Abstract
Background
Bronchial asthma is an inflammatory disease of the respiratory system. However, it may also induce systemic effects. Although reports suggest patients with asthma are at increased risk of cardiovascular events, the association between asthma and atherosclerosis is unclear. The aim of the present study was to compare the progression of atherosclerosis between patients with asthma treated with inhaled corticosteroids and healthy controls.
Material/Methods
In 102 adult patients with asthma, markers of arterial stiffness (pulse wave velocity and augmentation index) were evaluated by applanation tonometry. Structural atherosclerotic changes (intima-media complex thickness and presence of atherosclerotic plaque) were assessed sonographically. Lipid profile and fasting glucose level were measured. Clinical data concerning the course of asthma, its severity, and management strategy were obtained. A group of 102 healthy, age-matched controls were examined according to the same protocol.
Results
The majority of patients presented well-controlled asthma of moderate severity. When adjusted for weight, age, and systolic blood pressure, no significant differences were observed in pulse wave velocity, in augmentation index, or in intima-media complex thickness between groups. In controls, atherosclerotic plaque occurred significantly more often than in patients with asthma (p=0.0226). Moreover, in patients with asthma, the intima-media complex thickness of the right common carotid artery was significantly correlated with forced expiratory volume in 1 second (R2=−0.2951, p=0.0083). There was no significant difference in any of the atherosclerosis markers between different types and doses of administered inhaled corticosteroids.
Conclusions
Patients with bronchial asthma presented a decreased risk of atherosclerosis in comparison to healthy controls.
MeSH Keywords: Anti-Asthmatic Agents, Asthma, Atherosclerosis, Carotid Intima-Media Thickness, Pulse Wave Analysis
Background
Asthma and atherosclerosis are inflammatory diseases that affect the bronchi and arterial wall [1]. Vascular inflammation is noted early in the course of chronic systemic inflammatory diseases such as systemic lupus erythematosus, rheumatoid arthritis or psoriasis [1–3]. In these conditions, circulating pro-inflammatory cytokines and diminished nitric oxide production cause endothelial dysfunction, followed by propagation of the inflammatory cascade, premature atherosclerotic plaque formation, and, finally, increased risk of cardiovascular events [1,4–6]. Although the bronchi are affected in asthma, blood circulating through the lungs may “wash out” pro-inflammatory cytokines, promoting the progression of atherosclerosis. This pathway may be also indirect; for example, via the production of interleukin-6 (IL-6), which is a major signalling cytokine for C-reactive protein (CRP) expression in hepatocytes [5]. This may account for the elevated levels of serum CRP observed in patients with asthma [5]. However, the data concerning the association between asthma and atherosclerosis, which might be associated with lung function and asthma control, remains inconclusive [5,7–10].
Inhaled corticosteroids (ICSs) are first-line medications for the majority of patients with persistent asthma who require chronic treatment [5]. Although, in contrast to systemic glucocorticosteroids, ICSs have minimal/non-systemic adverse effects when appropriately dosed [11], they have been suggested to have an effect beyond the respiratory system [5]. By preventing the spilling-over of regional inflammation and decreasing risk of exacerbations, ICS may indirectly decrease the systemic inflammatory response, reducing the risk of atherosclerosis.
Early signs of atherosclerosis are a stiffening of the arterial wall and an increase of its thickness, particularly the intima-media complex thicknesses (IMCT). The criterion standard method for assessment of arterial stiffness is the evaluation of pulse wave velocity (PWv) [12], while the IMCT can be evaluated sonographically. Both increased arterial stiffness and atherosclerosis are risk factors for cardiovascular morbidity, and the latter remains the most significant cause of death worldwide [1,13,14].
Due to the inconclusive association between asthma and atherosclerosis, our aim was to compare the progression of atherosclerosis between adults affected by asthma treated with ICS and healthy controls. Our hypothesis was that patients with asthma present milder atherosclerotic changes.
Material and Methods
Patients
The study group comprised 109 patients diagnosed with asthma and 111 age-matched controls. Patients were recruited from the Pulmonology and Allergology Outpatient Clinics, and controls through an internet advertisement. Inclusion criteria for patients with asthma were as follows: age over 30 years old, diagnosis of asthma, and treatment with ICSs. The exclusion criteria comprised administration of oral glucocorticosteroids during the previous 6 months [15], current smoking, asthma-COPD overlap syndrome, diagnosis of diabetes mellitus or ischemic heart disease or atrial fibrillation, or a history of cardiovascular or cerebrovascular incidences. Controls were healthy, non-smoking adults over 30 years old who were not receiving treatment with ICS nor oral glucocorticosteroids, and were not affected by any of the aforementioned diseases. Current smoking was defined as consuming at least 1 cigarette daily. Diabetes mellitus (DM), ischemic heart disease, and atrial fibrillation were defined as being either self-reported, documented in patient records, or being treated with medication.
Based on the interview and analysis of medical records, the following characteristics of patients were evaluated:
The timespan and severity of asthma, and the level of asthma control (including last spirometry results). Diagnosis of asthma and its severity were determined according to the Global Initiative Strategy for Asthma Management (GINA) guidelines [16].
Present and former treatment of asthma: time of treatment onset, delay from diagnosis to onset of treatment (in years), types and doses of ICS administered during the previous 2 years. The daily dose of ICS was adjusted to an equipotent dose, with the dose of budesonide as reference, and equipotent doses of fluticasone and ciclesonide calculated with the conversion factor of 2.0 [17].
Past smoking history (determined by smoked pack-years) and presence of hypertension. Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg, or use of antihypertensive medications.
Following the evaluation, height and weight were measured without shoes and with the subject in light clothing to calculate the body mass index (BMI [kg/m2]). A sample of peripheral blood was then obtained from both groups, who had been fasting for least 8 hours. The concentrations of glucose (Gluc), total cholesterol (TCh) and its sub-fractions (high-density lipoprotein cholesterol [HDL-c] and low-density lipoprotein cholesterol [LDL-c]), and triglycerides (TG) were then measured.
Normal limits of glucose were 70–99 mg/dL [18]. Impaired fasting glucose was recognized when glucose level ranged between 100 and 125 mg/dL. If the level was ≥126 mg/dL, the patient was excluded from the study and referred to a specialist due to the potential risk of diabetes mellitus [18]. The normal limits of the lipogram were TCh <190 mg/dL; HDL-c ≥45 mg/dL; LDL-c ≤100 mg/dL; and TG ≤150 mg/dL [19].
All patients gave their written informed consent to participate in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Bioethics Committee of the Medical University of Lodz (approval number: RNN/41/13/KB).
Arterial stiffness assessment
The patient was placed in supine position. The distance from the jugular notch to the carotid artery sampling site (palpated place of its strongest pulsation) and the femoral artery sampling site (in the groin) were measured. The travel distance was estimated by subtracting the distance between the carotid sampling site and the jugular notch from the distance between the jugular notch and the femoral sampling site [20]. The ECG electrodes were placed on the patient and an ECG trace was obtained. After 5 minutes of rest in a semi-dark, quiet room, blood pressure was measured with an automatic sphygmomanometer. Then, the central aortic pressure waveform and augmentation index (AI) were evaluated based on the radial artery waveform. The augmentation index was defined as the proportional increase in systolic pressure due to the pulse wave reflected from peripheral arteries, expressed as a percentage of the central pulse pressure. This parameter is believed to be related to structural aortic atherosclerotic disease [14].
Following this, the PWv was measured by sequential recordings of the arterial pressure waveform at the carotid and femoral artery sampling sites. Aortic PWv was calculated as the ratio of the estimated travel distance in meters to the transit time of pulse wave in seconds. This parameter reflects central arterial stiffness, mainly that of the aorta [20]. The quality of measurement was evaluated by assessment of the operator index. The measurements were repeated if the result was not sufficient, i.e., lower than 75%. Measurements of the PWv and AI were performed by means of an applanation tonometer (SphygmoCor, AtCor Medical, New South Wales, Australia).
IMCT assessment
After tonometric examination, the patient remained in the same semi-dark, quiet room for at least 5 minutes. An ECG trace was then obtained and carotid ultrasound was performed. The patient was in a supine position with the head turned 45º opposite to the side of examination. Long axis images of both common carotid arteries (CCA) were obtained up to the carotid bulb. The IMCT was visualized and cine loops of 3 cardiac cycles were saved. To minimize the respiration-related motion artefacts, all readings were acquired during a short breath-hold at the end of expiration. Further analysis was performed on a workstation equipped with dedicated software (EchoPac PC, GE Medical System). The mean IMCT of the far wall was measured at the R wave on the ECG. Evaluation was performed with a semi-automated border-detection program at a distance of 200 points proximal from the carotid bulb, during each of 3 cardiac cycles in the cine loop. The mean value was calculated and used for further analyses. Atherosclerotic plaque was defined as a distinct area of increased IMCT 50% greater than that of neighboring sites, or as a focal region of IMCT greater than 1.5 mm that protrudes into the lumen of the artery [9].
Statistical analysis
The statistical analysis was performed using Statistica 12 software (StatSoft Polska, Cracow, Poland). Relationships with a p-value lower than 0.05 were considered significant. The results are presented as mean and standard deviation unless otherwise stated.
The normality of the continuous data distribution was checked with the Shapiro-Wilk test. The χ2 test was used for comparisons of nominal data. To evaluate correlations the Person or Spearman rank correlation coefficients were applied. When comparing the asthma and control groups with regard to continuous variables, the unpaired t test was used for normally-distributed data while the Mann-Whitney test was used for data with a skewed distribution. The 3 groups of ICS were compared with the one-way ANOVA or the Kruskal-Wallis ANOVA, according to the data distribution. When the F-omnibus test was significant, dedicated post hoc tests were performed. To evaluate the determinants of different atherosclerosis markers, the multiple regression analysis was performed. The following risk factors were included into the analysis: age, sex, group affiliation, blood pressure values, heart rate, laboratory test results, and BMI.
To evaluate the number of individuals required for the study, a pilot study was performed where PVW was assessed in 10 individuals from each group. The results indicated that 102 individuals needed to be included into each group, assuming α=5% and statistical power of 80%. This calculation was performed using PS software (Power and Sample Size Calculation Version 3.0; United States).
In 10 randomly-selected individuals (5 patients and 5 controls), all measurements were repeated twice: by the second investigator on the same day, and during a 1-week period by the primary investigator. The intraclass correlation coefficient for repeated scans of the same investigator were 0.97, 0.81, and 0.77 for evaluation of IMCT, PWv, and AI, respectively. The interclass correlation coefficient between repeated measurements performed by different readers were 0.95, 0.80, and 0.75 for the evaluation of IMCT, PWv, and AI, respectively.
Results
Seven of the initial group of 109 patients with asthma and 9 of the 111 controls were excluded due to the presence of a fasting glucose plasma level over 125 mg/dL. Hence, the study population comprised 102 patients with asthma and 102 controls.
The patients with asthma demonstrated significantly increased BMI and systolic blood pressure in comparison to the control group. The asthma group also included more hypertensive patients and more former smokers; however, this difference was not significant. Moreover, all of past-smokers had ceased smoking at least 10 years before the study date and the number of smoked pack-years did not differ significantly. All the characteristics of both groups are presented and compared in Table 1.
Table 1.
Comparison of group characteristics. The results are presented as mean and SD or as a number and percentage value.
| Asthma | Control | p | ||
|---|---|---|---|---|
| Age [years] | 56.9 (7.6) | 55.3 (6.9) | 0.1903 | |
| Gender [n (%)] | Female | 70 (68.6) | 80 (78.4) | 0.0806 |
| Male | 32 (31.4) | 22 (21.6) | ||
| BMI [kg/m2] | 28.1 (5.0) | 26.2 (4.2) | 0.0016 | |
| SBP [mmHg] | 134.1 (16.0) | 128.8 (18.2) | 0.0250 | |
| DBP [mmHg] | 83.7 (10.6) | 83.2 (11.7) | 0.7191 | |
| HR [1/min] | 70 (10) | 68 (9) | 0.1716 | |
| Hypertension [n (%)] | 15 (14.7) | 8 (7.8) | 0.1212 | |
| Former smokers [n (%)] | 17 (16.7) | 9 (8.8) | 0.0930 | |
| Smoked pack years | 20.3 (10.5) | 22 (12.9) | 0.7821 | |
| Total cholesterol | 216.2 (40.6) | 221.6 (37.8) | 0.3404 | |
| HDL | 61.6 (15.5) | 61.7 (15.1) | 0.9512 | |
| LDL | 132.2 (36.3) | 136.3 (31.3) | 0.3884 | |
| TG | 124.7 (66.7) | 119.7 (55.7) | 0.5713 | |
| Glucose | 95.5 (10.1) | 99.2 (14.9) | 0.0516 | |
BMI – body mass index; SBP and DBP – systolic and diastolic blood pressure; HR – heart rate; HDL and LDL – high and low density lipoproteins; TG – triglycerides.
In analysis of structural changes the atherosclerotic plaque in the RCCA was present significantly more often in controls than in patients with asthma (Table 2). Although patients with asthma presented significantly increased PWv than controls, multivariate regression analysis found the group affiliation to be an insignificant determinant when weight, age and systolic blood pressure were included (Table 3). These 3 variables explained 44.9% of the whole PWv variance (R2=0.4486, p<0.001). The difference in aortic AI was not significant (Table 2).
Table 2.
Comparison of vasculature condition parameters between groups.
| Asthma | Control | P | ||
|---|---|---|---|---|
| PWv [m/s] | 8.49 (1.86) | 7.85 (1.51) | 0.0098 | |
| AI [%] | 30.1 (10.9) | 32.4 (9.9) | 0.1307 | |
| IMCT [mm] | Left CCA | 0.66 (0.16) | 0.64 (0.15) | 0.3188 |
| Right CCA | 0.64 (0.15) | 0.62 (0.15) | 0.3186 | |
| Plaque in LCCA [n (%)] |
Present | 12 (11.8) | 10 (9.8) | 0.6517 |
| Non | 90 (88.2) | 92 (91.2) | ||
| Plaque in RCCA [n (%)] |
Present | 4 (3.9) | 13 (12.7) | 0.0226 |
| Non | 98 (96.1) | 89 (87.3) | ||
PWv – pulse wave velocity; AI – augmentation index; IMCT – intima media complex thickness; LCCA and RCCA – left and right common carotid artery.
Table 3.
Results of multivariate regression analysis for assessed parameters.
| PWv [m/s] R2=0.4486; p<0.0001 |
AI [%] R2=0.32093; p<0.0001 |
IMCT for LCCA [mm] R2=0.2560; p<0.0001 |
IMCT for RCCA [mm] R2=0.3196; p<0.0001 |
|||||
|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | |
| Group | −0.276 | 0.1812 | 2.542 | 0.0640 | −0.003 | 0.8644 | −0.013 | 0.5057 |
| Gender | 0.449 | 0.0851 | −3.923 | 0.0739 | −0.029 | 0.3650 | −0.028 | 0.3479 |
| Age [years] | 0.074 | 0.0001 | 0.350 | 0.0001 | 0.007 | 0.0000 | 0.009 | 0.0000 |
| SBP [mmHg] | 0.041 | 0.0001 | 0.107 | 0.0313 | 0.001 | 0.0782 | 0.001 | 0.2113 |
| DBP [mmHg] | −0.014 | 0.2280 | −0.017 | 0.8213 | 0.001 | 0.3397 | 0.001 | 0.3842 |
| HR [1/min] | 0.013 | 0.2314 | −0.155 | 0.0354 | 0.000 | 0.8821 | −0.000 | 0.9586 |
| BMI [kg/m2] | 0.006 | 0.7339 | −0.192 | 0.1100 | −0.001 | 0.5868 | 0.002 | 0.2890 |
| TCh [mg/dL] | −0.008 | 0.2957 | 0.260 | 0.0211 | −0.001 | 0.4431 | −0.003 | 0.0674 |
| HDL [mg/dL] | −0.008 | 0.6185 | −0.213 | 0.0454 | −0.000 | 0.8886 | 0.001 | 0.7054 |
| LDL [mg/dL] | 0.009 | 0.2941 | −0.268 | 0.0217 | 0.002 | 0.3111 | 0.003 | 0.0402 |
| TG [mg/dL] | −0.000 | 0.8448 | −0.028 | 0.1705 | −0.000 | 0.9484 | 0.000 | 0.1997 |
| Gluc [mg/dL] | 0.012 | 0.1611 | −0.060 | 0.2698 | −0.001 | 0.4073 | −0.000 | 0.7242 |
PWv – pulse wave velocity; AI – augmentation index; IMCT – intima media complex thickness; LCCA and RCCA – left and right common carotid artery; SBP and DBP – systolic and diastolic blood pressure; HR – heart rate; BMI – body mass index; TCh – total cholesterol; HDL and LDL – high and low density lipoproteins; TG – triglycerides; Gluc – glucose.
In patients with asthma, the mean timespan of the disease was 15.7 years (SD=12.2) and the mean time of ICS intake was 11.3 (SD=9.0) years. The mean delay from diagnosis to treatment was 4.3 years (SD=8.3). Most patients maintained good asthma control (mean ACT=22.6 points, SD=2.9, range: 12–25). None of the aforementioned parameters associated with the course of asthma were significantly correlated with markers of atherosclerosis (Table 4).
Table 4.
Correlation between asthma related features and atherosclerosis marker.
| PWv [m/s] | AI [%] | IMCT for LCCA [mm] | IMCT for RCCA [mm] | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | P | R2 | p | |
| Timespan of asthma [years] | 0.0896 | 0.4063 | 0.0488 | 0.6499 | −0.0251 | 0.7916 | 0.0445 | 0.6413 |
| Timespan of ICS intake [years] | 0.1290 | 0.2366 | −0.0827 | 0.4463 | −0.0088 | 0.9275 | 0.0646 | 0.5047 |
| Delay from diagnosis to treatment [years] | 0.1978 | 0.2780 | −0.2173 | 0.2322 | 0.0372 | 0.7233 | −0.0833 | 0.4301 |
| ACT [points] | −0.0101 | 0.9256 | 0.1382 | 0.1964 | −0.0138 | 0.8862 | 0.1356 | 0.1599 |
PWv – pulse wave velocity; AI – augmentation index; IMCT – intima media complex thickness; LCCA and RCCA – left and right common carotid artery; R – correlation coefficient; ICS – inhaled corticosteroids; ACT – asthma control test.
All patients were treated with ICS. The majority received budesonide (50 patients) in a mean dose of 584 μg/day (SD=276 μg/day). The remaining patients were treated with ciclesonide (31 patients, mean dose 585 μg/day; SD=349 μg/day) and fluticasone (21 patients, mean dose 804 μg/day; SD=310 μg/day). In all cases, therapy with ICS was augmented with long-acting β2-agonists, and 93 patients were provided with short-acting β2-agonist for quick relief. No differences in ACT score nor in atherosclerosis related markers were observed between groups treated with different ICSs (Table 5). Furthermore, equipotential doses of ICS did not correlate significantly with levels of atherosclerosis surrogates (PWv: R2=0.0975, p=0.3925; AI: R2=−0.1396, p=0.2170; IMCT for LCCA: R2=0.1236, p=0.1861; IMCT for RCCA: R2=−0.0166, p=0.8612).
Table 5.
Comparison of effect of different types of ISCs for asthma control and progression of atherosclerosis.
| Budesonide (n=50) [mean (SD)] | Cyclesonide (n=31) [mean (SD)] | Fluticasone (n=21) [mean (SD)] | p | ||
|---|---|---|---|---|---|
| ACT [points] | 22.6 (3.0) | 22.7 (2.9) | 21.8 (3.5) | 0.5907 | |
| PWv [m/s] | 8.50 (2.01) | 8.44 (1.83) | 8.39 (1.60) | 0.9995 | |
| AI [%] | 30.0 (11.0) | 26.4 (10.9) | 33.1 (10.11) | 0.2671 | |
| IMCT [mm] | LCCA | 0.66 (0.14) | 0.66 (0.14) | 0.71 (0.18) | 0.6976 |
| RCCA | 0.65 (0.16) | 0.62 (0.17) | 0.65 (0.14) | 0.7278 | |
ACT – asthma control test; PWv – pulse wave velocity; AI – augmentation index; IMCT – intima media complex thickness; LCCA and RCCA – left and right common carotid artery.
Most of the patients presented moderate severity of asthma (65 patients, 63.7%); however, no significant relationship was found between the severity of asthma and atherosclerosis markers (data not presented). The mean forced expiratory volume in the first second (FEV1) (% of predicted value for age) differed significantly between each of the subgroups (FEV1 was 106.5±12.7, 84.1±11.1 and 56.9±14.1 for mild, moderate and severe asthma, respectively, p=0.0001). Moreover, FEV1 was significantly negatively correlated with IMCT of the RCCA (R2=−0.2951, p=0.0083) (Figure 1).
Figure 1.
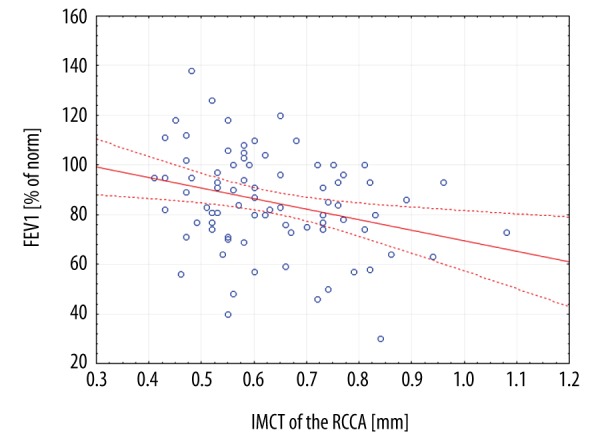
Scatterplot of correlation between forced expiratory volume in the first second (FEV1) and intima-media thickness (IMCT) in a right common carotid artery (RCCA).
Discussion
Our evaluation of IMCT and arterial stiffness parameters indicates that despite increased BMI and SPB, asthma patients treated with ICSs may be less prone to develop atherosclerotic changes than are healthy controls.
Inflammation of the arterial wall, resulting in the deterioration of the endothelium, initiates atherosclerosis. Patients with asthma are known to demonstrate elevated concentrations of serum pro-inflammatory cytokines such as IL-6, tumor necrosis factor α, CRP, IL-8, and fibrinogen [13,21]. However, the association between asthma and atherosclerosis remains inconclusive.
Atherosclerosis may manifest either as structural changes, particularly increased IMCT or atherosclerotic plaque formation, or as functional abnormalities, concerning arterial stiffening. As the latter may precede morphological changes, it is considered as an early marker of atherosclerosis [14]. Our comparison of the 2 groups revealed changes only in IMCT, which may indicate that the majority of patients have already exceeded a subclinical level of atherosclerosis (probably due to age) and reached the stage of progressive morphological changes. However, the less frequent occurrence of atherosclerotic plaques in patients with asthma may indicate that atherosclerosis progresses slower in this group of patients. This hypothesis is further supported by a trend towards lower AI (aortic atherosclerosis marker) in patients with asthma and the looser correlation between morphological and structural changes noted in this group.
Nevertheless, recent nationwide population-based studies in Taiwan demonstrated that asthma was associated with a 1.66-fold greater hazard for acute coronary syndrome and a 1.34-fold greater risk of peripheral artery disease [7,22]. In a previous cohort study, patients with asthma displayed increased risks of congestive heart disease, cerebrovascular disease, and heart failure in comparison to their counterparts with no history of asthma [23]. As far as the arterial stiffness is concerned, Sun et al. [13], in a comparison of 170 patients affected by asthma with 85 healthy controls, observed increased PWv in patients with asthma. Moreover, in these patients, PWv was negatively correlated with lung function [13]. In addition, a Fludeoxyglucose-PET CT study by Vijayakumar et al. [21] found that patients with asthma tended to have increased arterial inflammation and that its severity was inversely correlated with lung function. This is in accordance with our results in which lung function (FEV1) was inversely correlated with the severity of atherosclerosis (RCCA IMCT).
In contrast, a comparison of 150 patients with asthma with healthy controls showed the prevalence of atherosclerotic changes in the carotid arteries was significantly lower in patients with asthma, even those with a significantly higher smoking status [9]. Moreover, in contrast to our results, the authors found that carotid IMCT was significantly decreased in patients with asthma in comparison to controls. The authors did not find any significant difference in atherosclerotic plaque presence between groups; however, the site-specific findings were not reported.
Further reports regarding the decreased risk of atherosclerosis in patients with asthma concerned the administration of ICS. Treatment with oral glucocorticosteroids was associated with an increased risk of fatal and non-fatal myocardial infarction (odds ratio for myocardial infarct: OR=0.73; 95% CI 0.33–1.64); however, the administration of ICS was not associated with any increased risk of this event (OR=0.96; 95% CI 0.77–1.17) [15]. Moreover, several studies have reported anti-inflammatory benefits of ICSs beyond those affecting the airway [5]. In a 5-year follow-up study of 2671 nurses with asthma, Camargo et al. [5] reported significantly lower cardiovascular and all-cause mortality among woman with asthma who used ICSs compared to those who did not. On the other hand, lack of ICSs in treatment strategy may increase the risk of atherosclerosis. Another study found that atherosclerotic changes were more abundant in patients who had received significantly lower mean daily doses of ICS in the preceding 2 years [9].
The risk of atherosclerosis may be decreased by administration of ICS in 2 ways: by absorption across the lungs and exerting a direct effect on the vessel wall, or indirectly by reducing the airway inflammatory response, thus preventing the “washing out” of the pro-inflammatory cytokines to the systemic circulation. Yao et al. [22] noted that the indirect route may be also related to the crucial role of ICSs in preventing asthma exacerbations associated with major oxidative stress. Moreover, as asthma exacerbations are often precipitated by infections which may directly contribute to atherosclerosis by infecting vascular cells, or indirectly so through the action of cytokines, a dose-response relationship may exist between the number of exacerbations and the development of peripheral artery disease [22]. Our findings suggest that the type of ICSs did not influence aggravation of atherosclerotic process. All these drugs at appropriate dose effectively reduce inflammation in the respiratory system; however, they differ in systemic bioavailability. This was not reflected in the levels of atherosclerosis markers, which may suggest that they act indirectly by preventing exacerbations and the spilling-over of local inflammation.
This study has a few limitations. Firstly, as assessment of asthma severity and its management concerned only the preceding 2 years, it was not possible to evaluate the asthma control and effect of changing ICS types and doses during the entire course of the disease. This may hinder evaluation of the interplay between asthma and atherosclerosis. However, the majority of patients (86.3%) presented with mild/moderate severity of asthma with a stable course, and neither the type of ICS nor its dose were found to influence the atherosclerosis markers. Hence, we may assume that the moment of examination was representative of the whole course of asthma and a reliable time-point for evaluation of its association with atherosclerosis.
Secondly, the phenotype of asthma was not evaluated. It is widely accepted that asthma is a heterogeneous disease [16]. Although IMCT has been associated with adult-onset asthma in women, this was not found to be true for the childhood-onset phenotype [8]. However, as only 10 of the study group patients were under 18 years of age when asthma was diagnosed, the majority of patients included in the present study presented a phenotype that might predispose them to atherosclerosis. On the other hand, GINA [16] found no strong relationship between specific phenotype and particular clinical patterns or treatment responses.
Thirdly, women predominated in the study sample. Although this may bias conclusions derived for the whole population, this sex distribution does resemble general trends among patients with asthma, reducing the effect of any such bias.
Finally, the actual effect of asthma control could not be evaluated because most of the patients included in our study demonstrated good control of asthma. To further elucidate the role of ICS beyond the respiratory system, future studies should prospectively compare the progression of atherosclerosis in more diverse groups of patients with asthma, especially those with different frequencies of exacerbations and levels of asthma control.
Conclusions
In patients aged 50–60 years who are affected by chronic bronchial asthma, structural atherosclerotic changes may progress more slowly than in healthy controls, and the rate of progression may be associated with lung function. The degree of asthma control seems to have a greater impact on atherosclerotic changes than the type of applied ICS, which suggests that these drugs have an indirect protective effect.
Footnotes
Source of support: This work was supported by the Polish Ministry of Science and Higher Education as a research project within the “Diamentowy Grant (Diamond Grant)” program. Research number: DI2012 007742
Role of the funding source
The Polish Ministry of Science and Higher Education covered costs of diagnostic and laboratory examinations, salary of the main researcher (Michał Podgórski), and costs of publication of results.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Khan R, Spagnoli V, Tardif JC, L’Allier PL. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis [Internet]. Elsevier Ltd. 2015;240:497–509. doi: 10.1016/j.atherosclerosis.2015.04.783. [DOI] [PubMed] [Google Scholar]
- 2.Knoflach M, Kiechl S, Mayr A, et al. Allergic rhinitis, asthma, and atherosclerosis in the Bruneck and ARMY studies. Arch Intern Med. 2005;165:2521–26. doi: 10.1001/archinte.165.21.2521. [DOI] [PubMed] [Google Scholar]
- 3.Klingenberg R, Hansson GK. Treating inflammation in atherosclerotic cardiovascular disease: Emerging therapies. Eur Heart J [Internet] 2009;30:2838–44. doi: 10.1093/eurheartj/ehp477. [cited 2016 Mar 20] [DOI] [PubMed] [Google Scholar]
- 4.Yildiz P, Oflaz H, Cine N, et al. Endothelial dysfunction in patients with asthma: The role of polymorphisms of ACE and endothelial NOS genes. J Asthma. 2004;41:159–66. doi: 10.1081/jas-120026073. [DOI] [PubMed] [Google Scholar]
- 5.Camargo CA, Barr RG, Chen R, Speizer FE. Prospective study of inhaled corticosteroid use, cardiovascular mortality, and all-cause mortality in asthmatic women. Chest. 2008;134:546–51. doi: 10.1378/chest.07-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cakmak A, Zeyrek D, Cece H, Erel O. The relationship between carotid intima media thickness and oxidative stress in asthmatic children. Asian Pac J Allergy Immunol. 2010;28:256–61. [PubMed] [Google Scholar]
- 7.Chung WS, Shen TC, Lin CL, et al. Adult asthmatics increase the risk of acute coronary syndrome: A nationwide population-based cohort study. Eur J Intern Med. 2014;25:941–45. doi: 10.1016/j.ejim.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Onufrak S, Abramson J, Vaccarino V. Adult-onset asthma is associated with increased carotid atherosclerosis among women in the atherosclerosis risk in communities (ARIC) study. Atherosclerosis. 2007;195:129–37. doi: 10.1016/j.atherosclerosis.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otsuki M, Miyatake A, Fujita K, et al. Reduced carotid atherosclerosis in asthmatic patients treated with inhaled corticosteroids. Eur Respir J. 2010;36:503–8. doi: 10.1183/09031936.00090009. [DOI] [PubMed] [Google Scholar]
- 10.Akyüz Özkan E, Serin Hİ, Khosroshahi HE, et al. Arterial stiffness, distensibility, and strain in asthmatic children. Med Sci Monit. 2016;22:251–57. doi: 10.12659/MSM.895502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307–17. doi: 10.1016/j.rmed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Husmann M, Jacomella V, Thalhammer C, Amann-Vesti BR. Markers of arterial stiffness in peripheral arterial disease. Vasa. 2015;44(5):341–48. doi: 10.1024/0301-1526/a000452. [DOI] [PubMed] [Google Scholar]
- 13.Sun WX, Jin D, Li Y, Wang RT. Increased arterial stiffness in stable and severe asthma. Respir Med. 2014;108:57–62. doi: 10.1016/j.rmed.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi G, Brown R, Salciccioli L, et al. Relationship between aortic atherosclerosis and non-invasive measures of arterial stiffness. Atherosclerosis. 2007;195:190–94. doi: 10.1016/j.atherosclerosis.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Varas-Lorenzo C, Rodriguez LAG, Maguire A, et al. Use of oral corticosteroids and the risk of acute myocardial infarction. Atherosclerosis. 2007;192:376–83. doi: 10.1016/j.atherosclerosis.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Initiative G. Global strategy for asthma management and prevention © 2015. 2015. [Google Scholar]
- 17.Kramer S, Rottier BL, Scholten RJPM, Boluyt N. Ciclesonide versus other inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2013;(2):CD010352. doi: 10.1002/14651858.CD010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 19.Palazón-Bru A, Gil-Guillén VF, Orozco-Beltrán D, et al. Is the physician’s behavior in dyslipidemia diagnosis in accordance with guidelines? Cross-sectional ESCARVAL study. PLoS One. 2014;9:e91567. doi: 10.1371/journal.pone.0091567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber T, Ammer M, Rammer M, et al. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: A comparison with invasive measurement. J Hypertens. 2009;27:1624–30. doi: 10.1097/HJH.0b013e32832cb04e. [DOI] [PubMed] [Google Scholar]
- 21.Vijayakumar J, Subramanian S, Singh P, et al. Arterial inflammation in bronchial asthma. J Nucl Cardiol. 2013;20:385–95. doi: 10.1007/s12350-013-9697-z. [DOI] [PubMed] [Google Scholar]
- 22.Yao C-W, Shen T-C, Lu C-R, et al. Asthma is associated with a subsequent risk of peripheral artery disease. Medicine (Baltimore) 2016;95:e2546. doi: 10.1097/MD.0000000000002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iribarren C, Tolstykh IV, Miller MK, et al. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: A prospective study of 2 matched cohorts. Am J Epidemiol. 2012;176:1014–24. doi: 10.1093/aje/kws181. [DOI] [PubMed] [Google Scholar]


