Abstract
This Commentary highlights the article by Baluk et al (in this issue), who described that the overexpression of IL-1β stimulates lymphangiogenesis, but not angiogenesis, in mouse airways.
CME Accreditation Statement: This activity (“ASIP 2013 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2013 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
There are both significant similarities and differences between lymphangiogenesis and angiogenesis. In this issue of The American Journal of Pathology, Baluk et al1 add another observation to the column of differences with their finding that overexpression of IL-1β stimulates the growth of lymphatic but not blood vessels in the mouse airway. A Clara cell–specific promoter (Clara cell secretory protein) was used in a reverse tetracycline transactivator system to overexpress human IL-1β in airways. Induction of IL-1β was followed by influx of neutrophils and macrophages and was associated with the formation of lymphatic vessels that persisted after the cessation of IL-1β expression; however, there was no evidence of angiogenesis. The dependence of this effect on vascular endothelial growth factor (VEGF)-C and/or VEGF-D was demonstrated when adenovirally expressed soluble VEGF receptor-3 blocked the lymphangiogenesis. The absence of neutrophils did not influence the IL-1β induction of lymphatic vessels. The identification of IL-1β receptor on some epithelial basal cells and neuroendocrine cells in the trachea led the authors to suggest that these cells might be targets of the IL-1β.
Although the processes of angiogenesis and lymphangiogenesis are often coincident, the details of their development and basic mechanics of growth and sprouting are distinct.2, 3 The inherent differences in the structure of blood capillaries and lymphatic capillaries dictate this variation. Vascular capillaries join arteries and veins in a closed system to allow for the circular flow of blood throughout the body. In contrast, the open-ended lymphatic system has a unidirectional flow in which interstitial fluid consisting of protein-rich plasma is absorbed and transported via larger collecting ducts and lymph nodes and returned to the venous system, thereby helping to maintain fluid homeostasis within the body.
Capillaries
Blood capillaries are small in caliber: their luminal diameter is composed of just one or two endothelial cells (ECs), with just enough space for red blood cells to squeeze through in single file. This small gauge ensures the close approximation of the red blood cell membrane and the endothelial membrane necessary for gas exchange and diffusion. The wall of the capillary is one to two cell layers thick, depending on the site, and is composed of a simple squamous epithelium referred to as endothelium; pericytes are associated abluminally, with their frequency differing among various microvascular beds. ECs are connected via a variety of junctions to form a barrier that also varies depending on the tissue bed. Specialized capillaries in tissues, such as liver sinusoid, kidney glomerulus, pancreatic islet, or choroid plexus, are composed of fenestrated ECs to allow the exchange of larger molecules, proteins, or, in some cases, even cells.4
A basement membrane composed of collagen IV, laminin, and proteoglycans, produced by the EC and pericyte, surrounds and envelopes the capillary, providing both mechanical support and biochemical information. Pericytes are smooth muscle–like cells that ensheath portions of the circumference of the vessel, aiding in endothelial cell differentiation and survival.5 Blood flow and blood pressure are regulated by the pumping heart, larger-caliber vessels, such as arteries, that are composed of multiple layers of smooth muscle and venules that contain valves to prevent retrograde flow capillaries contribute little to the regulation of flow.
Lymphatic Capillaries
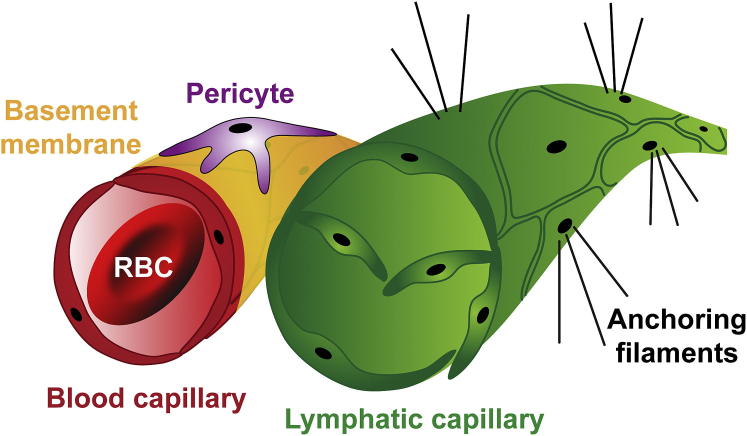
The terminal lymphatic capillary is blind ended, irregularly shaped, and larger in diameter than a blood capillary. Lymphatic capillaries do not contain blood; rather, they contain plasma and immune cells, such as lymphocytes. Lymphatic endothelial cells (LECs) are simple squamous epithelium, similar to vascular endothelial cells, except that their nuclei protrude slightly into the lumen. LECs lack a continuous basement membrane, and adjacent cells lack junctions and instead overlap at their edges. The abluminal side of the LEC is attached to the extracellular matrix via elastic anchoring filaments. Lymphatic capillaries function to drain excess fluid; as fluid accumulates and interstitial pressure increases, the anchoring filaments separate the LEC, generating gaps between adjacent cells and allowing for fluid drainage into the lymphatic vessel. Once pressure decreases, the LEC moves back into close apposition, thus preventing fluid from moving back into the interstitium. Although lymphatic capillaries are not associated with pericytes, larger collecting lymphatic ducts have a smooth muscle covering. Lymph is not propelled by a pump (heart), as in the vascular system; rather, fluid pressure and skeletal muscle movement regulate flow and bileaflet valves present in the lymphatic capillaries and collectors prevent backflow. Lymphatic capillaries drain into larger collecting ducts and enter the lymph node via the afferent lymphatic duct. In the lymph node, the fluid is cleansed of debris and pathogens and lymphatic fluid is returned to the venous system at the subclavian veins on either side of the neck. Figure 1 is a diagram comparing blood capillary and lymphatic capillary structure.
Figure 1.
Capillaries differ in their architecture. Blood capillaries are composed of endothelial cells, surrounded by basement membrane and pericytes, and contain blood and red blood cells (RBCs). Lymphatic capillaries are composed of lymphatic endothelial cells and lack basement membrane or pericytes. Anchoring filaments attach to the abluminal side of lymphatic capillaries and enable neighboring lymphatic endothelial cells to separate and allow fluid to enter the vessel. Lymphatic capillaries contain valves to prevent retrograde flow.
The Tracheal Model
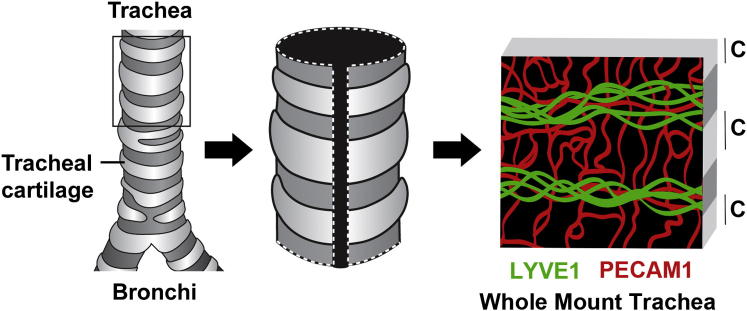
Many models are used to study angiogenesis6 and lymphangiogenesis7 in vivo, and the McDonald laboratory has optimized the mouse trachea whole mount assay for the purpose of investigating multiple aspects of these processes. The murine trachea model, depicted in Figure 2, allows the visualization of a two-dimensional network of blood vessels and lymphatic vessels in a consistent and reproducible manner. Tracheas are dissected and stained in whole mount preparations for lymphatic vessel endothelial hyaluronan receptor 1 to detect lymphatic vessels and platelet endothelial cell adhesion molecule 1 to detect blood vessels; then, confocal images are captured, analyzed, and quantified. Larger blood vessels and lymphatic vessels are found in the mucosal areas between cartilage rings, whereas blood capillaries are located over the cartilage in a ladder-like arrangement. This predictable pattern enables detection of subtle differences in vascular morphology characteristics after manipulation, such as the addition of drugs [eg, vascular endothelial growth factor receptor (VEGFR) inhibitors],8 or the induction of a transgene (eg, VEGF transgenic).9
Figure 2.
The tracheal model. The trachea is surrounded by C-shaped rings of cartilage. The trachea is dissected, cut longitudinally, and stained by whole mount with antibodies to lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) and platelet endothelial cell adhesion molecule 1 (PECAM1). Lymphatic vessels and larger blood vessels are located between cartilage regions, whereas blood capillaries cross the cartilage-covered regions in a ladder-like pattern.
The laboratory of McDonald and colleagues has used this system to study the architecture and remodeling of vessels,10 the angiogenic response to inflammation and infection,11 the development and maturation of the vascular plexus,12 and the inhibition of lymphangiogenesis.13 In this issue of The American Journal of Pathology, their laboratory reports the induction of IL-1β expression by CCSP-secreting cells using a doxycycline (Dox)-inducible system.1 After 2 weeks of IL-1β expression, the tracheas were removed and stained as whole mounts for lymphatic and blood vessels. Lymphatic capillaries were easily detected over the cartilage of IL-1β–overexpressing mice but not in the control mice, in which only blood capillaries were found.1 Whether the inflammation or lymphangiogenesis that is observed in the trachea in this model reflects what is occurring throughout the airway, such as in the cartilage-covered bronchi or the noncartilagenous bronchioles or alveoli, is unclear.
Lymphatic Stabilization
Although there is a growing body of information regarding the mechanisms that regulate microvascular regression or stabilization, much less is known about the control of those processes for lymphatic vessels. For newly forming vasculature, withdrawal of an angiogenic stimulus has been shown to lead to rapid vessel regression, unless the microvasculature has been stabilized by the recruitment and association with pericytes and the subsequent production of a basement membrane.14 However, lymphatic capillaries have neither pericytes nor a basement membrane, so the determinants of their stability remain unclear.
In the current study, the lymphatic vessels that were induced by IL-1β overexpression persisted for at least several weeks (longer time points were not examined) once the IL-1β expression was ended. In fact, there are few reports of the regression of lymphatic vessels. Adenoviral expression of VEGF164 in the mouse ear led to the formation of both blood vessels and lymphatics.15 Although the vasculature regressed as VEGF-A levels returned to normal, the lymphatics remained for at least 1 year after VEGF-A expression ceased. In contrast, the induction of inflammation in the cornea using a suture model led to parallel growth of both blood and lymphatic vessels, which was followed first by the regression of the lymphatics and then the blood vessels.16 It is possible that these distinct behaviors of lymphatic vessels are due to the different contexts of lymphatic induction: the former, an example of lymphangiogenesis in the absence of inflammation; and the latter, an example in which lymphatic growth occurred as part of the inflammatory process.
Macrophages and Lymphangiogenesis
The lack of effect of neutrophil deficiency on IL-1β induction of lymphangiogenesis in the current study led the authors to implicate macrophages in the process.1 This is a logical assumption given the long-standing role of the macrophage in lymphatic development during inflammation. There is growing evidence that macrophages play dual roles in the growth of lymphatic vessels, a paracrine function and a cell autonomous role.14 The paracrine role of macrophages involves their secretion of growth factors and cytokines, most notably VEGF-C and VEGF-D, which stimulate lymphangiogenesis from pre-existing lymphatics.
It is this paracrine mechanism that Baluk et al1 implicate as underlying the IL-1β induction of lymphatic vessels. The evidence for a paracrine role for macrophages, specifically, is circumstantial and involves immunohistochemical localization of VEGF-C in macrophages in proximity to the new lymphatics.1 A more conclusive demonstration would require macrophage deletion either genetically or pharmacologically (eg, clodronate).
Although the authors suggest that the IL-1β acts on the pulmonary epithelium, which, in turn, produces cytokines to recruit macrophages, there is also a possibility that the pulmonary epithelium is the source (or an additional source) of VEGF-C and VEGF-D. There are several reports of VEGF-C and VEGF-D expression by a wide range of epithelial cells, including myoepithelium of the mammary gland,17 conjunctival epithelial cells,18 retinal pigment epithelium,19 and the pulmonary epithelial cells themselves.20 In fact, a recent report indicates that conditional overexpression of hypoxia-inducible factor 1α in pulmonary epithelium led to increased lymphangiogenesis, presumably via elevated expression of VEGF-A and VEGF-C.20
The cell autonomous contribution is more recently described and entails the macrophages themselves incorporating into the forming lymphatic and has led to a consideration of macrophages as lymphatic endothelial cell progenitors. Although an investigation of this mechanism is clearly outside the scope of the current study, future examination would be appropriate, because macrophage incorporation into newly formed lymphatics has been documented in corneal inflammation,21 wound healing,22 and several tumor models.14
Lymphatics formed in the absence of inflammation and as the result of a paracrine event (eg, stimulated to form by cytokines) are permanent structures, whereas those formed in an inflammatory environment, such as seen in wound healing or in tumors, have a major component of direct macrophage incorporation and are more prone to regression. This idea is consistent with current published observations in models of corneal inflammation and wound healing. It also applies to the work of Baluk et al,1 because the simple overexpression of IL-1β is unlikely to be sufficient to recapitulate all aspects of inflammation.
Lack of Angiogenesis
One striking finding in this manuscript is that, although IL-1β induced lymphangiogenesis, there was an absence of angiogenesis. Although lymphangiogenesis typically follows neovascularization, lymphangiogenesis has been reported to occur independently as well. For example, in the corneal micropocket assay, low-dose (12.5 ng) fibroblast growth factor (FGF)-2 induced new lymphatic sprouts from the limbus without stimulating new capillary growth.23 The FGF-2 was shown to stimulate VEGF-C and VEGF-D expression in the cornea, and the lymphangiogenesis induced by the low-dose FGF-2 was blocked with VEGFR-3 neutralizing antibodies. This mechanism is similar to that for IL-1β–induced lymphangiogenesis, which the authors report was inhibited using soluble VEGFR-3.1 Thus, both FGF-2 and IL-1β stimulate lymphangiogenesis in an indirect manner.
VEGF-C and VEGF-D, the major lymphangiogenic growth factors, bind VEGFR-3 to elicit growth, survival, and migration of LECs. Both ligands are secreted as proproteins and can be cleaved by proprotein convertases, such as furin or PC5.24, 25 After cleavage, VEGF-C and VEGF-D can also bind to VEGFR-2, which is highly expressed on blood capillary endothelium.26 One possible explanation for the absence of angiogenesis in the trachea model would be a lack or paucity of proprotein convertase enzymes in this model, so that VEGF-C/VEGF-D could only act on lymphatic vessels through VEGFR-3.
Another possible explanation for the lack of angiogenesis observed by Baluk et al1 is the use of Dox to induce the transgene (IL-1β). Dox is a well-established inhibitor of matrix metalloproteinases and, therefore, also an inhibitor of angiogenesis.27, 28 Some studies using the Tet-On or Tet-Off systems use relatively low doses of Dox or tetracycline to avoid these associated adverse effects. For instance, Miao et al29 used 2.4 mg/kg per day to induce neuropilin 1 expression in tumor cells in a rat model; and Benjamin and Keshet30 gave 30 mg/kg per day to inhibit VEGF expression in a mouse tumor model. A higher dose of Dox (50 mg/kg per day) was shown to significantly inhibit angiogenesis in models of choroidal neovascularization.31 Similarly, Folkman and colleagues28 used 80 mg/kg per day of Dox to inhibit tissue swelling after delayed-type hypersensitivity reactions and to inhibit tumor growth. In fact, in a case report of a woman with lymphangioleiomyomatosis, Dox given at the ultra-low dose of 20 mg/day and dose escalated to 100 mg/day (approximately equivalent to 1.5 mg/kg per day) displayed decreased urinary matrix metalloproteinase levels and improved lung function.32 The current study1 used 150 mg/kg per day of Dox (0.5 mg/mL in drinking water; normal mouse, 20-g, drinks 6 mL/day) to induce IL-1β in Clara-like CCSP-secreting cells of the trachea, the same dose previously used for this transgenic model.33 It is possible that such high doses of Dox may be inhibiting angiogenesis normally induced by IL-1β. The effect of Dox on lymphangiogenesis, specifically, has not been reported.
Summary/Questions
Baluk and colleagues,1 using an elegant tracheal model, report the novel finding that IL-1β induces the growth of stable lymphatic vessels in the absence of angiogenesis. As with all good studies, this work stimulates several interesting questions, including the following:
-
1.
What mechanisms regulate the stability of lymphatic vessels?
-
2.
Can Dox be a complicating variable in mouse models using inducible systems?
-
3.
Is inflammation-associated lymphangiogenesis different from lymphatic vessel growth outside of inflammation?
-
4.
What is the relative contribution of macrophages, paracrine and cell autonomous, to inflammatory lymphangiogenesis?
Acknowledgment
We thank Kristin Johnson for her skills with graphic design.
Footnotes
See related article on page 1434.
References
- 1.Baluk P., Hogmalm A., Bry M., Alitalo K., Bry K., McDonald D.M. Transgenic overexpression of interleukin-1beta induces persistent lymphangiogenesis but not angiogenesis in mouse airways. Am J Pathol. 2013;182:1434–1447. doi: 10.1016/j.ajpath.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zwaans B.M., Bielenberg D.R. Potential therapeutic strategies for lymphatic metastasis. Microvasc Res. 2007;74:145–158. doi: 10.1016/j.mvr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norrmén C., Tammela T., Petrova T.V., Alitalo K. Biological basis of therapeutic lymphangiogenesis. Circulation. 2011;123:1335–1351. doi: 10.1161/CIRCULATIONAHA.107.704098. [DOI] [PubMed] [Google Scholar]
- 4.Pries A.R., Kuebler W.M. Normal endothelium. Handb Exp Pharmacol. 2006;176(Pt 1):1–40. doi: 10.1007/3-540-32967-6_1. [DOI] [PubMed] [Google Scholar]
- 5.Hirschi K.K., D’Amore P.A. Control of angiogenesis by the pericyte: molecular mechanisms and significance. EXS. 1997;79:419–428. doi: 10.1007/978-3-0348-9006-9_18. [DOI] [PubMed] [Google Scholar]
- 6.Auerbach R., Lewis R., Shinners B., Kubai L., Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 7.Bruyere F., Noel A. Lymphangiogenesis: in vitro and in vivo models. FASEB J. 2010;24:8–21. doi: 10.1096/fj.09-132852. [DOI] [PubMed] [Google Scholar]
- 8.Inai T., Mancuso M., Hashizume H., Baffert F., Haskell A., Baluk P., Hu-Lowe D.D., Shalinsky D.R., Thurston G., Yancopoulos G.D., McDonald D.M. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baluk P., Lee C.G., Link H., Ator E., Haskell A., Elias J.A., McDonald D.M. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol. 2004;165:1071–1085. doi: 10.1016/S0002-9440(10)63369-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baluk P., Raymond W.W., Ator E., Coussens L.M., McDonald D.M., Caughey G.H. Matrix metalloproteinase-2 and -9 expression increases in Mycoplasma-infected airways but is not required for microvascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2004;287:L307–L317. doi: 10.1152/ajplung.00404.2003. [DOI] [PubMed] [Google Scholar]
- 11.Ezaki T., Baluk P., Thurston G., La Barbara A., Woo C., McDonald D.M. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol. 2001;158:2043–2055. doi: 10.1016/S0002-9440(10)64676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni A., Lashnits E., Yao L.C., Baluk P., McDonald D.M. Rapid remodeling of airway vascular architecture at birth. Dev Dyn. 2010;239:2354–2366. doi: 10.1002/dvdy.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okazaki T., Ni A., Ayeni O.A., Baluk P., Yao L.C., Vossmeyer D., Zischinsky G., Zahn G., Knolle J., Christner C., McDonald D.M. Alpha5beta1 Integrin blockade inhibits lymphangiogenesis in airway inflammation. Am J Pathol. 2009;174:2378–2387. doi: 10.2353/ajpath.2009.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ran S., Montgomery K.E. Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) 2012;4:618–657. doi: 10.3390/cancers4030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy J.A., Vasile E., Feng D., Sundberg C., Brown L.F., Detmar M.J., Lawitts J.A., Benjamin L., Tan X., Manseau E.J., Dvorak A.M., Dvorak H.F. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cursiefen C., Maruyama K., Jackson D.G., Streilein J.W., Kruse F.E. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25:443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 17.Betterman K.L., Paquet-Fifield A., Asselin-Labat M.L., Visvader J.E., Butler L.M., Stacker S.A., Achen M.G., Harvery N.L. Remodeling of the lymphatic vasculature during mouse mammary gland morphogenesis is mediated via epithelial-derived lymphangiogenic stimuli. Am J Pathol. 2012;181:2225–2238. doi: 10.1016/j.ajpath.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara J., Kase S., Ohashi T., Ando R., Dong Z., Noda K., Ohguchi T., Kanda A., Ishida S. Expression of vascular endothelial growth factor C in human pterygium. Histochem Cell Biol. 2013;139:381–389. doi: 10.1007/s00418-012-1019-z. [DOI] [PubMed] [Google Scholar]
- 19.Puddu A., Sanguineti R., Durante A., Nicolò M., Viviani G.L. Vascular endothelial growth factor-C secretion is increased by advanced glycation end-products: possible implication in ocular neovascularization. Mol Vis. 2012;18:2509–2517. [PMC free article] [PubMed] [Google Scholar]
- 20.Bridges J.P., Lin S., Ikegami M., Shannon J.M. Conditional hypoxia inducible factor-1α induction in embryonic pulmonary epithelium impairs maturation and augments lymphangiogenesis. Dev Biol. 2012;362:24–41. doi: 10.1016/j.ydbio.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruyama K., Ii M., Cursiefen C., Jackson D.G., Keino H., Tomita M., Van Rooijen N., Takenaka H., D’Amore P.A., Stein-Streilein J., Losordo D.W., Streilein J.W. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama K., Asai J., Ii M., Thorne T., Losordo D.W., D’Amore P.A. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170:1178–1191. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang L.K., Garcia-Cardeña G., Farnebo F., Fannon M., Chen E.J., Butterfield C., Moses M.A., Mulligan R.C., Folkman J., Kaipainen A. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A. 2004;101:11658–11663. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McColl B.K., Paavonen K., Karnezis T., Harris N.C., Davydova N., Rothacker J., Nice E.C., Harder K.W., Roufail S., Hibbs M.L., Rogers P.A., Alitalo K., Stacker S.A., Achen M.G. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. FASEB J. 2007;21:1088–1098. doi: 10.1096/fj.06-7060com. [DOI] [PubMed] [Google Scholar]
- 25.Siegfried G., Basak A., Cromlish J.A., Benjannet S., Marcinkiewicz J., Chrétien M., Seidah N.G., Khatib A.M. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J Clin Invest. 2003;111:1723–1732. doi: 10.1172/JCI17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joukov V., Sorsa T., Kumar V., Jeltsch M., Claesson-Welsh L., Cao Y., Saksela O., Kalkkinen N., Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamin M.M., Khalil R.A. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS. 2012;103:209–279. doi: 10.1007/978-3-0348-0364-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fainaru O., Adini I., Benny O., Bazinet L., Pravda E., D’Amato R., Folkman J. Doxycycline induces membrane expression of VE-cadherin on endothelial cells and prevents vascular hyperpermeability. FASEB J. 2008;22:3728–3735. doi: 10.1096/fj.08-110494. [DOI] [PubMed] [Google Scholar]
- 29.Miao H.Q., Lee P., Lin H., Soker S., Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 30.Benjamin L.E., Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci U S A. 1997;94:8761–8766. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samtani S., Amaral J., Campos J.M., Fariss R.N., Becerra S.P. Doxycycline-mediated inhibition of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:5098–5106. doi: 10.1167/iovs.08-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moses M.A., Harper J., Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med. 2006;354:2621–2622. doi: 10.1056/NEJMc053410. [DOI] [PubMed] [Google Scholar]
- 33.Lappalainen U., Whitsett J.A., Wert S.E., Tichelaar J.W., Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]