Abstract
This Commentary highlights the article by Al-Sadi et al which reports on the molecular mechanisms and intracellular pathways involved in increased intestinal tight junction permeability mediated by TNF-α.
The dynamic nature of the intestinal epithelium is central to its roles in both intestinal homeostasis and human disease. Although often referred to as the mucosal or intestinal barrier for its ability to limit harmful molecules or antigens in the lumen from entering the body, the intestinal epithelium also allows for paracellular transport of fluid, ions, and nutrients that are crucial for normal gut function. A key structural component of the mucosal barrier that contributes to intestinal epithelial permeability is the intracellular tight junction, and defects in the tight junction barrier have been implicated in the etiology of chronic intestinal inflammation. In this issue of The American Journal of Pathology, Al-Sadi et al1 report on the molecular mechanisms and intracellular pathways involved in increased intestinal tight junction permeability mediated by the pro-inflammatory cytokine, tumor necrosis factor-α (TNF-α).
The hypothesis that increased intestinal permeability contributes to the pathogenesis of inflammatory bowel disease (IBD) was first described by Shorter et al2 in the early 1970s. Although several studies followed showing an association with decreased barrier function in patients with Crohn’s disease,3, 4, 5 it was Hollander et al6 who first demonstrated that both Crohn’s disease patients and a subset of their healthy relatives have a primary defect in intestinal permeability. Other groups expanded these findings and provided a genetic link (NOD2 3020insC) for the increased intestinal permeability observed in first-degree relatives of patients with Crohn’s disease.7, 8, 9 Collectively, these studies support the concept that increased intestinal permeability may predispose one to develop chronic intestinal inflammation through increased transport of luminal antigens.
TNF-α has been shown to induce intestinal epithelial cell permeability through the increased expression and activation of myosin light-chain kinase (MLCK).10, 11, 12, 13, 14 Monoclonal antibodies directed against TNF-α have shown improvement in clinical aspects of the disease,15, 16, 17 as well as a reduction in mucosal pro-inflammatory cytokine production.18 Furthermore, the work of Suenaert et al19 showed that the intestinal tight junction barrier in patients with active Crohn’s disease could be restored with anti-TNF treatment. Although monoclonal antibodies to TNF-α, such as infliximab, have shown promise in the treatment of intestinal inflammation, there is limited knowledge regarding the intracellular mechanisms involved in the TNF-α–mediated increase in intestinal epithelial cell permeability.
Bridging the Gap between TNF-α Signaling and Tight Junction Function in Vitro
In this issue of AJP, Al-Sadi et al1 reveal the counterparts of a signaling pathway initiated in the inflamed intestinal epithelium due to a rise in TNF-α. The group established the relevance of their in vitro model, colonic epithelial CaCo2 cells, for the study of TNF-α–induced tight junction dysfunction by showing that treatment of CaCo2 monolayers with this pro-inflammatory cytokine causes increased transepithelial flux. Moreover, the downstream target of TNF-α involved in epithelial barrier function, MLCK, was also increased in TNF-α treated CaCo2 monolayers.
Because TNF-α is known to activate mitogen-activated protein (MAP) kinases, the group went on to investigate the role of MAP kinases in epithelial barrier function. They showed that TNF-α activates the MAP kinases ERK1/2, but does not affect p38. Pharmacological inhibition or siRNA silencing of ERK1/2 reversed the effect of TNF-α on MLCK gene expression and subsequently restored transepithelial resistance in CaCo2 monolayers, suggesting that ERK1/2 has a key role in the regulation of intestinal barrier function.
In an effort to connect ERK1/2 signaling with the regulation of MLCK gene expression, this study focused on the transcription factor Elk-1, a known downstream target of MAP kinases. With the use of in silico analysis, the authors identified two Elk-1 binding motifs on the promoter region of the MLCK gene, with only one of them being adequate and sufficient to promote MLCK gene expression in CaCo2 cell monolayers. TNF-α induces Elk-1 activation in an ERK1/2-dependent manner, whereas both ERK1/2 and Elk-1 are necessary for MLCK gene expression and the reduction of tight junction function in CaCo2 monolayers, thus confirming the importance of this signaling pathway in the regulation of tight junction permeability in vitro. However, how relevant and important is this molecular pathway for the regulation of intestinal barrier function in vivo?
Confirming the Importance of the TNF-α/ERK1/2/Elk/MLCK-Dependent Barrier Function in Vivo: Tools for Future Therapeutic Targets
The final parts of this study include experiments confirming in vitro findings in an in vivo model. Here, the authors used a relatively new and sophisticated technique to silence intracellular molecules in vivo. An initial evaluation of the mouse model for relevance was performed to show that intestinal permeability was increased after intraperitoneal administration of TNF-α. The authors also showed that ERK1/2 and Elk-1 activity, as well as MLCK gene expression, were upregulated after TNF-α treatment.
Perhaps the most important findings of this study involve the application of an in vivo silencing approach using siRNA. This group has knocked down target molecules after in vivo transfection of siRNA with similar efficacy in previous studies.20, 21
In these experiments, the authors selectively knocked down the expression of ERK1/2 or Elk-1 in the intestine with siRNA in vivo, thus inhibiting the effect of TNF-α on intestinal barrier function.
Today, the pro-inflammatory effects of TNF-α are commonly treated with anti–TNF-α antibodies that have taken the lead in development of immune-modulating drugs because TNF-α is known to be an important cytokine in gut diseases such as Crohn disease and ulcerative colitis.17, 22, 23, 24
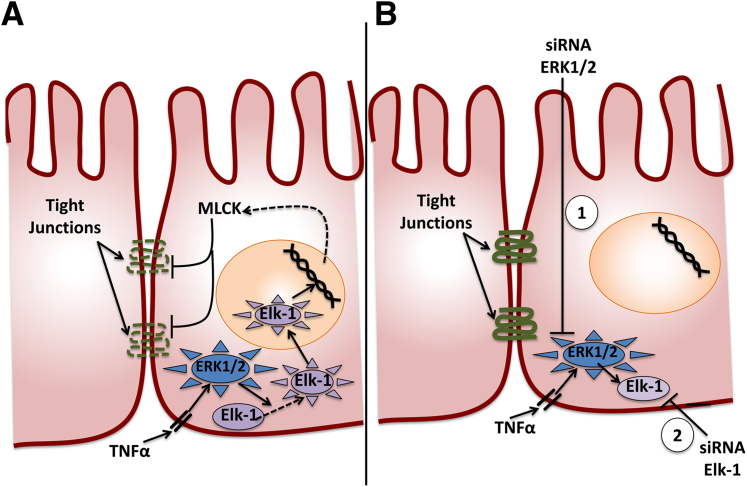
As intravenous administration of any drug presents with a number of limitations, a more localized approach could potentially optimize treatment options. Al-Sadi et al1 were able to block downstream targets of TNF-α and reverse pro-inflammatory effects in a localized manner, suggesting an alternative approach for treatment without the side effects of a systemically administered drug. In particular, the authors restored intestinal barrier function by targeting two separate TNF-α downstream molecules, directly to the mouse intestine. As shown in Figure 1, development of drugs that silence the expression of ERK1/2 or Elk-1 would prevent MLCK gene expression, thus restoring tight junction and intestinal barrier function.
Figure 1.
A: Schematic representation of the intracellular pathways involved in increased intestinal tight junction permeability mediated by the pro-inflammatory cytokine, TNF-α. B: Targeted silencing of ERK1/2 or Elk-1 expression prevents MLCK gene expression, thus restoring tight junctions and intestinal barrier function.
An important question that needs to be addressed after this study is whether in vivo silencing of ERK1/2 and/or Elk-1 ameliorates the colitis phenotype in relevant colitis mouse models. Both the trinitrobenzene sulfonic acid (TNBS) and the dextran sodium sulfate (DSS) colitis mouse models, as well as the immune models of intestinal inflammation, are widely accepted models for the study of inflammatory bowel disease. For example, future studies should address the extent of colitis in mice treated with siRNA against ERK1/2 or Elk-1 targeted to the intestine in vivo.
More importantly, the findings in the line of work discussed in this commentary suggest that similar in vivo silencing may be applied on other intracellular molecules exerting pro-inflammatory effects in the gut. The development of drugs that administer such silencing agents to the site of injury in the intestine should become a major focus in the field of gastroenterology.
Footnotes
Supported in part by a Crohns' and Colitis Foundation of America Fellowship (Ref. #3475) (K.B.).
See related article on page 1871
References
- 1.Al-Sadi R.G.S., Dongmei Y., Ma T.Y. TNF-α Modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol. 2013;183:1871–1884. doi: 10.1016/j.ajpath.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shorter R.G., Huizenga K.A., Spencer R.J. A working hypothesis for the etiology and pathogenesis of nonspecific inflammatory bowel disease. Am J Dig Dis. 1972;17:1024–1032. doi: 10.1007/BF02239143. [DOI] [PubMed] [Google Scholar]
- 3.Ukabam S.O., Clamp J.R., Cooper B.T. Abnormal small intestinal permeability to sugars in patients with Crohn’s disease of the terminal ileum and colon. Digestion. 1983;27:70–74. doi: 10.1159/000198932. [DOI] [PubMed] [Google Scholar]
- 4.Bjarnason I., O’Morain C., Levi A.J., Peters T.J. Absorption of 51chromium-labeled ethylenediaminetetraacetate in inflammatory bowel disease. Gastroenterology. 1983;85:318–322. [PubMed] [Google Scholar]
- 5.Pearson A.D., Eastham E.J., Laker M.F., Craft A.W., Nelson R. Intestinal permeability in children with Crohn’s disease and coeliac disease. Br Med J (Clin Res Ed) 1982;285:20–21. doi: 10.1136/bmj.285.6334.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollander D., Vadheim C.M., Brettholz E., Petersen G.M., Delahunty T., Rotter J.I. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 7.Teshima C.W., Dieleman L.A., Meddings J.B. Abnormal intestinal permeability in Crohn’s disease pathogenesis. Ann NY Acad Sci. 2012;1258:159–165. doi: 10.1111/j.1749-6632.2012.06612.x. [DOI] [PubMed] [Google Scholar]
- 8.Buhner S., Buning C., Genschel J., Kling K., Herrmann D., Dignass A., Kuechler I., Krueger S., Schmidt H.H., Lochs H. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irvine E.J., Marshall J.K. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology. 2000;119:1740–1744. doi: 10.1053/gast.2000.20231. [DOI] [PubMed] [Google Scholar]
- 10.Turner J.R., Angle J.M., Black E.D., Joyal J.L., Sacks D.B., Madara J.L. PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am J Physiol. 1999;277:C554–C562. doi: 10.1152/ajpcell.1999.277.3.C554. [DOI] [PubMed] [Google Scholar]
- 11.Turner J.R., Rill B.K., Carlson S.L., Carnes D., Kerner R., Mrsny R.J., Madara J.L. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 12.Ye D., Ma I., Ma T.Y. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 13.Zolotarevsky Y., Hecht G., Koutsouris A., Gonzalez D.E., Quan C., Tom J., Mrsny R.J., Turner J.R. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 14.Clayburgh D.R., Musch M.W., Leitges M., Fu Y.X., Turner J.R. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dullemen H.M., van Deventer S.J., Hommes D.W., Bijl H.A., Jansen J., Tytgat G.N., Woody J. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 16.Targan S.R., Hanauer S.B., van Deventer S.J., Mayer L., Present D.H., Braakman T., DeWoody K.L., Schaible T.F., Rutgeerts P.J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 17.D’Haens G., Van Deventer S., Van Hogezand R., Chalmers D., Kothe C., Baert F., Braakman T., Schaible T., Geboes K., Rutgeerts P. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn’s disease: a European multicenter trial. Gastroenterology. 1999;116:1029–1034. doi: 10.1016/s0016-5085(99)70005-3. [DOI] [PubMed] [Google Scholar]
- 18.Baert F.J., D’Haens G.R., Peeters M., Hiele M.I., Schaible T.F., Shealy D., Geboes K., Rutgeerts P.J. Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn’s ileocolitis. Gastroenterology. 1999;116:22–28. doi: 10.1016/s0016-5085(99)70224-6. [DOI] [PubMed] [Google Scholar]
- 19.Suenaert P., Bulteel V., Lemmens L., Noman M., Geypens B., Van Assche G., Geboes K., Ceuppens J.L., Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 20.Al-Sadi R., Guo S., Dokladny K., Smith M.A., Ye D., Kaza A., Watterson D.M., Ma T.Y. Mechanism of interleukin-1beta induced-increase in mouse intestinal permeability in vivo. J Interferon Cytokine Res. 2012;32:474–484. doi: 10.1089/jir.2012.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye D., Guo S., Al-Sadi R., Ma T.Y. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323–1333. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komatsu M., Kobayashi D., Saito K., Furuya D., Yagihashi A., Araake H., Tsuji N., Sakamaki S., Niitsu Y., Watanabe N. Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin Chem. 2001;47:1297–1301. [PubMed] [Google Scholar]
- 23.Braegger C.P., Nicholls S., Murch S.H., Stephens S., MacDonald T.T. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 24.Breese E.J., Michie C.A., Nicholls S.W., Murch S.H., Williams C.B., Domizio P., Walker-Smith J.A., MacDonald T.T. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]