Abstract
Respiratory syncytial virus (RSV) is the most common cause of severe respiratory infections worldwide, and an important cause of childhood bronchiolitis, pneumonia and mortality. Although prevention of RSV infection by immunoprophylaxis with palivizumab has proved effective, a precise understanding of the timing of RSV outbreaks is necessary to ensure that infants are protected when RSV is circulating. In this study a consistent shift in the seasonal patterns of RSV circulation in southeast Brazil (Sao Paulo) is reported based on the analysis of 15 years of viral surveillance. Surveillance was conducted from 1996 to 2010 and involved the collection of samples from children with symptoms of acute respiratory infection. Putative changes in school terms, in the proportion of RSV genotypes infecting children and in the seasonal dynamics of several climatic parameters during the period were also investigated. The results revealed a progression in the timing of RSV seasons, with a shift in the onset and peak of RSV epidemics from 2007 onwards. Although lower rainfall and temperatures were associated with the onset of outbreaks, there was no evidence of changes in climate, school terms or in the relative proportion of genotypes in the period analyzed. These findings have direct implications for improving the prophylactic use of palivizumab, and stress the importance of fine tuning prophylaxis with recent surveillance data. In the case of Sao Paulo, palivizumab prophylaxis should be initiated earlier than suggested currently. Similar adjustments may be necessary in other regions.
Keywords: respiratory syncytial virus, palivizumab, acute respiratory infections, Brazil
Introduction
Respiratory syncytial virus (RSV) is the most common cause of acute infections of the lower respiratory tract among young children worldwide [Bryce et al., 2005; CDC, 1999], although individuals from all age groups may be infected [Falsey et al., 2005]. RSV-associated infections are not only responsible for a high rate of hospital admissions, but are also an important cause of childhood bronchiolitis, severe pneumonia and mortality [Nair et al., 2010], mainly among premature and immunocompromised infants and individuals with chronic respiratory and cardiac conditions [Welliver, 2003].
Although a safe and effective vaccine is not yet available, prevention of RSV infection by passive immunoprophylaxis with the monoclonal antibody palivizumab has proved successful in reducing the burden of RSV disease among high-risk groups [Meissner and Long, 2003; Simões et al., 1998]. However, passive antibodies are expensive, representing a major barrier to the implementation of prevention programs. The high cost of drugs is particularly limiting in low-income countries, where the risk of severe viral respiratory infections is greatest [Stenballe et al., 2003]. Still, the cost-effectiveness of prophylaxis with palivizumab can be improved by restricting its use to those months of highest RSV circulation, with the first monthly dose administered just before the onset of RSV activity. A precise understanding of the dynamics and timing of RSV outbreaks is therefore crucial to ensure that the vulnerable population is protected when RSV is circulating.
In this study the evolution of the seasonal patterns of RSV circulation in the largest urban center of Brazil (São Paulo) was investigated by analyzing a long epidemiological series that includes 15 years of viral surveillance (1996 to 2010). The research was aimed at informing the necessary adjustments on the timing of RSV prophylaxis with palivizumab in Sao Paulo and potentially other cities in subtropical regions. Additionally, the dynamics of putative predictors of RSV outbreaks were also examined to determine the role of these factors in driving seasonal RSV patterns. RSV circulation has been shown to depend strongly on climate, with temperature in general, and rain in equatorial regions, positively associated with incidence rates [Checon et al., 2002; Nascimento et al., 1991; Stensballe et al., 2003]. School gatherings have been also implicated on the transmission of respiratory infections [Cintra et al., 2001]. Therefore, in addition to exploring the association between several climatic parameters and the seasonal characteristics of RSV, the role of school terms on the timing and peak of RSV outbreaks was examined. Similarly, the spectrum of RSV genotypes circulating from 1996 to 2010 was also investigated.
Material and Methods
Surveillance was conducted in the metropolitan area of Sao Paulo (23.4° S), the largest city in the southern hemisphere (approximately 20 million inhabitants). Sao Paulo is located 50 km from the coast and 760 m above sea level, and has a subtropical climate with mild winters and higher rainfall during summer.
From 1996 to 2010 clinical samples were collected throughout the year from 5 Children Hospitals (Sabará, Menino Jesus, Candido Fontoura, Darcy Vargas and Instituto da Criança) and 12 General Hospitals (Vila Nova Cachoeirinha, Vila Alpina, Hospital Universitario FMUSP, Cotoxó, Sao Camilo, Sao Luis, Santa Helena, Santa Marcelina, D. Antonio Alvarenga, Mandaqui, Albert Einstein, Sirio Libanes). Nasopharyngeal aspirates were collected for diagnosis purposes from all hospitalized children younger than 5 years of age presenting with symptoms of acute respiratory infection, namely fever, cough, sore throat, difficulty in breathing or fatigue when no other diagnosis was available. All children with a diagnosis of bronchiolitis or pneumonia were tested. Samples were collected at the physician’s request by health care workers trained on sampling and delivering methods. Respiratory samples (nasopharyngeal aspirates) obtained from two Sentinel Units (Hospital Infantil Menino Jesus and Hospital José Storopoli) of the National Influenza Virus Surveillance Network were also analyzed. The network was first established in Sao Paulo in 2002 to monitor the circulation of influenza and other respiratory viruses, and involved the systematic collection of five respiratory samples per week from children younger than 5 years of age presenting with symptoms of acute respiratory infection. The collection criteria were therefore the same as those used at the Children and General Hospitals.
All specimens were sent to the Respiratory Virus Laboratory (National Influenza Network) for rapid processing and screening. During the entire study period viral identification was performed with indirect immunofluorescence assays using monoclonal antibodies from Panel 1 Viral Screening and Identification Kit (Light Diagnostic™, Chemicon International Inc, Temecula, CA). Samples were collected with informed consent of parents or the guardians of each child. This study was approved by the Research Ethics Committee of the Adolf Lutz Institute.
Climatic, Genetic and Social Variables
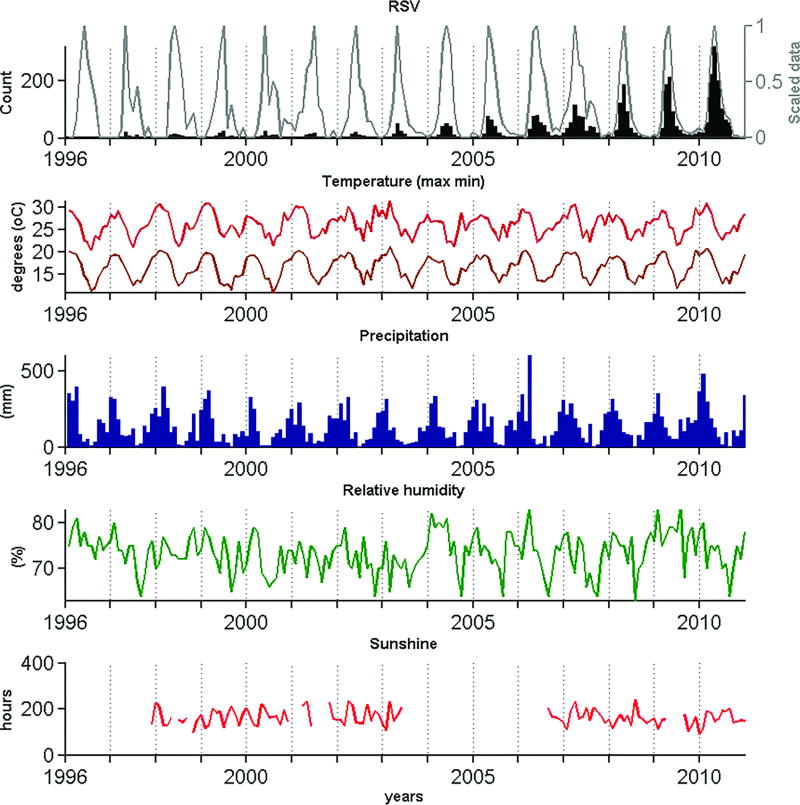
To determine the influence of potential drivers of RSV epidemics from 1996 to 2010 the seasonal dynamics of various climatic factors during the same period was analyzed. Monthly averages of precipitation, relative humidity, hours of sunshine and maximum and minimum temperatures from 1996 to 2010 were provided by the Brazilian Institute of Meteorology (INMET) upon request. With the exception of hours of sunshine (which were not available for some years), all series were continuous for the entire period (Figure 1).
Figure 1.
Time series showing RSV detection rates and seasonal variation in climatic parameters in the metropolitan area of São Paulo (1996–2010). In addition to showing original counts of RSV-positive samples (black, upper panel), the epidemiological series was also de-trended (scaled) to enable the visualization and comparison of the seasonal signal along the entire period (white, upper panel).
Since social gatherings may also affect the spread of RSV, the potential association between the timing of school terms and periods of highest RSV circulation was explored. The public school calendar was obtained from the Education Secretariat of the State of São Paulo [SESP, 2011]. Information was available for the period from 2001 to 2009. Finally, the possibility that differences in the relative proportion of RSV genotypes infecting children [Gaunt et al. 2011] affected the timing and size of RSV seasons was also addressed. Information on RSV genotypes in São Paulo city, as determined by PCR-RT jointly with nucleotide sequencing, was available from the literature [Carvalho, 2009] for the period from 2001 to 2007.
Data Analysis
The number of RSV-positive samples increased during the period studied due to the improvement of the vigilance network. Therefore, to enable the identification and comparison of the seasonal signal along the entire period, counts were scaled from 0 to 1 every year (Figure 1). Exploration of the seasonal patterns of each variable was performed both visually and using the average seasonal signature provided by summing the first three annual harmonics of each time series, as obtained by Fourier analysis [Rogers et al., 1996]. Briefly, the periodic variability of the time series is partitioned into harmonic functions that are summed to obtain a model of the periodic annual function (PAF). PAF can be considered an average seasonal signature of the original series, in which year-to-year variations are removed but seasonal variations within the year are preserved.
To identify putative associations between climatic parameters and viral circulation, Spearman correlations were conducted between the epidemiological time series (scaled RSV counts) and each climatic series with a temporal lag of 0 (contemporaneous) and 1 month. The latter case tests if changes in the value of a climatic factor in one month influence the timing of RSV outbreaks in a subsequent month. Climatic data was smoothed by log-transformation prior to the correlation analysis.
Given the paucity and (in the current case) mathematical simplicity of the school calendar and the genotypic data available, the analysis of their putative effect on seasonal RSV patterns was performed by comparing the time series.
Results
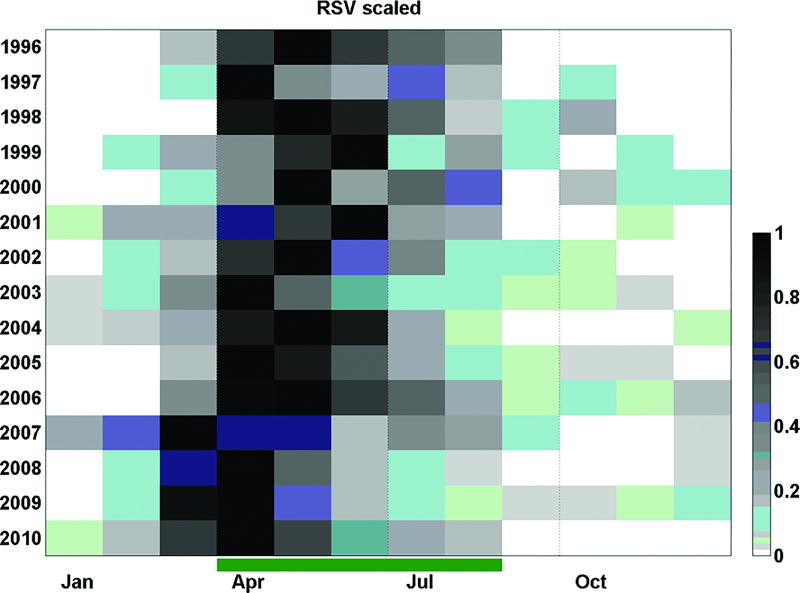
Figure 1 shows that the period of highest RSV activity extended from March to July (autumn-winter). The time series also revealed a progression in the timing of onset and peak of the epidemic season during the period studied. As shown in Figure 2, the highest detection rates (darker squares) occurred progressively earlier in more recent years (notice how the darker squares are aligned from the bottom left to the upper right of Figure 2). This pattern can be described in three stages: 1. (1996–2006) RSV detection rates was highest from March to August, peaking between April and July, 2. (2007) in this year there was an atypical season that started in December 2006, peaked in March 2007 and persisted until September (with a small decline in June) and 3. (2008–2010) viral detection was highest between February and August, peaking in April. This shift in the timing of the RSV season was therefore delimited by the 2007 outbreak. The results of the Fourier analysis also supported the observation of a shift in the timing of RSV circulation, as captured by the primary peak months, which from 1996 to 2006 were respectively May, April, May, May, May, May, May, April, May, May, May, in 2007 was March, and from 2008 to 2010 were respectively April, April and April. It is worth noticing that although the sampling effort was lower in the first years of the series, the seasonal patterns of RSV circulation in these years (white, upper panel of Fig.1) are consistent with those observed until 2006 (when 359 RSV-positive samples were identified). Interestingly, a low yet consistent number of RSV-positive samples were observed beyond the typical RSV circulation period, particularly from 2005 to 2010.
Figure 2.
Temporal distribution of RSV-positive samples. Palivizumab prophylaxis is currently recommended during the period indicated by the green bar on the x-axis. The number of RSV-positive samples was scaled from 0 to 1 (where 0 and 1 are respectively the minimum and maximum RSV monthly counts observed in the corresponding year).
The temporal association between the epidemiological and climatic time series was stronger when the series were contemporaneous, namely with no time lag (Table 1). To examine if climatic changes were associated to the observed shift in the timing of the RSV season, the correlation analyses were also performed for the period from January to April only, namely the period encompassing the earliest and latest onset of the epidemic season. Indeed, Table 1 shows that the highest absolute correlation coefficients (arbitrarily defined as r > 0.3) were obtained when the latter (reduced) period was considered. Except for sunshine hours, RSV was inversely associated with all climatic variables (all correlation coefficients were negative), circulating predominantly at lower levels of precipitation, relative humidity and lower temperatures. Precipitation and minimum temperature were the best predictors of RSV cycles in the period from January to April (r = −0.60 and r = −0.43, respectively). When all months were included in the analysis, precipitation and maximum temperature showed the highest negative association (r = −0.46 and r = −0.40, respectively) with RSV circulation.
Table 1.
Correlation coefficients (R, as determined by Spearman correlation) between the epidemiological (scaled RSV-positive counts) and each climatic times series (1996–2010; São Paulo, Brazil). Coefficients higher than 0.3 are shown in bold.
| All months included | January to April only | |||
|---|---|---|---|---|
|
| ||||
| Contemporaneous | Lag of 1 month |
Contemporaneous | Lag of 1 month |
|
| Precipitation | −.46 | −.10 | −.60 | .11 |
| Max. temperature | −.40 | −.03 | −.36 | .28 |
| Min. temperature | −.34 | .09 | −.43 | .25 |
| Relative Humidity | −.15 | .08 | −.33 | .03 |
| Sunshine | .25 | .10 | .45 | .03 |
From 1999 to 2010, primary public schools were opened, respectively, in the following days (February): 8, 9, 8, 6, 10, 9, 14, 13, 12, 18, 11 and 18. In the period until 2006 classes therefore commenced, on average, on February 9 (first day at school = 9.6 ± 2.5) and from 2007 to 2010 on February 14 (first day at school = 14.8 ± 3.3), hence a few days later. The differences between the two periods were therefore too small to drive the observed changed in the timing of RSV circulation. Moreover, they were in the opposite direction of what would be expected to explain the progressively earlier circulation of RSV.
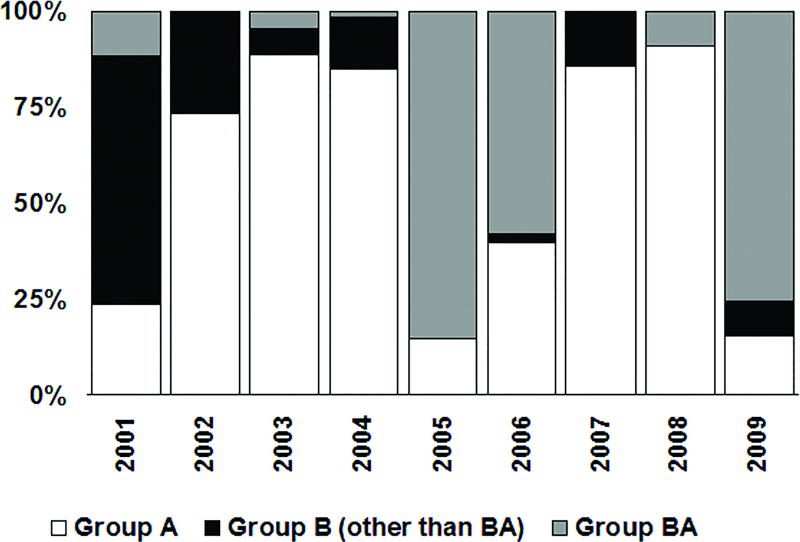
Figure 3 shows the relative proportion of RSV genotypes, as determined by RT-PCR and nucleotide sequencing of the glycoprotein (G) gene by Carvalho [2009], in the pediatric population in Sao Paulo. Genotypes GA2 and GA5 predominated in alternating years. The only exception to this pattern was observed in 2007, when genotype GA2 reappeared after remaining undetected during the previous year.
Figure 3.
Proportion of RSV genotypes detected in respiratory samples from children diagnosed with acute respiratory infections in Sao Paulo [Carvalho, 2009]. RSV genotypes were determined by Carvalho [2009] using RT-PCR and nucleotide sequencing of the glycoprotein (G) gene jointly with comparison to sequences representative of previously defined RSV genotypes.
Discussion
The retrospective analysis of data from 15 consecutive years of viral surveillance confirm the marked seasonal pattern of RSV outbreaks in São Paulo, with the highest incidence of laboratory-confirmed infections coinciding with autumn and winter in this city. To our knowledge, however, the present results represent the first description of a shift in the timing of RSV epidemics in recent years. Earlier results are consistent with the patterns revealed here in the early period, from 1996 to 2006, indicating that RSV outbreaks peak in mid-fall (May) and last until winter [Thomazelli et al., 2007; Vieira et al., 2001], an observation that has prompted the initiation of immunoprophylaxis in April in this city [SUS-SP, 2007]. Still, the present findings show a consistent shift in the onset and peak of RSV circulation in more recent years. Specifically, from 2007 to 2010 RSV circulated approximately one month earlier than in previous years, with the onset of the epidemic season overlapping with the end of summer. The peak in the number of RSV-confirmed cases was also altered by one month, occurring in May since 2007 as opposed to June in the period from 1999 to 2006.
The exploratory analysis on the association of RSV with several climatic factors strengthens the role of climate as a driver of RSV epidemics. In Brazil, a country with a marked climatic diversity, there is a marked latitudinal difference in the timing of the RSV seasons. While in the North and Northeast RSV predominates in the first months of the year, coinciding with the rainy season [Bezerra et al., 2011; Moura et al., 2006], in the South and Southeast RSV circulates predominantly during fall and winter [Cintra et al., 2001; Pecchini et al., 2008; Vieira et al., 2001]. Indeed, decreasing rainfall levels and temperatures during autumn and winter were strongly linked to the onset of the RSV season [Cintra et al., 2001]. Yet, there is no evidence of a change in the climatic patterns in São Paulo from 1996 to 2010, weakening the hypothesis that climate change affected the shift in the timing of RSV circulation. It has also been suggested that higher contact rates among children promoted by the return to school could affect RSV cycles by increasing transmission rates [Cintra et al., 2001]. Although the return of children to public schools in São Paulo occurs in February, the same month that marks the onset of RSV circulation in this city, there were no changes in the school calendar that could explain the present results. There is also no evidence of a change in the relative proportion of RSV genotypes circulating during the period, although it is not possible to rule out the possibility of genetic changes in viral strains.
Although further research is needed to explore putative drivers of the patterns described in this study, the present findings have direct implications for improving the prophylactic use of palivizumab in this city and potentially other regions. Previous studies have shown that the most cost-effective strategy is to initiate the administration of palivizumab just before the onset of the local RSV epidemic season. However, the results shown here stress the importance of constantly fine-tuning the implementation of prophylactic strategies with recent surveillance data. Indeed, the recommendations for the initiation of prophylaxis with palivizumab in the United States have been recently updated [American Academy of Pediatrics, 2009] to reflect the latest description of RSV seasonality in different locations within that country. In the case of Sao Paulo, findings indicate that RSV prophylaxis should be initiated earlier than currently suggested, ideally in January. Similar adjustments may be necessary in other regions to ensure that preventive strategies remain effective.
Acknowledgments
We thank Ana M.M. Lima, Juliana C. Pereira, Fabiana O. Burgos, Norio A. Sasaki and Renato S. Paulino from Adolfo Lutz Institute for laboratory support. We also thank the personnel of the Municipal and State Secretariat for Health (São Paulo, Brazil), the Public Health Services of the São Paulo State, the Influenza Virus Surveillance Network and the Brazilian Ministry of Health for logistic and administrative support and for the viral surveillance data. The authors also wish to thank the National Institute of Meteorology (INMET) for providing the climatic data and the São Paulo Secretariat for Education for providing the school calendar data. WJA was supported by the Fogarty International Center, National Institutes of Health (US). Financial support was also received from Adolfo Lutz Institute (Brazil), the State Secretariat for Health (São Paulo, Brazil) and the Brazilian Ministry of Health.
References
- American Academy of Pediatrics, Committee on Infectious Diseases. Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- Bezerra PGM, Britto MCA, Correia JB, Duarte MdCMB, Fonceca AM, Rose K, Hopkins MJ, Cuevas LE, McNamara PS. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS ONE. 2011;6:e18928. doi: 10.1371/journal.pone.0018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- Carvalho ACL. M.Sc. Thesis (Biotecnology) USP/Butantan/IPT; São Paulo: 2009. Genetic variability human respiratory syncytial virus in group B 60-nucleotide-duplication samples from children admitted in university hospital in São Paulo city. [Google Scholar]
- CDC. Update: respiratory syncytial virus activity: United States, 1998–1999 season. Morb Mortal Wkly Rep. 1999;48:1104–1115. [PubMed] [Google Scholar]
- Checon R, Siqueira MM, Lugon AK, Portes SRD. Short Report: Seasonal pattern of respiratory syncytial virus in a region with a tropical climate in southeastern Brazil. Am J Trop Med Hyg. 2002;67:490–491. doi: 10.4269/ajtmh.2002.67.490. [DOI] [PubMed] [Google Scholar]
- Cintra OAL, Owa MA, Machado AA, Cervi MC, Figueiredo LTM, Rocha GM, Siqueira MM, Arruda E. Occurrence and severity of infections caused by subgroup A and B respiratory syncytial virus in children in Southeast Brazil. J Med Virol. 2001;65:408–412. doi: 10.1002/jmv.2049. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- Gaunt ER, Jansen RR, Poovorawan Y, Templeton KE, Toms GL, Simmonds P. Molecular epidemiology and evolution of human respiratory syncytial virus and human metapneumovirus. PLoS ONE. 2011;6:e17427. doi: 10.1371/journal.pone.0017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H, Long S. Revised indications for the use of Palivizumab and respiratory syncytial virus immunoglobulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112:1447–1452. doi: 10.1542/peds.112.6.1447. [DOI] [PubMed] [Google Scholar]
- Moura FEA, Nunes IFS, Silva GB. Respiratory syncytial virus infections in northeastern Brazil: seasonal trends and general aspects. Am J Trop Med Hyg. 2006;74:165–167. [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi S, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EAF, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento JP, Siqueira MM, Sutmoller F, Krawczuk MM, Farias V, Ferreira V, Rodrigues M. Longitudinal study of acute respiratory disease in Rio de Janeiro: Occurrence of respiratory viruses during four consecutive years. Rev Inst Med Trop Sao Paulo. 1991;33:287–296. doi: 10.1590/s0036-46651991000400008. [DOI] [PubMed] [Google Scholar]
- Pecchini R, Berezin EN, Felício MCC, Passos SD, Souza MCOd, Lima LRdAVd, Ueda M, Matsumoto TK, Durigon EL. Incidence and clinical characteristics of the infection by the respiratory syncytial virus in children admitted in Santa Casa de São Paulo Hospital. Braz J Inf Dis. 2008;12:476–479. doi: 10.1590/s1413-86702008000600006. [DOI] [PubMed] [Google Scholar]
- Rogers DJ, Hay SI, Packer MJ. Predicting the distribution of tsetse flies in West Africa using temporal Fourier processed meteorological satellite data. Ann Trop Med Parasitol. 1996;90:225–241. doi: 10.1080/00034983.1996.11813049. [DOI] [PubMed] [Google Scholar]
- SESP. Information provided by the Secretariat of Education. State of São Paulo; 2011. Resolutions for the School Calendar for São Paulo State. (March/2011) [Google Scholar]
- Simões E, Sondheimer H, Top F., Jr Respiratory syncytial virus immune globulin for prophylaxis against respiratory syncytial virus in infants and children with congenital disease: the cardiac study group. J Pediatric. 1998;133:492. doi: 10.1016/s0022-3476(98)70056-3. [DOI] [PubMed] [Google Scholar]
- Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22:S21–32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- SUS-SP. Norma técnica relativa às diretrizes para a prevenção da infecção pelo virus sincicial respiratório. DOE. 2007;131 14/07/2007. Resolução SS - 249. [Google Scholar]
- Thomazelli LM, Vieira S, Leal AL, Sousa TS, Oliveira DBL, Golono MA, Gillio AE, Stwienr KE, Erdman DD, Durigon EL. Surveillance of eight respiratory viruses in clinical samples of pediatric patients in southeast Brazil. J Pediatria. 2007;83:422–428. doi: 10.2223/JPED.1694. [DOI] [PubMed] [Google Scholar]
- Vieira SE, Stewiew KE, Queiroz DAO, Durigon EL, Török T, Anderson LJ, Miyao CR, Hein N, Botoso VF, PAHL MM, Giglio AE, Ejzenberg E, Okay Y. Clinical patterns and seasonal trends in respiratory syncytial virus hospitalization in São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2001;43:125–131. doi: 10.1590/s0036-46652001000300002. [DOI] [PubMed] [Google Scholar]
- Welliver R. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112–S117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]