Abstract
Social value orientation (SVO) is a stable personality trait that reflects how people evaluate interdependent outcomes for themselves and others in social environments. Generally, people can be classified into two types: proselfs and prosocials. The present study examined how SVO affects the processing of outcome evaluation temporally using the event-related potential (ERP). Young adults with two different SVO types participated in a simple gambling task in which they received outcome distributions for themselves and others. The results showed that for the self outcomes, the feedback-related negativity (FRN) was more negative for self-loss than self-gain, and the P3 and late positive component (LPC) was larger for self-gain than self-loss in both prosocial and proself groups. For the other outcomes, however, the FRN, P3 and LPC were sensitive to other’s gain and loss only in the prosocial group but not in the proself group. These findings suggest that outcomes for oneself and others are processed differently at different stages of evaluation processing in the brains of individuals with distinct SVOs.
Keywords: social value orientation (SVO), outcome evaluation, event-related potential (ERP), feedback-related negativity (FRN), P3, late positive component (LPC)
Introduction
To efficiently navigate our social environment, people are required to make the right decisions, which demands us to evaluate available alternatives and take actions that are beneficial to ourselves (Daniel and Pollmann, 2014; Christopoulos and King-Casas, 2015). However, the circumstances become complicated when the outcomes of the decisions involve other social agents, because we usually make choices that affect not only our own outcomes but also those of other social agents (Poppe and Valkenberg, 2003). To smoothly solve such social interactions, the decision-makers need to evaluate the outcomes of decisions they have made for themselves and others depending on their own social motivations and preferences toward themselves and other social partners (Galvan et al., 2005). Researchers have defined this social preference or motivation as social value orientation (SVO). Extensive research regarding SVO suggests that this social preference is temporally stable and cannot be affected by situations (e.g. Kuhlman et al., 1986; Van Lange et al., 1997; Li et al., 2013). Moreover, Hilbig et al. (2014) associated SVO with basic personality traits and found that people with distinct SVOs scored differently in terms of the Honesty-Humility trait, which could effectively predict their pro-social behavior. Hence, SVO is a stable personality trait that reflects how people evaluate interdependent outcomes for themselves and others and plays an important role in the process of decision-making, especially outcome evaluation (Messick and McClintock, 1968; Van Lange, 2000).
Studies regarding SVO have revealed four main types of the social preferences, competition orientation (maximizing their relative advantage over other’s outcomes), individualism orientation (maximizing their own outcomes without considering other’s outcomes), cooperation orientation (maximizing the joint outcomes of both sides) and equality orientation (minimizing the difference in outcomes between themselves and others) (e.g. Kuhlman and Wimberley, 1976; McClintock and Liebrand, 1988). Broadly speaking, people in these four orientations can be classified into two categories. People of the competition and individualism orientations can be classified as proself individuals because they place their own interests above those of others. For the people of the cooperation and equality orientations, they can be classified as prosocial individuals because they are interested in enhancing the joint outcomes and pay a certain amount of attention to others’ outcomes (e.g. Joireman and Duell, 2005; Stouten et al., 2005). The integrative model of SVO assumes that the prosocials differ from the proselfs in two aspects: the weight assigned to other's outcomes and the weight assigned to the equality of outcomes (Van Lange, 1999, 2000). Thus, this model implies that relative to proselfs, the prosocials’ evaluation of outcomes are influenced by considerations of others’ outcomes and fairness, and the prosocials tend to pay more attention to outcomes for others (De Cremer and Van Lange, 2001; Bieleke et al., 2016).
Behavioral studies have indicated that the evaluation of social outcomes (i.e. outcomes involving other social agents) can vary across individuals with different SVOs. For instance, proselfs only care for their own interests so they seek outcomes benefiting themselves without considering the social partner, whereas prosocials care for collective interests and hence seek outcomes benefiting both themselves and others (Murphy and Ackermann, 2014). Research regarding the ultimatum game has revealed that proselfs strategically use fairness as a way to increase their own outcomes, and prosocials accept the unfair offer more frequently than proselfs due to the use of emotion regulation strategies (Van Dijk et al., 2004; Karagonlar and Kuhlman, 2013).
With the rise of social neuroscience in recent years, a growing number of studies have focused on the neural underpinnings of SVO. In their fMRI study, Haruno and Frith (2010) found that amygdala activity could predict the degree of inequity aversion in prosocials, which indicated that the prosocials’ emotion was largely influenced by other’s rewards, and they disliked large differences in the outcome distribution between themselves and others. Alternatively, in this study the proselfs were unaffected by others’ rewards and they preferred higher rewards for themselves. More recently, an fMRI study on SVO dependent learning signals suggested that reinforcement learning signals for other’s outcomes were identified in the medial prefrontal cortex, and more importantly, the magnitude and valence of the other’s outcomes learning signal depends strongly on a person’s prosocial or proself orientation toward others (Christopoulos and King-Casas, 2015). In addition, a study on the effects of exogenous oxytocin (OT) on cooperation has revealed that OT and social cues significantly interact to enhance the cooperative behavior of proselfs, whereas this effect was not observed for prosocials (Declerck et al., 2014).
In our daily life one needs to evaluate the outcome of his/her choice on many occasions as quickly as possible in order to assess the success of their actions, and has to be able to use positive or negative outcome feedback to adjust their anticipations and to guide their future behaviors (Yang et al., 2015). Previous studies using the event-related potential (ERP) have found three ERP components related to the processing of outcome evaluation: the feedback-related negativity (FRN), the P3, and the late positive component (LPC) (e.g. Miltner et al., 1997; Schupp et al., 2000; Gehring and Willoughby, 2002; Yeung et al, 2004). The FRN is a negative deflection in the fronto-central regions of the scalp and reaches its peak between 200 and 300 ms post-onset of the feedback stimulus. It is larger for negative outcomes, such as the loss of money or incorrect responses, than positive outcomes (e.g. Gehring and Willoughby, 2002; Hajcak et al., 2005; Paul and Pourtois, 2017) and sensitive to the violation of expectancy (Hajcak et al., 2006). The P3 is a centro-parietal positivity with a peak in the time window of 300–600 ms following the feedback onset. It is greater for positive feedback than for negative feedback and for a large reward than for a small reward (Hajcak et al., 2005, 2007; Holroyd et al., 2006). The LPC is a positive deflection over the centro-parietal scalp in the late time window between 300 and 900 ms post-stimulus, which is larger for feedback with high arousal levels and thus is considered to represent emotional arousal (e.g. Amrhein et al., 2004; Wu et al., 2012; Luo et al., 2015).
Although a considerable number of behavioral and neuroimaging studies have investigated the relationship between SVO and outcome evaluation, little is known about how the time course of the brain activities underlying the evaluation of self outcomes and other’s outcomes vary across individuals with distinct SVOs. Electroencephalography (EEG) is an excellent way to assess the spontaneous evaluation of outcomes involving self and others due to its high temporal resolution. Further, it allows for measurement of the variations in the degree to which people’s brains process the outcome distributions implicitly and rapidly (Scheepers and Derks, 2016). Therefore, in the present study, we attempted to explore the temporal processing of the effect of SVO on outcome evaluation using the ERP technique due to its unique advantages. According to previous ERP studies on outcome evaluation, we focused on the FRN, P3 and LPC, which represent the different stages of the processing of outcome evaluation. In this study, we first identified the participants’ social value orientation through a Triple-Dominance Scale (Van Lange et al., 1997). Then, a simple monetary gambling task was used to explore whether the FRN, P3 and LPC in response to the social outcomes would be impacted by different SVOs. Based on previous studies, we hypothesized that the three ERP components would be modulated by SVO in different outcome distributions. Specifically, we hypothesized that the FRN and P3 in the prosocial group would be affected by both the self outcome and other outcome, whereas those in the proself group would be only affected by the self outcome. In addition, we also predicted that SVO might have a lasting effect in the late stage of outcome evaluation indexed by the LPC with distinct emotional arousal levels.
Materials and methods
Overview of procedures
The present study consisted of two parts: the SVO assessment session and the ERP experiment session. Prior to the ERP experiment, the SVO of participants was assessed through the Triple-Dominance Measure of Social Values (Van Lange et al., 1997). Upon completion of the assessment, participants who could be classified as one of two types of the SVO, proselfs and prosocials, participated in the subsequent ERP experiment. Those who could not be classified as one of two types of the SVO were excluded from the study.
Participants
Fifty-seven healthy students (30 females; mean age 22.35 ± 1.36 years) from Renmin University of China participated in the study and were paid for their participation. In the first part of the study, five participants were excluded because they did not have a clear social preference. In the second session, two participants were excluded because there were not enough trials after artifacts were removed (<20 trials, the minimal number of trials required for a stable ERP component, Marco-Pallares et al., 2011). Thus, data from the remaining 50 participants (27 proselfs and 23 prosocials) were ultimately analyzed. All participants were right-handed, had normal or corrected-to-normal vision, and had no history of psychiatric, neurological or medical illness. Written informed consent was obtained from all participants. The study was approved by the local ethical committee.
First session: social value orientation assessment
In the first session of the present study, the SVO of participants was assessed using Triple-Dominance Measure (Van Lange et al., 1997), which has been widely used in the areas of sociology, economics and psychology for measuring social preferences (Murphy and Ackermann, 2014). The Triple-Dominance scale contains the most straightforward social value orientation measure and has been shown to have highly reliable results with remarkable stability, internal validity and construct validity (Van Lange, 1999; Murphy et al., 2011).
The Triple-Dominance SVO measurement contains nine items and each item consists of three optional outcome distributions of tokens allocated to the participant himself/herself and to an anonymous social other. Each outcome distribution represented a particular SVO: individualistic, competitive, or cooperative. The participant was required to choose the outcome distribution that he/she most preferred for each item. On completing the assessment, the participant was classified as one of three orientations (individualist, competitor, or cooperator) if he/she made six or more out of the nine possible choices that were consistent with the orientation. If the participant did not make at least six choices that were consistent with a particular orientation, then he/she was not categorized (Van Lange, 2000). In the present study, those who could not be classified as one of the three orientations were excluded and did not participate in the following ERP experiment. Studies regarding SVO usually combine individualistic orientation and competitive orientation into a single category called proself orientation, whereas the cooperation orientation is classified into a category called prosocial orientation (e.g. De Cremer and Van Lange, 2001; Steinel and De Dreu, 2004). To be consistent with previous studies, we also combined individualists and competitors into one group. Therefore, we defined two groups of students based on their SVO, 28 proselfs and 24 prosocials participated in the following ERP experiment.
Second session: simple gambling task (ERP experiment)
When both the participant and a same-gender confederate who was played by a lab assistant arrived at the laboratory, they were informed that they would play a gambling game in which one of them would be the decision maker, and the other would be the recipient. The decision maker would make choices for both of them by choosing cards in the gambling task, and the bonus for both was based on the choices that the decision maker made in the task. Each participant was told that the baseline payment was 50 Chinese Yuan. The more points that the decision maker earned for himself/herself and the recipient, the greater the bonus that they would receive at the end of the ERP experiment. Ultimately, they were paid an amount of money between 68 and 72 Yuan. To increase the credibility of the game, the participant and the confederate were required to draw lots to decide their roles, and it was deliberately designed that the true participant always drew the lot of being the decision maker.
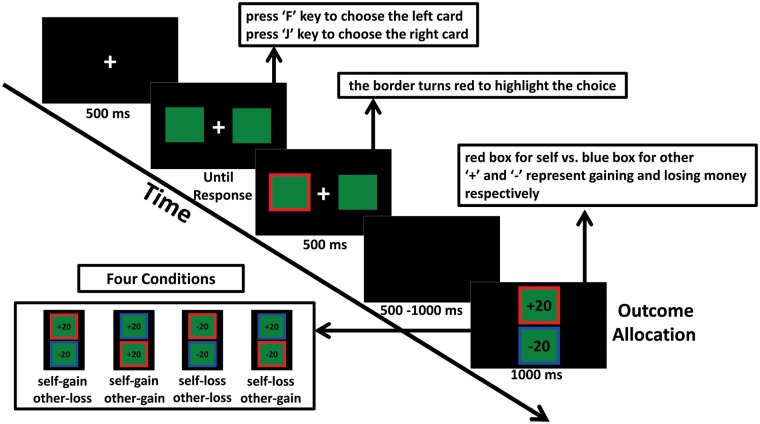
During the gambling task, the participant sat comfortably in an electromagnetic shielding room approximately 75 cm from a desktop computer monitor. As illustrated in Figure 1, each trial began with a white fixation cross presented for 500 ms on a black background. Then, two green squares (2.5° × 2.5°) representing two cards appeared on the left and right side of the fixation point. The participant was required to choose the left or right card by pressing the F or J keys on the keyboard with their left or right index fingers. Pressing the F key represented selecting the left card, and pressing the J key represented selecting the right card. When the participant responded, the chosen card was highlighted by a thickening of a red border for 500 ms, and then after an interval of 500–1000 ms, the outcome allocation behind the card chosen for himself/herself and the other was displayed on two squares, respectively, in a vertical array for 1000 ms. The participant’s own outcome was presented in the square with the red border, and the other’s outcome was presented in the square with the blue border. The vertical position of the two squares was random across trials. There were four outcome distributions in the gambling task, +20 and −20, +20 and +20, −20 and −20, or −20 and +20, which related to monetary losses or gains for the decision-maker and the recipient. The inter-trial interval lasted for 500 ms, and all conditions differed only in terms of valence; the magnitude of the reward was the same for both individuals. In the ‘self-gain other-loss condition’ (+20 and −20), the participant gained 20 points, while the other lost 20 points. In the ‘self-gain other-gain condition’ (+20 and +20), the two individuals both gained 20 points. In the ‘self-loss other-loss condition’ (−20 and −20), both individuals lost 20 points. In the ‘self-loss other-gain condition’ (−20 and +20), the participant lost 20 points, while the other gained 20 points. Unbeknownst to the participants, all the feedback was presented randomly, and each participant received equal times for each feedback condition.
Fig. 1.
An illustration of a single trial of the gambling task. Each trial began with a fixation cross. Participants viewed two squares and were required to choose one of the squares by pressing the corresponding key. Their choice was then highlighted for 500 ms. After an interval of 500–1000 ms, the outcome feedback was presented for 1000 ms, with the participant's own outcome shown in the square with the red border and the other's outcome in the square with the blue border. There were four outcome distributions resulting in four experimental conditions.
The entire gambling task consisted of 320 trials divided into eight blocks, so that each condition involved 80 trials. Before the formal task, participants practiced the task for 12 trials with 3 trials of each condition. The whole task lasted for approximately 25–30 min. Upon finishing the experiment, all participants were asked about the credibility of the stranger scenario and the cover story, and no one raised doubts about it. The stimulus presentation and the behavioral data acquisition were conducted by E-Prime 2.0 software (PST, Inc., Pittsburgh, PA, USA).
EEG recording and analyses
EEG was recorded from 64 cap-mounted tin electrodes arranged according to the 10/20 international placement system (Neuroscan Inc., Herndon, VA, USA), with an online reference to the left mastoid and an offline re-reference to the average of the left and right mastoids. The horizontal electro-oculogram (EOG) was recorded from the electrodes placed 1.5 cm lateral at the outer canthi of both eyes. Vertical EOG was recorded from the electrodes placed above and below the right eye. All inter-electrode impedance was kept below 5 kΩ during the recording. Signals were amplified using a 0.01–100 Hz band-pass filter and continuously sampled at 1000 Hz/channel for the offline analysis.
Offline analysis of the EEG data was performed using Neuroscan 4.5 software. Ocular artifacts were removed using a regression procedure implemented in the Neuroscan software (Semlitsch et al., 1986). The EEG data were low-pass filtered below 30 Hz (24 dB/oct) and were segmented into epochs from 200 ms before to 800 ms after the onset of the outcome presentation. The data were baseline-corrected according to 200 ms pre-feedback baseline. Epochs containing artifacts exceeding ±70 μV were excluded from further analysis. Then, epochs were averaged separately for each condition of each participant.
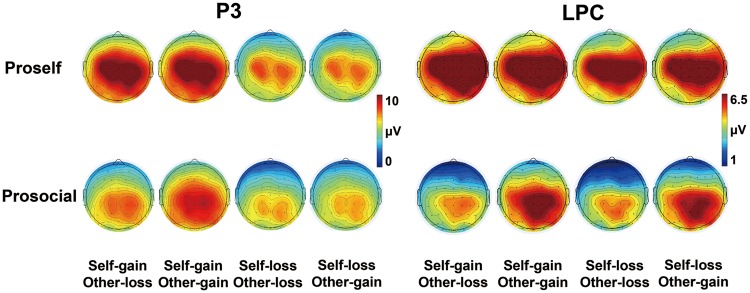
The ERP components that were analyzed included the FRN, P3 and LPC. The FRN was evaluated as the mean amplitude within the time window 250–320 ms following the outcome allocation presentation. The P3 was defined as the most positive peak in the period 350–450 ms after the feedback stimulus onset. The LPC was measured as the mean amplitude within the time window 500–700 ms following the feedback presentation. In preliminary analyses, the anteroposterior and lateral scalp locations were considered as topographic factors. Based on the topographical distribution of each ERP components (Figures 2C and 3) and previous studies (e.g. Gehring and Willoughby, 2002; Yeung and Sanfey, 2004), the FRN was calculated across 9 electrode locations (F3, FC3, C3, Fz, FCz, Cz, F4, FC4 and C4), and the P3 and LPC were quantified across 9 electrode locations (C3, CP3, P3, Cz, CPz, Pz, C4, CP4 and P4) that were chosen to cover scalp areas corresponding with these components. The results indicated that the effect of FRN was greatest at the Fz site, and the effects of P3 and LPC were largest at the CPz site. Hence, we focused on the Fz and CPz sites for more detailed analyses in which the ERP effects were maximal.
Fig. 2.
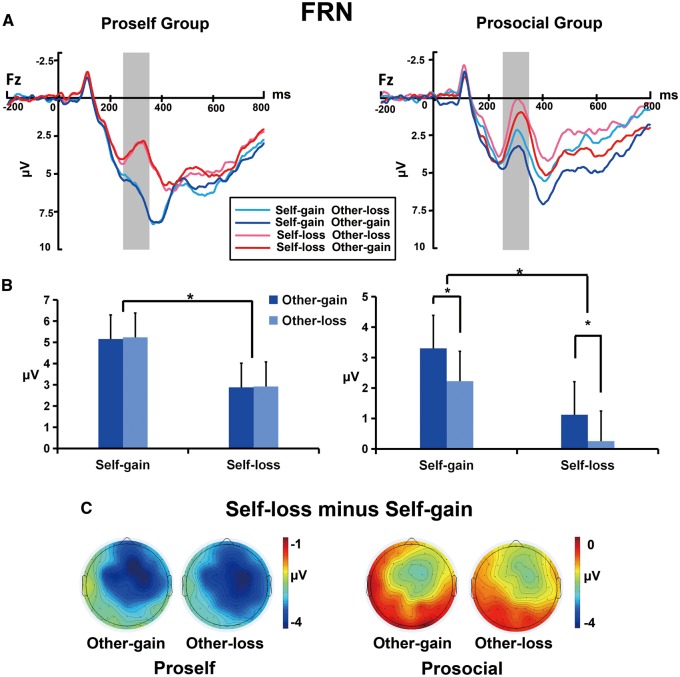
(A) Grand-average ERP waveforms from the Fz electrode site. The gray areas highlight the time window of the FRN (250–320 ms) used for statistical analysis. (B) The bar graphs show the mean value of the FRN amplitude for each condition. Error bars indicate standard error of the mean (SEM). *P < 0.001. (C) Topographies of the voltage differences between the self-loss and self-gain outcomes in the FRN time interval (250–320 ms), separately for trials involving other-gain and other-loss outcomes.
Fig. 3.
Topographical voltage distributions of the P3 (left panel) and LPC (right panel) for each condition, separately for the SVO groups (top row for proselfs and bottom row for prosocials).
Behavioral and ERP data were statistically analyzed using SPSS software (version 22.0, SPSS Inc., Chicago, IL, USA). The FRN, P3 and LPC amplitudes were each analyzed using a mixed three-way repeated measure analysis of variance (ANOVA) of 2 (SVO group: proself vs. prosocial) × 2 (Self-outcome: self-gain vs. self-loss) × 2 (Other-outcome: other-gain vs. other-loss). The SVO group was a between-subjects factor and the latter two were within-subjects factors. The significance level was set at 0.05 for all the analyses in the present study. The Greenhouse–Geisser correction was conducted to account for sphericity violations whenever appropriate. Post-hoc testing of the significant main effects was applied with the Bonferroni adjustments. Partial eta-squared (ηp2) values were conducted to examine the effect size in ANOVA models, such that 0.05 represents a small effect, 0.1 represents a medium effect, and 0.2 represents a large effect (Cohen, 1973).
Results
Behavioral results
In the gambling task, the mean (±SD) reaction times taken for decision-making by the proself group and prosocial group were 973 ± 79 ms and 1046 ± 113 ms, respectively. An independent sample t-test was conducted to compare the reaction time between two groups. The result showed that the proselfs reacted significantly faster than the prosocials, t(48) = −2.67, P = 0.04, indicating that during the process of decision-making execution (Paulus, 2005), the prosocials spent more time making choices than the proselfs did.
ERP results
Feedback-related negativity
Figure 2A shows grand-average ERP waveforms at the Fz electrode site. For the FRN amplitude, the main effect of the self-outcome was significant (F(1,48) = 394.02, P < 0.001, ηp2 = 0.89), indicating that the FRN was more negative when all participants received a self-loss outcome (1.80 μV) than a self-gain outcome (3.98 μV). The main effect of the other-outcome (F(1,48) = 88.14, P < 0.001, ηp2 = 0.65) was also significant, suggesting that in general the FRN was more negative for the other-loss outcomes (2.64 μV) than for the other-gain outcome (3.14 μV). In addition, the main effect of SVO group was significant (F(1,48) = 23.38, P < 0.001, ηp2 = 0.33), indicating that on average the FRN was more negative in the prosocial group (1.73 μV) than in the proself group (4.05 μV). Further post-hoc testing showed that the FRN was more negative in the prosocial group than in the proself group in each condition (Ps < 0.01). That is, the FRN for both the self and other outcomes were larger in the prosocial group than in the proself group.
In addition to the main effects, there was a significant interaction effect for the SVO group × other-outcome (F(1,48) = 81.58, P < 0.001, ηp2 = 0.63). Consequently, a simple effect analysis was applied to investigate this interaction. The results showed that the other-outcome effect in the proself group was not significant (F(1,48) = 1.83, P = 0.18, ηp2 = 0.003), such that the FRN amplitude did not show differences between the other-gain (4.06 μV) and other-loss outcomes (4.04 μV). This indicates that the proselfs did not distinguish between other’s gain and loss at the early stage of the outcome evaluation. In contrast, the other-outcome effect was significant in the prosocial group (F(1,48) = 76.81, P < 0.001, ηp2 = 0.63), such that the FRN was more negative for the other-loss outcome (1.24 μV) than other-gain outcome (2.21 μV). This finding suggested that prosocials would process the other’s outcome when they received the outcome allocations.
To further examine the different process for self and other’s outcome between individuals with different SVO, we compared the FRN amplitudes between the self-gain and self-loss outcomes, as well as the other-gain and other-loss outcomes using paired-samples t-tests in each group. Then, we found that in the proself group, the FRN was more negative for self-loss than for self-gain (t(26) = 26.17, P < 0.000, Cohen's d = 7.19), but it did not show differences between other’s loss and other’s gain (t(26) = 0.35, P > 0.05, Cohen's d = 0.10). However, in the prosocial group, the FRN was more negative for self-loss than for self-gain (t(22) = 13.41, P < 0.001, Cohen’s d = 4.00), as well as more negative for other-loss than for other-gain (t(22) = 10.11, P < 0.001, Cohen′s d = 3.01) (see Figure 2B). These results suggest that at the early stage of outcome evaluation, people with the proself orientation primarily process their own outcome and not the other’s outcome, whereas individuals with the prosocial orientation process both their own and the other’s outcome.
P3
Figure 4A shows grand-average ERP waveforms at the CPz electrode site. For the P3 amplitude, the main effect of the self-outcome was significant (F(1,48) = 397.36, P < 0.001, ηp2 = 0.89), indicating that the P3 was larger when the participants received a self-gain outcome (9.30 μV) than a self-loss outcome (7.05 μV). The main effect of the other-outcome (F(1,48) = 42.67, P < 0.001, ηp2 = 0.47) was also significant, suggesting that on average the P3 was larger for the other-gain outcome (8.35 μV) than the other-loss outcome (8.00 μV). In addition, there were significant interaction effects for the SVO group × other-outcome (F(1,48) = 76.75, P < 0.001, ηp2 = 0.62), self-outcome × other-outcome (F(1,48) = 56.05, P < 0.001, ηp2 = 0.54) and SVO group × self-outcome × other-outcome (F(1,48) = 54.42, P < 0.001, ηp2 = 0.53).
Fig. 4.
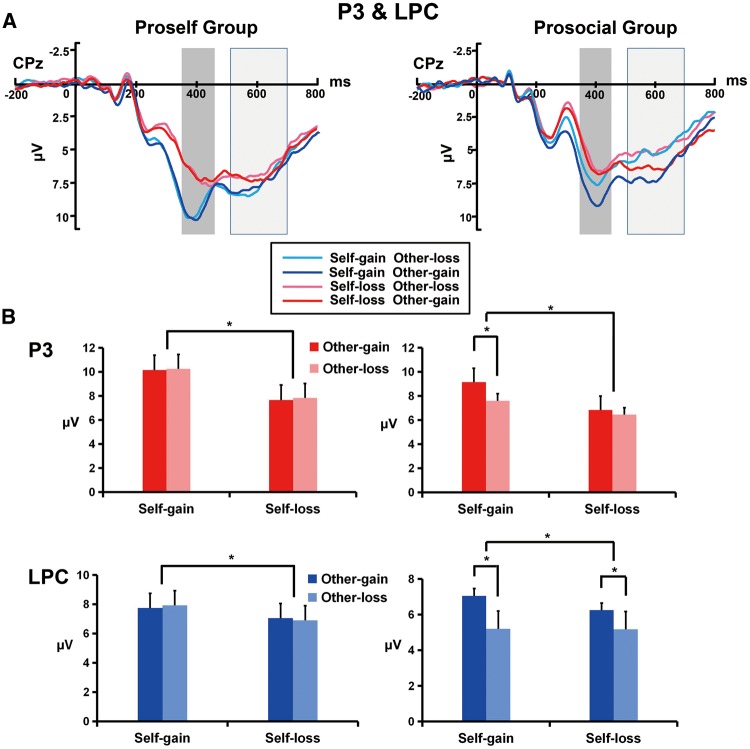
(A) Grand-average ERP waveforms from the CPz electrode site. The gray areas highlight the time window (350–450 ms) in which the peak amplitude of the P3 was measured. The light gray areas highlight the time window (500–700 ms) in which the mean amplitude of the LPC was measured. (B) The bar graphs show the mean value of the P3 amplitude (red) and LPC amplitude (blue) for each condition. Error bars indicate SEM (standard error of the mean). *P < 0.001.
A simple effect analysis was conducted to investigate the three-way interaction for the SVO group × self-outcome × other-outcome. The results revealed that in the prosocial orientation group, the other-outcome effect of the P3 was significant only when their self-outcome was gain (F(1,22) = 165.12, P < 0.001, ηp2 = 0.88) but not when their self-outcome was loss (F(1,22) = 1.44, P = 0.24, ηp2 = 0.06), indicating that the P3 was larger for the other-gain outcome than the other-loss outcome only when their own outcome was gain. However, in the proself group, there were no P3 differences between other’s gain and loss regardless of whether their own outcome was gain (F(1,26) = 1.45, P = 0.24, ηp2 = 0.05) or loss (F(1,26) = 1.42, P = 0.24, ηp2 = 0.05) (see Figure 4B). These results indicate that compared to the proselfs, the prosocials process others’ outcomes at a later stage of the outcome evaluation.
Late positive component
For LPC amplitude, the main effect of the self-outcome was significant (F(1,48) = 18.06, P < 0.001, ηp2 = 0.27), indicating that the LPC was larger when the participants received a self-gain outcome (7.17 μV) than a self-loss outcome (6.53 μV). The main effect of the other-outcome (F(1,48) = 68.09, P < 0.001, ηp2 = 0.58) was also significant, showing that the LPC was generally larger for the other-gain outcome (7.22 μV) than the other-loss outcome (6.48 μV). Further, there were significant interaction effects for the SVO group × other-outcome (F(1,48) = 68.94, P < 0.001, ηp2 = 0.59) and SVO group × self-outcome × other-outcome (F(1,48) = 9.185, P = 0.004, ηp2 = 0.16).
A simple effect analysis was conducted to investigate the three-way interaction for the SVO group × self-outcome × other-outcome. The results suggested that in the prosocial group, the LPC was larger for other-gain outcome than other-loss outcome no matter if their own outcome was gain (F(1,22) = 163.35, P < 0.001, ηp2 = 0.88) or loss (F(1,22) = 47.50, P < 0.001, ηp2 = 0.68). However, in the proself group, no LPC differences were observed between the other-gain and other-loss outcomes regardless of whether their own outcome was gain (F(1,26) = 0.299, P = 0.59, ηp2 = 0.01) or loss (F(1,26) = 0.11, P = 0.74, ηp2 < 0.001) (see Figure 4B). These results indicate that compared to the proselfs, the prosocials process others’ outcomes further at the final stage of the outcome evaluation.
Discussion
The present study used ERP to investigate the effect of SVO on the evaluation processing of outcomes involving others. Through three ERP indicators, the FRN, P3 and LPC, we found that individuals with distinct SVOs processed outcomes involving others differently in their brains. In line with our hypotheses, in the prosocial group, the three indicators were impacted by both the self-outcome and other-outcome. However, these ERP components were primarily impacted by the self-outcome rather than the other-outcome in the proself group. In addition, the FRN for both the self and other’s outcome was larger in the prosocial group than in the proself group. These findings revealed that the SVO modulated evaluation processing of outcomes involving others, which could be divided into three stages that are described subsequently.
The first stage
The first stage is represented by the FRN component, which is a fast and coarse assessment of ongoing events at the very early stage of the outcome evaluation process (Taylor et al., 2006; Bellebaum et al., 2010). In the present study, the FRN in both groups was larger when they received a loss than gain, which is consistent with the findings of previous studies (Gehring and Willoughby, 2002; Holroyd et al., 2004, 2007; Sidarus et al., 2017). Critically, the FRN effect can be modulated by people’s SVO. For the proselfs, their ideal reward expectation is self-interest maximization. Thus, the FRN was greater when proselfs received a loss than gain, regardless of the other’s outcomes, and the FRN amplitude only showed a difference between the self-loss and self-gain outcomes. In contrast, for the prosocials, their reward expectation is to maximize the interests of both sides; thus the FRN reached its maximum when they received the negative collective outcome, and conversely, it reached the minimum when they received the positive collective outcome. This supports previous findings that the prosocials seek outcomes benefiting both themselves and others and pursue maximization of the collective benefit (McClintock and Liebrand, 1988). Therefore, the FRN amplitude was affected by both the self and other outcome in prosocials. These distinct FRN effects indicate that proselfs and prosocials process personal interests and common interests differently at the very early stage of outcome evaluation.
In addition, the FRN of the prosocials was sensitive to the other’s outcome not only when they received a self-gain outcome but also when they received the self-loss outcome. This symmetry is inconsistent with previous study in which the FRN amplitude was found to be sensitive to other’s outcome only in the self-gain condition (Luo et al., 2015). This inconsistency might be interpreted as the impact of the SVO, because the previous study used a within-subjects design in which participants were not classified into different groups on the basis of their social preferences.
Another interesting finding was that the FRN amplitudes for both the self-outcome and other-outcome were larger in the prosocial group than in the proself group. The possible explanation for this is that the FRN also reflected the different responsibility levels between the prosocials and proselfs. Previous studies have reported that high responsibility would evoke a larger FRN in outcome processing (Li et al., 2010; Kimura and Katayama, 2013; Loehr et al., 2015). Accordingly, the FRN difference between groups indicated that the prosocials might have a higher sense of social responsibility for others, whereas the proselfs have low and even no sense of responsibility for others. These present findings provide electrophysiological evidences to support the integrative model of SVO, which suggests that relative to proselfs, prosocials are more concerned about social responsibility, which refers to a concern for both self and other (Fiske et al., 1998; Van Lange, 1999, 2000). Altogether, the FRN pattern in this task provides a preliminarily revelation of the cognitive significance of the feedback, which reflects people’s expectancy and preference for the outcomes.
The second stage
At the second stage, the processing of outcomes involving others was found to be more elaborate. The P3 component often reflects a more exquisite and precise evaluation especially in coding the motivational significance of outcomes (Taylor et al., 2006; Wu and Zhou, 2009). In the present study, the P3 amplitudes of the proselfs and prosocials were larger for self-gain than for self-loss, which is consistent with findings of previous studies regarding outcome evaluation (Bellebaum et al., 2010; Holroyd et al., 2006; Peterburs et al., 2017). Importantly, as proselfs tend to think and act in an individually rational manner, they will feel delighted when their own outcome is positive regardless of the outcome valence of others. Accordingly, their P3 reached a maximum when their own outcomes were a gain in the task. In contrast, prosocials are prone to think and act in a collectively rational manner and have a stronger sense of social responsibility (Van Lange, 2000; De Cremer and Van Lange, 2001), consequently, their P3 reached a maximum when both outcomes for self and other were positive. These findings suggest that SVO modulates people’s evaluations of outcome involving others at a more elaborate level.
Further, the results suggest that prosocials are sensitive to the other’s outcome only under the self-gain condition in the second evaluative stage, which is different from the symmetric pattern in the early stage. This difference might be interpreted as the effect of elaborate processing of the intrinsic psychological significance embodied in the outcomes (Johnston and Wang, 1991; Zhou et al., 2010). At the later stage, people tend to process feedback that is meaningful for themselves owing to their adaptive significance in receiving preferential access to attentional resources (Gray et al., 2004). Accordingly, relative to the self-loss outcome, people are prone to pay more attention to the self-gain outcome because it is more meaningful for them to maintain positive self-esteem, and we attribute this meaning in large part to the affection associated with self-interest and self-relevant information (Bargh, 1982). This positivity bias is consistent with previous studies indicating a greater weight on gains than losses in the social outcome evaluation process (Taylor et al., 2000; Ma et al., 2016). Additionally, when receiving a negative self-outcome, in order to address potential threat to the positive self-concept, people tend to avoid paying attention to another person’s outcome to protect themselves from the self-concept of getting hurt by comparing with others (Buunk and Gibbons, 2007; Powers et al., 2013). It seems that the positivity bias could be considered as an effective strategy to maintain a good self-esteem level for prosocials in social interactions, because they are more likely to be influenced by others. Therefore, the transformation in the P3 reveals a more sustained and elaborate processing stage in which people tend to attach more motivational and affective significance to positive self-outcomes in order to keep them positive and confident.
The third stage
The third stage of the evaluation processing of outcomes is indicated by the LPC, an ERP component associated with emotional arousal levels, which is typically taken to reflect the heightened processing related to increased motivated attention (Briggs and Martin, 2009). In our task, the gain-outcome elicited a larger LPC than the loss-outcome among all participants, which is in accordance with previous studies that consistently reported that enhanced LPC amplitudes were connected with positive, high-arousing stimuli (Schupp et al., 2004; van Hooff et al., 2011). Critically, people’s SVO modulated this effect of outcome evaluation involving others. For the proselfs, the LPC was larger for self-gain than for self-loss outcomes, but no difference was observed between the other-gain and other-loss outcomes. Whereas for prosocials, the LPC was not only greater for the self-gain condition than the self-loss condition, but it was also larger for the other-gain outcome than the other-loss outcome.
Prosocials tend to pursue maximization of common interests (De Cremer and Van Lange, 2001), hence the both-gain situation has the highest arousal level, which provides them more attentive capacity and maximizes their win-win motivation, as indicated by the largest LPC. This phenomenon was consistent with previous findings that the LPC was largest for the stimuli that were the most arousing or for stimuli with the strongest motivational relevance (Schupp et al., 2000).
In addition, some studies have demonstrated that the LPC is associated with the theory of mind (ToM), which is an ability to think about and reason the mental states of both themselves and others. Researchers have proposed that both the belief and desire for reasoning for others are connected with an enhanced LPC (Geangu et al., 2013; Jiang et al., 2016). Compared to proselfs, the prosocials have stronger ToM ability and altruistic motivations for others (Van Lange, 2000; Derks et al., 2015). Accordingly, the enhanced LPC evoked by others’ positive outcomes might reflect the altruistic motivation of prosocials. An intriguing finding was that the prosocials processing of the other’s outcome no matter whether their own outcome was gain or loss was different from the P3 pattern in the second stage in which they processed others’ outcomes only when their own outcome was gain. This transformation in the LPC suggested that at the last stage of outcome processing, the prosocials have changed their focus from self to other owing to their strong ability of ToM and helping motivations for others. In brief, the LPC patterns indicate the mental reasoning for others, emotional arousal and motivational significance of the outcomes in the last stage of outcome evaluation.
To summarize, using the ERP approach, the present study has revealed the SVO effect on the outcome evaluation involving others in three processing stages, suggesting from a neuro-psychological perspective that individuals with distinct SVOs have different social preferences, responsibility levels and altruistic motivations. Our findings extend the knowledge of the neural underpinning of SVO modulation of social outcome processing by providing a temporal description of the modulation.
Limitations and future research prospects
There are several limitations in our study. First, the experimental situation in our study is too pure to consider other social factors that might play an important role in social decision-making and outcome evaluation. In daily life, people usually make decisions and evaluate outcomes in more complex social contexts, and other individuals' attitudes and behaviors also have an impact on how we evaluate the outcomes. In addition, people with distinct SVOs may think and behave differently under different social situations. Hence, to obtain a more comprehensive understanding of the effect of SVO on social outcome evaluation, it would be interesting and meaningful to investigate how SVO modulates people’s process of outcome evaluation in various social contexts such as trust situations and cooperation situations.
Further, the present study did not take culture and gender factors into consideration. All participants in the current study were from China, which is praised as being a highly collectivist culture. In contrast, participants from the Western culture of individualist characteristics may process the social outcomes differently (Powers et al., 2013). Henceforth, future cross-cultural studies of SVO effects on social outcome evaluation are needed to verify the differences. Moreover, compared to males, females tend to consider others more and show stronger altruistic motivation (Eagly and Steffen, 1986). Thus, in the future studies it would be worthwhile to consider the interaction of gender and SVO on the evaluation processing of social outcomes.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31400888), and the Fundamental Research Funds for the Central Universities and the Research Funds of Renmin University of China (15XNLQ05).
Conflict of interest. None declared.
References
- Amrhein C., Múhlberger A., Pauli P., Wiedemann G. (2004). Modulation of event-related brain potentials during affective picture processing: a complement to startle reflex and skin conductance response? International Journal of Psychophysiology, 54(54), 231–40. [DOI] [PubMed] [Google Scholar]
- Bargh J.A. (1982). Attention and automaticity in the processing of self-relevant information. Journal of Personality and Social Psychology, 43(3), 425–36. [Google Scholar]
- Bellebaum C., Polezzi D., Daum I. (2010). It is less than you expected: the feedback-related negativity reflects violations of reward magnitude expectations. Neuropsychologia, 48(11), 3343–50. [DOI] [PubMed] [Google Scholar]
- Bieleke M., Gollwitzer P.M., Oettingen G., Fischbacher U. (2016). Social value orientation moderates the effects of intuition versus reflection on responses to unfair ultimatum offers. Journal of Behavioral Decision Making, 30(2), 569–81. [Google Scholar]
- Briggs K.E., Martin F.H. (2009). Affective picture processing and motivational relevance: arousal and valence effects on ERPs in an oddball task. International Journal of Psychophysiology, 72(3), 299–306. [DOI] [PubMed] [Google Scholar]
- Buunk A.P., Gibbons F.X. (2007). Social comparison: The end of a theory and the emergence of a field. Organizational Behavior and Human Decision Processes, 102(1), 3–21. [Google Scholar]
- Christopoulos G.I., King-Casas B. (2015). With you or against you: social orientation dependent learning signals guide actions made for others. Neuroimage, 104, 326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1973). Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educational and Psychological Measurement, 33(1), 107–12. [Google Scholar]
- Daniel R., Pollmann S. (2014). A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiology of Learning and Memory, 114(9), 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cremer D., Van Lange P.A.M. (2001). Why prosocials exhibit greater cooperation than proselfs: The roles of social responsibility and reciprocity. European Journal of Personality, 15, 5–18. [Google Scholar]
- Declerck C.H., Boone C., Kiyonari T. (2014). The effect of oxytocin on cooperation in a prisoner's dilemma depends on the social context and a person's social value orientation. Social Cognitive and Affective Neuroscience, 233(2), 545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks J., Van Scheppingen M.A., Lee N.C., Krabbendam L. (2015). Trust and mind-reading in adolescents: the moderating role of social value orientation. Frontiers in Psychology, 6, 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagly A.H., Steffen V.J. (1986). Gender and aggressive behavior. a meta-analytic review of the social psychological literature. Psychological Bulletin, 100(3), 309. [PubMed] [Google Scholar]
- Fiske A.P., Kitayama S., Markus H.R., Nisbett R.E. (1998). The cultural matrix of social psychology In: Gilbert D., Fiske S., Lindzey G., edirors. Handbook of Social Psycholog, 4th ed.,Vol. 2, pp. 915–981, Boston: McGraw-Hill. [Google Scholar]
- Galvan A., Hare T.A., Davidson M., Spicer J., Glover G., Casey B.J. (2005). The role of ventral frontostriatal circuitry in reward-based learning in humans. Journal of Neuroscience, 25(38), 8650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geangu E., Gibson A., Kaduk K., Reid V.M. (2013). The neural correlates of passively viewed sequences of true and false beliefs. Social Cognitive and Affective Neuroscience, 8(4), 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W.J., Willoughby A.R. (2002). The medial frontal cortex and the rapid processing of monetary gains and losses. Science, 295(5563), 2279–82. [DOI] [PubMed] [Google Scholar]
- Gray H.M., Ambady N., Lowenthal W.T., Deldin P. (2004). P300 as an index of attention to self-relevant stimuli. Journal of Experimental Social Psychology, 40(2), 216–24. [Google Scholar]
- Hajcak G., Holroyd C.B., Moser J.S., Simons R.F. (2005). Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology, 42(2), 161–70. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Holroyd C.B., Simons R.F. (2006). The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology, 71(2), 148–54. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Holroyd C.B., Simons R.F. (2007). It’s worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology, 44(6), 905–12. [DOI] [PubMed] [Google Scholar]
- Haruno M., Frith C.D. (2010). Activity in the amygdala elicited by unfair divisions predicts social value orientation. Nature Neuroscience 13(2),160–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbig B.E., Glöckner A., Zettler I. (2014). Personality and prosocial behavior: linking basic traits and social value orientations. Journal of Personality and Social Psychology, 107(3), 529–39. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Hajcak G., Larsen J.T. (2006). The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Research, 1105(1), 93–101. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Larsen J.T., Cohen J.D. (2004). Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology, 41(2), 245–53. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Wang Q., Li P., Li H. (2016). The neural correlates underlying belief reasoning for self and for others: evidence from ERPs. Frontiers in Psychology, 7, 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston V.S., Wang X.T. (1991). The relationship between menstrual phase and the P3 component of ERPs. Psychophysiology, 28(4), 400–9. [DOI] [PubMed] [Google Scholar]
- Joireman J., Duell B. (2005). Mother Teresa versus Ebenezer Scrooge: mortality salience leads proselfs to endorse self-transcendent values (unless proselfs are reassured). Personality and Social Psychology Bulletin, 31, 307–20. [DOI] [PubMed] [Google Scholar]
- Karagonlar G., Kuhlman D.M. (2013). The role of social value orientation in response to an unfair offer in the ultimatum game. Organizational Behavior and Human Decision Processes, 120(2), 228–39. [Google Scholar]
- Kimura K., Katayama J. (2013). Outcome evaluations in group decision making using the majority rule: an electrophysiological study. Psychophysiology, 50(9), 848–57. [DOI] [PubMed] [Google Scholar]
- Kuhlman D.M., Camac C.R., Cunha D.A. (1986). Individual differences in social orientation In: Wilke H., Messick D., Rutte C., editors. Experimental Social Dilemmas, pp. 151–176. New York, NY: Verlag Peter Lang. [Google Scholar]
- Kuhlman D.M., Wimberley D.L. (1976). Expectations of choice behavior held by cooperators, competitors, and individualists across four classes of experimental games. Journal of Personality and Social Psychology, 34(1), 69–81. [Google Scholar]
- Li J., Zhu L., Gummerum M., Sun Y. (2013). The development of social value orientation across different contexts. International Journal of Psychology, 48(4), 469–80. [DOI] [PubMed] [Google Scholar]
- Li P., Jia S., Feng T., Liu Q., Suo T., Li H. (2010). The influence of the diffusion of responsibility effect on outcome evaluations: electrophysiological evidence from an ERP study. Neuroimage, 52(4), 1727–33. [DOI] [PubMed] [Google Scholar]
- Loehr J.D., Kourtis D., Brazil I.A. (2015). It's not just my fault: neural correlates of feedback processing in solo and joint action. Biological Psychology, 111, 1–7. [DOI] [PubMed] [Google Scholar]
- Luo Y., Feng C., Wu T., et al. (2015). Social comparison manifests in event-related potentials. Scientific Reports, 5, 12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Li S., Wang C., et al. (2016). Distinct oxytocin effects on belief updating in response to desirable and undesirable feedback. Proceedings of the National Academy of Sciences of the United States of America, 113(33), 201604285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Pallares J., Cucurell D., Münte T.F., Strien N., Rodriguez-Fornells A. (2011). On the number of trials needed for a stable feedback-related negativity. Psychophysiology, 48, 852–60. [DOI] [PubMed] [Google Scholar]
- McClintock C.G., Liebrand W.B. (1988). Role of interdependence structure, individual value orientation, and another's strategy in social decision making: a transformational analysis. Journal of Personality and Social Psychology, 55(3), 396–409. [Google Scholar]
- Messick D.M., McClintock C.G. (1968). Motivational bases of choice in experimental games. Journal of Experimental Social Psychology, 4(1), 1–25. [Google Scholar]
- Miltner W.H., Braun C.H., Coles M.G. (1997). Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience, 9(6), 788–98. [DOI] [PubMed] [Google Scholar]
- Murphy R.O., Ackermann K.A. (2014). Social value orientation: theoretical and measurement issues in the study of social preferences. Personality and Social Psychology Review, 18(1), 13. [DOI] [PubMed] [Google Scholar]
- Murphy R.O., Ackermann K.A., Handgraaf M.J.J. (2011). Measuring social value orientation. Judgment and Decision Making, 6(8), 771–81. [Google Scholar]
- Paul K., Pourtois G. (2017). Mood congruent tuning of reward expectation in positive mood: evidence from FRN and theta modulations. Social Cognitive and Affective Neuroscience, 12(5), 765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M.P. (2005). Neurobiology of decision-making: Quo vadis? Cognitive Brain Research, 23(1), 2–10. [DOI] [PubMed] [Google Scholar]
- Peterburs J., Voegler R., Liepelt R., et al. (2017). Processing of fair and unfair offers in the ultimatum game under social observation. Scientific Reports, 7, 44062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe M., Valkenberg H. (2003). Effects of gain versus loss and certain versus probable outcomes on social value orientations. European Journal of Social Psychology, 33(3), 331–7. [Google Scholar]
- Powers K.E., Wagner D.D., Norris C.J., Heatherton T.F. (2013). Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Social Cognitive and Affective Neuroscience, 8(2), 151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepers D., Derks B. (2016). Revisiting social identity theory from a neuroscience perspective. Current Opinion in Psychology, 11, 74–8. [Google Scholar]
- Schupp H.T., Cuthbert B., Bradley M., Hillman C., Hamm A., Lang P. (2004). Brain processes in emotional perception: motivated attention. Cognition and Emotion, 18(5), 593–611. [Google Scholar]
- Schupp H.T., Cuthbert B.N., Bradley M.M., Cacioppo J.T., Ito T., Lang P.J. (2000). Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257–61. [PubMed] [Google Scholar]
- Semlitsch H.V., Anderer P., Schuster P., Presslich O. (1986). A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology, 23 (6), 695–703. [DOI] [PubMed] [Google Scholar]
- Sidarus N., Vuorre M., Haggard P. (2017). How action selection influences the sense of agency: an ERP study. Neuroimage, 150, 1–13. [DOI] [PubMed] [Google Scholar]
- Steinel W., De Dreu C.K.W. (2004). Social motives and strategic misrepresentation in social decision making. Journal of Personality and Social Psychology, 86(3), 419–34. [DOI] [PubMed] [Google Scholar]
- Stouten J., Cremer D.D., Dijk E.V. (2005). All is well that ends well, at least for proselfs: emotional reactions to equality violation as a function of social value orientation. European Journal of Social Psychology, 35(6), 767–83. [Google Scholar]
- Taylor S.E., Kemeny M.E., Reed G.M., Bower J.E., Gruenewald T.L. (2000). Psychological resources, positive illusions, and health. American Psychologist, 55(1), 99–109. [DOI] [PubMed] [Google Scholar]
- Taylor S.F., Martis B., Fitzgerald K.D., et al. (2006). Medial frontal cortex activity and loss-related responses to errors. Journal of Neuroscience, 26(15), 4063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk E., De Cremer D., Handgraaf M.J. (2004). Social value orientations and the strategic use of fairness in ultimatum bargaining. Journal of Experimental Social Psychology, 40(6), 697–707. [Google Scholar]
- van Hooff J.C., Crawford H., Van V.M. (2011). The wandering mind of men: ERP evidence for gender differences in attention bias towards attractive opposite sex faces. Social Cognitive and Affective Neuroscience, 6(4), 477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lange P.A.M. (2000). Beyond self-interest: a set of propositions relevant to interpersonal orientations. European Review of Social Psychology, 11(1), 297–331. [Google Scholar]
- Van Lange P.A.M. (1999). The pursuit of joint outcomes and equality in outcomes: an integrative model of social value orientation. Journal of Personality and Social Psychology, 77(2), 337–49. [Google Scholar]
- Van Lange P.A.M., Otten W., de Bruin E.M.N., Joireman J.A. (1997). Development of prosocial, individualistic, and competitive orientations: theory and preliminary evidence. Journal of Personality and Social Psychology, 73(4), 733–46. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang D., Elieson B., Zhou X. (2012). Brain potentials in outcome evaluation: when social comparison takes effect. International Journal of Psychophysiology, 85(2), 145–52. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhou X. (2009). The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Research, 1286, 114–22. [DOI] [PubMed] [Google Scholar]
- Yang Q., Tang P., Gu R., Luo W., Luo Y.J. (2015). Implicit emotion regulation affects outcome evaluation. Social Cognitive and Affective Neuroscience, 10(6), 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N., Botvinick M.M., Cohen J.D. (2004). The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review, 111(4), 931–59. [DOI] [PubMed] [Google Scholar]
- Yeung N., Sanfey A.G. (2004). Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience, 24(28), 6258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Yu R., Zhou X. (2010). To do or not to do? action enlarges the FRN and P300 effects in outcome evaluation. Neuropsychologia, 48(12), 3606–13. [DOI] [PubMed] [Google Scholar]