Abstract
The use of heat shock protein 90 inhibitors like 17-allylamino-17-demethoxy-geldanamycin (17-AAG) has been recently introduced as an attractive anticancer therapy. It has been shown that 17-AAG may potentiate the inhibitory effects of some classical anticolorectal cancer (CRC) agents. In this study, two panels of colorectal carcinoma cell lines were used to evaluate the effects of 17-AAG in combination with capecitabine and oxaliplatin as double and triple combination therapies on the proliferation of CRC cell lines. HT-29 and all HCT-116 cell lines were seeded in culture media in the presence of different doses of the mentioned drugs in single, double, and triple combinations. Water-soluble tetrazolium-1 (WST-1) assay was used to investigate cell proliferation 24 h after treatments. Then, dose-response curves were plotted using WST-1outputs, and IC50 values were determined. For double and triple combinations respectively 0.5 × IC50 and 0.25 × IC50 were used. Data was analyzed with the software CompuSyn. Drug interactions were analyzed using Chou-Talalay method to calculate the combination index (CI).The data revealed that 17-AAG shows a potent synergistic interaction (CI < 1) with oxaliplatin and capecitabine in double combinations (0.5 × IC50) in both cell lines. In the case of triple combinations, the findings showed an antagonistic interaction (CI > 1) in HT-29 and a synergistic effect (CI < 1) in HCT-116 (0.25 × IC50) cell lines. It was concluded that double combinations of 17-AAG with oxaliplatin or capecitabine might be effective against HCT-116 and HT-29 cell lines. However, in triple combinations, positive results were seen only against HCT-116. Further investigation is suggested to confirm the effectiveness of these combinations in clinical trials.
Keywords: Colorectal cancer, Capecitabine, Oxaliplatin, 17-AAG, HT-29, HCT-116
INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of death worldwide(1,2). Treatment of CRC depends on the location, size, and extent of cancer spread as well as the health of the patient.
One major target of cancer therapy is to predict patient response to chemotherapeutic agents using drug response assays or drug sensitivity assays(3). Oxaliplatin and capecitabine alone or in combination with other cytotoxic agents are clinically active in the treatment of CRC(4,5).
Oxaliplatin, classified as an alkylating agent, is an anti-cancer (cytotoxic) chemo-therapy drug for the treatment of metastatic CRC(6). This drug is effective in combination with other anticancer drugs 5-fluorouracil (5-FU) or capecitabine and leucovorin(7). The cytotoxic agent capecitabine, which metabolizes to 5-FU, is a thymidylate synthase inhibitor and interferes with DNA synthesis(8,9,10). Although these drugs are routinely used in the treatment of CRC, some problems such as drug resistance and side effects raise new challenges to find novel cytotoxic drugs to improve patient outcomes(11,12).
17-allylamino-17-demethoxygeldanamycin (17-AAG) is a new chemotherapeutic agent that inhibits heat shock protein 90 (Hsp90), deactivates oncogenic proteins, induces apoptosis, and displays antitumor effects(13).
In the present study, it was hypothesized that using the minimum tolerable doses of 17-AAG, oxaliplatin, and capecitabine in groups of double and triple combinations might have better antitumor effects in human colon carcinoma cell lines of HT-29 and HCT-116. The present study evaluated the impact of two common drugs in combination with the novel cytotoxic drug 17-AAG on CRC cell lines.
MATERIALS AND METHODS
Cell culture and treatments
HT-29 (NCBI Code: C-466) and HCT-116 (NCBI Code: C-570) colon cancer cells were purchased from Iran Pasteur Institute (Tehran). Cultures were maintained in a humidified incubator at 37 °C in 5% CO2-95% air in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum and 0.5% Pen-Strep. Cell culture materials were obtained from Biowest (USA). Capecitabine was purchased from Sigma- Aldrich (USA). Oxaliplatin and 17-AAG were procured from LC Corporation (USA). Stock solutions of drugs were prepared in water at concentrations of 10 mg/mL capecitabine and oxaliplatin and 50 μg/mL 17-AAG and stored at -80 °C until use. Cells were seeded in a 96-well plate at a density of 1 × 104/well 24 h before the treatment. Single dose and combined doses of drugs were diluted in free DMEM, and the solution was mixed by pipetting. Then, both cell lines treated with different doses of each single drug for 24 h. The cytotoxic effect of each single drug was tested at six different concentrations 0.5, 1, 2, 4, 8, and 16 μM/mL for capecitabine and oxaliplatin, and 5, 10, 20, 40, 80, and 160 nM/mL for 17AAG in each type of cell line. Double-combination drugs (capecitabine and oxaliplatin, capecitabine and 17-AAG, oxaliplatin and 17-AAG) were tested at four different concentrations (2 × IC50, 1 × IC50, 0.5 × IC50, and 0.25 × IC50) for each cell line. The effects of triple combinations (capecitabine, oxaliplatin, and 17-AAG) were also examined at three different concentrations (1 × IC50, 0.5 × IC50, and 0.25 × IC50) on each cell line. Each test was performed in triplicate for each drug concentration.
Water-soluble tetrazolium-1 assay
The cell proliferation assay kits were obtained from TAKARA BIO INC (Japan). Cell viability was assayed based on the cleavage of the tetrazolium salt, water-soluble tetrazolium-1 (WST-1), to dark red formazan. Twenty-four hours after single, double, or triple drug combination therapy, 10 μL of WST-1 solution was added to each well. After two hours of incubation, absorbance was determined at 420 nm with a reference wavelength > 650 using the enzyme-linked immunosorbent assay microplate reader.
Dose-effect analysis
IC50 values were determined on the basis of dose response curves from the WST-1 assay and calculated using CompuSyn software, V. 2.0 (Biosoft, Cambridge, UK). Drug interactions were classified by determining a combination index (CI) recognized as the standard measure of combination effect based on the Chou-Talalay method. This method shows two and three drug interactions. The CI values were obtained over a range of fractional cell kill levels (Fa) from 0.05 to 0.95 (5-95% cell kill). Based on the Chou-Talalay method(14,15,16,17), CI < 1 means synergism, CI = 1 means additivity, and CI > 1 is interpreted as antagonism. When the interaction between two drugs leads to an increase in the effects of one or both drugs, the interaction is called a synergistic effect. An additive effect is defined as the situation in which the final effect is equal to the sum of the effects of the two drugs. Drug interactions are interpreted as antagonistic when they lead to a decrease in the effects of one or both drugs(14,15,16,17). Data obtained from the CI method was used to determine the dose-reduction index (DRI) for two and three drug combinations. The DRI indicated the fold-decrease of each single agent when two and three drugs were used in combination to achieve a particular Fa. To assay the combination effect, lower combination doses among the groups having a synergistic response (CI < 1) were chosen, and the cytotoxic effects of double or triple combination therapies with single drug therapy (IC50 value) were compared.
Statistical analysis
Data are presented as the mean (± SD) of at least three replicates. IC50 values were calculated from concentrations vs. cell viability using Compusyn software (Combusyn, Inc., Paramus, USA) and GraphPad Prism version 4.0 (GraphPad Software Inc., San Diego, California). The differences between more than two means were detected using one-way analysis of variance (ANOVA) with SPSS software, V. 10.0 (SPSS Inc, Chicago, Illinois) and P < 0.05 was considered as the significance level.
RESULTS
Effects of oxaliplatin, capecitabine, and 17-AAG on cell proliferation
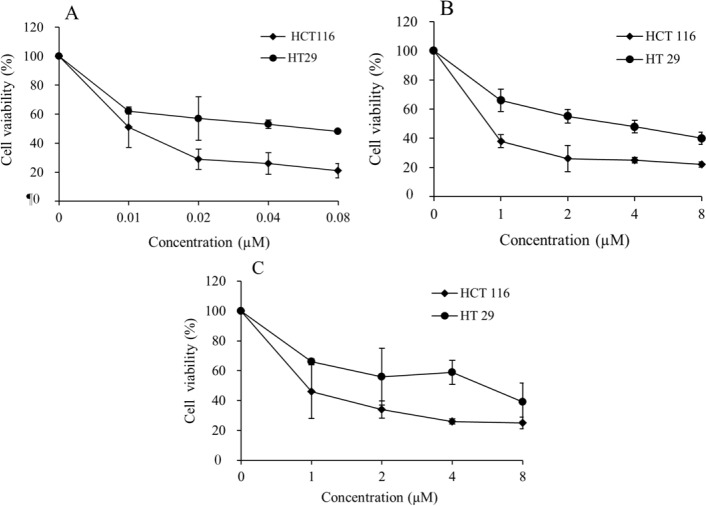
The cytotoxic effects of the tested drugs after 24 h of exposure in a panel of two cancer cell lines were plotted as the percentage of viable cells to the control cells and are presented in Fig. 1. As shown in Fig. 1, the exposure of these cells to different concentrations of a single drug (oxaliplatin, capecitabine, or 17-AAG) increased the growth inhibitory effect significantly in a dose-dependent manner. Based on the results, the HT-29 and HCT-116 cell lines had different levels of sensitivity to the treatment. Higher IC50 values for each of the three examined drugs in HT-29 compared with HCT-116 might be a sign of chemoresistance in the HT-29 cell line.
Fig. 1.
Cytotoxic effects of (A), 17-allylamino-17-demethoxygeldanamycin; (B), capecitabine, and (C), oxaliplatin in single drug treatments with different doses on HT-29 and HCT-116 cell proliferation. Sensitivity to three antineoplastic agents was determined by cell viability test, water-soluble tetrazolium-1 on HT-29 and HCT-116 cells. Each plot represents the average of at least 3 experiments. Data presented as mean ± standard deviation.
The IC50 values of oxaliplatin, capecitabine, and 17-AAG in the mentioned cell lines were determined using CompuSyn software, Chou-Talalay method(14,15), and on the basis of dose response curves from the WST-1 assay; the results are presented in Table 1.
Table 1.
Ratio of IC50 between oxaliplatin, capecitabine, and 17-AAG in HT-29 and HCT-116.

Effects of 17-AAG, capecitabine, and oxaliplatin combinations
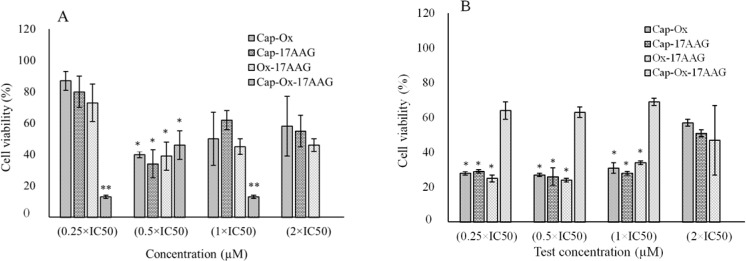
Different concentrations of oxaliplatin, capecitabine, and 17-AAG were selected based on the initial experiments mentioned in the methods section to assay the effects of drug combinations on the HT-29 and HCT-116 cell lines. WST-1 results from double and triple combination cases are presented in Fig. 2.
Fig. 2.
Water-soluble tetrazolium-1 (WST-1, cell viability assay) results of capecitabine, oxaliplatin, and 17-AAG in double combinations (2 × IC50, 1 × IC50, 0.5 × IC50, and 0.25 × IC50) and triple combinations (1 × IC50, 0.5 × IC50, and 0.25 × IC50) at different concentrations of each drug on (A), HCT-116 and (B), HT-29 cells. Data are presented as mean ± standard deviation. (17-AAG), 17-allylamino-17-demethoxygeldanamycin; (Cap), capecitabine; (Ox), oxaliplatin. * Significant differences between double combination compared with single treatments of each individual drug (P < 0.05). ** Significant differences of triple drug treated cases in compared to double combinations of each drugs (P < 0.05).
The tested concentrations 0.5 × IC50 in double and 0.25 × IC50 in triple combination showed synergistic responses after 24 h of treatment with the exception of triple combination on HT-29 cell line.
In the case of double combination treatments, drug concentrations were 0.5 × IC50, which were 1.7 and 0.75 μM of capecitabine, 1.9 and 0.75 μM of oxaliplatin, and 35 and 9.45 nM of 17-AAG for HT-29 and HCT-116 cells, respectively. The combinations of oxaliplatin-capecitabine, capecitabine-17AAG, and oxaliplatin-17AAG in 0.5 × IC50 concentration of each drug showed enhanced cytotoxicity in both cell lines compared with higher concentrations of each single agent (IC50).
Cell viability decreased significantly in all double combinations (0.5 × IC50 concentration for each drug) compared to single treatment (P < 0.05). As shown in Fig. 2, 17-AAG- capecitabine and 17-AAG-oxaliplatin at 0.5 × IC50 concentrations had highest effects compared to other double combinations at the same concentrations in both HCT-116 and HT-29 cell lines (P < 0.05).
In both cell lines oxaliplatin-capecitabine (conventional drug combination in CRC treatment) had lower efficacy than 17-AAG combinations with oxaliplatin and capecitabine and did not reach to significant levels (P > 0.05). According to our results double combinations exhibited more effects than monotherapy at 0.5 × IC50 concentrations in both cell lines (P < 0.05).
The drug concentrations for triple combinations were 0.25 × IC50, i.e. 0.85 and 0.37 μM of capecitabine, 0.95 and 0.37 μM of oxaliplatin, and 17.5 and 4.73 nM of 17-AAG for HT-29 and HCT-116 cells. The combination of three drugs in very low concentrations (0.25 × IC50) had higher efficacy than single and double treatments in HCT-116 (P < 0.05) (Fig. 2A). For HT-29 cells treated with triple combination, cell viability decreased non-significantly (P > 0.05) and a sort of antagonistic effect was observed (Fig. 2B).
Although double drug combinations in the current study showed synergism in both cell lines, notably better results (higher DRI and Fa values) were obtained in HT-29 than HCT-116 cell line under similar combinations. Furthermore, in HT-29 cell line under double combinations, even other examined concentrations (1 × IC50 and 0.25 × IC50) indicated greater activity compared to single treatments (P < 0.05).
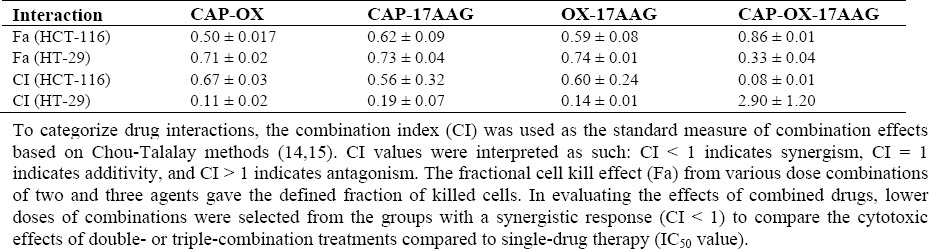
As shown in Tables 2 and 3, higher DRI and Fa values were obtained from double combinations, especially against the HT-29 cell line. Triple combinations showed an antagonistic effect in all utilized concentrations against HT-29 cells. However, all concentrations of triple combinations showed synergistic effects against HCT-116 cells. Moreover, the triple combination with 0.25 × IC50 had a greater effect (higher DRI and Fa values) against HCT-116 cell line.
Table 2.
Dose reduction index in double and triple combinations of 17-AAG, capecitabine, and oxaliplatin in HCT-116 and HT-29 cell lines.
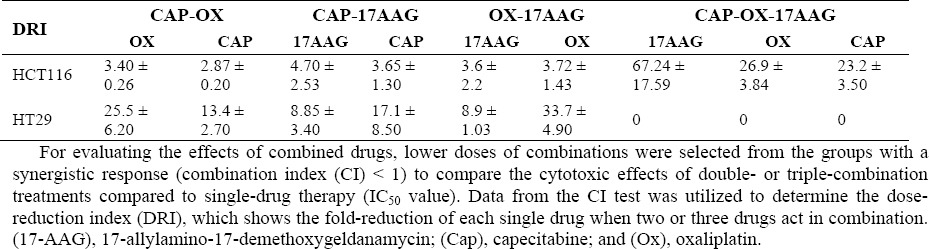
Table 3.
Interaction of fractional cell kill effect and combination index between 17-AAG, capecitabine, and oxaliplatin in HCT-116 and HT-29 cell lines.
DISCUSSION
Capecitabine plus oxaliplatin therapy is considered to be the first-line treatment for metastatic CRC in clinics(18,19). Some newer agents like 17-AAG (as HSP90 inhibitors), have been proposed to decrease problems such as drug resistance. In this regard, studies of the clinical effectiveness of HSP90 inhibitors have shown encouraging results(13,20,21,22). 17-AAG has shown moderate efficacy as a single agent in colorectal cell lines. Therefore, the current study examined whether the addition of 17-AAG to capecitabine and oxaliplatin could improve therapeutic indices of the HT-29 and HCT-116 cell lines. According to the results, the cytotoxic effect of each agent alone increased with increasing concentrations. Moreover, higher IC50 values were obtained in all single-drug treatment groups against HT-29 compared with HCT-116. Therefore, it can be deduced that HT-29 is more resistant to these drugs. Richard, et al., reported that in single-drug treatments by 5-FU and oxaliplatin, the HT-29 cell line had a higher IC50 values. Thus, HT-29 is more resistant than HCT-116(23). In a survey by Gaur, et al., monotherapy with oxaliplatin and dovitinib (IC50 doses) increased the proapoptotic cell population 1.8- fold in HCT-116 cells, but their combination increased this value to approximately 3-fold. In contrast to the current results, HT-29 showed no cell death in the presence of oxaliplatin alone, which points to HT-29 resistance to oxaliplatin(24). This incoherence between the results of Gaur, et al., and those of the current study may be due to the different dose regimens and treatment times in the two studies. In another study by Davis, et al., it was shown that 17-AAG as a single agent did not induce apoptosis in HT-29 and HCT-116, which is in contrast with the results of the current study which found decreased cellular viability by 17-AAG in both cell lines. However, Davis, et al. reported that the combination of 17-AAG with oxaliplatin and fluorouracil increased the fraction of apoptotic cells in these cells. The concentrations of 17-AAG in these combinations were 3-fold lower than the single-agent treatment for 17-AAG in both cell lines(25).
Rakitina, et al. evaluated the effects of 17-AAG and oxaliplatin against HT-29 and HCT- 116 cells. In single-drug treatments, they found HT-29 cells to be more sensitive than HCT-116 cells; in contrast to the current results, however, they reported antagonistic relationships in the case of the oxaliplatin-17-AAG combination on the HT-29 cell line. They also reported the additive effect of this combination against HCT-116 which is consistent with data from the current study(26). In another study, Vasilevskaya, et al. evaluated the cytotoxic effects of combinations of 17-AAG or geldanamycin (GA) with cisplatin (DDP) against HT-29 and HCT-116 cells. Against both cells the effects of GA and 17-AAG with DDP were additive, but against HT-29, both GA and 17-AAG antagonized DDP effects(27). Su, et al. evaluated the effects of Hsp90 inhibitors (NVP-AUY922) alone and in combination with berberine against multiple oncogenic signaling pathways for the treatment of CRC. They reported that Hsp90 inhibitors have a high therapeutic potential in CRC based on a combinatory analysis Gene Expression Omnibus repository and chemical genomic database of Connectivity Map. Their results also revealed that second generation Hsp90 inhibitors significantly down-regulated the regulating cell growth arrest and death kinases activity in NVP-AUY922-sensitive CRC cells. Combining berberine with HSP90 inhibitor had a potential inhibitory effect in survivin (member of the inhibitor of apoptosis) expression. This agent in combination with NVP-AUY922 resulted in synergistic antiproliferative effects for NVP-AUY922- sensitive and -insensitive CRC cells(28). In another study, Mohammadi, et al., showed that HSP90 inhibitors improve the antiproliferative and apoptotic properties of celecoxib on HT- 29 CRC cells by increasing the Bcl-2- associated X protein (BAX)/B-cell lymphoma 2 (BCL-2) ratios. Based on MTT results, they showed an increase in the inhibitory effects of celecoxib when combined with 17-AAG, particularly at low celecoxib concentrations. Flow cytometry analysis indicated that apoptosis induction is possibly a mechanism of the additive inhibitory effects of 17-AAG in combination cases. Furthermore, BAX and BCL-2 protein levels were determined by western blotting. The BAX/BCL-2 ratio in combination therapy was increased compared with the single-drug therapy(29).
Some possible mechanisms of the synergistic effect of 17-AAG combined with oxaliplatin and capecitabine have been proposed. It is proven that HSP90-dependent proto-oncogene tyrosine-protein kinase Src (Src) activation plays a key role in suppressing thymidylate synthase expression and tumor growth in CRC cell line. Based on these results, it is proposed that HSP90-dependent Src activation after 5-FU treatment led to an increase in thymidylate synthase expression in mRNA and protein levels, leading to 5-FU resistance. It seems that 17-AAG as an HSP90 inhibitor caused the suppression of Src and then led to 5-FU sensitivity (synergism effects)(30,31). Conversely, survivin plays an important role in the suppression of apoptosis through a caspase-dependent mechanism. In the case of synergism effects in oxaliplatin combination cases, it could be suggested that the oxaliplatin treatment significantly decreased survivin expression in cancer cells. Also, the oxaliplatin treatment led to an increase in apoptotic cells and caspase-3 activity with a decrease in cell viability(32,33). The results of previous studies have indicated that patients with survivin-positive tumors had a decreased apoptotic index and decreased survival rates compared with patients with survivin-negative tumors(34,35). Based on these findings, the double combinations examined in the current study seem to be more effective against the HT-29 cells. The synergistic effect of all double combinations on HCT-116 and HT-29 cell lines indicates that combination of 17-AAG with oxaliplatin or capecitabine could be a promising treatment for CRC. However, in the case of HT-29, it is proposed that future studies be conducted to find more effective doses in double combinations which do not cause antagonist effects as proposed by Rakitina, et al.(26). Surprisingly, antagonistic effects were found in triple combinations of all different doses against HT-29 cell line. Moreover, triple combinations used against HCT-116 cells showed synergistic effects in all doses. Thus, triple combinations could be considered as effective treatments in HCT-116 CRC. Using a 0.25 × IC50 dose of every single drug in the triple combination resulted in higher DRI and Fa values and acceptable results comparing IC50 doses of single agents. Davis, et al. showed that the combination of 17-AAG, oxaliplatin, and fluorouracil increased the fraction of apoptotic cells in HT- 29 and HCT-116 cell lines(25). Data from the current study revealed that the antagonistic effects weren't completely relevant by only WST-1 test. This result should be confirmed with complementary tests such as the clonogenic cell survival assay and various methods for detection of apoptotic cells. However, some probable mechanism may be involved in antagonistic effects in triple drug combinations.
The antagonistic effects seen in triple drug combinations could suggest that HSP90 inhibitor agents such as 17AAG induce the survivin expression in CRC cell lines. The upregulation of survivin in HT-29 at high level might have led to inhibiting of apoptosis in these situations. Suppression of survivin contributed by increasing drug sensitivity to HSP90 inhibitor agents in HT-29 cells(36). However, HT-29 compared with HCT-116 had higher expression levels of EPHB2, ITGβ-1, and Myc; overexpression of these genes is related to the acquisition of anticancer drug resistance(37). To the best of the authors' knowledge, no study has reported the effects of 17AAG/oxaliplatin/capecitabine triple combinations in any cell lines. However, some previous surveys have shown the efficacy of other triple combinations against CRC. Flis, et al. showed that the addition of sulindac sulfide as a cyclo-oxygenase inhibitor to oxaliplatin and 5-FU synergistically raised the inhibitory effects of 5-FU and oxaliplatin on CRC survival, parallel to the induction of apoptosis(16). Fischel, et al. studied two CRC cell lines (WIDR and SW620) and reported the synergistic effect of fluorouracil-folinic acid and oxaliplatin. In the current study, the combination of folinic acid-fluorouracil and SN38 (the active metabolite of irinotecan) had an antagonistic effect on WIDR and SW620 CRC cell lines(38). Vamvakas, et al. proved the effectiveness of combination therapy with capecitabine, oxaliplatin, and bevacizumab on elderly metastatic CRC patients(39). One major problem for current anticancer therapies is chemotherapy-induced high levels of toxicity(40). So the findings of the present study suggest that adding 17-AAG (at lower doses) in triple combinations to the mentioned classical antitumor agents resulted in greater cytotoxic effects in HCT-116 CRC, which was probably accompanied by a reduction in side effects and in the patient's burden.
The present survey had some limitations. Financial limitations and a lack of equipment prevented the evaluation of more than two CRC cell lines with further cell viability and apoptosis tests such as DNA fragmentation gel electrophoresis assay. The lack of financial support also prevented researchers from confirming the antagonistic effects by further assessment such as clonogenic survival assay. No prior, detailed studies assessing triple drug combinations related to factors which could affect treatment outcome were found. Subsequently, more studies are required to establish the efficacy of clinical treatments using 17-AAG in combination with other drugs for clinical use.
CONCLUSION
Data presented in this study supports the clinical use of double combinations of 17-AAG and oxaliplatin or capecitabine against HCT-116 and HT-29 CRC cell lines. As an HSP90 inhibitor, 17-AAG might have the potential to enhance oxaliplatin and capecitabine cytotoxicity in triple combinations against HCT-116 cells. However, future experiments are needed to evaluate the effect of these combinations in diverse doses and times. As the clinical use of double and triple combination therapies presented in this survey may cause combined toxicity in patients, further investigations are needed to investigate their efficacy in clinical treatments.
ACKNOWLEDGEMENTS
The authors are thankful to all members of Cellular and Molecular Research Center for their valuable suggestions and technical support.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden. Globocan 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Ulukaya E. Drug response assay: an increasing trend in designation of trailored-chemotherapy for more rational management of cancer patients. Adv Mol Med. 2006;2(2):53–58. [Google Scholar]
- 4.Cao Y, Liao C, Tan A, Liu L, Mo Z, Gao F. Capecitabine plus oxaliplatin vs. fluorouracil plus oxaliplatin as first line treatment for metastatic colorectal cancer meta-analysis of six randomized trials. Colorectal Dis. 2010;12(1):16–23. doi: 10.1111/j.1463-1318.2009.01803.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao G, Gao P, Yang KH, Tian JH, Ma B. Capecitabine/oxaliplatin as first-line treatment for metastatic colorectal cancer: a meta-analysis. Colorectal Dis. 2010;12(7):615–623. doi: 10.1111/j.1463-1318.2009.01879.x. [DOI] [PubMed] [Google Scholar]
- 6.Ehrsson H, Wallin I, Yachnin J. Pharmacokinetics of oxaliplatin in humans. Med Oncol. 2002;19(4):261–265. doi: 10.1385/MO:19:4:261. [DOI] [PubMed] [Google Scholar]
- 7.Lubner SJ, Loconte NK, Holen KD, Schelman W, Thomas JP, Jumonville A, et al. A phase II study of oxaliplatin, 5-fluorouracil, leucovorin, and high-dose capecitabine in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2010;9(3):157–161. doi: 10.3816/CCC.2010.n.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmoll HJ, Arnold D. Update on capecitabine in colorectal cancer. Oncologist. 2006;11:1003–1009. doi: 10.1634/theoncologist.11-9-1003. [DOI] [PubMed] [Google Scholar]
- 9.Cao S, Durrani FA, Rustum YM. Synergistic antitumor activity of capecitabine in combination with irinotecan. Clin Colorectal Cancer. 2005;4(5):336–343. doi: 10.3816/ccc.2005.n.007. [DOI] [PubMed] [Google Scholar]
- 10.Kolinsky K, Shen BQ, Zhang YE, Kohles J, Dugan U, Zioncheck TF, et al. In vivo activity of novel capecitabine regimens alone and with bevacizumab and oxaliplatin in colorectal cancer xenograft model. Mol Cancer Ther. 2009;8(1):75–82. doi: 10.1158/1535-7163.MCT-08-0596. [DOI] [PubMed] [Google Scholar]
- 11.Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8(1):57–84. doi: 10.1177/1758834015614530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Canc Netw. 2009;7(8):883–893. doi: 10.6004/jnccn.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayor-Lopez L, Tristante E, Carballo-Santana M, Carrasco-Garcia E, Grasso S, Garcia-Morales P, et al. Comparative study of 17-AAG and NVP-AUY922 in pancreatic and colorectal cancer cells: are there common determinants of sensitivity. Transl Oncol? 2014;7(5):590–604. doi: 10.1016/j.tranon.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 15.Chou TC, Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci. 1983;4:450–454. [Google Scholar]
- 16.Flis S, Splawinski J. Inhibitory effects of 5- fluorouracil and oxaliplatin on human colorectal cancer cell survival are synergistically enhanced by sulindac sulfide. Anticancer Res. 2009;29(1):435–441. [PubMed] [Google Scholar]
- 17.Flis S, Gnyszka A, Misiewicz-Krzeminska I, Spławinski J. Decytabine enhances cytotoxicity induced by oxaliplatin and 5-fluorouracil in the colorectal cancer cell line Colo-205. Cancer Cell Int. 2009;9:10. doi: 10.1186/1475-2867-9-10. doi: 10.1186/1475-2867-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22(11):2084–2091. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 19.Moreno-Solorzano I, Ibeas-Rollan R, Monzo- Planella M, Moreno-Solorzano J, Martinez-Rodenas F, Pou-Sanchis E, et al. Two doses of oxaliplatin with capecitabine (XELOX) in metastatic colorectal cancer. Clin Colorectal Cancer. 2007;6(9):634–640. doi: 10.3816/CCC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 20.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 22.Haupt A, Joberty G, Bantscheff M, Frohlich H, Stehr H, Schweiger MR, et al. Hsp90 inhibition differentially destabilises MAP kinase and TGF-beta signalling components in cancer cells revealed by kinase-targeted chemoproteomics. BMC Cancer. 2012;12:38–50. doi: 10.1186/1471-2407-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richard SM, Martinez Marignac VL. Sensitization to oxaliplatin in HCT116 and HT29 cell lines by metformin and ribavirin and differences in response to mitochondrial glutaminase inhibition. J Cancer Res Ther. 2015;11(2):336–340. doi: 10.4103/0973-1482.157317. [DOI] [PubMed] [Google Scholar]
- 24.Gaur S, Chen L, Ann V, Lin WC, Wang Y, Chang VH, et al .Dovitinib synergizes with oxaliplatin in suppressing cell proliferation and inducing apoptosis in colorectal cancer cells regardless of RAS-RAF mutation status. Mol Cancer. 2014;13:21. doi: 10.1186/1476-4598-13-21. doi: 10.1186/1476-4598-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis LE, Rakitina TV, VasilevskayaI IA, O'Dwyer PJ. 17-AAG enhances cytotoxicity of the oxaliplatin/fluorouracil combination in colon cancer cell lines. Cancer Res. 2005;65(9):145. [Google Scholar]
- 26.Rakitina TV, Vasilevskaya IA, O'Dwyer PJ. Additive interaction of oxaliplatin and 17- allylamino-17-demethoxygeldanamycin in colon cancer cell lines results from inhibition of nuclear factor κB signaling. Cancer Res. 2003;63(24):8600–8605. [PubMed] [Google Scholar]
- 27.Vasilevskaya IA, Rakitina TV, O'Dwyer PJ. Geldanamycin and its 17-allylamino-17-demethoxy analogue antagonize the action of cisplatin in human colon adenocarcinoma cells: differential caspase activation as a basis for interaction. Cancer Res. 2003;63(12):3241–3246. [PubMed] [Google Scholar]
- 28.Su YH, Tang WC, Cheng YW, Sia P, Huang CC, Lee YC, et al. Targeting of multiple oncogenic signaling pathways by Hsp90 inhibitor alone or in combination with berberine for treatment of colorectal cancer. Biochim Biophys Acta. 2015;1853(10 Pt A):2261–2272. doi: 10.1016/j.bbamcr.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Mohammadi A, Yaghoobi MM, Gholamhoseynian Najar A, Kalantari-Khandani B, Sharifi H, Saravani M. HSP90 inhibitor enhances anti-proliferative and apoptotic effects of celecoxib on HT-29 colorectal cancer cells via increasing BAX/BCL-2 ratio. Cell Mol Biol (Noisy-le-grand) 2016;62(12):62–67. doi: 10.14715/cmb/2016.62.12.11. [DOI] [PubMed] [Google Scholar]
- 30.Ahn JY, Lee JS, Min HY, Lee HY. Acquired resistance to 5-fluorouracil via HSP90/Src-mediated increase in thymidylate synthase expression in colon cancer. Oncotarget. 2015;6(32):32622–32633. doi: 10.18632/oncotarget.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaraju GP, Alese OB, Landry J, Diaz R, El-Rayes BF. HSP90 inhibition downregulates thymidylate synthase and sensitizes colorectal cancer cell lines to the effect of 5-FU-based chemotherapy. Oncotarget. 2014;5(20):9980–9991. doi: 10.18632/oncotarget.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu HF, Hu HC, Chao JI. Oxaliplatin down- regulates survivin by p38 MAP kinase and proteasome in human colon cancer cells. Chem Biol Interact. 2010;188(3):535–545. doi: 10.1016/j.cbi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Khan Z, Khan N, Tiwari RP, Patro IK, Prasad GB, Bisen PS. Down-regulation of survivin by oxaliplatin diminishes radioresistance of head and neck squamous carcinoma cells. Radiother Oncol. 2010;96(2):267–273. doi: 10.1016/j.radonc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58(22):5071–5074. [PubMed] [Google Scholar]
- 35.Lee MA, Park GS, Lee HJ, Jung JH, Kang JH, Hong YS, et al. Survivin expression and its clinical significance in pancreatic cancer. BMC Cancer. 2005;4(5):127. doi: 10.1186/1471-2407-5-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung CH, Chen HH, Cheng LT, Lyu KW, Kanwar JR, Chang JY. Targeting Hsp90 with small molecule inhibitors induces the over-expression of the anti-apoptotic molecule, survivin, in human A549, HONE-1 and HT-29 cancer cells. Mol Cancer. 2010;9:77. doi: 10.1186/1476-4598-9-77. doi:10.1186/1476-4598-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Khoury F, Corcos L, Durand S, Simon B, Le Jossic-Corcos C. Acquisition of anticancer drug resistance is partially associated with cancer stemness in human colon cancer cells. Int J Oncol. 2016;49(6):2558–2568. doi: 10.3892/ijo.2016.3725. [DOI] [PubMed] [Google Scholar]
- 38.Fischel JL, Rostagno P, Formento P, Dubreuil A, Etienne MC, Milano G. Ternary combination of irinotecan, fluorouracil-folinic acid and oxaliplatin: results on human colon cancer cell lines. Br J Cancer. 2001;84(4):579–585. doi: 10.1054/bjoc.2000.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vamvakas L, Matikas A, Karampeazis A, Hatzidaki D, Kakolyris S, Christophylakis C, et al. Capecitabine in combination with oxaliplatin and bevacizumab (AXELOX) as 1st line treatment for fit and vulnerable elderly patients (aged >70 years) with metastatic colorectal cancer (mCRC): a multicenter phase II study of the Hellenic Oncology Research Group (HORG) BMC Cancer. 2014;14:277. doi: 10.1186/1471-2407-14-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ataee R, Ataie A, Shadifar M, Nasri N, Hagghi H, Hayati E. Synergic effect of curcumin and melatonin on proliferation and apoptosis of HT29 colorectal cancer cell. Res Pharm Sci. 2017;7(5):S117. [Google Scholar]