Abstract
Purpose/Objectives
Radiation therapy (RT) is the principal modality in the treatment of patients with brain metastases (BM). However, given the activity of EGFR tyrosine kinase inhibitors in the central nervous system (CNS), it is uncertain whether upfront brain RT is necessary for patients with EGFR-mutant lung adenocarcinoma with BM.
Methods and Materials
Patients with EGFR-mutant lung adenocarcinoma and newly diagnosed BM were identified.
Results
222 patients were identified. Exclusion criteria included prior erlotinib use, presence of a de novo erlotinib resistance mutation, or incomplete data. Of the remaining 110 patients, 63 were treated with erlotinib, 32 with whole-brain RT (WBRT), and 15 with stereotactic radiosurgery (SRS). Median OS for the whole cohort was 33 months. There was no significant difference in OS between the WBRT and erlotinib groups (median 35 vs. 26 months, p = .62), while patients treated with SRS had a longer OS compared with the erlotinib group (median, 64 months, p = .004). Median time to ICP was 17 months. There was a longer time to ICP in patients who received WBRT vs. erlotinib upfront (median 24 vs. 16 months, p = .04). Patients in the erlotinib or SRS group were more likely to fail intracranially as a component of first failure, while WBRT patients were more likely to fail outside the brain (p = .004).
Conclusions
The survival of patients with EGFR-mutant adenocarcinoma with BM is notably long, whether they receive upfront erlotinib or brain RT. We observed longer intracranial control with WBRT, even though the WBRT patients had a higher burden of intracranial disease. Despite the equivalent survival between the WBRT and erlotinib group, this study underscores the role of WBRT in producing durable intracranial control in comparison to a targeted biologic agent with known CNS activity.
Keywords: EGFR mutations, brain metastases, whole brain radiation therapy
INTRODUCTION
Lung cancer is the leading cause of death in the United States with an estimated 226,000 diagnoses and 160,000 deaths in 2012 [1]. Non-small cell lung cancer (NSCLC) comprises the majority of lung cancer diagnoses. Approximately 20–40% of patients with NSCLC develop brain metastases (BM) during the course of their illness [2]. Historically, whole-brain radiation therapy (WBRT), alone or in combination with surgery and stereotactic radiosurgery (SRS), has been the standard of care for BM. In historical series of WBRT for solid tumors, median OS is only 4.5 months [3]. More recent data examining survival in patients with BM in a population selected for patients with EGFR mutations have shown survival rates of 14.5–17 months from the time of BM development [4–6].
In recent years, EGFR tyrosine kinase inhibitors (TKI) have replaced cytotoxic chemotherapy as first-line therapy for patients with metastatic EGFR-mutant disease, based on randomized trials demonstrating improved survival with EGFR-TKI compared with cytotoxic chemotherapy [7–9]. Yet the management of patients with EGFR mutations and BM remains controversial. There are lower rates of central nervous system (CNS) progression in EGFR-mutant advanced NSCLC patients treated with an EGFR-TKI compared with chemotherapy [10]. Phase II trials have demonstrated efficacy of EGFR-TKI as treatment for BM in patients with EGFR-mutant NSCLC without the upfront use of radiation [5, 11]. Other Phase I and Phase II trials have explored the concurrent use of WBRT and erlotinib [12, 13] and a Phase III trial investigated the combination of WBRT, SRS, and erlotinib [14]. However, the relative benefits of radiation therapy (RT) vs. EGFR-TKI in EGFR-mutated patients with BM have not been determined. Because brain RT is associated with potential neurocognitive long-term toxicities, it is of significant clinical interest whether EGFR-TKI therapy is sufficient to manage BM in this population.
We therefore conducted a retrospective review of patients at our institution who were diagnosed with EGFR-mutated adenocarcinoma with BM. To our knowledge, this is the first analysis that directly compares RT against EGFR-TKI as first-line therapy for BM in EGFR-mutant adenocarcinoma. We hypothesized that there would be no difference in overall survival (OS) between patients treated with upfront WBRT vs. EGFR-TKI, and that rates of intracranial progression (ICP) would be lower in the WBRT group.
MATERIALS AND METHODS
Study design and patients
Using an institutional query system, we identified all patients with BMs and lung adenocarcinoma that harbored EGFR mutations treated at our institution from 2006 to 2012. 2006 was when our institution initiated reflex testing for EGFR mutations in all NSCLC patients. Since our goal was to compare RT and EGFR-TKI in the treatment of EGFR-TKI–naïve patients, we excluded all patients who developed BM while already receiving EGFR-TKI. The majority of these patients had already developed resistance to EGFR-TKI and thus their inclusion would have introduced bias in the comparison of EGFR-TKI to RT. For similar reasons, we also excluded patients with de novo EGFR-TKI resistance mutations. Finally, we excluded patients who came to our institution for consultation only, or who did not have any pretreatment brain imaging that could be used to measure ICP.
The remaining patients were classified into three groups: 1) patients treated with erlotinib upon diagnosis of BM, either alone or in combination with cytotoxic chemotherapy; 2) patients treated with WBRT, with or without the addition of erlotinib after completion of radiation; and 3) patients treated with SRS, either in a single fraction or in 3–5 fractions.
Information was collected on baseline variables such as age at diagnosis of BM, sex, stage at diagnosis, Graded Prognostic Assessment (GPA) at diagnosis of BM [15], smoking history, neurologic symptoms, type of EGFR mutation, and the number and size of BMs on pretreatment imaging. All variables were compared between treatment groups using a global test by Chi-square or Fisher’s exact test for categorical data and one-way analysis of variance for continuous data.
Mutation analysis
Mutation analysis was conducted by extracting DNA and identifying EGFR exon 19 deletions and exon 21 L858R mutations by standard sequencing and/or fragment analysis as previously described [16–18]. Beginning in January 2009, 92 specific point mutations in multiple genes were identified using a mass spectrometry-based mutation profiling assay (Sequenom, San Diego, CA) [18]. Mutation analysis was performed on subsequent biopsies to identify the mechanism of resistance to EGFR-TKI therapy.
Statistical methods and design
OS and ICP were estimated using the Kaplan-Meier method and Cox proportional hazards regression was used to determine factors associated with OS and ICP. All endpoints were calculated from date of BM diagnosis. For analyses of OS, patients in all three treatment groups were included. For analyses of ICP, only patients treated with erlotinib or WBRT were included, since intracranial failure in SRS patients may represent progression in untreated brain and not treatment failure. ICP was determined by reviewing all MRI and computed tomography (CT) scans of the head subsequent to development of BM. ICP was defined as radiographic progression of pre-existing BM and/or the development of new BM. All calculations of ICP utilized the date of progression for patients who progressed intra-cranially or date of last scan for patients who did not progress, thereby eliminating the need to consider death as a competing risk. All patients had follow-up imaging available for analysis of ICP. Leptomeningeal failure was analyzed using Gray’s and Fine-Gray competing-risks methods with death without event as a competing risk. Progression of disease after diagnosis of BM was characterized as extracranial, intracranial, or both simultaneously. MRI scans were reviewed to determine if intracranial failure was due to growth of pre-existing lesions, development of new lesions, or both. Chi-square or Fisher’s exact test was used to compare site of first failure and nature of intracranial failure between the different treatment groups. The statistical analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC) and R 3.0.1 package “cmprsk.”
RESULTS
Patient characteristics
Between January 1, 2006 and December 31, 2012, 222 patients were identified with metastatic EGFR-mutated adenocarcinoma with BM. Fifty-seven patients were excluded because they received EGFR-TKI prior to the development of BM and 7 patients were excluded with de novo EGFR-TKI resistance mutations. Forty-eight additional patients were excluded because they came to our institution for consultation only, or because there was no pretreatment imaging. The remaining 110 patients were treated for BM with upfront erlotinib (n = 63), WBRT (n = 32), or SRS (n = 15). Twenty-one of the patients in the WBRT group also received erlotinib within 2 months of completing RT. No patients were treated concurrently with WBRT and erlotinib. Table 1 shows the baseline characteristics of the patients according to treatment group. Patients were predominantly female (68%) and never-smokers (62%) with KPS ≥80 (83%). Most patients were stage IV at initial diagnosis (92%). The majority of patients in the erlotinib and WBRT groups were GPA 0.0–2.0 whereas the majority of patients in the SRS group were GPA 2.5–4.0, though this difference was not statistically significant (p=0.10). In the erlotinib group, only 3% of patients were symptomatic, with higher percentages of symptomatic patients in the WBRT and SRS groups (31% and 53%, respectively). Patients had a median of 4 BM with a median largest metastasis diameter of 11 mm. Patients in the WBRT group were more likely to have a greater number of BM and larger-sized BM. There were no significant differences in the subtype of EGFR mutation among the three groups.
Table 1.
Baseline characteristics
| Factor | Treatment, n (%)
|
p value1 | ||
|---|---|---|---|---|
| Erlotinib (n=63) |
Whole-brain radiation therapy (n=32) |
Stereotactic radiosurgery (n=15) |
||
| Age at BM diagnosis (year), | ||||
| mean ± SD | 62 ± 13 | 58 ± 11 | 61 ± 11 | 0.32 |
| Gender | 0.69 | |||
| Female | 44 (70) | 20 (63) | 11 (73) | |
| Male | 19 (30) | 12 (38) | 4 (27) | |
| Stage | 0.77 | |||
| I–III | 5 (8) | 2 (6) | 2 (13) | |
| V | 58 (92) | 30 (94) | 13 (87) | |
| Symptomatic from BM | <0.0001 | |||
| No | 61 (97) | 22 (69) | 7 (47) | |
| Yes | 2 (3) | 10 (31) | 8 (53) | |
| Smoking status | 0.23 | |||
| Non-smoker/<100 cigs lifetime | 43 (69) | 17 (53) | 8 (53) | |
| Smoker | 19 (31) | 15 (47) | 7 (47) | |
| NA1 | 1 (2) | 0 (0) | 0 (0) | |
| Graded Prognostic Assessment | 0.10 | |||
| 0.0–2.0 | 40 (64) | 19 (59) | 5 (33) | |
| 2.5–4.0 | 23 (37) | 13 (41) | 10 (67) | |
| Number of BMs | ||||
| ≤3 | 33 (52) | 4 (13) | 12 (80) | |
| >3 | 30 (48) | 28 (88) | 3 (20) | <0.0001 |
| Largest size of BMs | ||||
| ≤10 mm | 46 (73) | 5 (16) | 4 (27) | |
| >10 mm | 17 (27) | 27 (84) | 11 (73) | <0.0001 |
| EGFR mutation | ||||
| Exon 19 deletion | 36 (57) | 25 (78) | 7 (47) | |
| Exon 21 L858R | 26 (41) | 7 (22) | 6 (40) | 0.12 |
| Other1 | 1 (2) | 0 (0) | 2 (13) | |
Excluded from analysis due to small number.
BM, brain metastasis; NA, not available; SD, standard deviation.
Treatment characteristics
Patients treated with erlotinib upfront were primarily treated with erlotinib monotherapy (n = 59). Four patients were treated with erlotinib plus cytotoxic chemotherapy. Median dose for patients treated with WBRT was 3000 cGy in 10 fractions (range, 3000c–3750 cGy). Of the 15 patients treated with SRS, 12 (80%) were treated with a single-fraction to one (58%) or multiple (42%) lesions. Median dose of single-fraction SRS was 2000 cGy (range, 1500–2100). Eight patients underwent craniotomy prior to initiation of erlotinib (n = 1), WBRT (n = 2), or SRS (n = 5).
Of the patients treated with erlotinib upfront, 35% (n=22) were seen by a radiation oncologist prior to the decision to treat with erlotinib. Sixty-two percent never received brain radiation. The patients who eventually received RT (n = 24) did so at a median of 17 months (range, 5–40 months) after the diagnosis of BM.
Outcomes
Median follow-up for all patients was 20 (range 3–75) months. Median OS from diagnosis of BM was 33 months (95% confidence interval [CI], 23.3–39.9 months). The Kaplan-Meier curve for OS is shown in Fig. 1. OS did not differ significantly between the erlotinib and WBRT group, with a median OS of 26 months and 35 months, respectively (p = .62) (Fig. 2). The SRS group had significantly longer OS with a median of 64 months (vs. erlotinib group, p = .006). The only other factor associated with longer OS on univariate analysis (UVA) was GPA class. The effect of treatment group on OS retained significance on multivariate analysis (MVA) (SRS vs. erlotinib: HR 0.26, 95% CI 0.09–0.78, p=0.02). There was a trend that higher GPA class was associated with improved OS after adjusting for the treatment type (2.5–4.0 vs. 0.0–2.0: HR 0.59, 95% CI 0.33 – 1.04, p=0.07)
Figure 1.
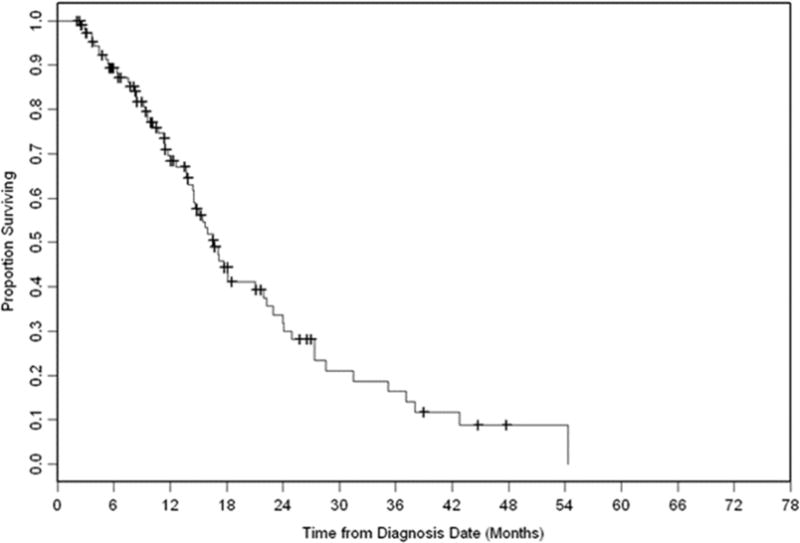
Overall survival of whole cohort.
Figure 2.
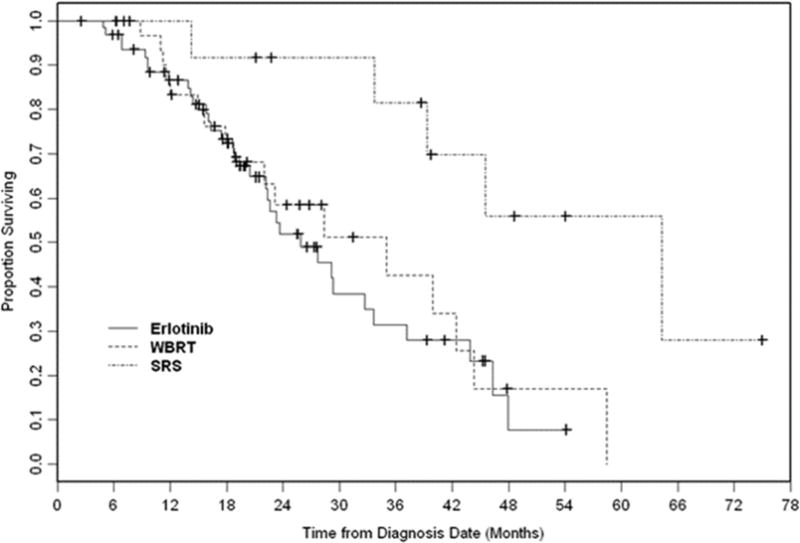
Overall survival by treatment group (erlotinib, whole-brain radiation therapy [WBRT], stereotactic radiosurgery [SRS]).
Sixty-four out of all patients progressed intracranially (58%) with a median time to ICP of 17 months. As shown in Fig. 3, there was a longer time to ICP in the WBRT group with a median time of 24 months compared with median of 16 months in the erlotinib group (p = .04). Two-year ICP-free survival was 26% in the erlotinib and 52% in the WBRT group. In addition to receipt of WBRT, larger size of brain metastases (>10 mm) was also associated with a longer time to ICP (p = .003). On MVA, the size of brain metastases remained significant (p = .003). Type of EGFR mutation did not correlate with time to ICP.
Figure 3.
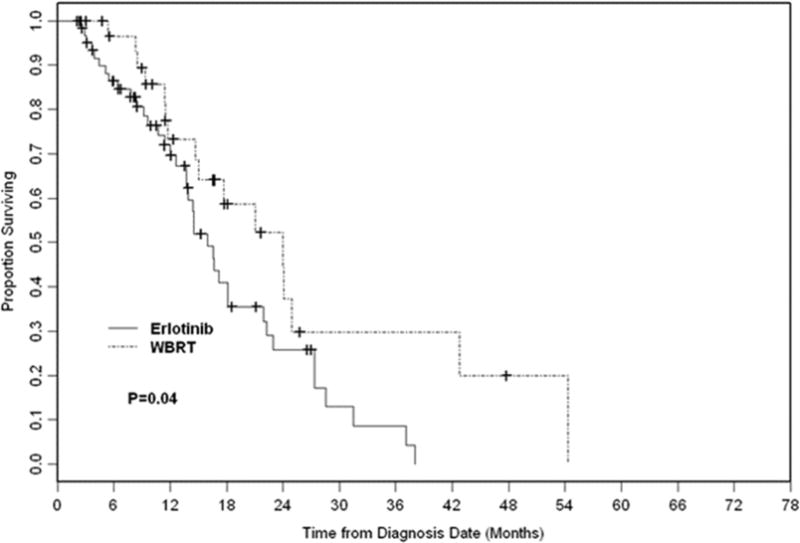
Intracranial progression in whole-brain radiation therapy (WBRT) vs. erlotinib.
Among patients in the WBRT group who received erlotinib within 2 months of completing radiation (n=21), median time to ICP was 25 months with a two-year ICP-free survival of 64% (vs. 52% in the entire WBRT group). When compared to patients treated with erlotinib alone, receipt erlotinib within 2 months of WBRT was associated with improved intra-cranial control on UVA (p=.01), but this effect lost significance on MVA (p=0.20).
Patterns of both intra- and extracranial failure differed significantly by treatment group during the follow-up period (p = .004). As shown in Table 2, among patients who progressed (n = 90), patients treated with WBRT were less likely to fail in the brain as a component of first failure (24%), compared with 58% of patients treated with erlotinib and 71% of patients treated with SRS (p = .004). Patterns of failure within the brain differed significantly for the 63 patients who experienced ICP (p = .03). As shown in Table 3, patients treated with WBRT or SRS were much less likely to have progression of pre-existing lesions (53% and 30%, respectively) compared with 79% of patients treated with erlotinib.
Table 2.
First site of progression by treatment type
| Factor | Treatment, n (%)
|
p value | ||
|---|---|---|---|---|
| Erlotinib (n=52) |
Whole-brain radiation therapy (n=25) |
Stereotactic Radiosurgery (n=14) |
||
| First site of progression | 0.004 | |||
| Outside of the brain | 21 (41) | 19 (76) | 4 (29) | |
| Brain or both at same time | 30 (59) | 6(24) | 10 (71) | |
Table 3.
Patterns of intracranial progression
| Factor | Treatment, n (%)
|
p value | ||
|---|---|---|---|---|
| Erlotinib (n=3 8) |
Whole-brain radiation therapy (n=151) |
Stereotactic radiosurgery (n=10) |
||
| Intracranial progression | 0.03 | |||
| New lesion | 8 (21) | 7 (47) | 7 (70) | |
| Old lesion | 11 (29) | 2 (13) | 2 (20) | |
| Both | 19 (50) | 6 (40) | 1(10) | |
Thirty-nine patients were biopsied at time of progression (intracranial or extracranial) with 18 patients harboring an acquired EGFR T790M mutation and one patient with an EGFR G719S mutation. The median time between erlotinib initiation and development of biopsy-proven resistance was 13 months.
Eighteen patients (16%) developed leptomeningeal disease at a median of 15 months. There was no difference between the erlotinib and WBRT groups with regard to cumulative incidence of leptomeningeal disease (p = .41). There were no leptomeningeal failures in the SRS group (vs. erlotinib, p = .09).
DISCUSSION
To our knowledge, this is the first report directly comparing EGFR-TKI treatment to brain RT in a molecularly selected group of patients with NSCLC and BM. We found similar rates of survival, but a significantly increased time to ICP among patients treated with WBRT vs. erlotinib, with 1- and 2-year rates of intracranial control of 73% and 52%. This difference in intracranial control lost significance on MVA.
EGFR-TKIs are well established as first-line systemic therapy for patients with metastatic EGFR-mutant NSCLC [7–9], but whether additional brain-directed therapy is necessary for treatment of BM has not yet been determined prospectively. This question is particularly important in the context of preliminary evidence that patients with EGFR-mutant lung cancers may be more likely to develop BMs than patients with wild-type tumors [9, 19, 20]. The long OS observed for patients with EGFR-mutant lung cancer and brain metastases, both in our series and other studies, further underscores the need to define the optimal therapy for these patients, who are more likely to experience late neurotoxicity from treatment such as WBRT.
There is prospective evidence that EGFR-TKIs are effective as treatment for BM. In a Phase II trial in molecularly selected patients, EGFR-TKI resulted in an intracranial disease control rate of 93%, with 83% having a partial response and 11% demonstrating stable disease [5]. In a second Phase II trial, patients with EGFR-mutant NSCLC and BM treated with EGFR-TKI had a longer intracranial median progression-free survival of 15.2 months compared with wild-type patients (median progression-free survival of 4.4 months) (p = .02) [11].
Other studies have investigated the combination of WBRT and erlotinib with the rationale that EGFR-TKI are radiosensitizing, due to reduction of proliferation, inhibition of antiapoptotic pathways, inducing cell-cycle redistribution leading, and inhibition of DNA repair [21, 22]. A retrospective analysis showed higher response rates to WBRT in patients with EGFR-mutant lung adenocarcinoma compared with those with wild-type tumors. The administration of an EGFR-TKI during WBRT was independently associated with response [6]. Several Phase I and Phase II trials have explored concurrent erlotinib and WBRT [12, 13]. A Phase II trial conducted in a molecularly unselected population demonstrated that the combination of erlotinib and WBRT was both safe and efficacious with an overall response rate of 86% and no increase in neurotoxicity or enhancement of the erlotinib-related rash in the treatment portal area [13]. Patients with EGFR-mutant lung cancers had increased survival with a median survival of 19.1 months vs. 9.3 months for wild-type patients (p=.53). Though no patients were treated concurrently with WBRT and erlotinib in our series, those who received erlotinib within 2 months of WBRT had better intracranial control compared to the WBRT group as a whole. This suggests the additive and potentially synergistic activity of erlotinib and WBRT but we cannot be conclusive, since the effect was no longer significant on MVA.
A recent study comparing the combination of SRS and WBRT alone vs. SRS and WBRT with erlotinib showed inferior results in the erlotinib group with a shorter OS (13.4 vs. 6.1 months, respectively) and no improvement in time to CNS progression [14]. This trial is surprising in light of other data showing improved results when erlotinib is combined with radiation. That the population was not selected for patients with EGFR-mutant lung cancer is the most likely explanation for these surprisingly poor results. Also, the shorter OS is possibly due to excess toxicity of SRS, WBRT, and erlotinib (49% grade 3–5 toxicity) compared with WBRT and SRS alone (11% grade 3–5 toxicity).
The median OS in our series of 32.7 months is comparable to other trials that segregate patients by EGFR mutation status [13, 23]. Of note, patients treated with SRS had a significantly longer OS. We would expect this difference to reflect the better performance status and fewer BM in the SRS group compared to the erlotinib and WBRT groups. On MVA, GPA, which captures both the number of BM and performance status, trended towards significance for improved OS (p=0.07) and SRS remained significant even after adjusting for GPA (p=0.02). Given the small number of patients in this study and its retrospective nature, it is hard to draw definitive conclusions on the association between SRS and OS independent of GPA though we make note of this finding. In the randomized RTOG 9508 trial, patients with NSCLC had a trend towards improved OS when SRS was added to WBRT (p=0.0508). Though this trial was comparing WBRT with or without SRS, it does represent a randomized trial in which SRS improved OS in NSCLC patients [24]. A retrospective study with similar design to our study compared systemic chemotherapy, upfront WBRT, and SRS in an unselected NSCLC population and also showed a survival advantage in the SRS group in a subgroup analysis of patients with adenocarcinoma. A MVA was not performed in this subgroup to examine confounding factors such as GPA class or extent of intracranial disease [25].
In addition to finding a difference in time to ICP between the erlotinib and WBRT groups on univariate analysis, we also found differences in patterns of failure, with patients treated with erlotinib or SRS much more likely to fail intracranially as a component of first failure and WBRT patients more likely to fail extracranially. There was a significant association between BM size and treatment group, with 84% of patients in the WBRT group having BM greater than 10 mm and only 27% of patients in the erlotinib group having BM greater than 10 mm. However, BM size, and not treatment group, remained significant on MVA for time to intracranial progression. This is contrary to what one would expect, as larger BMs, in the absence of treatment considerations, seem more likely to progress intracranially. One possible explanation is that larger BMs represent a more indolent disease process. Their larger size at diagnosis of BM may be a result of their being slow growing and thus asymptomatic, leading to delayed time to diagnosis and larger size at diagnosis. We did not find a difference in rates of intracranial progression based on EGFR mutation type, in contrast to a prior series that found higher rates of CNS progression in patients with exon 19 deletions compared with patients with L858R mutations [23].
This study highlights the need for multidisciplinary management of patients with EGFR-mutated NSCLC and BM. Only 35% of patients in the erlotinib group saw a radiation oncologist prior to the decision to defer radiation. Regardless of the ultimate decision made for each patient, the input of radiation oncology is crucial, particularly in light of the findings of this study.
The results of this study are limited by the retrospective nature of the analysis. A large number of patients were excluded from the analysis due to lack of baseline imaging or other relevant data in the electronic medical record. It is possible that the excluded population introduces bias that we are unable to account for and is not representative of the larger sample. Furthermore, each treatment group is not homogenous with regard to baseline characteristics, dose of radiation, and the sequencing of erlotinib, radiation, and cytotoxic chemotherapy. The reported patterns and rates of progression both intra- and extracranially were limited by the frequency of imaging and clinical evaluation and the length of follow-up. Finally, this data represents patients from a single institution, which may limit its applicability.
Until data matures from ongoing prospective trials including the TRACTS trial (clinical trials.gov NCT01763385), comparing concurrent WBRT and erlotinib to erlotinib alone with WBRT at time of progression, or the TACTIC trial (clinicaltrials.gov NCT00554775), comparing WBRT and erlotinib with WBRT alone, this study represents the only data directly comparing upfront erlotinib with radiation for the treatment of BM in patients with EGFR-mutant lung cancers. Patients treated with WBRT had a longer duration of intracranial control compared with those treated with erlotinib, even though the WBRT patients had a greater burden of intracranial disease. Although this effect lost significance on MVA, this study underscores the role of WBRT in intracranial control when compared to targeted biologic agents with known CNS activity. Whether there are subsets of patients treated with erlotinib in whom WBRT can be omitted remains to be proven in future studies.
Summary.
This is a retrospective comparison of erlotinib and radiation therapy (RT) for patients with EGFR-mutant lung adenocarcinoma and brain metastases who are naïve to erlotinib. We found a longer time to intracranial progression among patients treated with whole-brain RT as compared to patients treated with erlotinib, with no difference in overall survival between these two groups. This study underscores the role of RT even when targeted biologic agents with known CNS activity are available.
Acknowledgments
The authors would like to thank Lawrence Herman for his editorial assistance with this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None. For full disclosure information, please see attached disclosure forms.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–72. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 3.Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21(13):2529–36. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 4.Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12(11):1193–9. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77(3):556–60. doi: 10.1016/j.lungcan.2012.05.092. [DOI] [PubMed] [Google Scholar]
- 6.Gow CH, Chien CR, Chang YL, et al. Radiotherapy in lung adenocarcinoma with brain metastases: effects of activating epidermal growth factor receptor mutations on clinical response. Clin Cancer Res. 2008;14(1):162–8. doi: 10.1158/1078-0432.CCR-07-1468. [DOI] [PubMed] [Google Scholar]
- 7.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 8.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 9.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 10.Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18(16):4406–14. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803) Ann Oncol. 2013;24(4):993–9. doi: 10.1093/annonc/mds529. [DOI] [PubMed] [Google Scholar]
- 12.Lind JS, Lagerwaard FJ, Smit EF, et al. Phase I study of concurrent whole brain radiotherapy and erlotinib for multiple brain metastases from non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;74(5):1391–6. doi: 10.1016/j.ijrobp.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31(7):895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85(5):1312–8. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with bran metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7(3):396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Angelo SP, Park B, Azzoli CG, et al. Reflex testing of resected stage I through III lung adenocarcinomas for EGFR and KRAS mutation: report on initial experience and clinical utility at a single center. J Thorac Cardiovasc Surg. 2011;141(2):476–80. doi: 10.1016/j.jtcvs.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–7. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto S, Takahashi K, Iwakawa R, et al. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer. 2006;119(6):1491–4. doi: 10.1002/ijc.21940. [DOI] [PubMed] [Google Scholar]
- 20.Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 2012;18(4):938–44. doi: 10.1158/1078-0432.CCR-11-2529. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Munshi A, Brooks C, et al. Gefitinib radiosensitizes non-small cell lung cancer cells by suppressing cellular DNA repair capacity. Clin Cancer Res. 2008;14(4):1266–73. doi: 10.1158/1078-0432.CCR-07-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva) Cancer Res. 2005;65(8):3328–35. doi: 10.1158/0008-5472.CAN-04-3547. [DOI] [PubMed] [Google Scholar]
- 23.Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16(23):5873–82. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–72. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 25.Kim KH, Lee J, Lee JI, et al. Can upfront systemic chemotherapy replace stereotactic radiosurgery or whole brain radiotherapy in the treatment of non-small cell lung cancer patients with asymptomatic brain metastases? Lung Cancer. 2010;68(2):258–63. doi: 10.1016/j.lungcan.2009.06.008. [DOI] [PubMed] [Google Scholar]


