Abstract
Background: Malaria mosquitoes form mating swarms around sunset, often at the same locations for months or years. Unfortunately, studies of Anopheles swarms are rare in East Africa, the last recorded field observations in Tanzania having been in 1983.
Methods: Mosquito swarms were surveyed by trained volunteers between August-2016 and June-2017 in Ulanga district, Tanzania. Identified Anopheles swarms were sampled using sweep nets, and collected mosquitoes killed by refrigeration then identified by sex and taxa. Sub-samples were further identified by PCR, and spermatheca of females examined for mating status. Mosquito ages were estimated by observing female ovarian tracheoles and rotation of male genitalia. GPS locations, types of swarm markers, start/end times of swarming, heights above ground, mosquito counts/swarm, and copulation events were recorded.
Results: A total of 216 Anopheles swarms were identified, characterized and mapped, from which 7,142 Anopheles gambiae s.l and 13 Anopheles funestus were sampled. The An. gambiae s.l were 99.6% males and 0.4% females, while the An. funestus were all males. Of all An. gambiae s.l analyzed by PCR, 86.7% were An. arabiensis, while 13.3% returned non-amplified DNA. Mean height (±SD) of swarms was 2.74±0.64m, and median duration was 20 (IQR; 15-25) minutes. Confirmed swarm markers included rice fields (25.5%), burned grounds (17.2%), banana trees (13%), brick piles (8.8%), garbage heaps (7.9%) and ant-hills (7.4%). Visual estimates of swarm sizes by the volunteers was strongly correlated to actual sizes by sweep nets (R=0.94; P=<0.001). All females examined were nulliparous and 95.6% [N=6787] of males had rotated genitalia, indicating sexual maturity.
Conclusions: This is the first report of Anopheles swarms in Tanzania in more than three decades. The study demonstrates that the swarms can be identified and characterized by trained community-based volunteers, and highlights potential new interventions, for example targeted aerosol spraying of the swarms to improve malaria control.
Keywords: Anopheles swarms, mating behavior of mosquitoes, malaria, vector control
Background
Recent successes in malaria control have been mostly attributed to vector control measures, in particular, long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) 1. These tools have contributed to ~78% of all gains achieved since 2000 1, 2. However, effectiveness of these interventions is compromised, partly due to spread of insecticide resistance 3 and increased outdoor exposure to mosquito bites among other challenges 4– 6. Consequently, persistent malaria transmission still occurs in many places, even where high and sustained coverage of LLINs has been achieved.
To develop new options for malaria vector control, it is important to re-examine the overall ecology of the malaria vectors 7, including not only the blood-feeding and resting habits commonly targeted by LLINs and IRS, but also other mosquito habits indoors and outdoors. Mating behavior is one of the most important aspects in the maintenance of species 8, yet it is a widely under-investigated aspect of the mosquito biology. Improved understanding mosquito mating systems could possibly provide new opportunities for expanding vector control options. Most insects mate in swarms, whereby dispersed populations aggregate at specific times and places 9, 10. In mosquitoes, including the malaria vectors, swarming flights are influenced by presence of visual markers on the ground 11, 12. Targeting swarms to deplete mosquito densities indeed offer unrivalled opportunities to drastically reduce mosquito-borne pathogen transmission 13. Such an approach has proven effective against some Anopheles mosquitoes on a limited scale in Burkina Faso 14, but needs to be validated for other vector species in other areas. Besides, mosquito swarms are known to occur perpetually in the same locations at approximately the same time each day 10, 15, thus targeting them could be easily achieved by trained community volunteers. The concentrations of males in swarms, predictability and accessibility of the swarming sites and the fact that swarms can be artificially manipulated 16, makes the male mosquitoes vulnerable and an easy control target.
While swarms have been commonly identified in west and central Africa 17– 19, observations have rarely been reported in East and Southern Africa, most probably because mosquito swarming has not been as thoroughly investigated in this area, as it has been in West Africa. Prior to this current report, there was only a handful of reports, including the seminal work by R.P. Marchand in Tanzania in the early 1980s 20, the work by J.D. Charlwood in Mozambique in the early 2000s 21, and unpublished observations of An. arabiensis swarms in the Kilombero valley in south eastern Tanzania (Dr. Kija Nghabi and Japhet Kihonda, Personal Communication).
This current study was designed to explore the Anopheles mating systems in rural south-eastern Tanzania, by assessing occurrence of natural swarming and outdoor nocturnal behaviors of Anopheles mosquitoes. The assessment involved accurate identification, mapping and characterizing of mosquito swarms in three study villages in Ulanga district, Tanzania. We assumed that despite lack of previous records, Anopheles swarms do indeed occur in the area, and that they can be readily identified and characterized. We used a combination of crowd-sourced community knowledge 22, intensive field surveys and expert advice. Our initial objective was therefore to demonstrate natural occurrence of swarms of malaria vectors in rural, south eastern Tanzania and characterize these swarms.
Methods
Study area
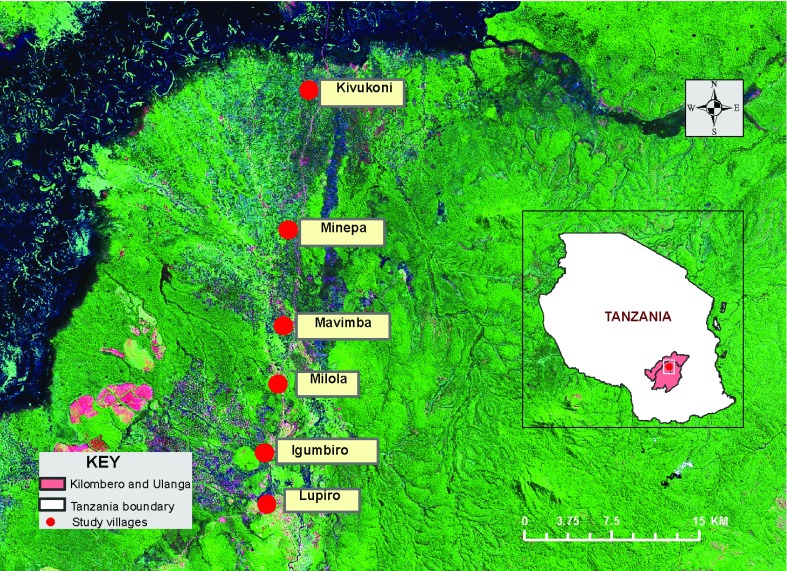
The study was conducted in rural Ulanga and Kilombero districts, in south eastern Tanzania. Initially, the surveys covered multiple villages in the two districts, but we eventually focused on just three villages of Kivukoni (8.2135°S, 36.6879°E), Minepa (8.2710°S, 36.6771°E) and Mavimba (8.3124°S, 36.6771°E) in Ulanga district, on the Kilombero river floodplain ( Figure 1). The main malaria vectors in the area include Anopheles funestus and An. arabiensis with minor contributions from An. rivulorum 23. Overall EIR was last estimated at 4.2 and 11.7 infectious bites/person/year by An. arabiensis and An. funestus respectively 23. The main vector control approach used in the area is LLINs, and the villages benefited from universal LLIN coverage campaigns both in 2011 and in 2016. The main malaria vectors are resistant to pyrethroids used in the LLINs, but still susceptible to organophosphates 23, 24. Mean household size is 4.2 25. Most of the houses are mainly mud and brick walled, with thatched or iron-sheet roofs. The communities rely mainly on substance farming, cultivating rice and maize but also fishing.
Figure 1. Map of the study area, showing villages in south-eastern Tanzania, where swarm surveys were conducted.
Image description: Pan-sharpened mosaic, acquired by Enhanced Thematic Mapper Plus (ETM+) sensor on Landsat 7 Satellite (Courtesy of Donall Cross, University of Aberystwith, UK)).
Trainings on swarm surveys and characterization
Ten participants were selected to support the project in the three study villages, with the help of community leaders. All participants were male adults aged 18 to 35 years, who had provided written informed consent prior to being enrolled in the study. Initially, the project leaders and research assistants were trained on how to identify mosquito swarms and swarm markers by experienced entomologists from Burkina Faso. The first brief training was conducted in Ifakara, Tanzania, in December 2014, at which stage this project had just been conceived. The second training was done for project leaders during a visit to Burkina Faso in September 2016. All participants in this study, including volunteers searching the swarms, provided written informed consent prior to being enrolled.
In April 2016, five months before the second training of project leaders, exploratory swarm surveys were initiated, as an extension of ongoing entomological studies in the study area. During and after these exploratory surveys, the project leaders trained the local community-based swarm searching volunteers on how swarms are identified and characterized, focusing on major features of both the swarms and their associated markers ( Figure 2). This training was conducted over several weeks, and included: i) swarm searching techniques; ii) swarm sampling techniques; iii) swarm characterization and iv) various entomological procedures essential for examining male and female Anopheles mosquitoes collected from swarms. All trainings for local community volunteers were conducted in Kiswahili language, which is the common language in Tanzania, and in this specific study area. These volunteers were then relied upon to identify swarms, which the researchers would then follow up for species confirmation.
Figure 2. Pictures of volunteers and researchers learning how to identify and sample mosquito swarms in one of the study villages.
All participants in the picture provided consent to being photographed.
Further advanced training on swarm surveys and characterization was conducted in Tanzania by a team of experts from Institut de Recherche en Sciences de la Santé (IRSS), Burkina Faso. This advanced training covered: i) swarm searching techniques in dry and wet seasons; ii) swarm visualization techniques; iii) GIS-based analysis of swarms iv) swarm markers and associated environmental features; v) swarm sampling techniques vi) photographic survey of swarms, vii) swarm characterization (including key features to consider); and viii) identification of mating couples in swarms and examination of male and female mosquitoes to identify mated and non-mated individuals.
Initial exploratory surveys of mosquito swarms
To aid logistical assignment of the swarm searching activities, volunteers were assigned one sub-village to search and report the occurrence of swarms. The trained community volunteers were dispatched to search and report any types of insect swarms in their areas, and report these to the researchers. Every time a swarm was reported by the volunteers, the researchers visited the site immediately (if nearby) or the next day (if in far locations): i) first, to verify whether the swarms were actually present, ii) second, to determine whether they were swarms of mosquitoes as opposed to other insects, and iii) third, to assess whether they were actually Anopheles swarms. The first confirmed Anopheles mosquito swarm was identified by a community-based swarm searcher in mid-August 2016 in Minepa Village. This provided our initial verified evidence of presence of Anopheles swarms in this area. As a result of this initial discovery, the team was reorganized for more intensive swarm searching expeditions. From this point forward, the key objectives now included demonstrating that local community members could be trained to readily identify and characterize Anopheles swarms and conduct comprehensive mapping and characterization of Anopheles swarms in selected villages. Subsequent efforts were restricted to just three villages (Mavimba, Kivukoni and Minepa) in Ulanga district ( Figure 1), to enable detailed swarm studies.
Detailed mapping and characterization of Anopheles mosquito swarms and swarm markers in three selected villages
After the initial swarm searching, a comprehensive mapping and characterization of the Anopheles swarms was conducted in three selected villages, i.e. Minepa, Mavimba and Kivukoni. We worked with volunteers from the different sub-villages, who had been trained on how to spot mosquito swarms and swarm markers, time of day when swarms typically occur, and how to collect mosquitoes using sweep nets to identify the swarming mosquitoes. We relied on lessons learned during the initial exploratory surveys, and progressively optimized our swarm survey techniques. The training provided by experts from Burkina Faso significantly improved the swarm characterization capabilities of the Tanzania team.
Volunteers were assigned specific area (sub-villages) to search for mosquito swarms, by first identifying potential swarm markers in these areas during the day, and then actively searching of mosquito swarms across the area every evening from 18:00Hrs, with the aim of identifying swarms. During the swarm surveys, the volunteers continued to receive supportive supervision and training on how to identify, locate and sample mosquitoes using sweep nets. This continuous training was important since this is the first time Anopheles swarms were being recorded in this area. To facilitate the swarm surveys, we also identified, characterized and recorded all potential swarm markers in the study villages. The swarm markers were classified based on the criteria developed by Diabate et al., 26, into four categories as follows: a) flat-contrast markers, such as bare land and footpaths, b) flat no-contrast markers, such as grasses and rice fields, c) elevated contrast markers, such as anthill, woodpiles, bricks and garbage bin, and d) elevated no-contrast markers such as banana tree. Then these markers were subsequently monitored in the evenings to identify and record those with swarms.
Following the initial identification and mapping, each verified swarm was comprehensively characterized by the expert team, by repeatedly visiting and examining the swarms at least three times in different days. In addition to recording date, month, and name of village, we followed a systematic process and recorded various pre-determined characteristics as follows: a) heights at which the swarms occurred, (measured as a distance between the base of the swarm and the ground level), b) time of day when the swarming started appearing, recorded to the nearest minute, c) time of night when the swarms were completely dispersed, also measured to the nearest minute; d) physiological status of a sample of mosquitoes collected in the swarm, e.g. mating status and parity, e) any other mosquito species and insects occurring in the same swarm, f) exact geo-location of the swarm marker; measured using handheld GPS receiver (Magellan eXplorist GC, USA), g) ratio of males to females collected in the sample; h) number of copulation events observed in the swarm; i) number and proportion of females caught that have evidence of having been inseminated, j) type of swarm marker; k) parity status of female mosquitoes collected in the swarms, including number and proportion of females that were parous or nulliparous; l) morphological and molecular identification of the species of Anopheles mosquitoes collected in the swarm; m) any other unique observations made at the swarm site or on the swarms; and n) approximate number of mosquitoes collected in each swarm. Swarm sizes were estimated in two ways: by sampling with a large sweep net (190 cm diameter), approximately 10 minutes after the start of the swarm, and visually, by the trained volunteers counting the flying mosquitoes and approximating the counts. Lastly, we observed other features which may affect the occurrence of mosquito swarms, time of sunset such as human movements and other insects.
Laboratory processing of mosquitoes collected in the swarms
Collected mosquitoes (dead or alive) were aspirated from the sweep nets, kept in separate paper cups and maintained on 10% glucose solution so that those still alive could survive till the next morning. The following morning, all mosquitoes were killed in a closed container by freezing, then identified morphologically by taxa and sex, following keys developed by Gillies and Coetzee 27. A sub-sample from each collection was further identified by multiplex polymerase chain reaction (PCR) to identify sibling species that were morphologically indistinguishable. The PCR assays were conducted using protocols developed by Scott et al., 28.
Physiological age of the wild-caught female malaria vectors was approximated based on the status of their ovaries, i.e. whether they had previously laid eggs or not, by observing the coiling or uncoiling of the ovariole tracheoles 29. Each unfed female mosquito was first anesthetized in a refrigerator. A drop of distilled water was added to a slide and each specimen kept still on the slide, then the seventh and eighth abdominal segment was pulled using fine needles under stereo dissecting microscope. The ovarial tracheoles were then observed under a compound microscope at 10X objective lens magnification, to determine whether the mosquitoes were parous (tracheoles uncoiled and stretched) or nulliparous (with tracheolar skeins, i.e. coiled) 29.
Assessment of female insemination status and sexual maturity in males:
To determine the insemination rate, female An. arabiensis were dissected under a dissecting microscope and their spermathecae examined for the presence of spermatozoon under a compound microscope using 10X objective lenses 30, 31. To assess whether males had matured sexually, genitalia of An. arabiensis males were also observed for signs of rotation 32.
Data analysis
Data analysis was done by using R software version 3.0 33. Number of mosquitoes per swarm, duration of swarms, proportion inseminated, proportion parous (or nulliparous) or proportion of males with rotated genitalia were modeled as a function of different variables as follows: village, swarm marker type, month, height of swarms, time of swarming, and volunteer identification. Geolocations of the swarms relative to households and other landmarks were visualized using the ArcGIS 10.0 (ESRI, USA). Percentages of parity status, insemination rate, mean heights of swarms above ground, median duration of swarms, and time of day when swarms started or ended at the different months of surveys, were also calculated. Swarm markers were classified following the classification category developed by Diabate et al., 26. Visualized volunteer estimates of swarm sizes, and sweep net swarm size estimates were compared and their linear correlation coefficients estimated.
Ethics statement
Meetings with local leaders were held in the study areas and the main aims of the study were explained by the research team. Written and signed informed consent were obtained from all volunteers participating in the study. All information was given in Kiswahili, the local language. Ethical approval for the study was obtained from Ifakara Health Institute Institutional Review Board (IHI/IRB/No: 38-2016), and from the Medical Research Coordinating Committee (MRCC) at the National Institutes of Medical Research (NIMR), Ref: NIMR/HQ/R.8a/Vol.IX/2428. Approval was also obtained from the Human Research Ethics Committee (Medical) clearance at the University of the Witwatersrand, where EK is registered for PhD (Ethics approval certificate No: M160806). Approval for publishing the manuscript was obtained from the National Institutes of Medical Research (NIMR), Ref: NIMR/HQ/P.12VOL.XXII/24. Printed copies and web links to the publication will be provided to NIMR after publication.
Results
Results of exploratory surveys: initial evidence of Anopheles swarms
The initial exploration, conducted in multiple villages, revealed that Anopheles swarms do indeed occur in villages along the Kilombero river valley in rural south-eastern Tanzania. The surveys also revealed that community members can be relied upon to identify these swarms and that by combining their local knowledge with expert knowledge, the Anopheles swarm surveys could be effectively characterized. The first Anopheles arabiensis swarm was observed on 15 th August 2016, in the village of Minepa, by one of the local volunteers. To the best of our knowledge, this is the first recorded Anopheles swarm in this specific part of Tanzania, and the first in the country since the last report from north-eastern part of the country in 1983 20. In the initial exploration, several non-mosquito swarms and also culicine mosquito swarms were incorrectly reported by the volunteers as Anopheles swarms, but were dismissed by the expert verification teams. The reliability of the volunteers however increased over the course of the surveys.
Anopheles mosquito swarms in the three selected villages
After the initial exploratory surveys, and confirmation of occurrence of Anopheles swarms, subsequent surveys were concentrated in just the three selected villages of Minepa, Mavimba and Kivukoni ( Figure 1). A total of 216 swarms were observed from August 2016 to June 2017. The distribution of mosquito swarms in the study villages is shown ( Figure 3), and was as follows; 58.3% of the swarms (n=126) were found in Minepa village, 11.1% (n=24) were found in Mavimba village and 30.5% (n=66) were found in Kivukoni village. There were more swarms distributed in areas close to the rice fields, at the edges of the villages, compared to areas near human settlements ( Figure 3). All the swarms observed consisted exclusively of An. gambiae s.l. We analyzed a sub-sample of 112 An. gambiae s.l mosquitoes by PCR to determine actual sibling species, out of which we obtained 97 successful amplifications (86.6%) and 15 non-amplifications (13.4%). All the successful amplifications were determined to be An. arabiensis (100%). In one instance in the early days of the surveys, we also collected 13 male An. funestus mosquitoes, in an area where we had An. arabiensis swarms. Since no repeat observation of An. funestus, we are unable to verify presence of swarms of this species here, until further investigations are completed.
Figure 3. Comparison of swarm distribution between the three study villages (Original figure created by authors of this article).
Start times, end times, duration and height of Anopheles swarms
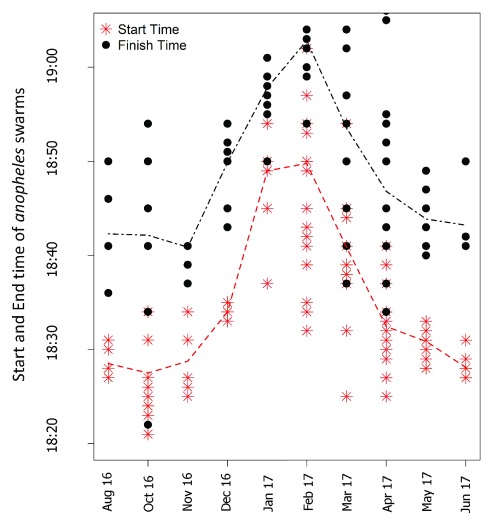
It was observed that swarms began with two to three mosquitoes congregating after sunset, and flying above a swarm marker. Then the numbers increased over the next 10 to 15 minutes, with multiple males and slowly decreased in size then disappeared after 20 (IQR;15-25) minutes. Flying mosquitoes were observed by viewing them against the sunset. Mosquitoes tended to congregate at the mean height (±SD) of 2.74±0.64m from the ground. The swarm size increased and became compact above the marker but they disappeared when it became dark, which was also when swarms observations became impossible. Furthermore, we observed that the start and end time of swarming periods varied across months. Swarms were appearing earlier in the period between August 2016 and January 2017, but later in the period between February and June. Specific start and finish times for swarms during each of the sampling months is shown in Figure 4.
Figure 4. Observed start and end times of Anopheles arabiensis swarms in the study villages.
According to weather information archived online by timeanddate.com, the latest time of sunset in the study area (Kilombero Valley, Tanzania) varied in a similar pattern as the start and end times of the observed Anopheles swarm sessions. We considered sunset times for all the months of our data collection, starting August, 2016 to June 2017. Between August and November 2016, the latest time sunset time ranged between 18:32Hrs and 18:42Hrs. However, between December 2016 and March 2017, sunset occurred much later, with the latest sunset times between 18:56Hrs and 19:03Hrs. The sunset time was early again between April 2017 and August 2017, when the latest sunset times over the valley ranged from 18:26Hrs and 18:35Hrs: https://www.timeanddate.com/sun/@157407.
Estimated swarm sizes: data collected through direct visualization by community volunteers compared to data collected using sweep nets
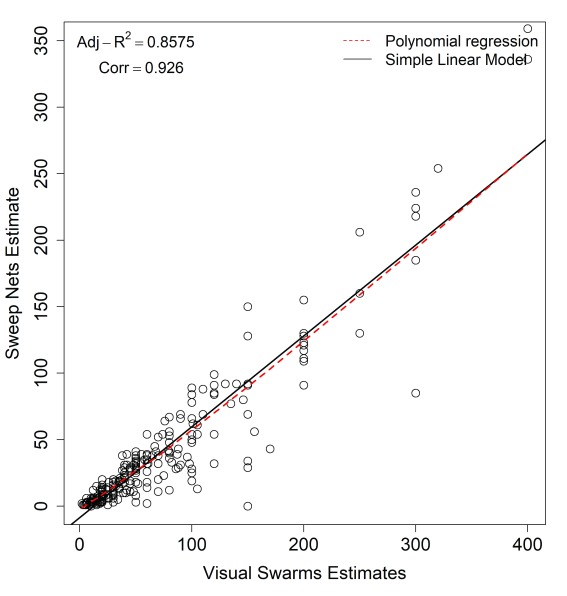
We estimated swarm sizes from two data sources. First, we used data provided from visual assessments of the volunteers looking at swarms and estimating how many mosquitoes were flying in them at that time. Second, we used data from collections done by the large standardized sweep nets, with which trained volunteers collected the mosquitoes approximately 10 minutes after the start of swarming. Though the values provided from the two data sources were not equal (i.e. visual estimates being consistently higher than sweep net counts), there was a significant linear correlation ( Table 1 and Figure 5) between the visual estimates provided by the community volunteers and estimates obtained from sweep net counts (R 2 = 0.94; P <0.001). There were also variations of swarm sizes observed in different villages, with Minepa village having the largest swarms followed by Mavimba and Kivukoni villages ( Table 1).
Table 1. Swarm sizes estimated by either visual observations by trained volunteers, or sweep net sampling in the three study villages (a total of 216 swarms were observed).
C.I: Confidence Interval.
| Total number of
swarms (% of all swarms observed) |
Visual estimates
by volunteers (No. mosquitoes/swarm) |
Sweep net sampling
estimates (No. mosquitoes/swarm) |
|||
|---|---|---|---|---|---|
| Mean | C.I | Mean | C.I | ||
| Kivukoni | 66 (30.5%) | 34.0 | 31.4 - 36.6 | 13.1 | 11.5 - 14.9 |
| Mavimba | 24 (11%) | 38.7 | 36.3 - 41. 3 | 20.6 | 19.9 - 22.5 |
| Minepa | 126 (58.3%) | 95.5 | 93.8 - 97.3 | 56.1 | 54.8 - 57.4 |
Figure 5. Estimated swarm sizes; and correlation between estimates done by visualization and estimates done using sweep nets.
Results of swarm markers survey
The commonest swarm markers were rice fields (25.6%), burned grounds (17.2%), banana trees (13%), brick piles (8.8%), grass (7.9%) garbage heaps (7.9%), ant hill (7.4%), wood piles (5.6%), demolished houses (1.8%),footpath (1.3%), trenches (1.3%), toilets (1.3) and cowshed (0.5). Table 2 shows the common swarm markers and the frequency of swarms observed in each marker. Most of the swarm locations remained the same, several months after the swarms were first identified and verified.
Table 2. Common types swarm markers observed in the three study villages.
In brackets, the actual number of swarm markers that had Anophele s swarms over them.
| Flat contrast
swarm markers |
Flat no-contrast
markers |
Elevated contrast
markers |
Elevated no-contrast
markers |
|
|---|---|---|---|---|
| Minepa Village | Burned ground (17)
Well (0) Cowshed (0) Trench (0) |
Rice fields (36)
Footpath (1) Grass (8) |
Bricks (17)
Garbage heaps (10) Woodpile (1) Demolished house (2) Ant hill (7) Toilets (3) |
Banana trees (24) |
| Mavimba Village | Burned ground (4)
Well (0) Cowshed (0) Trench (2) |
Rice fields (1)
Footpath (1) Grass (5) |
Bricks (0)
Garbage heaps (4) Woodpile (1) Demolished house (0) Ant hill (6) Toilets (0) |
Banana trees (0) |
| Kivukoni Village | Burned ground (16)
Well (0) Cowshed (1) Trench (1) |
Rice fields (18)
Footpath (1) Grass (4) |
Bricks (2)
Garbage heaps (3) Woodpile (10) Demolished house (2) Ant hill (3) Toilets (1) |
Banana trees (4) |
Copulation events, mating status, sexual maturity and parity rates in mosquitoes collected from the swarms
Throughout the study period, we observed and collected a total of 22 copulation events in the swarms. The proportion of female to male An. arabiensis mosquitoes in the swarm was 0.004 (28/7114). Of the 28 female anopheles arabiensis mosquitoes dissected, 54% (N=15) were determined as nulliparous. The remaining 46% (N=13) were not examinable since the specimen had dried up. We also assessed the male An. arabiensis collected from the swarms for sexual maturity. Of these, 95.4% (N=6787) had rotated male genitalia, indicating sexual maturity. We assessed the 28 females recovered from the swarms, to assess whether their spermatheca were already filled with male sperms or not. Of all females dissected, 42.9% (N=12) had evidence of having been inseminated, while the remaining 57.1% (N=16) were not inseminated.
Discussion
Malaria vector control remains constrained by lack of comprehensive understanding of mosquito ecology, yet such ecological studies are considered a prerequisite for improved control and eventual disease elimination 7. Though not widely studied, the mating behavior of malaria mosquitoes may presents some unique opportunities for improved vector control 13. In a recent trial in Burkina Faso, it has been demonstrated that targeting Anopheles mosquito swarms with effective aerosol insecticide spraying could rapidly crash vector populations in local communities 14. Though mating and swarming behaviours of Anopheles mosquitoes are among the most important components of the vector reproduction biology 8, there have been limited field studies of mosquito swarming in Tanzania, the last published field observations having been in 1983 20. This neglect is mainly because most scientist have not considered swarming behaviour as having any potential opportunities for disease prevention and vector control. On the contrary, studies on mating and swarming behaviours of Anopheles mosquitoes in West Africa have already demonstrated that it could possibly be relied upon to control mosquito densities and associated pathogen transmission, by killing mosquito swarms with effective insecticides 13, 14.
Our study has yielded evidence of occurrence of the swarms of An. arabiensis mosquitoes in the rural south-eastern Tanzania. Furthermore, the study proves that trained volunteers are able to successfully identify and locate swarms in their villages. A map of all swarm locations so far identified has been built-up with the help of the locally recruited volunteers ( Figure 3). Interestingly, most of the swarm locations remained the same, several months after they were first identified. This phenomenon of swarms occurring in the same location is widely reported by other scientists, particularly in west Africa 26. In this current study, we characterized the Anopheles swarms repeatedly to obtain an initial profile of malaria vector swarms in this area; first evidence of such swarms in the study area.
The commonest swarm markers are shown in Table 2, and were found on landscapes that were not blocked by either trees or houses. Several other studies have also reported the roles of such markers on mosquito swarming 15, and here, we relied on similar swarm marker classification as previously used in Burkina Faso 26. Mosquitoes preferred to swarm on areas such as rice fields, burned grounds, banana trees, brick piles, garbage heaps and ant hills. It may therefore be possible to predict the distributions of Anopheles swarms based on the spatial distribution of the common swarm markers in the village. In this study, once we had an accurate representation of the main swarm markers, it became easier to conduct training programs for the volunteers, who relied heavily on these swarm markers as a guide to search for mosquito swarms in their assigned areas. Second, with a clear definition of swarm markers, it could be possible, as has been demonstrated in some studies to manipulate swarm markers, or create artificial swarm markers to attract Anopheles mosquitoes and enhance mating 16.
Our study also demonstrated that locally recruited and trained non-entomologist volunteers from the study villages are capable of not only identifying the swarm but also estimating the swarm size and the associated swarm markers in the study village. This idea was derived from an earlier study conducted in the villages where community members were able to identify locations where mosquito densities were high 22. In this current study, we initially guided the volunteers on how to identify mosquito swarms and their associated environmental features. This approach confirmed that such community-based volunteers can be relied upon to identify, characterize and map Anopheles swarms across villages, so that these swarms can potentially be targeted for control and ensuring its sustainability. An added advantage of this approach is that it also increases local community knowledge on mosquitoes, and the level of community engagement in research increased. By using such participatory approach, we expect that in the time of targeting Anopheles swarms e.g. by aerosol spraying, high levels of acceptability could be achieved, and the volunteers could be relied upon to lower implementation costs.
This study did not observe any An. funestus swarms. However, 13 male An. funestus mosquitoes were collected near the An. arabiensis swarms, suggesting possible presence of An. funestus swarms in the area. Unfortunately, this was only one instance and could not be verified during this study. It may be possible that this was a separate distinct An. funestus swarm that was formed close to An. arabiensis. Since they were all male An. funestus, we speculate that An. funestus swarms also occur in the area, and that extended surveys could reveal greater details of their swarming behaviours. It is therefore recommended that more detailed surveys should be conducted to understand the swarming behaviours of An. funestus in the area. Evidence by other studies in Africa suggest that swarms of An. funestus appear in a similar way to those of An. gambiae 21. Since it is know that An. funestus contributes to the significant amount of the remaining malaria transmission in this area 23, it is therefore suggested that any control measures targeting An. gambiae without affecting An. funestus will have little impact on the ongoing malaria transmission in this area. More attention should be put on how to identify swarms of An. funestus and their associated environmental features.
Consequently presence of dragonflies, though not initially targeted for observation, was an indication of swarm presence in many locations, though in this case we have not considered it as a physical swarm marker. Similar observations were also reported by Sawadogo et al., 15 in their studies in Burkina Faso. Furthermore, human movements, sounds, strong wind and rainfall were found to affect mosquito swarming behaviours. For instance, in some cases when swarm sampling was conducted during the rainfall, we rarely observed swarms even in the previously confirmed swarms. Dragon flies and other predators could thus be considered good indicators of presence of mosquito swarms in certain areas. Lastly, in this study, most of the swarms were found and confined at the edge of the village and in the rice fields, this could mostly be attributed to the fact that swarms prefers to the location that are not disturbed by human activities such as movements.
Overall, the swarming behavior of An. arabiensis in the valley is now clearly tractable. Further investigations should explore opportunities on whether new interventions exploiting this phenomenon, such as targeted aerosol spraying of swarms, could significantly disrupt malaria transmission.
Conclusions
This is the first report of Anopheles swarms in Tanzania, in more than three decades. The study provides evidence that the swarms can be identified, characterized and quantified by trained community-based volunteers; and opens potential new opportunities for targeting male malaria mosquitoes to improve disease control. Further investigations are recommended to further characterize the swarms in the area and to verify whether other residual malaria vector populations such as An. funestus also form similar mating swarms. More importantly, future studies should seek to demonstrate whether new interventions exploiting this phenomenon, such as targeted aerosol spraying of swarms, could significantly disrupt malaria transmission.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2017 Kaindoa EW et al.
The data used in this manuscript have been uploaded online to the Ifakara Health Institute, DOI, 10.17890/ihi.2017.06.99 34, under a CC0 1.0 license.
The terms and conditions that users need to agree to in order to download the data, can be viewed here.
Acknowledgements
We thank the local communities of Ulanga district for allowing us to work in their compounds. We thank Mr. Maxeclin Ngakuka (Kivukoni village) and all volunteers for their assistance during swarm searching and sampling. We also thank the project team Felician Meza (Ifakara Health Institute) and Said Abbasi (Ifakara Health Institute). We also extend our thanks to the team from Burkina Faso; Dr. Abdoulaye Niang (IRSS, Bobo-Dioulasso, Burkina Faso), Mr. Simon Sawadogo (IRSS, Bobo-Dioulasso, Burkina Faso), Mr. Azizie Millogo (IRSS, Bobo-Dioulasso, Burkina Faso), Mr. Abdourasmane Noufou Diabate (IRSS, Bobo-Dioulasso, Burkina Faso) for provide training on swarm searching. Dr. Jane Bonds, Latham and Dr. Frederic Tripet, all participate in initial discussions where the protocols for this study were developed. Their support is greatly appreciated. All persons acknowledged have been informed. Lastly, we thank all reviewers of this article for their comments and suggestions.
Funding Statement
This work was supported by the Wellcome Trust [100540, 102350 and 087547], the Innovative Vector Control Consortium (IVCC) [Project No: 43 – Mating Swarms], the Carnegie Corporation of New York [B 8606.R02], and Sida [54100029].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 1 approved, 2 approved with reservations]
References
- 1. World Health Organization: World Malaria Report 2016. WHO.2016. Reference Source [Google Scholar]
- 2. Bhatt S, Weiss DJ, Cameron E, et al. : The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–211. 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ranson H, N’Guessan R, Lines J, et al. : Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27(2):91–98. 10.1016/j.pt.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 4. Reddy MR, Overgaard HJ, Abaga S, et al. : Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184. 10.1186/1475-2875-10-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russell TL, Govella NJ, Azizi S, et al. : Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. 10.1186/1475-2875-10-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Govella NJ, Ferguson H: Why Use of Interventions Targeting Outdoor Biting Mosquitoes will be Necessary to Achieve Malaria Elimination. Front Physiol. 2012;3:199. 10.3389/fphys.2012.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferguson HM, Dornhaus A, Beeche A, et al. : Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7(8):e1000303. 10.1371/journal.pmed.1000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howell PI, Knols BG: Male mating biology. Malar J. 2009;8(Suppl 2):S8. 10.1186/1475-2875-8-S2-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuval B: Mating systems of blood-feeding flies. Annu Rev Entomol. 2006;51:413–440. 10.1146/annurev.ento.51.110104.151058 [DOI] [PubMed] [Google Scholar]
- 10. Downes JA: The swarming and mating flight of Diptera. Annu Rev Entomol. 1969;14:271–298. 10.1146/annurev.en.14.010169.001415 [DOI] [Google Scholar]
- 11. Charlwood JD, Pinto J, Sousa CA, et al. : The swarming and mating behaviour of Anopheles gambiae s.s. (Diptera: Culicidae) from São Tomé Island. J Vector Ecol. 2002;27(2):178–183. [PubMed] [Google Scholar]
- 12. Diabate A, Baldet T, Brengues C, et al. : Natural swarming behaviour of the molecular M form of Anopheles gambiae. Trans R Soc Trop Med Hyg. 2003;97(6):713–716. 10.1016/S0035-9203(03)80110-4 [DOI] [PubMed] [Google Scholar]
- 13. Diabate A, Tripet F: Targeting male mosquito mating behaviour for malaria control. Parasit Vectors.Parasites & Vectors;2015;8:347. 10.1186/s13071-015-0961-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sawadogo SP, Niang A, Bilgo E, et al. : Targeting male mosquito swarms to control malaria vector density. PLoS One. 2017;12(3):e0173273. 10.1371/journal.pone.0173273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sawadogo PS, Namountougou M, Toé KH, et al. : Swarming behaviour in natural populations of Anopheles gambiae and An. coluzzii: review of 4 years survey in rural areas of sympatry, Burkina Faso (West Africa). Acta Trop. 2014;132(Suppl):S42–52. 10.1016/j.actatropica.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 16. Facchinelli L, Valerio L, Lees RS, et al. : Stimulating Anopheles gambiae swarms in the laboratory: application for behavioural and fitness studies. Malar J.BioMed Central;2015;14:271. 10.1186/s12936-015-0792-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diabaté A, Dabire RK, Kengne P, et al. : Mixed Swarms of the Molecular M and S Forms of Anopheles gambiae (Diptera: Culicidae) in Sympatric Area from Burkina Faso. J Med Entomol. 2006;43(3):480–483. 10.1093/jmedent/43.3.480 [DOI] [PubMed] [Google Scholar]
- 18. Assogba BS, Djogbénou L, Saizonou J, et al. : Characterization of swarming and mating behaviour between Anopheles coluzzii and Anopheles melas in a sympatry area of Benin. Acta Trop.Elsevier B.V;2014;132(Suppl):S53–S63. 10.1016/j.actatropica.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 19. Dabiré KR, Sawadogo PS, Hien DF, et al. : Occurrence of natural Anopheles arabiensis swarms in an urban area of Bobo-Dioulasso city, Burkina Faso, West Africa. Acta Trop. 2014;132(Suppl):S35–41. 10.1016/j.actatropica.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 20. Marchand RP: Field Observations On Swarming and Mating in Anopheles gambiae Mosquitoes in Tanzania1. Netherlands J Zool. 1983;34(3):367–387. 10.1163/002829684X00209 [DOI] [Google Scholar]
- 21. Charlwood JD, Thompson R, Madsen H: Observations on the swarming and mating behaviour of Anopheles funestus from southern Mozambique. Malar J. 2003;2:2. 10.1186/1475-2875-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mwangungulu SP, Sumaye RD, Limwagu AJ, et al. : Crowdsourcing Vector Surveillance: Using Community Knowledge and Experiences to Predict Densities and Distribution of Outdoor-Biting Mosquitoes in Rural Tanzania. PLoS One. 2016;11(6):e0156388. 10.1371/journal.pone.0156388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaindoa EW, Matowo NS, Ngowo HS, et al. : Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS One. 2017;12(5):e0177807. 10.1371/journal.pone.0177807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okumu FO, Chipwaza B, Madumla EP, et al. : Implications of bio-efficacy and persistence of insecticides when indoor residual spraying and long-lasting insecticide nets are combined for malaria prevention. Malar J. 2012;11:378. 10.1186/1475-2875-11-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geubbels E, Amri S, Levira F, et al. : Health & Demographic Surveillance System Profile: The Ifakara Rural and Urban Health and Demographic Surveillance System (Ifakara HDSS). Int J Epidemiol. 2015;44(3):848–861. 10.1093/ije/dyv068 [DOI] [PubMed] [Google Scholar]
- 26. Diabaté A, Yaro AS, Dao A, et al. : Spatial distribution and male mating success of Anopheles gambiae swarms. BMC Evol Biol. 2011;11:184. 10.1186/1471-2148-11-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gillies MT, Coetzee M: A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region). Publications of the South African Institute for Medical Research.1987;1–143. Reference Source [Google Scholar]
- 28. Scott JA, Brogdon WG, Collins FH: Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49(4):520–529. 10.4269/ajtmh.1993.49.520 [DOI] [PubMed] [Google Scholar]
- 29. Detinova TS: Age-grouping methods in Diptera of medical importance with special reference to some vectors of malaria. Monogr Ser World Health Organ. 1962;47:13–191. [PubMed] [Google Scholar]
- 30. Mahmood F, Reisen WK: Anopheles stephensi (Diptera: Culicidae): changes in male mating competence and reproductive system morphology associated with aging and mating. J Med Entomol. 1982;19(5):573–588. 10.1093/jmedent/19.5.573 [DOI] [PubMed] [Google Scholar]
- 31. Tripet F, Touré YT, Taylor CE, et al. : DNA analysis of transferred sperm reveals significant levels of gene flow between molecular forms of Anopheles gambiae. Mol Ecol. 2001;10(7):1725–1732. 10.1046/j.0962-1083.2001.01301.x [DOI] [PubMed] [Google Scholar]
- 32. Dahan YL, Koekemoer LL: Analysis of the genitalia rotation in the male Anopheles funestus (Diptera: Culicidae). Acta Trop.Elsevier B.V;2014;132(Suppl):S20–S25. 10.1016/j.actatropica.2013.07.023 [DOI] [PubMed] [Google Scholar]
- 33. R Development Core Team R: R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.2011. Reference Source [Google Scholar]
- 34. Kaindoa EW, Ngowo HS, Limwangu A, et al. : Tanzania - Targeting swarms and mosquito nocturnal behaviours to control outdoor malaria transmission. Data Source [Google Scholar]