Seemingly, the p120ctn/Cdc42/Rac1 pathway could be activated by constitutively active EGFR mutant in the absence of smoke exposure, eventually causing lung adenocarcinoma in patients who have never smoked. In addition to EGFR,42, 43 Rac1 and its downstream effects on cell motility could be activated by platelet-derived growth factor receptor49 and insulin receptor,50 as previously reported. Thus, the EGFR/p120ctn-dependent and EGFR/p120ctn-independent pathways may be operative in smokers, former smokers, and nonsmokers.
Further exploration of Rac1 activation in the absence of p120ctn may provide clues as to the early tumorigenic/metastatic events that ultimately inhibit the effectiveness of conventional chemotherapeutics, such as those directed against EGFR. In line with this study, increased Rac1 activity was reported to be associated with trastuzumab resistance of breast cancer cells, a humanized monoclonal antibody targeting the ErbB2 receptor (EGFR).51 Thus, this study revealed Rac1 as an essential regulator of smoke-induced cell migration, suggesting its potential as an omnipotent therapeutic target in reversing early and late events of lung cancer.
Acknowledgments
We dedicate this article to the memory of Dr. Carol Basbaum, our friend and mentor, who first described the EGFR signaling pathway linking smoke to mucin production.
Footnotes
Supported by American Cancer Society grant 115502-RSG-08-136-01-CNE.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2013.02.008.
Supplemental Data
Constitutively active Rac1 Q61L mutant, but not dominant-negative Rac1 T17N mutant, salvaged smoke-induced cell migration in Rac1-knockdown HBE cells in the presence and absence of p120ctn. HBE cells were transfected with plasmid expressing Rac1 siRNA (Rac1 KD), p120ctn and Rac1 siRNA (p120ctn KD, Rac1 KD), and Rac1 Q61L or Rac1 T17N. A: Forty-eight hours after transfection, HBE cells were treated with Ctrl and Smk for 4 hours. Cell lysates were obtained and analyzed by Western blot with Cof-P. Equal expression of Rac1 mutants was shown by Rac1. Equal loading was confirmed with glyceraldehyde-3-phosphate dehydrogenase (GAP). Relative fold change of Cof-P in Smk-exposed cells was normalized to unexposed Ctrl (designated onefold) and reported as means ± SEM fold change. B: Forty-eight hours after transfection, siRNA- and Rac1 Q61L– or Rac1 T17N–transfected cells were loaded on Boyden chambers and incubated with Ctrl or Smk for 4 hours. Migrating cells on the bottom side of the membrane were quantified by measuring the OD at 595 nm and plotted as the means ± SD of three independent chambers. *P < 0.01, Smk-treated cells versus the respective Ctrl.
References
- 1.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Tobacco smoke and involuntary smoking. IARC MonogrEval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 2.Proctor R.N. The global smoking epidemic: a history and status report. Clin Lung Cancer. 2004;5:371–376. doi: 10.3816/CLC.2004.n.016. [DOI] [PubMed] [Google Scholar]
- 3.Hecht S.S., Kassie F., Hatsukami D.K. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer. 2009;9:476–488. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasari V., Gallup M., Lemjabbar H., Maltseva I., McNamara N. Epithelial-mesenchymal transition in lung cancer: is tobacco the “smoking gun”? Am J Respir Cell Mol Biol. 2006;35:3–9. doi: 10.1165/rcmb.2006-0051SF. [DOI] [PubMed] [Google Scholar]
- 5.Gomperts B.N., Spira A., Massion P.P., Walser T.C., Wistuba I.I., Minna J.D., Dubinett S.M. Evolving concepts in lung carcinogenesis. Semin Respir Crit Care Med. 2011;32:32–43. doi: 10.1055/s-0031-1272867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polyak K., Weinberg R.A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 7.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M., Campbell L.L., Polyak K., Brisken C., Yang J., Weinberg R.A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds A.B., Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–7956. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 9.Davis M.A., Ireton R.C., Reynolds A.B. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishiyama N., Lee S.H., Liu S., Li G.Y., Smith M.J., Reichardt L.F., Ikura M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Soto E., Yanagisawa M., Marlow L.A., Copland J.A., Perez E.A., Anastasiadis P.Z. p120 catenin induces opposing effects on tumor cell growth depending on E-cadherin expression. J Cell Biol. 2008;183:737–749. doi: 10.1083/jcb.200805113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anastasiadis P.Z., Moon S.Y., Thoreson M.A., Mariner D.J., Crawford H.C., Zheng Y., Reynolds A.B. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- 13.Noren N.K., Liu B.P., Burridge K., Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosheva I., Shtutman M., Elbaum M., Bershadsky A.D. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114:695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y.T., Gallup M., Nikulina K., Lazarev S., Zlock L., Finkbeiner W., McNamara N. Cigarette smoke induces epidermal growth factor receptor-dependent redistribution of apical MUC1 and junctional beta-catenin in polarized human airway epithelial cells. Am J Pathol. 2010;177:1255–1264. doi: 10.2353/ajpath.2010.091129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L., Gallup M., Zlock L., Finkbeiner W., McNamara N.A. p120-catenin modulates airway epithelial cell migration induced by cigarette smoke. Biochem Biophys Res Commun. 2012;417:49–55. doi: 10.1016/j.bbrc.2011.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L., Gallup M., Zlock L., Basbaum C., Finkbeiner W.E., McNamara N.A. Cigarette smoke disrupts the integrity of airway adherens junctions through the aberrant interaction of p120-catenin with the cytoplasmic tail of MUC1. J Pathol. 2013;229:74–86. doi: 10.1002/path.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoreson M.A., Reynolds A.B. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation. 2002;70:583–589. doi: 10.1046/j.1432-0436.2002.700911.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Wang Y., Zhang Y., Miao Y., Zhao Y., Zhang P.X., Jiang G.Y., Zhang J.Y., Han Y., Lin X.Y., Yang L.H., Li Q.C., Zhao C., Wang E.H. Abnormal expression of p120-catenin: E-cadherin, and small GTPases is significantly associated with malignant phenotype of human lung cancer. Lung Cancer. 2009;63:375–382. doi: 10.1016/j.lungcan.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Burridge K., Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 21.Burridge K., Doughman R. Front and back by Rho and Rac. Nat Cell Biol. 2006;8:781–782. doi: 10.1038/ncb0806-781. [DOI] [PubMed] [Google Scholar]
- 22.Baum B., Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobes C.D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 24.Du B., Leung H., Khan K.M., Miller C.G., Subbaramaiah K., Falcone D.J., Dannenberg A.J. Tobacco smoke induces urokinase-type plasminogen activator and cell invasiveness: evidence for an epidermal growth factor receptor dependent mechanism. Cancer Res. 2007;67:8966–8972. doi: 10.1158/0008-5472.CAN-07-1388. [DOI] [PubMed] [Google Scholar]
- 25.Yamadori T., Ishii Y., Homma S., Morishima Y., Kurishima K., Itoh K., Yamamoto M., Minami Y., Noguchi M., Hizawa N. Molecular mechanisms for the regulation of Nrf2-mediated cell proliferation in non-small-cell lung cancers. Oncogene. 2012;31:4768–4777. doi: 10.1038/onc.2011.628. [DOI] [PubMed] [Google Scholar]
- 26.Lemjabbar-Alaoui H., Dasari V., Sidhu S.S., Mengistab A., Finkbeiner W., Gallup M., Basbaum C. Wnt and Hedgehog are critical mediators of cigarette smoke-induced lung cancer. PLoS One. 2006;1:e93. doi: 10.1371/journal.pone.0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaya M., Finkbeiner W.E., Chun S.Y., Widdicombe J.H. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 28.Ji C.M., Plopper C.G., Witschi H.P., Pinkerton K.E. Exposure to sidestream cigarette smoke alters bronchiolar epithelial cell differentiation in the postnatal rat lung. Am J Respir Cell Mol Biol. 1994;11:312–320. doi: 10.1165/ajrcmb.11.3.8086168. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Li Q.C., Miao Y., Xu H.T., Dai S.D., Wei Q., Dong Q.Z., Dong X.J., Zhao Y., Zhao C., Wang E.H. Ablation of p120-catenin enhances invasion and metastasis of human lung cancer cells. Cancer Sci. 2009;100:441–448. doi: 10.1111/j.1349-7006.2008.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatin F., Varon C., Genot E., Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–781. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- 31.Wildenberg G.A., Dohn M.R., Carnahan R.H., Davis M.A., Lobdell N.A., Settleman J., Reynolds A.B. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 32.Sander E.E., ten Klooster J.P., van Delft S., van der Kammen R.A., Collard J.G. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warner S.J., Longmore G.D. Cdc42 antagonizes Rho1 activity at adherens junctions to limit epithelial cell apical tension. J Cell Biol. 2009;187:119–133. doi: 10.1083/jcb.200906047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bamburg J.R. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh M., Song X., Mouneimne G., Sidani M., Lawrence D.S., Condeelis J.S. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- 36.Moriyama K., Iida K., Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells. 1996;1:73–86. doi: 10.1046/j.1365-2443.1996.05005.x. [DOI] [PubMed] [Google Scholar]
- 37.Edwards D.C., Sanders L.C., Bokoch G.M., Gill G.N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 38.Maekawa M., Ishizaki T., Boku S., Watanabe N., Fujita A., Iwamatsu A., Obinata T., Ohashi K., Mizuno K., Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 39.Anastasiadis P.Z., Reynolds A.B. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol. 2001;13:604–610. doi: 10.1016/s0955-0674(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 40.Guo J., Ibaragi S., Zhu T., Luo L.Y., Hu G.F., Huppi P.S., Chen C.Y. Nicotine promotes mammary tumor migration via a signaling cascade involving protein kinase C and CDC42. Cancer Res. 2008;68:8473–8481. doi: 10.1158/0008-5472.CAN-08-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshii S., Tanaka M., Otsuki Y., Fujiyama T., Kataoka H., Arai H., Hanai H., Sugimura H. Involvement of alpha-PAK-interacting exchange factor in the PAK1-c-Jun NH(2)-terminal kinase 1 activation and apoptosis induced by benzo[a]pyrene. Mol Cell Biol. 2001;21:6796–6807. doi: 10.1128/MCB.21.20.6796-6807.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurokawa K., Itoh R.E., Yoshizaki H., Nakamura Y.O., Matsuda M. Coactivation of Rac1 and Cdc42 at lamellipodia and membrane ruffles induced by epidermal growth factor. Mol Biol Cell. 2004;15:1003–1010. doi: 10.1091/mbc.E03-08-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Itoh R.E., Kiyokawa E., Aoki K., Nishioka T., Akiyama T., Matsuda M. Phosphorylation and activation of the Rac1 and Cdc42 GEF Asef in A431 cells stimulated by EGF. J Cell Sci. 2008;121:2635–2642. doi: 10.1242/jcs.028647. [DOI] [PubMed] [Google Scholar]
- 44.Nimnual A.S., Taylor L.J., Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 45.Ebbert J.O., Yang P., Vachon C.M., Vierkant R.A., Cerhan J.R., Folsom A.R., Sellers T.A. Lung cancer risk reduction after smoking cessation: observations from a prospective cohort of women. J Clin Oncol. 2003;21:921–926. doi: 10.1200/JCO.2003.05.085. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y., Ren Y., Fang Z., Li C., Fang R., Gao B., Han X., Tian W., Pao W., Chen H., Ji H. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010;28:4616–4620. doi: 10.1200/JCO.2010.29.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taga M., Mechanic L.E., Hagiwara N., Vahakangas K.H., Bennett W.P., Alavanja M.C., Welsh J.A., Khan M.A., Lee A., Diasio R., Edell E., Bungum A., Jang J.S., Yang P., Jen J., Harris C.C. EGFR somatic mutations in lung tumors: radon exposure and passive smoking in former- and never-smoking U.S. women. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:988–992. doi: 10.1158/1055-9965.EPI-12-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Calvez F., Mukeria A., Hunt J.D., Kelm O., Hung R.J., Taniere P., Brennan P., Boffetta P., Zaridze D.G., Hainaut P. TP53 and KRAS mutation load and types in lung cancers in relation to tobacco smoke: distinct patterns in never, former, and current smokers. Cancer Research. 2005;65:5076–5083. doi: 10.1158/0008-5472.CAN-05-0551. [DOI] [PubMed] [Google Scholar]
- 49.Hawkins P.T., Eguinoa A., Qiu R.G., Stokoe D., Cooke F.T., Walters R. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Current Bio. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 50.Nobes C.D., Hawkins P., Stephens L., Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- 51.Dokmanovic M., Hirsch D.S., Shen Y., Wu W.J. Rac1 contributes to trastuzumab resistance of breast cancer cells: rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer. Mol Cancer Ther. 2009;8:1557–1569. doi: 10.1158/1535-7163.MCT-09-0140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Constitutively active Rac1 Q61L mutant, but not dominant-negative Rac1 T17N mutant, salvaged smoke-induced cell migration in Rac1-knockdown HBE cells in the presence and absence of p120ctn. HBE cells were transfected with plasmid expressing Rac1 siRNA (Rac1 KD), p120ctn and Rac1 siRNA (p120ctn KD, Rac1 KD), and Rac1 Q61L or Rac1 T17N. A: Forty-eight hours after transfection, HBE cells were treated with Ctrl and Smk for 4 hours. Cell lysates were obtained and analyzed by Western blot with Cof-P. Equal expression of Rac1 mutants was shown by Rac1. Equal loading was confirmed with glyceraldehyde-3-phosphate dehydrogenase (GAP). Relative fold change of Cof-P in Smk-exposed cells was normalized to unexposed Ctrl (designated onefold) and reported as means ± SEM fold change. B: Forty-eight hours after transfection, siRNA- and Rac1 Q61L– or Rac1 T17N–transfected cells were loaded on Boyden chambers and incubated with Ctrl or Smk for 4 hours. Migrating cells on the bottom side of the membrane were quantified by measuring the OD at 595 nm and plotted as the means ± SD of three independent chambers. *P < 0.01, Smk-treated cells versus the respective Ctrl.