In the article entitled “Increased Expression of 14-3-3β Promotes Tumor Progression and Predicts Extrahepatic Metastasis and Worse Survival in Hepatocellular Carcinoma” (Volume 179, pages 2698-2708 of the December 2011 issue of The American Journal of Pathology), the affiliation of the third author was incorrect. Bor-Sheng Ko is from the Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan.
In the article entitled “Lactobacillus rhamnosus GG Treatment Potentiates Intestinal Hypoxia-Inducible Factor, Promotes Intestinal Integrity and Ameliorates Alcohol-Induced Liver Injury” (Volume 179, pages 2866-2875 of the December 2011 issue), Table 2 contained errors in units of measure; Plasma LPS were measured in EU/mL. The correct Table 2 appears below.
Table 2.
Characteristics and Biochemical Changes in LGG-Treated Mice
| Characteristic | PF | AF | AF + LGG |
|---|---|---|---|
| Body weight (g) | 28.1 ± 0.64 | 25.96 ± 0.72⁎ | 26.68 ± 0.48 |
| Plasma ALT (U/L) | 28.88 ± 2.93 | 43.66 ± 2.02⁎ | 32.74 ± 2.67⁎⁎ |
| Plasma LPS (EU/mL) | 0.13 ± 0.04 | 0.37 ± 0.17⁎ | 0.17 ± 0.04⁎⁎ |
| Liver TG (mg/dL) | 39.93 ± 5.59 | 100.2 ± 8.17⁎ | 89.7 ± 3.87⁎⁎ |
| Free fatty acid (mE/g liver) | 0.14 ± 0.04 | 0.21 ± 0.08⁎ | 0.13 ± 0.03 |
| Cholesterol (mmol/g liver) | 12.75 ± 0.78 | 17.33 ± 0.99⁎ | 14.9 ± 0.46 |
Values are reported as means ± SD.
AF, alcohol-fed; ALT, alanine aminotransferase; LGG, L. rhamnosus GG; LPS, lipopolysaccharide; PF, pair-fed; TG, triglyceride.
P < 0.05 versus PA;
P < 0.05 versus AF.
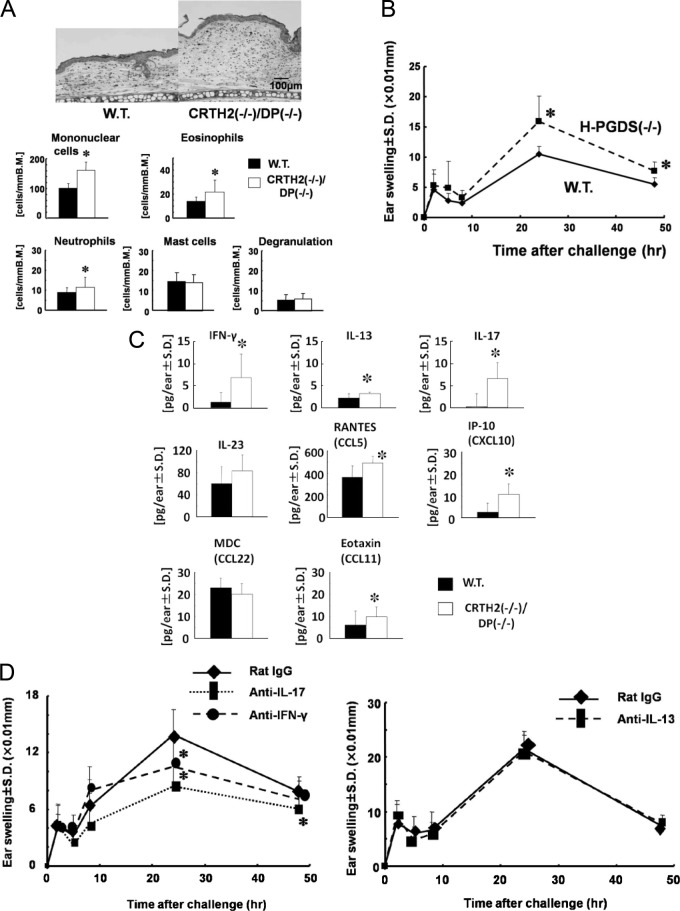
In the article entitled “Dual Functions of Prostaglandin D2 in Murine Contact Hypersensitivity via DP and CRTH2” (Volume 179, pages 302-314, of the July 2011 issue), error bars in Figure 2C (IFN-γ graph) were inadvertently switched between open and closed bars. The authors state that the results and statistical significance do not change. The corrected Figure 2 with legend appears on the next page.
Figure 2.
Modulation of parameters in contact hypersensitivity (CHS) in mutant mice. A: Histological features and cell populations in inflammatory skin. Wild-type (W.T.) and chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2–/–)/D prostanoid (DP–/–) mice were challenged with 2,4,6-trinitrochlorobenzene (TNCB) and inflammatory ears at 24 hours after challenge were subjected to histological observation and cell counting. Cells were counted in five distinct areas and determined as numbers per 1 mm of basement membrane (B.M.). Means ± SD. B: CHS responses in mice lacking the H-PGDS gene. W.T. and hematopoietic-type PGD synthase (H-PGDS–/–) mice were challenged with TNCB. Ear thickness was measured at the times indicated. C: Cytokine and chemokine production in CHS. W.T. and CRTH2–/–/DP–/– mice were challenged with TNCB. Ear lobes were punched 24 hours after challenge and levels of cytokines and chemokines were determined by enzyme-linked immunosorbent assay. Means ± SD. D: Ear thickness in CHS treated with anti-cytokine antibodies. CRTH2–/–/DP–/– mice were sensitized with TNCB. Blocking Abs against cytokines interferon [(IFN)- γ, IL-13, and IL-17 at doses of 200 μg/mouse] were administered i.p. 1 day before challenge. Ear thickness was measured at the times indicated. *P < 0.05. Numbers of mice in each group were four to six. Representative results from at least two independent experiments are shown.