Abstract
There is evidence both for and against Na+- and Cl−-dependent neurotransmitter transporters forming oligomers. We found that cross-linking the human dopamine transporter (DAT), which is heterologously expressed in human embryonic kidney 293 cells, either with copper phenanthroline (CuP) or the bifunctional reagent bis-(2-methanethiosulfonatoethyl)amine hydrochloride (bis-EA) increased the apparent molecular mass determined with nonreducing SDS/PAGE from ≈85 to ≈195 kDa. After cross-linking, but not before, coexpressed, differentially epitope-tagged DAT molecules, solubilized in Triton X-100, were coimmunoprecipitated. Thus, the 195-kDa complex was a homodimer. Cross-linking of DAT did not affect tyramine uptake. Replacement of Cys-306 with Ala prevented cross-linking. Replacement of all of the non-disulfide-bonded cysteines in the extracellular and membrane domains, except for Cys-306, did not prevent cross-linking. We conclude that the cross-link is between Cys-306 at the extracellular end of TM6 in each of the two DATs. The motif GVXXGVXXA occurs at the intracellular end of TM6 in DAT and is found in a number of other neurotransmitter transporters. This sequence was originally found at the dimerization interface in glycophorin A, and it promotes dimerization in model systems. Mutation of either glycine disrupted DAT expression and function. The intracellular end of TM6, like the extracellular end, is likely to be part of the dimerization interface.
The dopamine transporter (DAT) is responsible for the re-uptake of dopamine released into the synaptic cleft, and thus it represents the major mechanism for the termination of dopaminergic neurotransmission (1, 2). DAT is a member of the family of Na+- and Cl−-dependent neurotransmitter transporters that includes transporters for other neurotransmitters, norepinephrine, serotonin, γ-aminobutyric acid (GABA), glycine, and a number of other small molecules (3, 4). These transporters couple the movement of sodium down its concentration gradient to the transport of substrate, but the molecular mechanism of this process is understood only in general terms. The Na+- and Cl−-dependent neurotransmitter transporters are thought to have 12 transmembrane segments (TMs) with intracellular N and C termini, and this general topology has been supported by the accessibility of putative extracellular and intracellular loop residues to chemical modification (5, 6). Although the packing of the 12 TMs is unknown, some distance constraints have been established in DAT through the identification of an endogenous zinc-binding site and subsequent use of engineered metal-binding sites (7, 8). These constraints place the large extracellular loop between TM3 and TM4 in proximity to the extracellular ends of TM7 and TM8.
The serotonin transporter (SERT) has been inferred to be a homo-oligomer, based in part on coimmunoprecipitation studies of differentially epitope-tagged SERT constructs (9). In addition, in cells in which two SERT constructs, only one of which was sensitive to chemical modification, were expressed in different ratios, the effect of modification on uptake lagged behind the fraction of the sensitive SERT construct (9). This implied that SERT is in a complex and that it is necessary to inhibit more than one SERT to inhibit uptake. The demonstration of fluorescence resonance energy transfer between different green fluorescent proteins fused to the N termini of SERT molecules also supports the idea of dimerization (10). In contrast, Horiuchi et al. (11) showed that mature glycine transporter in the plasma membrane was not oligomeric, whereas immature intracellular transporter was oligomeric.
By using cysteine cross-linking, we have now explored the possibility that DAT exists as a dimer or higher-order oligomer in the plasma membrane. We show that DAT is at least a homodimer, with the extracellular end of TM6 at a symmetrical dimer interface. Moreover, we have identified the presence of a dimerization motif in TM6 of DAT and of many related neurotransmitter transporters.
Materials and Methods
Bis-(2-methanethiosulfonatoethyl)amine Hydrochloride (bis-EA) Synthesis.
The approach was that of Bruice and Kenyon (30), as adapted by Stauffer and Karlin (12). Sixty millimoles of sodium methanethiosulfonate and 30 mmol of bis-(2-chloroethyl)amine hydrochloride were dissolved in absolute ethanol and refluxed overnight under argon. An initial precipitate was removed by filtration, and the filtrate was cooled. A second precipitate was collected and recrystallized from absolute ethanol. The purity was 95% by assay with 5-thio-2-nitrobenzoic acid (12); the melting point was 122–125°C. The structure was confirmed by H NMR.
Stable Transfection of DAT.
We used EM4 cells, which are human embryonic kidney 293 cells stably transfected with macrophage scavenger receptor to increase their adherence to tissue culture plastic (13). These were additionally stably transfected with a synthetic human DAT gene that was tagged at the amino terminus with tandem epitopes. One was the Flag epitope fused to the hemagglutinin epitope (Flag-HA; 18 residues); another was the c-Myc-epitope fused to 11 histidines (Myc-His; 23 residues). These epitope tags replaced the first 22 aa of the protein and did not significantly affect expression or transport (ref. 14 and data not shown). Mutations were made by PCR and confirmed by sequencing. EM4 cells were grown in DMEM supplemented with 10% FBS at 37°C and 5% CO2. Thirty-five-millimeter dishes of EM4 cells at 70–80% confluence were transfected with 2 μg of wild-type (WT) or mutant DAT cDNA in the bicistronic expression vector pCIHyg (14) with 9 μl of Lipofectamine (Invitrogen) and 500 μl of OPTIMEM (Invitrogen). Five hours after transfection the solution was removed and fresh media added. Twenty-four hours after transfection the cells were split to a 100-mm dish and 250 μg/ml hygromycin was added to select for a stably transfected pool of cells. To create a pool of cells expressing both Flag-HA-DAT and Myc-His-DAT, a pool of EM4 cells stably expressing Flag-HA-DAT was stably transfected with Myc-His-DAT in a bicistronic vector expressing the bleomycin resistance gene, and a pool of cells was selected by using 200 μg/ml zeocin. A pool expressing only Myc-His-DAT was created by transfecting EM4 cells with this construct.
Cross-Linking.
EM4 cells, stably transfected with the appropriate DAT construct and adherent to the plate, were washed with PBS (154 mM NaCl/8.1 mM Na2HPO4/1.9 mM NaH2PO4, pH 7.4) and reacted for 5 min at room temperature either with 100 μM copper sulfate and 400 μM 1,10-phenanthroline (CuP) or with 20 μM bis-EA in PBS. The cells were washed twice with 2 ml of PBS, and reacted 20 min at room temperature with 10 mM N-ethylmaleimide (NEM) to block free sulfhydryl groups. The cells were scraped into PBS-PI buffer (PBS supplemented with 2 μg/ml Pefablock, 2 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, and 10 mM NEM) and pelleted at 800 × g for 5 min at 4°C. The pellet was suspended and incubated in 0.2% digitonin in PBS-PI for 20 min at 4°C. Cells were pelleted at 2,000 × g for 10 min and stirred in 0.2 ml of 1% Triton X-100 in PBS-PI (lysis buffer) per 60-mm plate at 4°C for 1 h. The mixture was centrifuged at 14,000 × g for 30 min at 4°C. Twenty microliters of extract was mixed with an equal volume of 4× Laemmli sample buffer without reducing agent and held for 30 min at room temperature.
Coimmunoprecipitation.
EM4 cells, stably transfected with Flag-HA-DAT, Myc-His-DAT, or both, were reacted with cross-linker and solubilized in Triton X-100 as above. Five microliters of anti-Myc 9E10 (Santa Cruz Biotechnology) was added to 0.2 ml of Triton X-100 extracts, and the mixture was incubated for 1 h at 4°C. Twenty microliters of rec-protein G Sepharose (Zymed) was added, and the mixture was incubated for 1 h at 4°C, washed three times in 0.5 ml of lysis buffer, and eluted in 45 μl of 2× Laemmli sample buffer without reducing agent.
Immunoblotting.
Samples were applied to 1.5-mm, 15-well 7.5% acrylamide gels prepared and run according to Laemmli (15). The bands were transferred to a poly(vinylidene difluoride) (PVDF) membrane (Millipore), which was blocked for 1 h in 5% nonfat milk, 1% BSA, 0.1% Tween-20 in TBS (50 mM Tris⋅HCl/150 mM NaCl, pH 7.4). To detect Flag-HA-DAT, we typically incubated the blot with anti-FLAG M2 monoclonal antibody (Sigma) diluted to 4 μg/ml in blocking buffer for 1 h at room temperature, washed four times for 15 min in TBS containing 0.1% Tween 20, incubated in horseradish peroxidase-conjugated rabbit anti-mouse-antibody (Santa Cruz Biotechnology), diluted 1:15,000 in blocking buffer for 1 h at room temperature, washed six times for 10 min with TBS containing 0.1% Tween, and reacted with ECL-Plus reagent (Amersham Pharmacia). Fluorescence was detected and quantitated on a Storm 840 System (Molecular Dynamics).
Uptake of [3H]Tyramine.
Tyramine was used instead of dopamine for uptake assays because it is a substrate for DAT but not a substrate for endogenous catechol O-methyltransferase present in human embryonic kidney 293 cells (16). EM4 cells, expressing the appropriate construct, were seeded in a 96-well plate and used 48 h later, when they had grown to confluence. The cells were washed in PBS, cross-linked as above, washed three times with 200 μl PBS, and incubated for 5 min at room temperature with 56 nM [3H]tyramine (American Radiolabeled Chemicals, St. Louis) in uptake buffer (130 mM NaCl/1.3 mM KCl/10 mM Hepes/1.2 mM MgSO4/1.2 mM KH2PO4/2.2 mM CaCl2/10 mM glucose, pH 7.4. The uptake was terminated by aspiration and washing once with ice-cold uptake buffer. Cells were permeabilized with 50 μl of 1% Triton X-100, 200 of μl Optiphase Supermix scintillation fluid (Wallac, Gaithersburg, MD) was added, and radioactivity was determined in a Wallac 1450 microbeta counter. Nonspecific uptake was determined in the presence of 2 mM unlabeled tyramine.
Preparation of Mouse Striatal Membranes.
Striata from male C57BL/6 mice (Charles River Breeding Laboratories) were dissected on ice. Thirty milligrams of striatal tissue was homogenized in 5 ml of PBS-PI buffer with a Polytron at a setting of 6 for 10 s. The membranes were centrifuged at 19,500 × g for 15 min at 4°C. The pellet was resuspended in 0.5 ml of PBS-PI. Aliquots of 180 μl were reacted with CuP or bis-EA in a final volume of 200 μl for 5 min at room temperature as described above. The reaction was stopped by adding 1 ml of PBS-PI buffer containing 10 mM NEM. The mixture was centrifuged at 14,000 × g for 5 min at 4°C. The pellet was dissolved in 125 μl of 2× Laemmli sample buffer and held for 1 h at room temperature. Twenty-five micrograms of protein was loaded per sample for SDS/PAGE and immunoblotting as described above.
Results
To test whether DAT was expressed as a spontaneously disulfide cross-linked dimer, we analyzed EM4 cells stably expressing Flag-HA-DAT. The cells were solubilized and the proteins were separated by SDS/PAGE. Both before and during solubilization, the cells were exposed to 10 mM NEM to alkylate all free sulfhydryls and thereby prevent both the formation of new disulfide bonds and sulfhydryl–disulfide interchange. DAT migrated as a heterogeneously glycosylated monomer of ≈85 kDa in nonreducing SDS/PAGE (Fig. 1). Thus, if DAT is oligomeric, the oligomer dissociates in SDS. Clearly, DAT is not a disulfide-linked dimer in the plasma membrane.
Figure 1.
Cross-linking of Flag-HA-DAT. Adherent EM4 cells stably expressing Flag-HA-DAT were treated as described in Materials and Methods with bis-EA or CuP, alone or followed by DTT (50 mM for 30 min at room temperature) or N-glycosidase F (PNGase F; 2 μl/20-μl sample for 2 h at 37°C; New England Biolabs). Immunoblotting and detection were performed as described in Materials and Methods.
A small amount of immaturely glycosylated DAT at ≈57 kDa was also present (Fig. 1). This immature species in intact cells is not labeled by impermeant reagents and is therefore not present on the cell surface (data not shown). Removing all of the N-linked sugars by treatment with N-glycosidase F (PNGase F) decreased the apparent mass of both the mature and the immature bands to ≈53 kDa (Fig. 1).
After coexpression of Flag-HA-DAT and Myc-His DAT, we attempted to coimmunoprecipitate Flag-HA-DAT with an anti-Myc monoclonal antibody and protein G. Although we sometimes coimmunoprecipitated a very small amount of Flag-HA-DAT, more often we observed none. If DAT is oligomeric in the membrane, the oligomer does not survive Triton X-100 solubilization.
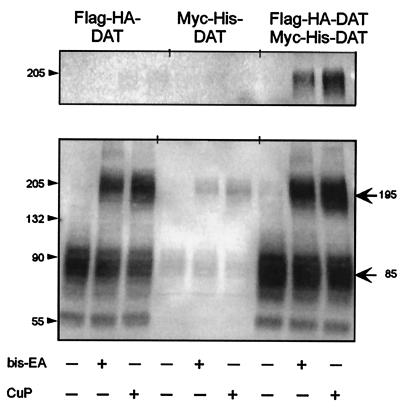
We attempted to trap possible oligomeric forms of DAT in the membrane by disulfide cross-linking. Because we showed previously that two extracellular cysteines in DAT are accessible to charged methanethiosulfonate (MTS) reagents (6), we first tried cross-linking by means of native cysteines. We used the bifunctional reagent bis-EA. This flexible cross-linker can form a disulfide bond with two cysteines, adding —SCH2CH2NH CH2CH2S— between the two cysteine sulfurs, which are then held 5–10 Å apart. Treatment of Flag-HA-DAT with 20 μM bis-EA for 5 min produced a new band at ≈195 kDA, about twice the size of the DAT monomer (Fig. 1). We integrated the density of the ≈85-kDa and ≈195-kDa bands; the ≈195-kDa band density was 37% ± 5% of the total density (mean ± SD, n = 4). We also reacted Flag-HA-DAT in intact cells with CuP, an oxidizing reagent that promotes the formation of disulfide bonds directly between cysteines (17, 18). CuP also produced a cross-linked band of ≈195 kDa (Fig. 1); the ≈195-kDa band density was 46% ± 5% of the total density (mean ± SD, n = 4).
CH2CH2S— between the two cysteine sulfurs, which are then held 5–10 Å apart. Treatment of Flag-HA-DAT with 20 μM bis-EA for 5 min produced a new band at ≈195 kDA, about twice the size of the DAT monomer (Fig. 1). We integrated the density of the ≈85-kDa and ≈195-kDa bands; the ≈195-kDa band density was 37% ± 5% of the total density (mean ± SD, n = 4). We also reacted Flag-HA-DAT in intact cells with CuP, an oxidizing reagent that promotes the formation of disulfide bonds directly between cysteines (17, 18). CuP also produced a cross-linked band of ≈195 kDa (Fig. 1); the ≈195-kDa band density was 46% ± 5% of the total density (mean ± SD, n = 4).
Treatment with 50 mM DTT in PBS (pH 7.4) for 30 min eliminated the ≈195-kDA band produced by bis-EA and greatly reduced the intensity of the band produced by CuP (Fig. 1), consistent with the reduction of at least one of the disulfides in the bis-EA-treated DAT and of the disulfide in the CuP-treated DAT.
The size of the cross-linked species was consistent with its being a homodimer of DAT or a heterodimer of DAT with another protein of similar size. Deglycosylation of the cross-linked species produced a major species of ≈150 kDa, and a minor species of ≈110 kDa (Fig. 1). The 150-kDA species could be the product of deglycosylation of just one of the monomers in a dimer, whereas the 110-kDa species is approximately the size expected of a completely deglycosylated DAT homodimer.
The partners in the cross-linked species were definitively identified by coimmunoprecipitation of Flag-HA-DAT coexpressed with Myc-His-DAT. After reaction with either bis-EA or CuP, the product of immunoprecipitation with anti-Myc antibody ran as an approximately 195-kDa band that was recognized by anti-HA antibody (Fig. 2). In contrast, when Flag-HA-DAT and Myc-His-DAT were expressed separately and cross-linked, precipitation with anti-Myc antibody did not produce a species recognized by anti-HA antibody (Fig. 2). These results establish that the ≈195-kDa band is a dimer containing both Flag-HA-DAT and Myc-His DAT.
Figure 2.
Coimmunoprecipitation of Flag-HA-DAT and Myc-His DAT. Cells expressing Flag-HA-DAT, Myc-His DAT, or both were treated with bis-EA or CuP as described in Materials and Methods. (Lower) An immunoblot of the extracts. (Upper) The elution after immunoprecipitation with anti-Myc antibody, performed as described in the Materials and Methods. Flag-HA-DAT was detected in these experiments with anti-HA rabbit polyclonal antibody (0.2 μg/ml, Santa Cruz Biotechnology) and horseradish peroxidase-coupled anti-rabbit antibody (1:15,000; Santa Cruz Biotechnology), to avoid labeling the residual mouse anti-Myc antibody.
To explore whether the susceptibility of DAT to cross-linking might be a result of its high-level expression in heterologous cells, we performed similar studies with mouse striatal membranes. Under nonreducing conditions, mouse DAT migrated on SDS/PAGE as a monomer of ≈75 kDa (in contrast to ≈85 kDa in EM4 cells), identified by binding of a specific antibody to mouse DAT (Fig. 3). After the membranes were reacted with CuP or bis-EA we observed a band at ≈175 kDa, a size compatible with dimer, as well as a larger band. The appearance of the dimer and the larger oligomeric species containing DAT in mouse striatum is consistent with the dimeric or oligomeric nature of DAT in its native membrane.
Figure 3.
Cross-linking of DAT in mouse striatal membranes. Mouse striatal membranes were reacted with bis-EA or CuP and immunoblotted as described in Materials and Methods, but with a rabbit polyclonal anti-DAT antibody (5 μg/ml; Chemicon) and horseradish peroxidase-coupled anti-rabbit antibody (1:15,000).
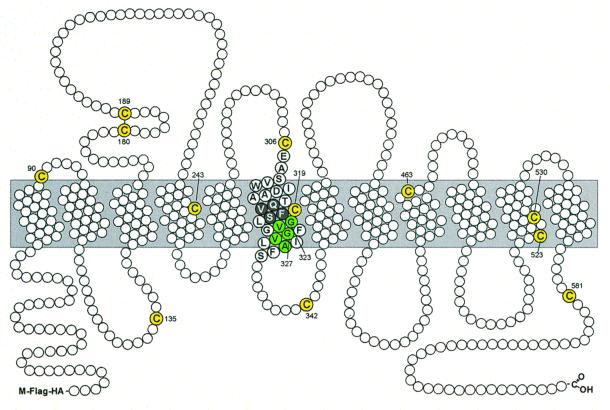
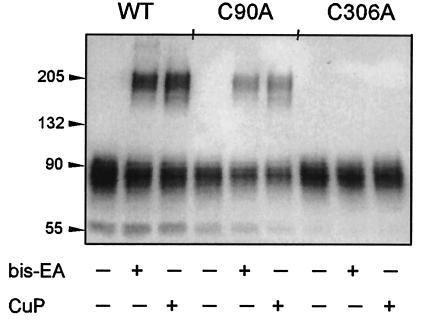
There are 13 cysteines in human DAT (Fig. 4). According to our current view of the topology, 4 cysteines are extracellular, with 2 of these likely being disulfide-bonded (19, 20). Another 4 cysteines are in intracellular loops, and the remaining five cysteines are in the transmembrane domain. Pretreatment with methanethiosulfonate ethyltrimethylammonium, an impermeant, positively charged MTS reagent (21), prevented subsequent bis-EA or CuP from cross-linking Flag-HA-DAT (data not shown), indicating that the cysteine involved in the cross-link is exposed to the extracellular milieu. Because we previously demonstrated that Cys-90 and Cys-306 are accessible to impermeant MTS reagents applied extracellularly (6), we hypothesized that one or both of these cysteines participate in the cross-link. Mutation of Cys-90 to Ala did not prevent cross-linking, whereas mutation of Cys-306 to Ala completely prevented the formation of dimer by either reagent (Fig. 5). Both C90A and C306A were functional and expressed at the cell surface (data not shown).
Figure 4.
Schematic representation of the topology of Flag-HA-DAT. The extracellular side of the membrane is on the top of the diagram and the cytoplasmic side is on the bottom. The endogenous cysteines are shown in yellow. The GVXXGVXXA motif is shaded green. The stretch of residues from Gln-317 to Ser-321 that were mutated to cysteine is shown with black circles with white letters.
Figure 5.
Cross-linking of WT DAT, C90A, and C306A Flag-HA-DAT. Wild-type (WT), C90A, and C306A Flag-HA-DAT were reacted with bis-EA or CuP, and immunoblotting was performed as described in Materials and Methods.
Although these results established that Cys-306 is involved in the formation of the disulfide-linked dimer, it did not prove that the cross-link is between a Cys-306 on each member of the dimer, because the partner cysteine could be another of the endogenous cysteines in DAT. To explore this possibility, we simultaneously mutated C90A, C243A, C306A, C319M, C463S, C523S, C530A, and C581L in the Flag-HA-DAT background. Because Cys-6 is part of the short N-terminal fragment truncated and replaced with Flag-HA or Myc-His in our background construct, the only cysteines remaining in this construct are Cys-180 and Cys-189, which likely form a native disulfide bond (19, 20), and Cys-135 and Cys-342, which are in cytoplasmic loops (6). Thus, this cysteine-depleted construct (CD-DAT) is predicted to have no extracellular or transmembrane free sulfhydryls. CD-DAT is expressed at the cell surface and transports dopamine and tyramine (data not shown) but is not cross-linked by either bis-EA or CuP (Fig. 6). Restoration of Cys-306 into CD-DAT (CD-DAT-A306C) produced a transporter that was cross-linked by bis-EA and CuP (Fig. 6), demonstrating that Cys-306 is both necessary and sufficient for cross-linking, and further demonstrating that the cross-link is symmetrical between Cys-306 at the extracellular end of TM6 in each dimer partner.
Figure 6.
Cross-linking of WT DAT, a cysteine-depleted DAT (CD-DAT), CD-DAT-A306C, and CD-DAT-M319C. Wild-type (WT) DAT, CD-DAT, CD-DAT-A306C, and CD-DAT-M319C, all in the Flag-HA background, were cross-linked and immunoblotted as described in Materials and Methods.
We also replaced cysteines in TM6 at positions 317–321 (Fig. 4), in the vicinity of the endogenous Cys-319, into the CD-DAT background. Only CD-DAT-V318C and CD-DAT-M319C expressed at the cell surface, and neither of these constructs cross-linked on treatment with bis-EA or CuP (Fig. 6 and data not shown). Replacement of Gln-317, Phe-320, or Ser-321 with cysteine was not tolerated (at least in the CD-DAT background), consistent with the conservation of these residues in all Na+- and Cl−-dependent neurotransmitter transporters (see Fig 7).
Figure 7.
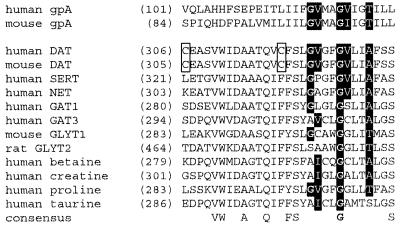
Sequence alignment of TM6 of DAT, other Na+- and Cl−-dependent neurotransmitter transporter family members, and glycophorin A (gpA). The number of the first residue of each sequence shown is given in parentheses. Cys-306, the endogenous cysteine that is cross-linked in DAT, and Cys-319, the endogenous cysteine in TM6, are enclosed within a box. The conserved residues in the dimerization motif are shown with white font on a black background. Residues that are completely conserved in all of the aligned transporters are shown on the consensus line.
Cross-linking Flag-HA-DAT with bis-EA or with CuP produced no significant inhibition of substrate transport (92.6% ± 5.6% and 86.4% ± 6.5% of control in Flag-HA-DAT for bis-EA and CuP, respectively, mean ± SD, n = 3). Moreover, the effect of the reagents was quite similar in C306A, a mutant that was not cross-linked by these reagents (99.3% ± 12.6% and 89.7% ± 16.0% of control in Flag-HA-DAT-C306A for bis-EA and CuP, respectively, mean ± SD, n = 3).
Our findings implied that the extracellular end of TM6 might be part of a dimer interface in DAT and led us to search for other indications of dimerization. We identified in the intracellular half of TM6 a well characterized dimerization motif, G(V/I)XXG(V/I)XX(A/T), originally identified in glycophorin A (22–24). This dimerization motif is found not only in DAT, but in other neurotransmitter transporters as well (Fig. 7). The second glycine is 100% conserved in all Na+- and Cl−-dependent neurotransmitter transporters, and this was the only residue in glycophorin A found to be indispensable for dimerization (22). Alanine was tolerated as a substitution for the first glycine in glycophorin A, and serine was not studied at this position (22). Similarly, the first glycine is highly conserved in Na+- and Cl−-dependent neurotransmitter transporters, but is replaced in several sequences by alanine or serine (Fig. 7).
Mutation of Gly-323 to valine completely blocked tyramine transport (Fig. 8), and mutation to either valine or cysteine dramatically lowered expression of the mature form of DAT (Fig. 9). The small amount of mature, mutated DAT that was expressed was susceptible to reaction with an impermeant biotinylation reagent (data not shown), indicating that it was on the cell surface. Furthermore, some of this mutant DAT on the cell surface was still susceptible to cross-linking (Fig. 9), albeit nonfunctional as a transporter. Mutation of the second glycine, Gly-327, to either cysteine or valine completely prevented expression of the mature transporter (Fig. 9). In contrast, DAT, in which Val-324 or Val-328 was mutated to either cysteine or glycine transported tyramine like WT (Fig. 8) and was cross-linked by CuP and bis-EA (Fig. 9). Similarly, glycophorin A formed significant amounts of dimer after mutation of these valines (22).
Figure 8.
Tyramine uptake in dimerization motif DAT mutants. Tyramine uptake was measured as described in Materials and Methods. Total uptake was normalized to maximal uptake in WT and is shown for WT (solid squares), for G323V (open squares), G327V (open circles), V324C (solid circles), and V328C (solid triangles) Flag-HA-DAT, and for untransfected EM4 cells (×).
Figure 9.
Cross-linking of dimerization motif DAT mutants. G323V, G327V, V324C, and V328C Flag-HA-DAT were cross-linked and immunoblotted as described in Materials and Methods.
Discussion
The major finding of this study is that DAT in the plasma membrane can be cross-linked into a dimer by the flexible cross-linker bis-EA or by CuP-generated oxidation. The cross-linked residue, Cys-306, is located at the putative extracellular end of TM6, presumably at a symmetrical dimer interface. DAT in mouse striatal membranes was also cross-linked. The efficiency of cross-linking was relatively high. Because cross-linking requires that only one of the two cysteines involved is modified initially by the reagent, and the derivatized cysteine then reacts by collision with the second unmodified cysteine, the rate of collision must be faster than the rate of initial modification. This is consistent with the cysteines being close initially. Thus, it is likely that DAT exists as a dimer in the membrane not treated with cross-linker, but that this dimer does not survive solubilization in nonionic detergent. This characteristic is in contrast to SERT, in which a dimer survives such solubilization (9).
DAT dimer in the plasma membrane of EM4 cells might associate further to form a dimer of dimers, but only one endogenous cysteine in each DAT, Cys-306, forms a covalent cross-link. In two radiation-inactivation studies, DAT was inferred to be a dimer (25) and a tetramer (26). Experiments with concateners of SERT supported a dimeric or tetrameric, but not a trimeric, quaternary structure (27). Cross-linking of SERT was also consistent with an oligomeric structure of SERT, although the residues responsible for cross-linking were not identified (28).
The site of covalent cross-linking of DAT, Cys-306, is completely conserved in all DAT species, but it is not present in any other homologous neurotransmitter transporter (Fig. 7). Although there is no evidence for basal cross-linking of DAT in our cells or in mouse striatal membranes, it is conceivable that, under oxidizing conditions, DAT might cross-link in vivo. The results of such oxidation would be of unclear import, given that Cys-306 can be cross-linked without apparent effect on uptake. Thus, whatever conformational movement is necessary for transport can apparently still take place not only with the constraint of the 5- to 10-Å cross-link by bis-EA, but even with the constraint of the direct 2.5-Å S—S cross-link formed by CuP.
The glycine transporters Glyt1 and Glyt2, which are homologous to DAT, have been shown to exist in the plasma membrane exclusively as monomers (11). In contrast, a significant fraction of intracellular Glyt1 and Glyt2 was oligomeric (11). The DAT dimer, however, is present on the cell surface: cross-linking can be blocked by membrane-impermeant methanethiosulfonate ethyltrimethylammonium, and the cross-linked dimer can be labeled with an impermeant biotinylation reagent (data not shown).
In all known DAT sequences, TM6 contains a perfect match of the dimerization sequence in glycophorin A (22). This same dimerization motif predominated in random peptides found to dimerize in a membrane domain (23). The existence of this motif in DAT TM6, the extracellular end of which is susceptible to disulfide cross-linking, suggests that this motif functions in DAT as it does in glycophorin A, although the DAT dimer is not as stable as the glycophorin dimer. In glycophorin A, the second glycine in the motif was the only position that was indispensable for dimerization (22), and in DAT, mutation of the second glycine, Gly-327, completely prevented surface expression. In glycophorin A, mutation of the first glycine to a large residue disrupted dimerization, but mutation to alanine was tolerated, and mutation to cysteine or serine was not tested. In DAT, mutation of the first glycine, Gly-323, to valine greatly decreased surface expression and completely blocked all transport. The small amount that was expressed on the surface, however, was still susceptible to disulfide cross-linking, albeit nonfunctional as a transporter. In other oligomeric proteins, blocking oligomerization blocks transport to the cell surface (29), and a functional dimerization motif in TM6 of DAT may be necessary as well for efficient trafficking to the cell surface.
Acknowledgments
We are grateful to Jasmine Ferrer and Lei Shi for making some of the constructs used in these experiments, to Jiayun Chen for preliminary work on this project, to Vernice Jackson for mouse striata, to David Stauffer and Kenji Monde for synthesis and characterization of bis-EA, and to Myles Akabas, Irache Visiers, and Harel Weinstein for helpful discussion. This work was supported by National Institutes of Health Grants DA12408, DA11495, MH57324, and NS07065.
Abbreviations
- DAT
dopamine transporter
- CuP
copper phenanthroline
- bis-EA
bis-(2-methanethiosulfonatoethyl)amine hydrochloride
- HA
hemagglutinin epitope
- CD
cysteine-depleted
- MTS
methanethiosulfonate
- NEM
N-ethylmaleimide
- TMn
transmembrane segment n
- SERT
serotonin transporter
- WT
wild type
References
- 1.Giros B, Caron M G. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 2.Giros B, el Mestikawy S, Bertrand L, Caron M G. FEBS Lett. 1991;295:149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- 3.Amara S G, Kuhar M J. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 4.Kanner B I. J Exp Biol. 1994;196:237–249. doi: 10.1242/jeb.196.1.237. [DOI] [PubMed] [Google Scholar]
- 5.Chen J G, Liu-Chen S, Rudnick G. J Biol Chem. 1998;273:12675–12681. doi: 10.1074/jbc.273.20.12675. [DOI] [PubMed] [Google Scholar]
- 6.Ferrer J V, Javitch J A. Proc Natl Acad Sci USA. 1998;95:9238–9243. doi: 10.1073/pnas.95.16.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norregaard L, Frederiksen D, Nielsen E O, Gether U. EMBO J. 1998;17:4266–4273. doi: 10.1093/emboj/17.15.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loland C J, Norregaard L, Gether U. J Biol Chem. 1999;274:36928–36934. doi: 10.1074/jbc.274.52.36928. [DOI] [PubMed] [Google Scholar]
- 9.Kilic F, Rudnick G. Proc Natl Acad Sci USA. 2000;97:3106–3111. doi: 10.1073/pnas.060408997. . (First Published March 14, 2000; 10.1073/pnas.060408997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid J A, Scholze P, Kudlacek O, Freissmuth M, Singer E A, Sitte H H. J Biol Chem. 2001;276:3805–3810. doi: 10.1074/jbc.M007357200. [DOI] [PubMed] [Google Scholar]
- 11.Horiuchi M, Nicke A, Gomeza J, Aschrafi A, Schmalzing G, Betz H. Proc Natl Acad Sci USA. 2001;98:1448–1453. doi: 10.1073/pnas.041329498. . (First Published February 6, 2001; 10.1073/pnas.041329498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stauffer D A, Karlin A. Biochemistry. 1994;33:6840–6849. doi: 10.1021/bi00188a013. [DOI] [PubMed] [Google Scholar]
- 13.Robbins A K, Horlick R A. Biotechniques. 1998;25:240–244. doi: 10.2144/98252st04. [DOI] [PubMed] [Google Scholar]
- 14.Saunders C, Ferrer J V, Shi L, Chen J, Merrill G, Lamb M E, Leeb-Lundberg L M, Carvelli L, Javitch J A, Galli A. Proc Natl Acad Sci USA. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. . (First Published May 23, 2000; 10.1073/pnas.110035297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Eshleman A J, Stewart E, Evenson A K, Mason J N, Blakely R D, Janowsky A, Neve K A. J Neurochem. 1997;69:1459–1466. doi: 10.1046/j.1471-4159.1997.69041459.x. [DOI] [PubMed] [Google Scholar]
- 17.Careaga C L, Falke J J. J Mol Biol. 1992;226:1219–1235. doi: 10.1016/0022-2836(92)91063-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Kaback H R. Proc Natl Acad Sci USA. 1996;93:14498–14502. doi: 10.1073/pnas.93.25.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J B, Moriwaki A, Uhl G R. J Neurochem. 1995;64:1416–1419. doi: 10.1046/j.1471-4159.1995.64031416.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen J G, Liu-Chen S, Rudnick G. Biochemistry. 1997;36:1479–1486. doi: 10.1021/bi962256g. [DOI] [PubMed] [Google Scholar]
- 21.Akabas M H, Stauffer D A, Xu M, Karlin A. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- 22.Lemmon M A, Treutlein H R, Adams P D, Brunger A T, Engelman D M. Struct Biol. 1994;1:157–163. doi: 10.1038/nsb0394-157. [DOI] [PubMed] [Google Scholar]
- 23.Russ W P, Engelman D M. J Mol Biol. 2000;296:911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- 24.Senes A, Gerstein M, Engelman D M. J Mol Biol. 2000;296:921–936. doi: 10.1006/jmbi.1999.3488. [DOI] [PubMed] [Google Scholar]
- 25.Berger S P, Farrell K, Conant D, Kempner E S, Paul S M. Mol Pharmacol. 1994;46:726–731. [PubMed] [Google Scholar]
- 26.Milner H E, Beliveau R, Jarvis S M. Biochim Biophys Acta. 1994;1190:185–187. doi: 10.1016/0005-2736(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 27.Chang A S, Starnes D M, Chang S M. Biochem Biophys Res Commun. 1998;249:416–421. doi: 10.1006/bbrc.1998.9158. [DOI] [PubMed] [Google Scholar]
- 28.Jess U, Betz H, Schloss P. FEBS Lett. 1996;394:44–46. doi: 10.1016/0014-5793(96)00916-7. [DOI] [PubMed] [Google Scholar]
- 29.Reddy P S, Corley R B. BioEssays. 1998;20:546–554. doi: 10.1002/(SICI)1521-1878(199807)20:7<546::AID-BIES5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Bruice T W, Kenyon G L. J Prot Chem. 1982;1:47–58. [Google Scholar]