Abstract
Background and aims
Biopsy of the ampulla of Vater may be performed to evaluate for ampullary adenomas, suspected ampullary tumors and immunohistological staining for autoimmune pancreatitis. Ampullary biopsies are commonly performed at the time of endoscopic retrograde cholangiopancreatography (ERCP). Due to the well-established complication rate following ERCP, the contribution of ampullary biopsy as a potential independent risk factor would require a controlled comparison.
Methods
A matched-pairs, case-control analysis was performed for patients undergoing ERCP with or without ampullary biopsy. The analysis involved a retrospective review of adult patients at a tertiary-care center who underwent ampullary biopsies during ERCP compared (via procedural complexity) with a matched control group who underwent ERCP without ampullary biopsies.
Results
Of 159 procedures involving ampullary biopsy, 54 ERCPs that met the inclusion criteria were performed with ampullary biopsy and included in the analysis cohort. This cohort was compared with 54 patients undergoing ERCP without ampullary biopsy, matched by American Society for Gastrointestinal Endoscopy (ASGE) grade of procedural complexity. There were no patients with sphincter of Oddi dysfunction. Ampullary biopsies suggested a diagnosis in 75.9% of the procedures including 12 adenomas, 5 adenocarcinomas and 1 intraductal papillary mucinous neoplasm. Including major and minor complications, the overall complication rate with biopsy (9.3%) was equivalent to the complication rate in the control group without ampullary biopsy (9.3%, P>0.99). The incidence of post-procedure pancreatitis was not significantly different between the two groups (5.6% vs 3.7%, P=0.6). Age and pancreatic duct manipulation, but not ampullary biopsy, were associated with complications on multivariate analysis in the study population.
Conclusions
Ampullary biopsy performed during ERCP had a high diagnostic yield and was not associated with an increased rate of post-procedure complications or pancreatitis when compared with ERCP alone.
Keywords: endoscopic retrograde cholangiopancreatography, ampullary biopsy, complications
Introduction
Endoscopic examination of the ampulla of Vater may be performed during upper gastrointestinal endoscopic procedures, particularly those utilizing side-viewing duodenoscopy [1]. Ampullary abnormalities may be encountered during endoscopy to screen for duodenal adenomas in familial adenomatous polyposis or as part of endoscopy for other indications. More recently, ampullary biopsies may be performed to stain for IgG4 cells in patients undergoing evaluation for autoimmune pancreatitis and to differentiate IgG4 sclerosing cholangitis from primary sclerosing cholangitis (PSC) [2–5].
Ampullary biopsies may be performed at the time of endoscopic retrograde cholangiopancreatography (ERCP) wherein a side-viewing endoscope provides an improved view of the papilla. The complications following ERCP include pancreatitis, bleeding, infection and perforation, and have been evaluated in multiple series [6,7]. Complications following biopsy of the ampulla have not been systematically evaluated, with only small retrospective series and anecdotal experiences being reported in the literature [1,4,8–11]. Given the well-established complication rate following ERCP, a controlled comparison is necessary to understand the potential contribution of ampullary biopsy as an independent predictor of complications. The aim of this study was to evaluate the complication rate and diagnostic yield of ampullary biopsies performed at the time of ERCP compared with procedural complexity and physical condition in matched pair groups of patients undergoing ERCP without ampullary biopsies.
Methods
The institutional review board approved this study. Written informed consent was obtained from all patients in the study for all endoscopic procedures. This study was a case-controlled, retrospective analysis of adult patients undergoing ampullary biopsies during ERCP compared with a control group undergoing ERCP without ampullary biopsies at a tertiary-care center (matched on American Society for Gastrointestinal Endoscopy (ASGE) grade of procedural complexity). The ERCP procedures, during which biopsy of the ampulla of Vater was performed, were identified by querying the endoscopy database for all procedures performed between 1 December 2008 and 30 May 2013 at a major tertiary-care hospital and ambulatory surgery center. The search terms papillary biopsy(ies), papilla biopsy(ies), ampulla biopsy(ies), ampullary biopsy(ies) and periampullary biopsy(ies) were used.
The procedure notes, pathology results, clinical documentation, outpatient visits to the hospital and clinic, telephone encounters and discharge summaries were reviewed to determine indications for the procedure, the procedure’s complexity by ASGE grade, post-procedural complications and final pathologic diagnosis for ampullary biopsy. The exclusion criteria included patients undergoing endoscopic ampullectomy and ampullary biopsies obtained during non-ERCP endoscopic procedures. The control group of ERCP procedures without ampullary biopsies was obtained through the same database. The closest ERCP in time that was equivalent in ASGE grade procedural complexity and performed without biopsy was sought for the control. The ASGE grade for each procedure was determined based on the procedure note and clinical documentation. All procedures were performed with adult therapeutic duodenoscopes (Olympus TJF Q180V, Q160), and biopsies were obtained using standard endoscopic forceps (2.4 mm OD, Boston Scientific, Natick, MA).
Pancreatitis was defined as new onset abdominal pain lasting more than 24 hours, pain requiring admission or pain prolonging the duration of hospitalization with a serum lipase >3 times the upper limit of normal [6,7]. Post-ERCP bleeding was defined by overt gastrointestinal bleeding requiring hospitalization, drop in the patient’s hemoglobin or need for an intervention for hemostasis based on review of progress notes, procedure notes and discharge summaries. Infections following ERCP were defined as fever requiring antibiotics without an alternative source and were based on review of progress notes, patient communication or clinic visits.
The data were collected and stored in a secure database protected by the institutional firewall. The Chi-square test was used for comparison between the groups. The Fisher exact test was used for categorical variables, and the independent samples t-test was used for comparison of means. Multivariate logistic regression analysis was performed to determine predictors of complications. A P value <0.05 on a 2-tailed test was considered statistically significant. Data were analyzed using IBM SPSS version 22.0 statistical software (IBM Corporation, Armonk, NY).
Results
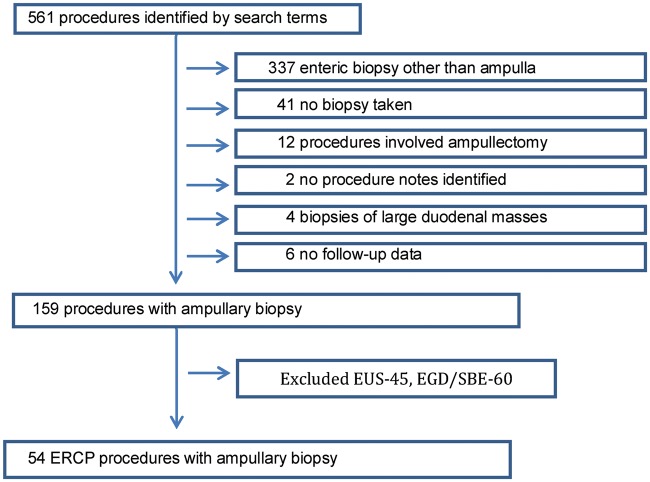
On the initial queries of the database, 561 procedures were identified. As shown in Figure 1, 159 of those cases involved ampullary biopsy. Of the 159 queries, 54 were ERCP, 45 were endoscopic ultrasonography (EUS), and 60 were esophagogastroduodenoscopy (EGD) / single balloon endoscopy (SBE). Cases not involving ERCP (EUS, EGD and SBE) were excluded. In total, 54 ERCPs were performed with ampullary biopsy that met the inclusion criteria and were included in the analysis cohort. A matched ERCP group was identified based on ASGE procedural complexity from patients who did not have ampullary biopsy.
Figure 1.
Flow diagram of 561 procedures identified.
Baseline characteristics of the two groups are reported in Table 1. As expected, the two groups were similar in clinical condition, procedural complexity and predictors of complications after ERCP. Both the ampullary biopsy group and the control group comprised ASGE grade 1 (31, 57%), grade 2 (19, 19%) and grade 3 (4, 7%) procedures. The group with biopsy had more procedures performed for ampullary or papillary tumors compared with the ERCP group without biopsy. The indications for ERCP in both groups can be seen in Table 2.
Table 1.
Baseline demographics and procedural risk factors for patients undergoing ERCP with and without ampullary biopsy
| ERCP with biopsy (N = 54) | ERCP without biopsy (N = 54) | P value | |
|---|---|---|---|
| Female, n (%) | 22 (40.7) | 29 (53.7) | 0.24 |
| Mean age, years | 62.9 | 56.7 | 0.07 |
| Common bile duct size, mm | 8.3 ± 2.7 | 7.5 ± 2.4 | 0.12 |
| Trainee involvement, n (%) | 43 (79.6) | 39 (72.2) | 0.5 |
| Total bilirubin level, μmol/L | 2.7 ± 3.2 | 3.3 ± 2.6 | 0.28 |
| Normal bilirubin, n (%) | 18 (33.3) | 13 (24.1) | 0.4 |
| Pancreatic duct injection, n (%) | 6 (11.1) | 4 (7.4) | 0.74 |
| Pancreatic duct stent, n (%) | 3 (5.6) | 3 (5.6) | 1.0 |
| Major papilla sphincterotomy, n (%) | 34 (63.0) | 40 (74.1) | 0.9 |
| ASA class, n (%) | 0.9 | ||
| II | 7 (13.0) | 12 (22.2) | |
| III | 46 (85.2) | 40 (74.1) | |
| IV | 1 (1.9) | 2 (3.7) | |
| ASGE grade, n (%) | 0.99 | ||
| 1 | 31 (57.4) | 31 (57.4) | |
| 2 | 19 (35.2) | 19 (35.2) | |
| 3 | 4 (7.4) | 4 (7.4) | |
| Periprocedural antibiotic use, n (%) | 8 (14.8) | 11 (20.4) | 0.6 |
| Periprocedural antiplatelet use, n (%) | 4 (7.4) | 7 (13.0) | 0.5 |
ASA: American Society of Anesthesiologists; ASGE: American Society for Gastrointestinal Endoscopy; ERCP: endoscopic retrograde cholangiopancreatography.
Table 2.
Indications for ERCP with ampullary and without ampullary biopsy
| Indication | Number of cases (%) |
|---|---|
| With ampullary biopsy (N = 54) | |
|
52 (96.2) |
|
16 (29.6) |
|
12 (22.2) |
|
7 (12.9) |
| Without ampullary biopsy (N = 54) | |
|
44 (81.5) |
|
21 (38.9) |
|
14 (25.9) |
Ampullary biopsies during ERCP suggested a diagnosis in 75.9% of the procedures including 12 adenomas, 5 adenocarcinomas and 1 intraductal papillary mucinous neoplasm (Table 3). The overall rate of complications was similar between the two groups (9.3% in each group) (Table 4). There was no significant difference in the complication rates on subgroup analysis. The rate of post-procedure pancreatitis (PEP) was 5.6% in the group with ampullary biopsy and 3.7% in the control (ERCP) group (P=0.6). Bleeding occurred in one patient (1.9%) in the ERCP group with biopsy and in two patients (3.7%) in the control group (P=0.9). Infectious complications were encountered in one patient (1.9%) in both the biopsy group and the control group. No perforations were seen in either group. Age and pancreatic duct manipulation—but not ampullary biopsy—were significantly associated with complications on multivariate analysis (Table 5).
Table 3.
Histopathological results from endoscopic ampullary biopsies performed at the time of ERCP (N = 54)
| Histopathologic biopsy results | Number of cases (%) |
|---|---|
| Enteric/ampullary mucosa | 13 (24.1) |
| Inflammation | 18 (33.3) |
| Amyloid | 1 (1.9) |
| Adenoma | 12 (22.2) |
| Tubulovillous | 2 (3.7) |
| Low grade | 1 (1.9) |
| High grade | 3 (5.6) |
| Not otherwise specified | 6 (11.1) |
| Adenocarcinoma | 5 (9.3) |
| Intraductal papillary mucinous neoplasm | 1 (1.9) |
| Other | 4 (7.4) |
Table 4.
Complication rates for patients undergoing ERCP with and without ampullary biopsy
| Complication | ERCP with biopsy (N = 54) | ERCP without biopsy (N = 54) | P value |
|---|---|---|---|
| Post-ERCP pancreatitis, n (%) | 3 (5.6) | 2 (3.7) | 0.6 |
| Bleeding, n (%) | 1 (1.9) | 2 (3.7) | 0.9 |
| Infection, n (%) | 1 (1.9) | 1 (1.9) | 0.99 |
| Total | 5 (9.3) | 5 (9.3) | 0.99 |
Table 5.
Multivariate logistic regression analysis of the complication rate by individual risk factors previously associated with post -RCP pancreatitis
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age | 0.96 | 0.92–1.00 | 0.034 |
| Body mass index | 0.92 | 0.81–1.06 | 0.259 |
| Female sex | 1.29 | 0.36–4.65 | 0.695 |
| Ampulla biopsy | 0.39 | 0.05–3.20 | 0.381 |
| Major papilla sphincterotomy | 2.47 | 0.59–10.30 | 0.213 |
| Pancreatic duct manipulation | 4.30 | 1.12–16.6 | 0.034 |
| Pancreatic stent placement | 5.41 | 0.51–57.34 | 0.161 |
| Total procedure time (in minutes) | 3.65 | 0.96–13.96 | 0.061 |
| Common bile duct size | 2.88 | 0.76–10.90 | 0.119 |
| Trainee involvement | 2.25 | 0.25–20.36 | 0.469 |
Discussion
Ampullary abnormalities on autopsy studies have a reported prevalence of approximately 0.04% to 0.12% cases per year [12]. Features that suggest a benign etiology include regular margins, no ulceration, soft consistency and no spontaneous bleeding [8]. Endoscopy has a prominent role in the management of ampullary lesions for tissue diagnosis, staging and treatment. Ampullary biopsies are frequently performed during ERCP; however, it is not known if ampullary biopsies performed during ERCP increase the complication rate. Given the increased complication rate with ERCP relative to non-therapeutic endoscopic procedures, understanding the contribution of ampullary biopsy to the procedural risk requires a matched controlled comparison. This study involved a matched pairs, case-controlled retrospective comparison of ERCP with and without ampullary biopsy. The data suggest that ampullary biopsy does not increase the risk of complications in patients undergoing ERCP and that ampullary biopsies performed during ERCP have a good diagnostic yield of 75.9%.
In our study, the overall rate of complications following ERCP with ampullary biopsy was 9.3% and did not differ significantly from the rate of 9.3% in the control group. The complication rate in both groups was consistent with the established complication rate with ERCP [6,7,13–15]. Another uncontrolled, smaller study [4], as well as case reports [9–11] on ampullary biopsy complications after ERCP, was in keeping with these findings. Our data agreed with the largest uncontrolled series reporting no complications with ampullary biopsies in a retrospective review of 62 patients undergoing ERCP for pancreatobiliary cancer or IgG-4 sclerosing cholangitis; however, post-procedure follow-up was not evaluated in that study [9]. No study has evaluated ampullary biopsy during ERCP as an independent risk factor in the context of post-ERCP complications.
Several risk factors have been identified for PEP including inadequate training, young age, female sex, normal bilirubin, prior PEP, sphincter of Oddi dysfunction and manometry, sphincterotomy, difficult cannulation, pancreatic duct injection and ampullectomy [6]. In most series, ERCP carries a 2–10% risk of PEP [16]. Similarly, there is concern that injury to the ampulla from forceps biopsy can lead to ampullary edema and thus increase the risk of PEP. In our study, there was a 5.6% risk of PEP in patients undergoing ERCP with ampullary biopsies, which was not significantly different from the 3.7% risk of PEP in our control group of patients undergoing ERCP without ampullary biopsies (P=0.6). The rates were well within the previously established PEP rate from other large studies [6,13,15]. The rate for other complications was also similar between groups, with no increased risk following ampullary biopsies. In this ampullary biopsy and matched ERCP pair population, age and pancreatic duct manipulation were predictors of complications on multivariate analysis. Ampullary biopsy was not independently associated with complications. Overall, these data suggest that ampullary biopsy during ERCP is safe and does not appear to increase the risk of PEP relative to ERCP without ampullary biopsy.
ASGE guidelines recommend endoscopic biopsy of ampullary abnormalities for tissue diagnosis prior to attempted resection to confirm the diagnosis and exclude a focus of cancer [3]. In this study, ampullary biopsy had a diagnostic yield of 75.9%, suggesting that diagnosis can be obtained in the majority of patients without a significant increase in the complication rate.
There are several limitations to this study. The study was conducted from a single center and was retrospective in nature. The use of ASGE procedural complexity grade as one of the matching variables is another limitation of our study. However both groups were similar in clinical condition and predictors of complications after ERCP. Although complications were extensively sought and the rates agreed with prior reports, some complications may have been missed or not captured in the electronic record. It is assumed that failed detection would be approximately equally distributed amongst the groups with and without biopsy. Rectal indomethacin was not administered during the trial period at the study institution. To the best of our knowledge, this is the first study evaluating ampullary biopsy as an independent predictor of complications occurring in the post-ERCP setting.
In summary, ampullary biopsy performed during ERCP appears to have a good diagnostic yield and does not appear to increase the risk of post-procedure complications or post-procedure pancreatitis relative to ASGE grade complexity-matched ERCP performed without ampullary biopsy. This matched controlled study supports the ASGE recommendation of ampullary biopsy for tissue diagnosis prior to endoscopic resection.
Presentation: The data contained in this manuscript were presented in part at Digestive Disease Week (DDW 2014) in Chicago, IL.
Funding
This manuscript was supported in part by a development grant from the Department of Medicine at Emory University School of Medicine.
Conflict of interest statement: none declared.
References
- 1. Hajj EI II, Coté GA.. Endoscopic diagnosis and management of ampullary lesions. Gastrointest Endosc Clin N Am 2013;23:95–109. [DOI] [PubMed] [Google Scholar]
- 2. Kubota K, Kato S, Akiyama T, et al. Differentiating sclerosing cholangitis caused by autoimmune pancreatitis and primary sclerosing cholangitis according to endoscopic duodenal papillary features. Gastrointest Endosc 2008;68:1204–8. [DOI] [PubMed] [Google Scholar]
- 3. Standards of Practice Committee; Adler DG, Qureshi W, Davila R, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc 2006;64:849–54. [DOI] [PubMed] [Google Scholar]
- 4. Kawakami H, Zen Y, Kuwatani M, et al. IgG4-related sclerosing cholangitis and autoimmune pancreatitis: histological assessment of biopsies from Vater's ampulla and the bile duct. J Gastroenterol Hepatol 2010;25:1648–55. [DOI] [PubMed] [Google Scholar]
- 5. Chini P, Draganov PV.. Diagnosis and management of ampullary adenoma: The expanding role of endoscopy. World J Gastrointest Endosc 2011;3:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freeman ML. Complications of endoscopic retrograde cholangiopancreatography: avoidance and management. Gastrointest Endosc Clin N Am 2012;22:567–86. [DOI] [PubMed] [Google Scholar]
- 7. Woods KE, Willingham FF.. Endoscopic retrograde cholangiopancreatography associated pancreatitis: A 15-year review. World J Gastrointest Endosc 2010;2:165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han J, Kim MH.. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc 2006;63:292–301. [DOI] [PubMed] [Google Scholar]
- 9. Ishida Y, Okabe Y, Tokuyasu H, et al. A case of acute pancreatitis following endoscopic biopsy of the ampulla of vater. Kurume Med J 2013;60:67–70. [DOI] [PubMed] [Google Scholar]
- 10. Morales TG, Hixson LJ.. Acute pancreatitis following endoscopic biopsy of the ampulla in a patient with Gardner's syndrome. Gastrointest Endosc 1994;40:367–9. [DOI] [PubMed] [Google Scholar]
- 11. Gincul R, Ciocirlan M, Dumortier J, et al. Severe acute pancreatitis following endoscopic biopsy of the minor duodenal papilla. Endoscopy 2009;41 Suppl 2:E195–6. [DOI] [PubMed] [Google Scholar]
- 12. Martin JA, Haber GB.. Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am 2003;13:649–69. [DOI] [PubMed] [Google Scholar]
- 13. Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006;101:139–47. [DOI] [PubMed] [Google Scholar]
- 14. Testoni PA, Mariani A, Giussani A, et al. Risk factors for post-ERCP pancreatitis in high- and low-volume centers and among expert and non-expert operators: a prospective multicenter study. Am J Gastroenterol 2010;105:1753–61. [DOI] [PubMed] [Google Scholar]
- 15. Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001;54:425–34. [DOI] [PubMed] [Google Scholar]
- 16. Thaker AM, Mosko JD, Berzin TM.. Post-endoscopic retrograde cholangiopancreatography pancreatitis. Gastroenterol Rep (Oxf) 2015;3:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]