Abstract
Ribonucleotide reductases (RNRs) catalyze the conversion of nucleotides to deoxynucleotides. Class I RNRs are composed of two homodimeric subunits: R1 and R2. R1 is directly involved in the reduction, and R2 contains the diferric-tyrosyl radical (Y⋅) cofactor essential for the initiation of reduction. Saccharomyces cerevisiae has two RNRs; Y1 and Y3 correspond to R1, whereas Y2 and Y4 correspond to R2. Y4 is essential for diferric-Y⋅ formation in Y2 from apoY2, Fe2+, and O2. The actual function of Y4 is controversial. Y2 and Y4 have been further characterized in an effort to understand their respective roles in nucleotide reduction. (His)6-Y2, Y4, and (His)6-Y4 are homodimers, isolated largely in apo form. Their CD spectra reveal that they are predominantly helical. The concentrations of Y2 and Y4 in vivo are 0.5–2.3 μM, as determined by Western analysis. Incubation of Y2 and Y4 under physiological conditions generates apo Y2Y4 heterodimer, which can form a diferric-Y⋅ when incubated with Fe2+ and O2. Holo Y2Y4 heterodimer contains 0.6–0.8 Y⋅ and has a specific activity of 0.8–1.3 μmol⋅min⋅mg. Titration of Y2 with Y4 in the presence of Fe2+ and O2 gives maximal activity with one equivalent of Y4 per Y2. Models for the function of Y4 based on these data and the accompanying structure will be discussed.
Ribonucleotide reductases (RNRs) play an essential role in DNA replication and repair by providing all of the monomeric deoxynucleotides required for these processes. The genome sequencing projects have revealed that both prokaryotes and eukaryotes possess multiple RNRs, whose functions remain to be elucidated (1). Class I RNRs are composed of two homodimeric subunits: R1 (α2) and R2 (β2). Both subunits are essential for activity. R1 contains the binding sites for the nucleoside diphosphate substrates and the deoxynucleotide and ATP allosteric effectors that govern which nucleotide is reduced and its rate of reduction. R2 possesses a diferric-tyrosyl radical (Y⋅) cofactor essential for initiation of nucleotide reduction on R1 (2, 3).
Four genes have been identified that encode RNR subunits in Saccharomyces cerevisiae: RNR1 and RNR3 (their gene products designated Y1 and Y3) are R1 homologues, and RNR2 and RNR4 (their gene products designated Y2 and Y4) are R2 homologues (4–9). Y1 and Y3 share 80% sequence identity (4). RNR1 expression is cell cycle regulated, and the gene is essential for mitotic viability (5, 10). In contrast, RNR3 is not expressed under normal growth conditions. Transcription of RNR1 and RNR3 is inducible by DNA damage; the mRNA of the latter is up-regulated >500-fold (5). Y2 and Y4 share 56% sequence identity. Y4 contains several unusual features relative to a canonical R2. It lacks about 50 amino acid residues from the N terminus, and of the 16 amino acid residues conserved in almost all class I R2s, six have been replaced in Y4. The most notable substitutions are two histidines and a glutamate, ligands of the di-iron center, which have been replaced by two tyrosines and an arginine. Such substitutions would be expected to disrupt the ability of Y4 to bind iron. RNR2 is essential for mitotic viability (10, 11). RNR4 also appears to be important for mitotic viability, but its essentiality varies with genetic background (8, 9, 12). In the one case where a Y4 knockout was shown to be lethal, the lethality could be suppressed by overexpression of Y1 and Y3 (8).
The transcriptional regulation of the yeast RNR genes has been studied extensively. However, little is known about the biochemical properties of yeast RNR proteins, their structures, or their complex posttranscriptional and allosteric regulation. Recently, we (13) and Chabes et al. (14) reported the isolation and purification of the yeast RNR proteins. One of the most notable results from our in vitro studies was that Y4 was essential for the assembly of the diferric-Y⋅ cofactor of apo Y2. Furthermore, coimmunoprecipitation studies by using Y4 antibodies on crude yeast cell extracts suggested that Y2 and Y4 can form a complex in vivo, substantiating earlier studies by using myc-tagged proteins and a myc antibody (8). On the basis of these results, we suggested that Y4 plays an important function in the cofactor maturation of Y2. We speculated that this role might be as a metallo-chaperone, based on recent discoveries of copper-chaperone proteins in yeast (15).
The most notable result from the recent studies of Chabes et al. (14) was the isolation of a stable heterodimer of Y2Y4 expressed in Escherichia coli. The heterodimer was active in nucleotide reduction and contained 0.4 radicals and 1.2 irons. On the basis of these studies, they concluded that the active form of yeast R2 is the heterodimer both in vitro and in vivo. In the same article, they reported that recombinant Y2 expressed in E. coli was only partially folded based on its chromatographic properties and its CD spectrum. These observations allowed them to suggest that Y4 functions as a chaperone protein assisting the folding of apoY2 and, consequently, cofactor assembly on Y2 to form the active protein.
We now report that recombinant Y2 and Y4 expressed in E. coli are homodimers and under physiological conditions in vitro can assemble into a heterodimer. The heterodimer is moderately stable in its apo form, and upon addition of ferrous ion and O2 rapidly generates 0.6–0.8 equivalents (eq) of Y⋅ per Y2Y4. The significance of these in vitro observations to the active form of R2 in vivo has been addressed by Western blotting, which has revealed that both proteins are present at micromolar concentrations. Their localization, however, remains to be established. Studies are presented that suggest Y4 is not essential for Y2 to fold, nor does it appear to bind iron and deliver it to Y2. Thus, further studies of Y4, essential for the formation of diferric-Y⋅ cofactor in vitro, are required to define its function in vivo. Several models for the function of Y4 are put forth based on our studies and the recent structure of the Y2Y4 heterodimer (16).
Materials and Methods
Competent E. coli CodonPlus BL21(DE3) RIL cells were purchased from Stratagene. Complete EDTA-free protease inhibitor tablets were purchased from Roche Biochemicals. Iron content was determined by the ferrozine assay (17). Yeast strain PS0799 (MATa, ade2–1, his3–11,15, leu2–3,112, ura3, trp1–1, can1–100) was a gift of P. K. Soger (Massachusetts Institute of Technology). Oligonucleotides were ordered from the Massachusetts Institute of Technology Biopolymers Laboratory or Research Genetics. Plasmids pHis-Y2, pY1A, pY2 M, and pY4J have been described (13). Protein concentrations were determined by using extinction coefficients (ɛ280–310 nm) per dimer [105,600 M−1⋅cm−1 for (His)6-Y2, 94,000 M−1⋅cm−1 for Y4, and 99,800 for M−1⋅cm−1 for (His)6-Y2:Y4]. The concentration of Y1 was determined by Bradford assay with BSA as standard.
Expression Plasmids for Y2 and Y4 in E. coli.
The Y2 gene (1.2 kb) was obtained by digesting pY2 M with NdeI/BamHI (13). It was purified by agarose gel electrophoresis and ligated into the NdeI/BamHI sites of pET-14b to produce p(His)6-Y2. (His)6-Y2 contains the following N terminus: MGSSHHHHHHSSGLVPRGSH-native protein.
The Y4 (1.0 kb) and the ΔY4 (UAA stop codon at Glu-338) genes were amplified by PCR by using pY4J as template with primers 5′-AGGAGATATCATATGGAAGCACAT-3′ and 3′-AAGATTCCTAGGCTTAAGCTC-5′, 5′-AGGAGATATCATATGGAAGCACAT-3′ and 3′-TTCATTTAATTGAAACTACTGAAGATTCCTAGGCTT-5′, respectively. The PCR products were digested with NdeI/BamHI, purified on agarose gel, and ligated into NdeI/BamHI sites of pET-14b to give pHis-Y4 and pHis-Y4Δ. All constructs were verified by sequencing.
Expression and Purification of RNR Subunits.
Y1, (His)6-Y2 from yeast, and Y4 were expressed and purified by using procedures described earlier (13) with the following modifications: E. coli BL21 CodonPlus (DE3) RIL replaced BL21(DE3) pLysS as the expression host; Y4 (pHis-Y4, pHis-Y4Δ, and pY4J) growth and expression were conducted at 37°C; bacterial cells were lysed in the presence of Complete EDTA-free protease inhibitors; (His)6-Y2 from yeast was purified with buffers that included 4 mM β-mercaptoethanol/2 μM leupeptin/5 μM pepstatin A; and an additional purification step using DEAE-Sepharose FastFlow chromatography (Amersham Pharmacia) on a BioCAD Sprint FPLC station (Perkin–Elmer) was added to the Y4 isolation. The newly constructed p(His)6-Y2 was expressed in E. coli BL21 CodonPlus (DE3) RIL as described for pY2 M (13).
Recombinant (His)6-tagged proteins [(His)6-Y2, (His)6-Y4, (His)6-Y4Δ] were purified by using the following general procedures. Frozen bacterial cell paste was resuspended in 4 vol of buffer A (50 mM Hepes/5% glycerol/1.0 mM PMSF, pH 7.4) at 4°C. One EDTA-free protease inhibitor tablet was added per 50 ml of cell suspension. The suspension was passed through the French press at 14,000–16,000 psi. The cell lysate was centrifuged at 19,800 × g for 30 min. The supernatant was treated with streptomycin sulfate to a final concentration of 1% (wt/vol), and the pellet was removed by centrifugation. The supernatant was loaded onto a TALON cobalt affinity column (2.5 × 5 cm), which had been pre-equilibrated with buffer A. The column was subsequently washed with 40 column volumes (CV) of buffer A containing 100 mM NaCl, followed by 16 CV of buffer A containing 10 mM imidazole. The protein was eluted first with 10 CV of buffer A containing 100 mM imidazole, then with 10 CV of buffer A containing 200 mM imidazole. Fractions containing protein monitored by A280 nm were combined and concentrated to >5 mg/ml. Imidazole was removed either by Sephadex G-25 chromatography or dialysis. All proteins were concentrated to >5 mg/ml, frozen in liquid nitrogen, and stored at −80°C.
Coexpression and Purification of (His)6-Y2:Y4 Complex.
Plasmids p(His)6-Y2 and pY4J were cotransformed into E. coli BL21 CodonPlus (DE3) RIL cells. The heterodimeric complex was expressed and isolated as described above for (His)6-Y2 with the following changes. If apo heterodimer was isolated, 1,10-phenanthroline (100 μM) was added to the growth media 10 min before induction with isopropylthio-β-D-galactoside (18). DNase I was added to remove DNA in place of the streptomycin sulfate precipitation step. In the isolation of holo heterodimer, Fe(NH4)2(SO4)2 was added to the crude lysate at a final concentration of 170 μM.
Isolation of Apo (His)6-Y2:Y4.
A 50-ml solution containing equal molar amounts of apo (His)6-Y2 (2.0 μM) and Y4 (2.0 μM) in buffer A was incubated for 1 h at 4°C. The mixture was loaded on a TALON column (2.5 × 5 cm) and washed with 20 CV of buffer A. The protein was eluted with 50 ml of buffer A containing 100 mM imidazole, followed by 50 ml of buffer A containing 200 mM imidazole. Fractions containing the heterodimer as monitored by A280 nm and SDS/PAGE were pooled and concentrated to >5 mg/ml. The protein solution was directly applied to a DEAE-Sepharose FF column (1.6 × 10 cm). The bound protein was eluted from the column by using a (100 × 100 ml) linear gradient of 0–500 mM NaCl in buffer A. Fractions were monitored by A280 nm and SDS/PAGE, and those containing a 1:1 (His)6-Y2:Y4 complex were collected.
Activation of Apo (His)6-Y2:Y4.
Stock solutions of (His)6-Y2 and Y4 [(His)6-Y4, or (His)6-Y4Δ] were deoxygenated by allowing 50-μl aliquots to equilibrate in an anaerobic glove box (M. Braun, Newburyport, MA) for >3 h at 4°C. Alternatively, samples of >500 μl were deoxygenated by repeated cycles of vacuum pumping and argon flushing on a Shlenk line. A 1:1 mixture of (His)6-Y2 and Y4 (2 μM each) was prepared from these solutions, to which 3 eq of Fe(II) per (His)6-Y2:Y4 heterodimer was added from a deoxygenated FeSO4 solution (2 mM in buffer A). The resulting mixture was incubated at 4°C for 1 h, then exposed to air for 15 min with mixing. EPR analysis, activity assays, and iron determination were carried out.
Isolation of Holo (His)6-Y2:Y4.
The heterodimer containing assembled iron cluster was purified by using procedures described above for apo heterodimer complex. Holo (His)6-Y2:Y4 was subsequently analyzed for Y⋅ by EPR methods as well as for enzymatic activity and iron content.
Size Exclusion Chromatography (SEC).
SEC was performed on a Bio-Silect SEC 250–5 column (7.6 × 300 mm, Bio-Rad) on a BioCAD Sprint station. Gel filtration standards from Bio-Rad were used for molecular weight calibration.
Selenomethionine (SeMet) Y4 Anion Exchange Chromatography.
SeMet Y4 was expressed in E. coli B834(DE3) in M9 minimal media supplemented with SeMet and purified as described for Y4 (13), except 10 mM DTT and 0.2 mM EDTA were added to all buffers. The protein was chromatographed on a POROS 20 HQ anion exchange column (4.6 × 100 mm) on a BioCAD Sprint station. The column was washed with 10 CV of 50 mM Tris⋅HCl, pH 6.5, and the protein was eluted with a (60 × 60 ml) linear gradient of 0–350 mM NaCl in the same buffer.
One Eq of Y4 Is Required for Maximal Nucleotide Reduction Activity of Y2.
(His)6-Y2 isolated from yeast or E. coli and Y4 were made anaerobic as described above. The proteins were mixed with a deoxygenated FeSO4 solution to give final concentrations of 2.0 μM (His)6-Y2, 0–10 μM Y4, and 12 μM FeSO4 (3 eq per heterodimer). After 1 h at 4°C, the samples were removed from the glove box and exposed to air on ice. Enzyme activity assays in the presence of 10 eq of Y1 per heterodimer were performed immediately after reconstitution.
Activity Assay.
A typical reaction mixture contained in a final volume of 0.28 ml was as follows: 50 mM Hepes buffer, pH 7.0/3.0 μM Y1/0.3 μM (His)6-Y2:Y4/2.0 mM ATP/1.0 mM [2-14C]CDP (specific activity 1.0 × 103 cpm/nmol)/100 μM E. coli thioredoxin/1.0 μM E. coli thioredoxin reductase/2.0 mM NADPH/20 mM Mg(OAc)2. The assay mixture was incubated at 30°C. Aliquots of 50 μl were removed over a 20-min time period and quenched in a boiling water bath. dCDP was analyzed by the method of Steeper and Stewart (19). One unit of activity is defined as 1 μmol of dCDP formed/min. The specific activity was calculated per mg of (His)6-Y2.
EPR Spectroscopy.
EPR spectra (139.5 GHz) were recorded at 12 K on a custom-designed pulsed EPR spectrometer equipped with an Oxford liquid helium flow cryostat (20). Before each measurement, the cavity was calibrated at room temperature with α,γ-bisdiphenylene-β-phenylallyl radical. All echo-detected EPR spectra were measured by using the stimulated echo sequence [(π/2)x − τ − (π/2)x − T − (π/2)x − τ − echo). π/2 pulses of 100 ns were used with spacing of τ = T = 200 ns. Each data point was averaged over 10 shots with a repetition rate of 400 ms/point. Spin concentration was determined by comparison of the signal intensities of yeast RNR samples to a calibration curve established with E. coli R2 Y⋅ (50–170 μM).
CD Spectroscopy.
CD spectra were recorded at 20°C on an Aviv CD 62 DS spectrometer by using a quartz cuvette with 1.0-mm path length. Samples (1.0–5.0 μM dimer) were diluted into 10 mM sodium phosphate buffer, pH 7.0. Five scans were averaged. Molar ellipticity [θ] was calculated with a macro program written by Aviv Associates (Lakewood, NJ).
Quantitative Western Blot.
A midlog phase culture of yeast cells (BJ5465 or PS0799) was harvested by centrifugation. The cells were washed with water and resuspended in ice-cold lysis buffer (50 mM Hepes, pH 7.4/200 mM NaCl/10 mM EDTA/5 μg/ml aprotinin/5 μg/ml leupeptin/2 μg/ml E-64/2.5 μg/ml pepstatin A/1 mM PMSF). Glass beads were added, and cells were cracked with eight 30-s rounds of vortexing and cooling on ice. Cell debris was removed by centrifugation. Extract was stored at −80°C. Protein concentration was determined by Lowry with BSA as standard.
A typical quantitative Western blot contained a standard curve of the purified RNR subunit (8–60 ng) and crude extracts (5–12 μg) and was carried out as described (13), except that the proteins were visualized with luminol chemiluminescence. Membranes were incubated in luminol solution (1.25 mM luminol/65 mM Tris, pH 8.0/0.2 mM coumaric acid/0.01% H2O2) for 1 min and then exposed to film. Bands were quantitated by using the public domain National Institutes of Health image program (http://rsb.info.nih.gov/nih-image/).
Results
Y2 and Y4 Are Homodimers.
(His)6-Y2 and Y4 were isolated as described (13). The native molecular weights of (His)6-Y2 and Y4 were determined by SEC. For comparison, apo and holo E. coli R2, both known to be homodimers, were characterized as well. (His)6-Y2 and Y4 exhibit apparent molecular masses of 133 kDa and 102 kDa (95 kDa and 80 kDa predicted), respectively. Holo and apo E. coli R2 exhibit apparent molecular masses of 120 kDa and 124 kDa (87 kDa predicted), respectively. The comparative data suggest that recombinant Y2 and Y4 are both homodimers. Furthermore, heterodimers of apo and holo (His)6-Y2:Y4, discussed subsequently, have apparent molecular masses of 99 kDa and 113 kDa, respectively.
Y4 Dimer Is Capable of Protomer Reorganization.
To make SeMet-labeled Y4 to facilitate its structure determination, a fortuitous observation was made that demonstrates that the Y4 dimer is capable of protomer reorganization. Purified SeMet Y4 was a mixture of full-length and clipped proteins (Fig. 1A, lane 1). When this mixture was analyzed on a DEAE anion exchange column, three protein-containing peaks were observed (Fig. 1B). SDS/PAGE analysis (Fig. 1 A and B) revealed clipped Y4 in peak a, a 1:1 mixture of clipped and unclipped Y4 in peak b, and unclipped Y4 in peak c. Mass spectrometric analysis and N-terminal sequencing of the clipped Y4 revealed that the C terminus had been cleaved between residues Ser-330 and Lys-331. The observed 1:1 ratio of proteins in peak b and the fact that the two polypeptides, full-length and clipped Y4, generated three different species provide additional support for the conclusion from SEC studies that Y4 is a dimer.
Figure 1.
Demonstration of the ability of Y4 protomers to reorganize. Anion exchange chromatography and SDS/PAGE of full-length and clipped SeMet Y4. (A) SDS/PAGE. Lane 1, full-length SeMet Y4 and clipped Y4 standards. Lanes 2–4 represent proteins found in peaks a, b, and c of the chromatogram shown in B. (B) Anion exchange chromatography of SeMet Y4. (C) Peak b in B was collected and rechromatographed.
The observation of a clipped and unclipped SeMet-Y4 dimer offered an opportunity to test the possibility that Y4 protomers are capable of re-equilibration. The protein eluted in peak b (Fig. 1B) was pooled, concentrated, and reinjected onto the same column. The results shown in Fig. 1C are almost identical to those in Fig. 1B. These results demonstrate a re-equilibration of the Y4 mixed dimer, although the mechanism of such equilibration through either monomers or tetramer has not yet been established.
Is Y4 a Catalytic or Stoichiometric Activator of Y2 for Nucleotide Reduction?
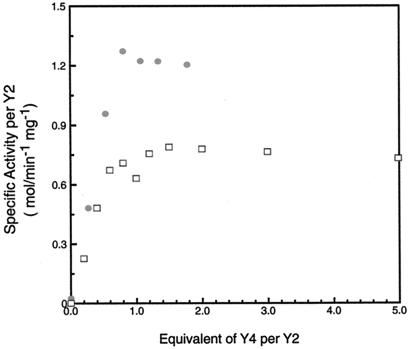
Y4 as isolated contains no iron and consequently no Y⋅ (13, 14). Our working hypothesis for the function of Y4 has been that it is a metallo-chaperone protein that delivers iron to Y2. If Y4 is a metallo-chaperone, one would predict that it may function catalytically in the assembly of diferric-Y⋅ cofactor in Y2. Experiments were carried out in which apo Y2 (2.0 μM) was incubated with 0–5 eq of Y4 in the presence of 3 eq of ferrous ion per Y2 monomer under anaerobic conditions. O2 was added to assemble the cluster, and the resulting mixture was assayed for nucleotide reduction ability. The results of these experiments are shown in Fig. 2. Approximately one eq of Y4 gives maximal activity of 1.3 and 0.8 μmol⋅min⋅mg for (His)6-Y2 expressed in yeast and in E. coli, respectively. These results suggest that, under the conditions investigated, Y4 acts stoichiometrically rather than catalytically and suggest that the active R2 may be a heterodimer.
Figure 2.
Reconstitution of (His)6-Y2 expressed in yeast and in E. coli with Y4, Fe(II), and O2. (His)6-Y2 expressed in S. cerevisiae (●) was reconstituted with 0–2 eq of Y4 and 6 eq of FeSO4 per Y2 dimer. (His)6-Y2 expressed in E. coli (□) was reconstituted with 0–5 eq of Y4 and 6 eq of FeSO4 per Y2 dimer.
Generation of Apo Y2Y4.
To test this model, a 1:1 mixture of (His)6-Y2 and Y4 (2–10 μM dimer) were incubated at 4°C. The (His)6 tag facilitates separation of the possible combinations of subunits by using a cobalt affinity column. Y4, if in excess, elutes during the extensive washes. As shown in Fig. 3, a 1:1 mixture of (His)6-Y2 and Y4 elutes with 100 mM imidazole. Additional (His)6-Y2 homodimer elutes with 200 mM imidazole. The fractions containing the heterodimer were rechromatographed on a DEAE anion exchange column [(His)6-Y2 and Y4 have isoelectric points of 5.5 and 5.1, respectively]. The heterodimer eluted at 250 mM NaCl using a linear NaCl gradient, and SDS/PAGE (Fig. 3) revealed a 1:1 mixture of Y2 and Y4. These studies indicate that Y2 and Y4 can re-equilibrate to form heterodimer and that in the apo form the interactions between these proteins is sufficiently strong to survive two chromatographic steps. As noted above, SEC studies verified that Y2Y4 is also a dimer.
Figure 3.
SDS/PAGE of (His)6-Y2:Y4 heterodimer in apo and holo forms reveal the stability of heterodimer through two chromatographic steps. Lane 1, low MW SigmaMarker; lane 2, apo (His)6-Y2:Y4 after TALON column; lane 3, apo complex after DEAE Sepharose column; lanes 4 and 5, holo (His)6-Y2:Y4 after TALON and DEAE Sepharose columns, respectively.
Generation of Active Heterodimer.
Optimized conditions were established for Y⋅ generation from apo heterodimer. A 1:1 mixture of apo (His)6-Y2 and Y4 (2 μM dimer of each protein) was mixed under anaerobic conditions with 3 eq of ferrous ion per (His)6-Y2 monomer. After 1 h at 4°C, the solution was aerated and then purified on a TALON column followed by an anion exchange column. The results shown in Fig. 3 reveal a 1:1 ratio in the heterodimer. This protein was analyzed by UV/Vis and EPR spectroscopy for Y⋅. The spectrum reveals a diferric cluster (features at 325 nm and 360 nm) and a very broad Y⋅ feature at 416 nm. The Y⋅ feature is similar to that reported for mouse M2 (21). Quantitation of the Y⋅ by high field EPR gave 0.6–0.8 radicals per heterodimer. These results contrast with the in vitro reconstitution experiments on E. coli apo R2 in which 1.0–1.2 radicals were observed per homodimer (22, 23).
Chabes et al. (14) reported that coexpression of Y2 and Y4 in E. coli resulted in an active heterodimer with 0.4 radicals and a specific activity of 2.5 μmol⋅min⋅mg. The assay conditions included ferric ion and used DTT as reductant. In our assay system, E. coli thioredoxin, thioredoxin reductase, and NADPH are used as reductant, and no iron is added. When we assayed our yeast expressed and reconstituted Y2 under the conditions of Chabes et al., the activity was 32% of the activity using our assay. We then coexpressed (His)6-Y2 and Y4 in E. coli and isolated heterodimer in the apo and iron-loaded forms. The Y⋅ and specific activity were similar to those obtained from the heterodimer that was assembled from individual homodimers, and there were 1.8 eq of iron per heterodimer.
CD Spectroscopy on Y2, Y4, and Y2Y4.
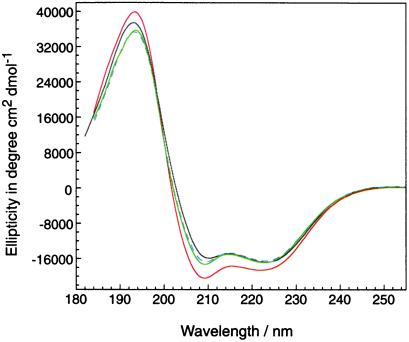
Studies of Chabes et al. (14) suggested that their recombinant (His)6-Y2 was partially denatured as shown by the CD spectrum. Therefore, we acquired the CD spectra of Y2, Y4, apo Y2Y4, and iron-loaded Y2Y4 under conditions identical to those reported (14). The CD spectra of Y4, (His)6-Y2, and the apo and holo heterodimers appear to be very similar and predominantly helical (Fig. 4). Thus, our (His)6-Y2 protein appears to be folded. Chabes et al. were unable to purify Y2 using strong anion exchange chromatography. We were able to chromatograph Y2 by using weak anion exchange chromatography, but the recoveries were poor, suggesting instability of the protein in high salt.
Figure 4.
CD spectra of (His)6-Y2, Y4, and apo and holo forms of the heterodimer. Red line represents (His)6-Y2 protein expressed in E. coli; black line represents Y4; green and dashed blue lines represent (His)6-Y2:Y4 heterodimer in apo and holo forms, respectively.
Iron Binding to Y4?
Several experiments were carried out to determine whether iron in the ferrous or ferric state can bind to Y4. Incubation of Y4 with a 5-fold excess of 55Fe2+ in an anaerobic box, followed by Sephadex G-25 chromatography, failed to detect any Fe(II) associated with the protein fractions. A similar experiment carried out with [55Fe]ferric citrate also failed to reveal any iron binding. A control experiment to determine ferrous ion binding to apo E. coli R2 revealed ≈0.2 eq of iron per monomer. Thus, if iron does bind to Y4, its Kd must be high.
One additional experiment was performed to test the model of iron delivery to Y2 by Y4. In the case of yCCS, the chaperone protein for superoxide dismutase (SOD), its C terminus (last 30 aa) has been shown to play a key role in copper delivery in vivo (15, 24). Examination of the C-terminal sequence of Y4 in comparison with other R2 sequences revealed a preponderance of carboxylates that might function as ligands to iron. Therefore, we constructed a Y4 truncation mutant [(His)6-Y4Δ] in which the last eight amino acid residues were deleted. This construct was examined for its ability to generate active heterodimer in vitro. (His)6-Y4Δ gave the same amount of Y⋅ per heterodimer as did Y4 [(His)6-Y4]. These results together suggest that Y4 homodimer does not bind iron tightly in either the Fe(II) or Fe(III) oxidation state and that Fe(II) can be incorporated into Y2Y4 heterodimer in the absence of the C-terminal region of Y4.
Determination of Y2 and Y4 Concentrations in Vivo Using Quantitative Western Blots.
We and others have shown that a Y2Y4 complex can be detected in vivo using antibodies (8, 13). Furthermore, Chabes et al. (14) recently proposed, based on in vitro studies, that the heterodimer is the active form of R2 in vivo. Our in vitro studies also support this model. However, this model requires that Y2 and Y4 be present in similar amounts in vivo and that they be localized in the same compartment inside the cell. Quantitative Western blot studies have been carried out to determine the Y2:Y4 ratio. Two different yeast strains, BJ5465 and PS0799, were examined. The results show that Y2 dimer is present at 0.4–2.3 μM and Y4 dimer at 0.5–1.3 μM, assuming a cell volume of 7 × 10−8 μl. This calculated concentration of Y4 is in good agreement with the amount of Y4 calculated from two-dimensional gel electrophoresis, which estimated a concentration of 44,000 copies per yeast cell or 0.5 μM if the same cell volume of 7 × 10−8 μl is assumed (25). These results suggest that Y2 and Y4 could exist as a heterodimer in vivo.
Discussion
The recent discovery of a second R2 subunit in mammalian systems linked to p53 (26) and the success of gemcitabine in the treatment of pancreatic cancer (27) makes understanding the regulation of RNRs, which play a central role in DNA replication and repair, of primary interest. We have chosen yeast as a model system to understand the complex regulation of these enzymes (25, 28–31).
Our initial efforts have been focused on identifying the active form of the R2 subunit and the function of Y4 in diferric-Y⋅ cofactor formation. This information is crucial for understanding the active form(s) of RNR in vivo. Our thinking about the mechanism of formation of the cofactor in R2 has been influenced by our in vitro reconstitution experiments of E. coli R2 and by the recent discovery of copper chaperone proteins (15, 32–34). The studies with apo E. coli R2 have demonstrated that the diferric-Y⋅ cofactor can be assembled in vitro by addition of ferrous ion, O2, and reductant (22, 35). Much has been learned about the chemistry and the kinetics of this assembly process. However, further studies have been hampered because of our inability to uniquely load ferrous ion into R2 and deliver the required reducing equivalent by a single pathway. Thus, to understand the details of cluster assembly requires knowledge of the proteins that deliver Fe(II) and the protein that provides the reducing equivalent.
Studies on copper delivery to proteins in yeast provided the basis for our proposal that Y4 may be involved in Fe(II) delivery to Y2. These studies revealed that no free copper ions exist inside yeast cells and that specific chaperone proteins deliver copper to specific targets (32, 36, 37). The chaperone protein yCCS, for example, delivers Cu(I) to copper-zinc SOD (15, 32–34). Crystallographic studies of yCCS and SOD revealed that they are structurally homologous (38). yCCS is not active as a SOD, as the active site ligands for the metal have been altered. It has been proposed that copper-loaded yCCS delivers copper to SOD by transient formation of a heterodimer (33) or heterotetramer (34). The analogy between the yCCS/SOD system and our Y2/Y4 system is striking. Our results show that Y4 is essential for diferric-Y⋅ cofactor formation in Y2 in vitro. Y4 is homologous to Y2 with three of the six conserved iron ligands replaced, presumably abolishing iron binding to the active site.
Recently, Chabes et al. (14) suggested, based on coexpression of Y2 and Y4 in E. coli, that Y4 is a structural chaperone protein assisting in the folding of Y2 to form an active heterodimer in vivo. The ability of chaperone proteins to function in the folding of other proteins is well documented. However, to our knowledge, there is no example of a structural homologue stoichiometrically assisting in folding. Our ability to chromatograph Y2 and its CD spectrum in comparison with other R2s (Fig. 4) suggest that Y2 does not require Y4 for folding. However, our Y2 protein contains a (His)6-tag that differs from the construct reported by Chabes et al., which could account for our different observations. At present, we believe that Y2 can fold independently of Y4 and that Y4 is not a folding chaperone.
A number of important conclusions can be drawn from the experiments described here, which have been designed to elucidate the function of Y4. It is clear that in vitro, Y2 and Y4 can form an active heterodimer as recently reported (14). Our experiments differ in that their active heterodimer was obtained only through coexpression of Y2 and Y4 in E. coli. In our case, Y2Y4 heterodimer can be generated by coexpression, activation of Y2 by Y4 with Fe(II)/O2, or activation of apo Y2Y4 by Fe(II)/O2. Our holo heterodimer possesses 0.6–0.8 tyrosyl radicals and is active in nucleotide reduction. The relevance of this heterodimer to the active R2 in vivo is supported by coimmunoprecipitation studies, as well as by the determination in two different yeast strains that Y2 and Y4 are present in similar amounts.
In our opinion, the active form of R2 in vivo still remains an open question (13, 14). First, we do not know whether Y2 and Y4 reside in the same location within the cell. Second, if the heterodimer is the active R2 in DNA replication, one would expect that the deletion of RNR4 would be lethal. Studies from the Elledge lab support this proposal (8). However, overexpression of either Y1 or Y3 in the deletion strain leads to cell survival. In addition, two recent independent reports show that RNR4 is important, but not essential, for yeast cell viability (12, 39). Third, studies of the mRNA levels of RNR2 and RNR4 as a function of cell cycle suggest that the Y2 mRNA level varies 2-fold over the cell cycle, whereas that of Y4 mRNA remains constant (7, 8). Thus, yeast cells can survive in the absence of Y4. This observation seems to be at odds with all proposals made thus far for the function of Y4.
Modified models for the function of Y4 can be proposed, influenced by recent studies of metallo-cofactor biosynthesis in a number of systems. Studies on iron–sulfur cluster assembly (40), nickel cofactor assembly in urease (41), copper assembly in copper zinc SOD (32, 33), and diferric-Y⋅ assembly in R2 (3, 22) have shown that reconstitutions of active cofactors from apo proteins can occur in the absence of additional accessory protein factors despite the requirement for proteins in vivo. In the first three cases, biosynthetic pathways for cofactor assembly have been identified and partially characterized. In the case of R2, protein factors are likely, but they have not yet been identified. One possibility is that apo heterodimer, Y2Y4, takes up iron from a transporter or small iron chaperone protein in vivo. Ferrous ion binding to Y2 could occur either by ferrous ion binding initially to Y4 followed by its delivery to Y2 or by Y4 altering the local conformation of Y2, thus facilitating direct access of iron into the Y2 active site. This reaction could be made irreversible by rapid addition of O2 once two irons are in place on Y2 and by the addition of one reducing equivalent from a yet unknown factor.
The model in which Y4 delivers ferrous ion is less appealing given that Y4Δ, with the truncated C-terminal tail (Y4 contains three Asp and one Glu in the last 15 residues, whereas Y2 contains two Glu, one Asp, and one Gln in a different sequence context), is active in cluster assembly in vitro. The kinetics of assembly, however, have not been studied, and it is possible that they are altered in comparison with the kinetics of cluster assembly with intact Y4. The importance of the C-terminal tail of all R2s in interacting with R1 suggests that this truncation may preclude testing our model in vivo (42, 43).
Y2Y4 heterodimer is clearly active and stable in vitro. An unresolved issue is whether this heterodimer can reorganize to form Y2 and Y4 homodimers. To provide insight into the differences between the homodimer and heterodimer interfaces, crystallization efforts have been undertaken for Y2Y4, Y2, and Y4. The structure of the heterodimer is presented in the accompanying article (16). This structure in comparison with the structures of E. coli R2 (44, 45) and mouse M2 (46) and structure-based sequence alignments with Y2 and Y4 have suggested an experimentally testable hypothesis about how the heterodimer could convert to the homodimers (16).
The most compelling argument at present for Y2Y4 being the active R2 is the detection of 0.6 to 0.8 Y⋅ per heterodimer. In no other R2 characterized so far have two Y⋅ been observed per homodimer. This putative half-site reactivity interestingly has been observed in many radical-using proteins including pyruvate formate lyase, galactose oxidase, and the oxygen-evolving complex in photosynthesis (3, 47). The observation of one radical per homodimeric molecule in these systems suggests that it is likely not an artifact of isolation or in vitro reconstitution. The half-site reactivity has very important implications in the actual form of active RNRs. This half-site reactivity would be preserved if Y2Y4 is the active species in vivo. Finally, the observation of one Y⋅ in the absence of a second iron-containing subunit suggests that the extra reducing equivalent required for cofactor assembly in yeast R2 cannot come from the active site of the second protomer, as previously proposed for E. coli R2 (23). Establishing whether the heterodimer is the active species in yeast during DNA replication and repair is the focus of our current efforts.
Abbreviations
- RNR
ribonucleotide reductase
- SeMet
selenomethionine
- Y⋅
tyrosyl radical
- SEC
size exclusion chromatography
- CV
column volume(s)
- eq
equivalents
- SOD
superoxide dismutase
References
- 1.Jordan A, Reichard P. Annu Rev Biochem. 1998;67:71–98. doi: 10.1146/annurev.biochem.67.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Licht S, Stubbe J. In: Comprehensive Natural Products Chemistry. Barton S D, Nakanishi K, Meth-Cohn O, Poulter C D, editors. Oxford: Elsevier Science; 1999. pp. 163–204. [Google Scholar]
- 3.Stubbe J, van der Donk W A. Chem Rev. 1998;98:705–762. doi: 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]
- 4.Yagle K, McEntee K. Mol Cell Biol. 1990;10:5553–5557. doi: 10.1128/mcb.10.10.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elledge S J, Davis R W. Genes Dev. 1990;4:740–751. doi: 10.1101/gad.4.5.740. [DOI] [PubMed] [Google Scholar]
- 6.Hurd H K, Roberts C W, Roberts J W. Mol Cell Biol. 1987;7:3673–3677. doi: 10.1128/mcb.7.10.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elledge S J, Davis R W. Mol Cell Biol. 1987;7:2783–2793. doi: 10.1128/mcb.7.8.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang M, Elledge S J. Mol Cell Biol. 1997;17:6105–6113. doi: 10.1128/mcb.17.10.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P J, Chabes A, Casagrande R, Tian X C, Thelander L, Huffaker T C. Mol Cell Biol. 1997;17:6114. doi: 10.1128/mcb.17.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Elledge S J. Genetics. 1992;131:851–866. doi: 10.1093/genetics/131.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns N, Grimwade B, Ross-Macdonald P B, Choi E Y, Finberg K, Roeder G S, Snyder M. Genes Dev. 1994;8:1087–1105. doi: 10.1101/gad.8.9.1087. [DOI] [PubMed] [Google Scholar]
- 12.Winzeler E A, Shoemaker D D, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke J D, Bussey H, et al. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen H H, Ge J, Perlstein D L, Stubbe J. Proc Natl Acad Sci USA. 1999;96:12339–12344. doi: 10.1073/pnas.96.22.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chabes A, Domkin V, Larsson G, Liu A, Graslund A, Wijmenga S, Thelander L. Proc Natl Acad Sci USA. 2000;97:2474–2479. doi: 10.1073/pnas.97.6.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenzweig A C. Acc Chem Res. 2001;34:119–128. doi: 10.1021/ar000012p. [DOI] [PubMed] [Google Scholar]
- 16.Voegtli W C, Ge J, Perlstein D L, Stubbe J, Rosenzweig A C. Proc Natl Acad Sci USA. 2001;98:10073–10078. doi: 10.1073/pnas.181336398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stookey L L. Anal Chem. 1970;42:779–782. [Google Scholar]
- 18.Parkin S E, Chen S, Ley B A, Mangravite L, Edmondson D E, Huynh B H, Bollinger J M., Jr Biochemistry. 1998;37:1124–1130. doi: 10.1021/bi9723717. [DOI] [PubMed] [Google Scholar]
- 19.Steeper J R, Steuart C D. Anal Biochem. 1970;34:123–130. doi: 10.1016/0003-2697(70)90092-8. [DOI] [PubMed] [Google Scholar]
- 20.Bar G, Bennati M, Griffin R G, Nguyen H, Stubbe J. J Am Chem Soc. 2001;123:3569–3576. doi: 10.1021/ja003108n. [DOI] [PubMed] [Google Scholar]
- 21.Mann G J, Graslund A, Ochiai E I, Ingemarson R, Thelander L. Biochemistry. 1991;30:1939–1947. doi: 10.1021/bi00221a030. [DOI] [PubMed] [Google Scholar]
- 22.Bollinger J M, Jr, Edmondson D E, Huynh B H, Filley J, Norton J R, Stubbe J. Science. 1991;253:292–298. doi: 10.1126/science.1650033. [DOI] [PubMed] [Google Scholar]
- 23.Elgren T E, Lynch J B, Juarez-Garcia C, Munck E, Sjöberg B M, Que L., Jr J Biol Chem. 1991;266:19265–19268. [PubMed] [Google Scholar]
- 24.Schmidt P J, Rae T D, Pufahl R A, Hamma T, Strain J, O'Halloran T V, Culotta V C. J Biol Chem. 1999;274:23719–23725. doi: 10.1074/jbc.274.34.23719. [DOI] [PubMed] [Google Scholar]
- 25.Futcher B, Latter G I, Monardo P, McLaughlin C S, Garrels J I. Mol Cell Biol. 1999;19:7357–7368. doi: 10.1128/mcb.19.11.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka H, Arakawa H, Yamaguchi T, Shiraishi K, Fukuda S, Matsui K, Takei Y, Nakamura Y. Nature (London) 2000;404:42–49. doi: 10.1038/35003506. [DOI] [PubMed] [Google Scholar]
- 27.Heinemann V. Oncology. 2001;60:8–18. doi: 10.1159/000055290. [DOI] [PubMed] [Google Scholar]
- 28.Chan A K, Persad S, Litchfield D W, Wright J A. Biochim Biophys Acta. 1999;1448:363–371. doi: 10.1016/s0167-4889(98)00115-3. [DOI] [PubMed] [Google Scholar]
- 29.Elledge S J, Zhou Z, Allen J B, Navas T A. BioEssays. 1993;15:333–339. doi: 10.1002/bies.950150507. [DOI] [PubMed] [Google Scholar]
- 30.Abid M R, Anthony C, DeBenedetti A. J Biol Chem. 1999;274:35991–35998. doi: 10.1074/jbc.274.50.35991. [DOI] [PubMed] [Google Scholar]
- 31.Chabes A, Thelander L. J Biol Chem. 2000;275:17747–17753. doi: 10.1074/jbc.M000799200. [DOI] [PubMed] [Google Scholar]
- 32.Rae T, Schmidt P, Pufahl R, Culotta V, O'Halloran T. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 33.Lamb A L, Torres A S, O'Halloran T V, Rosenzweig A C. Biochemistry. 2000;39:14720–14727. doi: 10.1021/bi002207a. [DOI] [PubMed] [Google Scholar]
- 34.Hall L T, Sanchez R J, Holloway S P, Zhu H, Stine J E, Lyons T J, Demeler B, Schirf V, Hansen J C, Nerissian A M, et al. Biochemistry. 2000;39:3611–3623. doi: 10.1021/bi992716g. [DOI] [PubMed] [Google Scholar]
- 35.Stubbe J, Riggs-Gelasco P. Trends Biochem Sci. 1998;23:438–443. doi: 10.1016/s0968-0004(98)01296-1. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan C, Posewitz M C, George G N, Winge D R. Biochemistry. 1998;37:7572–7577. doi: 10.1021/bi980418y. [DOI] [PubMed] [Google Scholar]
- 37.Pufahl R A, Singer C P, Peariso K L, Lin S J, Schmidt P J, Fahrni C J, Culotta V C, Penner-Hahn J E, O'Halloran T V. Science. 1997;278:853–856. doi: 10.1126/science.278.5339.853. [DOI] [PubMed] [Google Scholar]
- 38.Lamb A L, Wernimont A K, Pufahl R A, Culotta V C, O'Halloran T V, Rosenzweig A C. Nat Struct Biol. 1999;6:724–729. doi: 10.1038/11489. [DOI] [PubMed] [Google Scholar]
- 39.Kumar A, Cheung K-H, Ross-Macdonald P, Coelho P S R, Miller P, Snyder M. Nucleic Acids Res. 2000;28:81–84. doi: 10.1093/nar/28.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lill R, Kispal G. Trends Biochem Sci. 2000;25:352–356. doi: 10.1016/s0968-0004(00)01589-9. [DOI] [PubMed] [Google Scholar]
- 41.Soriano A, Colpas G J, Hausinger R P. Biochemistry. 2000;39:12435–12440. doi: 10.1021/bi001296o. [DOI] [PubMed] [Google Scholar]
- 42.Climent I, Sjöberg B M, Huang C Y. Biochemistry. 1992;31:4801–4807. doi: 10.1021/bi00135a009. [DOI] [PubMed] [Google Scholar]
- 43.Fisher A, Yang F D, Rubin H, Cooperman B S. J Med Chem. 1993;36:3859–3862. doi: 10.1021/jm00076a015. [DOI] [PubMed] [Google Scholar]
- 44.Nordlund P, Eklund H. J Mol Biol. 1993;232:123–164. doi: 10.1006/jmbi.1993.1374. [DOI] [PubMed] [Google Scholar]
- 45.Nordlund P, Sjöberg B-M, Eklund H. Nature (London) 1990;345:593–598. doi: 10.1038/345593a0. [DOI] [PubMed] [Google Scholar]
- 46.Kauppi B, Nielsen B B, Ramaswamy S, Larsen I K, Thelander M, Thelander L, Eklund H. J Mol Biol. 1996;262:706–720. doi: 10.1006/jmbi.1996.0546. [DOI] [PubMed] [Google Scholar]
- 47.Sjöberg B M, Karlsson M, Jornvall H. J Biol Chem. 1987;262:9736–9743. [PubMed] [Google Scholar]