Abstract
Transcription control is the foundation of gene regulation. Whereas a cell is fully equipped for this task, viruses often depend on the host to supply tools for their transcription program. Over the course of evolution and adaptation, viruses have found diverse ways to optimally exploit cellular host processes such as transcription to their own benefit. Just as cells are increasingly understood to employ nascent RNAs in transcription regulation, recent discoveries are revealing how viruses use nascent RNAs to benefit their own gene expression. In this review, we first outline the two different transcription programs used by viruses, i.e., transcription (DNA-dependent) and RNA-dependent RNA synthesis. Subsequently, we use the distinct stages (initiation, elongation, termination) to describe the latest insights into nascent RNA-mediated regulation in the context of each relevant stage.
Keywords: transcription, nascent RNA, HIV-1, influenza, Epstein-Barr virus, RNA polymerase II, RNA-dependent RNA polymerase, respiratory syncytial virus
1. Coevolution of Viral and Host mRNA Synthesis Pathways
All living organisms are fully equipped to synthesize messenger RNA (mRNA) using their endogenously encoded multi-subunit RNA polymerases (RNAPs). However, many viruses form an exception to this dogma and employ a parasitic lifestyle that exploit parts of the host transcription pathways. This co-option allows viruses to dramatically downsize their genomes to the minimum number of genes essential for successful infection. As a result, viruses and their hosts have been involved in an eternal battle of adaptation and counter-adaptation for RNA synthesis since far before the emergence of humans [1,2,3]. During this time, viruses coevolved many pathways to transcribe their own genetic material, meanwhile avoiding counter-adaptations of the host [4]. Weapons in the arsenal of the virus include virally-encoded proteins and micro RNAs (miRNAs), which alter transcription pathways in both host and virus via many different mechanisms. These mechanisms have been reviewed extensively elsewhere (for example [5,6,7,8]). However, one major, yet underappreciated, component of viral gene regulation occurs via nascent RNAs.
RNAs have many attractive properties that can be used by viruses to manipulate gene regulation pathways. First, RNAs, like DNAs, can specifically pair with complementary viral or host sequences using minimal investment of genetic material, thus allowing further minimization of the viral genome. Encoding a DNA-binding protein, on the other hand, consumes significantly more genetic information. A typical DNA-binding domain recognizes ~10 base pairs (bp) or more of DNA and comprises at least ~60 amino acids (aa), thereby requiring 180 bp of encoding DNA [9]. In contrast, RNA requires only the number of complementary nucleotides as the DNA/RNA region it recognizes. This can be beneficial for the virus where space constraints are dictated by the size of the viral capsid. Second, RNAs are less immunogenic compared to proteins. Whereas hosts developed many pathways to respond to foreign invading proteins, RNAs are dealt with by relatively easy to avoid RNAi machinery or certain Toll-like and retinoic acid-inducible gene I (RIG-I)-like receptors [10]. Furthermore, because RNAs can be smaller (see point one) it is easier to avoid recognition by the host’s immune system. Third, RNA can store information in its sequence, its secondary and tertiary structure, its ability to form ligand-binding platforms (e.g., riboswitches or ribozymes), and via chemical modification of bases and sugars. This makes RNA a flexible molecule, adaptive to multiple situations. Fourth, RNAs, like proteins, are modular and can use domains or different surfaces within one domain to interact with other molecules [11]. The modular nature of RNAs and the versatility of each module (see point three) dramatically expands the repertoire of regulatory RNAs.
For decades, the principal focus of gene regulation studies has been on protein regulators, but recent discoveries in virology highlight the ever-increasing appreciation that RNAs, including nascent RNAs, also play a major part in gene regulation by viruses. In this review, we will describe several recent discoveries of intriguing viral pathways that manipulate gene expression of the host for their own benefit via nascent RNA. To place these discoveries in context, we will first describe the current understanding of the distinct stages (initiation, elongation, termination) in the general mechanism of eukaryotic transcription, as used by the host and virus, and present examples of nascent RNA-mediated regulation in the context of each relevant stage.
2. The Viral RNA Synthesis Machinery
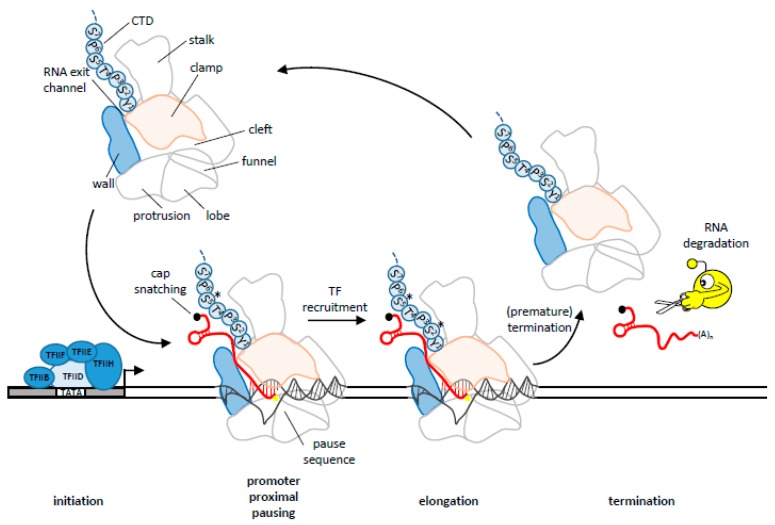
Cellular RNA is synthesized from a DNA template in a process called transcription by RNAP-containing transcription complexes. Eukaryotes employ three different classes of RNAP: RNAPI, II, III, while plants also encode for RNAPIV and V [12]. Of these, primarily RNAPII is used to synthesize cellular mRNA and viral mRNAs. RNAPII is a 550 kDa multi-subunit enzyme complex. These subunits are arranged in a claw-like manner (Figure 1), with a central cleft through which the template DNA is threaded. Nucleoside triphosphates (NTPs) enter the polymerase via the funnel and the newly transcribed RNA exits RNAPII through an RNA-exit channel formed by the mobile clamp and wall elements. Unique to RNAPII is an unstructured carboxy-terminal domain (CTD) that extends from the largest subunit and is composed of 52 heptapeptide repeats (consensus: Tyr1-Ser2-Pro3-Thr4-Ser5-Pro6-Ser7) [13]. The serine, threonine, and tyrosine residues can be reversibly phosphorylated [14]. Changes in the phosphorylation patterns of the CTD during transcription orchestrate the association of different sets of transcription-regulating factors with RNAP.
Figure 1.
Transcription cycle. A cartoon depiction of RNA polymerase II (RNAPII) is shown in the upper left, with several domains and modules labelled. The clamp and wall (flap in prokaryotes) modules that make up the RNA-exit channel are depicted in pink and blue, respectively. The first repeat of the RNAPII C-terminal domain (CTD) is shown as a string of amino acids with their respective numbers. Next, the stages of the transcription cycle are shown, with the viral interfering mechanisms. First, general transcription factors assemble at the promoter and direct RNAPII towards the transcription start site. Transcription factor (TF) IIH phosphorylates the RNAPII at Ser5 (black asterisk). TFIIH opens the DNA template, forming the transcription bubble, permitting RNAPII to begin transcribing RNA in its active center (yellow dot). Upon transcribing the first 20–60 nucleotides, most RNAP will pause. Transcription factor recruitment regulates these transcription kinetics and phosphorylate RNAPII at Ser2. When the correct signal is encountered, RNAPII will terminate transcription and release the RNA. Some viruses encode endonucleases (yellow sphere with scissor) that can cleave RNAs, causing RNA degradation.
In the early 1960s, researchers observed that a subset of viruses were resistant to actinomycin D, a drug that inhibits cellular DNA-directed RNA synthesis as performed by RNAPII [15]. This led to the discovery of RNA viruses. Like their DNA-containing counterparts, these RNA viruses are grouped into double stranded (ds) and single stranded (ss) virus classes. The ssRNA viruses are further subdivided into negative or positive sense single-stranded (−ss or +ss, respectively), depending on viral genomic RNA (vRNA) polarity. All RNA viruses encode an RNA-dependent RNA polymerase (RdRp) to copy the vRNA and synthesize the viral mRNA. RdRp synthesizes RNA complementary to the starting RNA template and is an essential protein of all RNA viruses that have no intermediate DNA-stage [16,17]. This process is often called “transcription”, but differs from the conventional definition of transcription by using RNA rather than DNA as a template for mRNA synthesis. To avoid confusion, we will refer to RNA-dependent mRNA (Rd-mRNA) synthesis in this review. Both transcription and Rd-mRNA synthesis occur in three stages: initiation, elongation and termination (Figure 1). During initiation, the polymerase machinery is recruited to the viral promoter. Initiation of Rd-mRNA synthesis begins at or near the 3′ end of the RNA template in a primer-independent (de novo), or a primer-dependent mechanism. Subsequently, 5′ to 3′ elongation starts but is frequently interrupted by pauses [18]. Finally, the mRNA is terminated and released from the elongation complex. During the elongation phase, a complementary mRNA product is generated by 5′ to 3′ processive nucleotidyl transfer [19,20]. The three stages of mRNA synthesis by both RNAP and RdRp, and their associated regulatory events, are described in detail below.
3. Transcription Initiation
Initiation is promoted by factors that recruit RNAP to the promoter, melt the DNA duplex and load the template DNA strand into the active site of RNAP. For RNAPII, these steps require the combined action of basal transcription factors (TF) (Figure 1) [21,22]. The minimal pre-initiation complex (PIC) includes RNAPII and six general transcription factors: TFIIA, TFIIB, TFIID, TFIIE, TFIIF, and TFIIH. First, TFIID containing the TATA-binding protein (TBP) is recruited to TATA box containing promoter sequences (Figure 1) [23,24,25]. The binding of TFIID to the TATA box in the promoter region of the gene initiates the recruitment of TFIIB, TFIIE, TFIIF and TFIIH, which position the double-stranded (ds) DNA above the cleft within RNAPII [26,27,28]. Local unwinding of the dsDNA by the Ssl2 helicase subunit of TFIIH delivers the template strand into the RNAPII active center and creates a DNA bubble downstream of the TATA box [29]. Efficient DNA opening requires TFIIE and TFIIH, but these factors are not required for low levels of transcription [30]. The TFIIK kinase module of TFIIH will subsequently phosphorylate RNAPII on the Ser5 of its CTD repeats, facilitating promoter clearance and starting the elongation stage [31].
4. Initiation of RNA-Dependent RNA Synthesis
Whereas DNA viruses only need to generate mRNA, RNA viruses without a DNA stage have to synthesize both vRNA and mRNA. The vRNA is generated through a replication intermediate, named antigenome which serves as a template for vRNA synthesis. ssRNA viruses evolved sophisticated approaches to generate both RNA species from one template. Using respiratory syncytial virus (RSV) as a model, it was illustrated that RdRp has two RNA synthesis activities which determine nascent RNA fate. RSV is a −ssRNA virus and the major cause of respiratory tract disease in infants and young children, causing between 66,000 and 199,000 deaths per year worldwide [32]. To differentiate between Rd-mRNA synthesis and vRNA replication, RdRp initiates at one of two different start sites within the promoter sequences at the 3′ end of the RSV RNA genome. Synthesis of the antigenome starts at the expected +1 site. However, the more abundant RNA species in infected cells was found to initiate at the +3 site [33]. RdRp’s that initiate at the +3 site are unable to enter a stable elongation mode and release the RNA after approximately 20–25 nucleotides. Many de novo transcription initiation events by RdRp are known to generate abortive transcripts during the initiation phase of RNA synthesis [34,35,36]. However, the function of this small RNA is yet unknown. It has been proposed that synthesis of this small abortive RNA allows the polymerase to break contacts with the promoter [37]. After releasing the small RNA, the RSV polymerase can now scan the template and locate the conserved gene start signal (~10–13 nt) of the first gene, however the mechanism behind this transition is unknown [38]. Here the polymerase will reinitiate RNA synthesis, cap the 5′ end of the mRNA and commit the RdRp to Rd-mRNA synthesis. The choice between the +1 and +3 initiation site by RSV RdRp seems to be determined by the loading of a specific nucleotide and subsequent creation of a dinucleotide primer complementary to the specific initiation site [37]. Remarkably, even when the priming site is mutated or absent, the enzyme is able to selectively synthesize the original dinucleotide priming site at the 3′ end of the viral RNA, explaining the strict conservation of the 5′- and 3′-end dinucleotides of both +ss and −ssRNA viruses [39]. It has been proposed that a domain, unique in de novo initiating RdRp’s, named “priming loop” (T794 to A799) regulates the accessibility of the active site of RdRp [40]. An essential role during initiation is played by the residue H798 of the priming loop, by providing a platform to which the base of the priming ATP could establish a stacking interaction [39]. These conservational mechanisms, mediated by the polymerase alone have been suggested to be used by several ssRNA viruses [41,42].
5. How to Obtain a Cap?
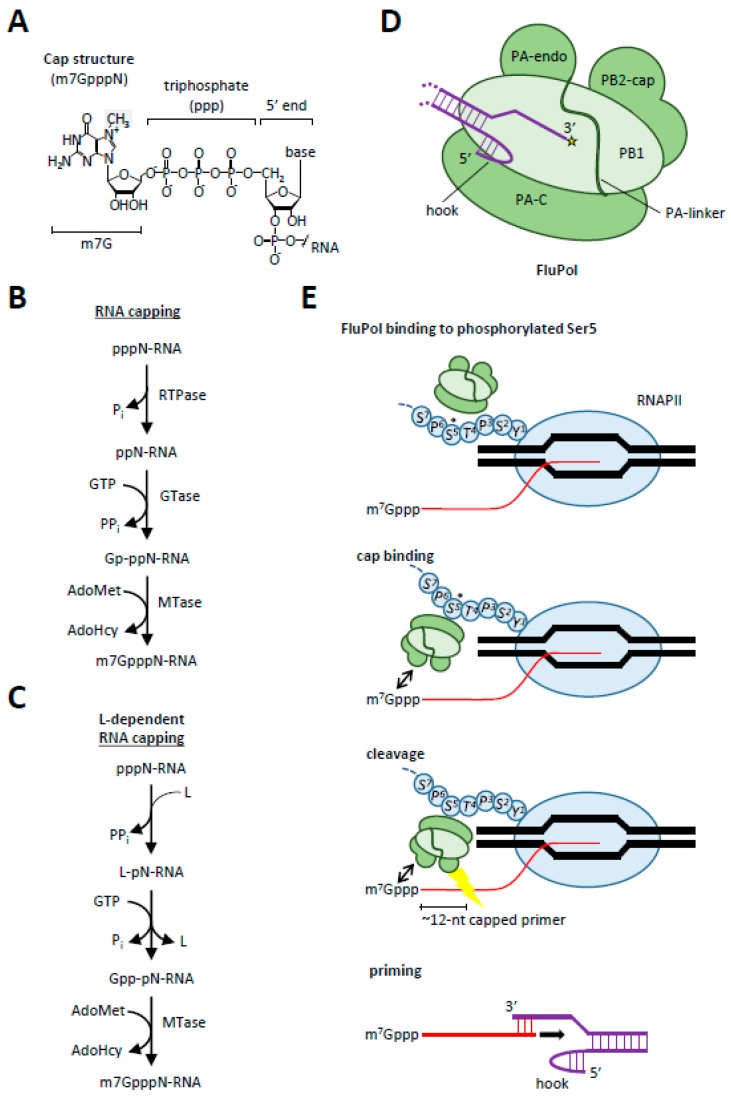
5′-terminal capping of eukaryotic mRNAs is probably the first co-transcriptional RNA processing event. Formation of the 5′-terminal cap structure (m7Gppp[5′]N-; where N is the first nt of the nascent RNA; Figure 2A) in eukaryotic mRNAs, in which 7-methylguanosine (m7G) is linked to the initiator nucleoside of mRNA through the 5′-5′ triphosphate bridge, is mediated by a series of enzymatic steps and is required for the efficient translation and stability of mRNAs. Eukaryotes and some DNA viruses (e.g., vaccinia virus) and dsRNA viruses (e.g., reovirus) obtain this cap by processing the 5′-triphosphate end (pppN-) of nascent RNA transcripts into a diphosphate (ppN-) using an RNA 5′-triphosphatase (RTPase) (Figure 2B). Subsequently, the guanosine monophosphate (GMP) moiety of a guanosine triphosphate (GTP) is transferred to the 5′ diphosphate by a GTP:RNA guanylyltransferase (GTase) to generate the cap core structure (Gp-ppN-). This core structure is then methylated at the guanine-7N position by S-adenosyl-l-methionine (AdoMet)-dependent cap (guanine-N7) methyltransferase (MTase) to produce the final cap structure (m7GpppN-). Higher eukaryotes and some viruses include an additional methylation step in which the 2′ OH position of the penultimate ribose is methylated by cap ribose-2′-O-MTase (m7GpppNm-).
Figure 2.
Capping mechanisms in RNA viruses. (A) The eukaryotic mRNA cap consists of a 7-methylguanosine linked to the initiator nucleoside of mRNA through the 5′-5′ triphosphate bridge. The methyl group at the N7 position of the guanosine is shaded gray; (B) the conventional RNA capping pathway; (C) the L-dependent capping pathway utilized by negative-sense ssRNA viruses; (D) the FluPol complex with the polymerase acidic endonuclease (PA-endo), PA C-terminal (PA-C), polymerase basic 2 cap-binding (PB2-cap) and PB1 domains indicated. The influenza viral RNA (vRNA; purple) is circularized with the extreme 5′ end docked in a pocket formed by PA-C and PB1 and the 3′ end loaded in the FluPol active center (yellow star); (E) the first step in cap-snatching is the recognition and binding of FluPol to the phosphorylated Ser5 of RNAPII through its PA-C domain. This will allow the PB2-cap domain to bind the cap and to direct the nascent RNA towards PA-endo. After cleavage the nascent RNA will be loaded into the active domain of FluPol where it will be used to prime the 3′ end of the vRNA, allowing elongation of the RNA primer from 5′ to 3′ direction (indicated with black arrow).
However, ssRNA viruses (e.g., influenza, Ebola, measles) do not have a DNA template and thus cannot rely on the cellular capping enzymes. For this reason, RNA viruses have evolved capping mechanisms that are different from capping during cellular transcription. Most +ssRNA viruses (e.g., flaviviruses and nidoviruses) encode capping enzymes that selectively cap the newly synthesized viral mRNAs [43,44,45]. For instance, the flavivirus-encoded nonstructural protein 3 (NS3) contains a triphosphatase that releases the terminal phosphate from the 5′-triphosphate end of nascent + stranded RNA, forming a diphosphorylated ppN-RNA [46]. Subsequently, a GMP moiety from GTP is transferred to the 5′ end of the ppN-RNA through the GTase activity of the viral NS5 MTase. The GpppA-capped RNA is further methylated by the NS5 MTase to form the final m(7)GpppA- and m(7)GpppNm-RNA products [47,48]. However, both the process of discrimination between −RNA and +RNA 5′-ends for capping and the precise timing of the capping process remain largely undefined [49].
On the other hand, unsegmented −ssRNA viruses (including rabies, measles, and Ebola) carry an RdRp termed “L” and a phosphoprotein “P”, which share extensive amino acid sequence similarity among all these viruses [50]. P is an essential co-factor that targets the L polymerase to the vRNA [51,52,53]. Here, the L polymerase covalently links with the 5′-monophosphorylated viral mRNA-start sequence to form an intermediate complex (Figure 2C) [54]. Subsequently, the RNA:GDP polyribonucleotidyltransferase activity of the L polymerase transfers the GDP moiety of GTP to the monophosphorylated viral mRNA forming the viral mRNA cap. Thus, in contrast to GTases which transfer a monophosphate to the 5′ diphosphate end of RNA, the L polymerase-mediated capping reaction transfers a diphosphate to the 5′ monophosphate end of RNA.
Another solution to the capping problem of the negative-sense RNA viruses comes from the influenza virus in the form of “cap-snatching” from nascent host pre-mRNAs [55]. To synthesize viral mRNA and vRNA from each of the eight single-stranded viral RNA segments, influenza uses a virally encoded heterotrimeric RdRp called FluPol (Figure 2D). FluPol consists of three virally encoded subunits: polymerase basic 1 (PB1), PB2, and polymerase acidic (PA) arranged in a globular structure [56,57,58]. Each genome segment is circularized by the base-pairing of the near complementary 5′ and 3′ ends, and individually packaged into viral ribonucleoprotein particles (vRNPs) together with one copy of FluPol. FluPol is bound to the duplex at the point of circularization, tightly docked into the 5′ end of the vRNA by a pocket formed between the PA and PB1 subunits, creating an intramolecular stem-loop (‘hook’; Figure 2A) [56,57]. The 3′ segment of the negative-sense vRNA enters the active-site cavity of FluPol, where it serves as a template (Figure 2E) [59,60].
After entry into the cell, the vRNPs are released and transported into the nucleus. After arrival in the nucleus, the C-terminal domain of the FluPol PA subunit will specifically target and bind the phosphorylated Ser5 of actively transcribing RNAPII CTD [61] (Figure 2E). Subsequently, the PB2 cap-binding domain associates with the 5′ end of the cellular mRNA [60]. The PA endonuclease domain next cleaves the mRNA. This creates a capped 11–12 nt small RNA primer that is loaded into the active site of FluPol [62,63,64] (Figure 2E). Processive Rd-mRNA synthesis is initiated by the addition of an NTP to the 3′ end of the capped primer, complementary to the second or third residue in the vRNA template. During elongation, the 5′-capped viral mRNA is separated from the vRNA template by threading into their respective exit channels. After elongation, the viral mRNA is polyadenylated at an oligo-U stretch near the vRNA 5′ end [65]. The capped viral mRNA can now be used to translate all the necessary viral proteins, whereas the vRNA can be packaged, re-used as template, or degraded. Small host non-coding RNAs (snRNAs), especially U1 and U2 snRNA, are the preferred targets for cap-snatching, rather than mRNAs or pre-mRNAs [62,63,64]. Why the virus prefers to use these snRNAs as targets has yet to be experimentally established, but it has been proposed that the selective de-capping of U1 and U2 RNAs in combination with the binding of the viral NS1 protein to U6 snRNA may serve to inhibit host pre-mRNA splicing [66].
6. Transcription Elongation
Elongation is the repeated addition of a nucleoside monophosphate (NMP) to the 3′ end of the growing RNA chain. Progressive RNA synthesis during the elongation stage can be affected by several hurdles, including transcriptional pause sites, that RNAPII must overcome [67]. These transcriptional pauses can be used to create a time window for co-transcriptional events such as splicing, nucleotide modification, or RNA processing. Pauses also yield opportunities to regulate transcription in response to intra- and extra-cellular cues [68]. At most eukaryotic promoters, major transcriptional pauses typically occur within 20–60 nt after transcription initiation [69]. These promoter-proximal pauses are induced by a myriad of factors, including negative elongation factor (NELF) and nascent RNA structures. These pauses give opportunities for the organism to regulate transcription at an early time point.
7. Promoter-Proximal Paused RNA Transcripts as a Scaffold for Transcription Regulators
A promoter-proximal pause will often allow the nascent RNA to fold a structure that can serve as a protein-recruiting scaffold [70]. Additional transcription factors (TFs) can be recruited during this step. Three distinct modes of action can be envisaged whereby TF-interacting RNAs might affect transcription. They could act as (i) scaffolds to recruit one or more TFs [71,72,73]; (ii) competitors, regulating the binding or release of a TF [74]; or (iii) transacting guides for interacting TFs by base-pairing with DNA or RNA in the vicinity of their target sites [75].
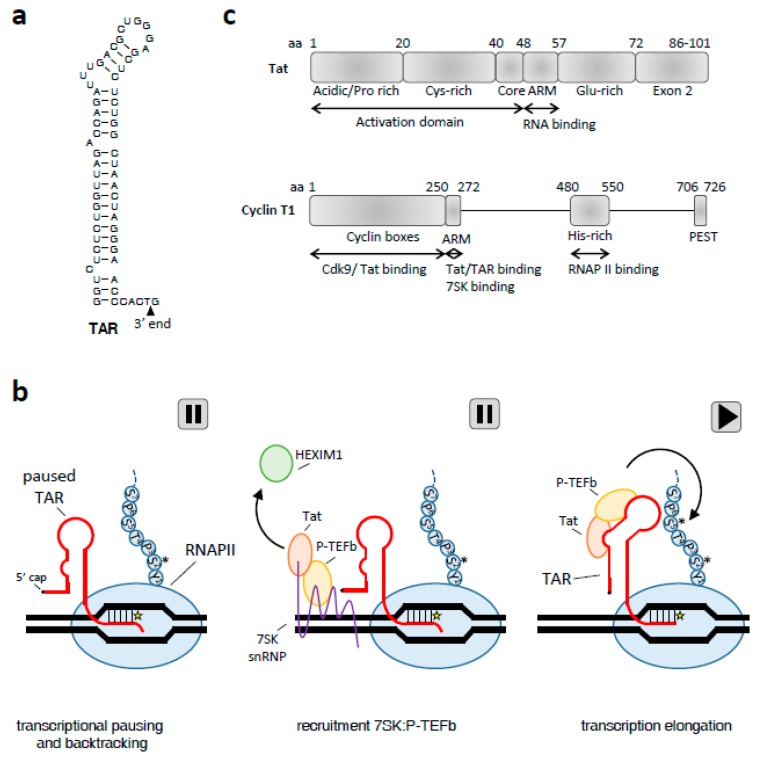
The best-studied case of a TF-recruiting RNA scaffold formed by promoter-proximal pausing is the human immunodeficiency virus type 1 (HIV-1) trans-acting region (TAR; Figure 3A) [76]. As a retrovirus, HIV-1 contains an RNA genome that is reverse transcribed into DNA upon entry of the host cell. This DNA integrates into the host genome as a provirus where it can be actively transcribed or enter a latent state. As in all retroviruses, the 5′ located long terminal repeat will act as the viral promoter. The viral promoter can be subdivided into the enhancer and core regions. The enhancer region contains binding sites for NF-κB [77,78], nuclear factor of activated T-cells (NFAT) [79], activator protein 1 (AP1) [80,81] and variable other TFs dependent on the HIV-1 subtype [82]. This enhancer upregulates HIV-1 transcription, but removal of its binding sites is not deleterious in certain cell lines [83]. In contrast, the core promoter is essential for transcription and contains three binding sites for SP1 [84] and a TATA box (CATATAA) element [81]. RNAPII, phosphorylated at Ser5 by TFIIH, will initiate transcription from this core region [85,86] (Figure 3B). However, most RNAPII complexes will pause after transcribing 62-nt, yielding short transcripts [87,88]. This is caused by the combined action of NELFs/5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) sensitivity inducing factor (DSIF) [89,90], the local RNA structure [88,91], microprocessor [70] and sequences within template DNA that promote transcriptional pausing [92]. The position of the promoter-proximal paused RNAPII on the 3′ end of the short transcript allows the nascent TAR transcript to fold into an initial, semi-stable RNA structure [88] (Figure 3B; paused TAR). This nascent hairpin allows the initial RNAPII complex to backtrack [91], such that the 3′ end of the nascent transcript is displaced from the RNAPII catalytic center [93] (Figure 3B). As a result, RNAPII is paused until it is reactivated, which may include refolding into the most stable TAR configuration.
Figure 3.
Factors involved in HIV-1 transcription and initiation. (a) Structure of the TAR hairpin; (b) model of RNAPII pausing (indicated with “II” button for pause) and reactivation (black triangle) upon TAR transcription. Phosphorylation of the CTD domain of RNAPII is indicated (black asterisk). The catalytic center of RNAPII is positioned at the yellow star. 7SK snRNA is depicted in purple. See the main text for more details; (c) domain organization of the viral Tat protein and positive transcription elongation factor b (P-TEFb) component Cyclin T1. Amino acid (aa) positions are indicated and the domain functions are described.
The folding of the TAR hairpin is key to the regulation of HIV-1 transcription [94] as it is used as a scaffold to recruit essential transcription factors, including the 86–101 amino acid (aa) viral trans-activator protein (Tat) [83] (Figure 3C). The interaction between Tat and TAR is necessary to recruit positive transcription elongation factor b (P-TEFb) from the 7SK small nuclear ribonucleoprotein (snRNP) [95]. P-TEFb consists of CyclinT1 (CycT1; Figure 3C) tightly associated with CDK9 kinase and is sequestered in an inactive form by the 7SK snRNP. Tat recruits P-TEFb by outcompeting the 7SK snRNP in several ways. First, the TAR-binding Arginine-rich motif (ARM) region of Tat (Figure 3C; aa 48–57) shows high similarity to the 7SK-binding of 7SK snRNP component hexamethylene bisacetamide-induced protein 1 (HEXIM1) [96]. Second, the HEXIM1-bound 7SK stem-loop 1 (SL1) is highly similar to the consensus minimal structure of the HIV-1 TAR element [97], and third, both Tat and HEXIM1 bind CycT1 in a mutually exclusive manner due to the shared CycT1 binding site [98,99]. However, the Tat activation domain (Figure 3C; AD; aa 1–47) has a 10-fold higher affinity for CycT1 [100]. Tat will thus bind to 7SK and CycT1 by displacing HEXIM1 (Figure 3B) [97]. As soon as TAR forms in the nascent viral transcript emerging from RNAPII, the Tat:P-TEFb complex will bind to TAR, resulting in the release of the inhibitory 7SK snRNP. This activates P-TEFb, allowing it to phosphorylate NELF, DSIF, and Ser2 on the RNAPII CTD, thus flipping the switch between RNAPII pausing and elongation [95].
Interestingly, the human T-lymphotropic virus type 1 transcriptional activator Tax also utilizes P-TEFb for viral transcription and displaces P-TEFb from 7SK snRNP through binding CycT1 [101,102], suggesting that P-TEFb liberation from 7SK snRNP could be a common theme developed by different viruses to support their replication in host cells.
8. Recruitment of Transcription Factors by Viral Noncoding RNAs
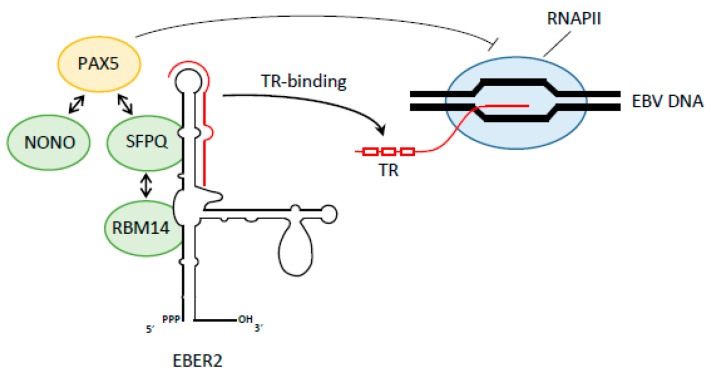
Instead of using promoter-proximal paused transcripts as cis-acting scaffolds to recruit TFs, viruses can also use noncoding RNAs. A recent example described that the Epstein-Barr virus (EBV) uses a virally encoded noncoding RNA named EBV-encoded RNA 2 (EBER2) that acts as a trans-acting guide to promote its own transcription [75] (Figure 4). EBV is a human γ-herpesvirus that persists as a mini-chromosome in the nucleus of B lymphocytes [103]. Herpesviruses are large enveloped ds DNA viruses that encode more than 80 genes. They are classified into three subfamilies (α-, β-, and γ-herpesviruses). EBV is the causative agent of mononucleosis and is associated with several types of tumors, including lymphomas and carcinomas [104]. Two long ncRNAs called EBER1 (167 nt) and EBER2 (173 nt) are expressed in infected cells at high levels (EBER1: ~106 copies; EBER2: ~2.5 × 105) [105,106] and localize exclusively to the nucleoplasm [107]. Due to their high copy number, these ncRNAs were used as diagnostic tool for EBV infection [108], but their function remained unknown. Employing a technique named ‘capture hybridization analysis of RNA targets’ (CHART), Lee et al. [75] found that EBER2 localizes to the terminal repeats (TRs) in the latent EBV genome. TRs are tandem direct repeats of approximately 550 bp that flank both ends of the linear genome present in virions and are the site of circularization to form the viral episome after its entry into the host cell [109]. EBER2 localizes in the vicinity of a binding site for the paired box protein 5 (PAX5) host transcription factor [110]. This co-localization suggested that these factors collaborate in some way, and Lee et al. show that EBER2 interacts with PAX5, albeit indirectly through an as-yet unidentified bridging factor. PAX5 is known to downregulate viral latent membrane protein 1/2A/2B (LMP1/2) gene expression and entry into EBV lytic replication [111,112]. Top candidates for this bridging factor include three host proteins: splicing factor proline and glutamine rich (SFPQ), non-POU domain-containing octamer-binding protein (NONO), and RNA-binding motif protein 14 (RBM14) [112]. SFPQ and RBM14 contact EBER2 directly as shown by RNA-protein crosslinking, and binding studies show that SFPQ and NONO associate with PAX5 (Figure 4). EBER2 depletion abrogates PAX5 recruitment to the TR, but PAX5 depletion has no effect on EBER2 localization, suggesting that this ncRNA is used to recruit PAX5 to the TR. Indeed, EBER2 base pairs with nascent transcripts generated from the TR regions, thus potentially targeting the interacting PAX5 TF to its consensus sequence sites within the TRs and facilitating its binding.
Figure 4.
Factors involved in Epstein-Barr virus-encoded RNA (EBV EBER) transcription regulation. RNA structure of EBER2 non coding RNA (black) with the terminal region (TR) binding domain indicated in red. EBER2 recruits several protein factors to the nascent EBV RNA. See the text for more details.
9. Transcription Termination
The last stage of transcription is termination, which leads to the release of the RNA transcript and the dissociation of RNAP from the template DNA. Upon encountering sequence-encoded termination signals (e.g., the canonical polyadenylation signal, AAUAAA), factors will be recruited that cleave and modify the RNA 3′ end. Eukaryotic mRNA undergoes multiple types of modifications at its 3′ end throughout its life cycle [113]. For example, newly synthesized mRNA acquires a long poly (A) tail (up to ∼250 nt) through canonical transcription-coupled polyadenylation, which facilitates mRNA export from the nucleus [114,115]. In the cytoplasm, poly(A) tails are associated with poly(A)-binding proteins (PABPCs) that stabilize the mRNA by acting as a safeguard against multiple decay machineries and promote protein synthesis [116,117]. mRNAs carrying short poly(A) tails (<25 nt) are recognized and poly-uridylated by terminal uridylyl transferases (TUTases) 4 and 7, which labels the mRNA for degradation [118].
Many +ssRNA viruses lack a poly (A) tail at the 3′ end of their genome, but are still efficiently translated. For instance, flaviruses (e.g., dengue virus, West Nile virus) have a capped RNA genome which contains conserved sequences at the 5′ and 3′ ends, allowing for circularization and efficient translation [119,120]. Other examples that follow the same strategy include rotaviruses [121], barley yellow dwarf virus [122], and possibly hepatitis C virus [123].
Additionally, premature transcription termination events are being uncovered that allow viruses to produce another layer of transcripts encoded by the same genome. By using these sub-genomic transcripts, viruses can create regulatory RNA molecules that undergo a different fate than the main vRNA. This can for instance be miRNA production by retroviruses [6] or the above described EBER small ncRNAs produced by EBV. Retroviruses have to produce an alternative transcript as a miRNA-source to prevent cleavage of the vRNA genome during miRNA processing by the Microprocessor complex [6,124]. Most of these sub-genomic transcripts are RNAPIII-encoded [125,126], but can also result from transcription termination of promoter-proximal paused RNAPII [127]. This nascent RNA will subsequently be exported to the cytoplasm, where it can enter the RNAi pathway at the Dicer step.
10. Viral Nucleases Induce mRNA Degradation in the Host
Viruses can downregulate host transcription by targeted mRNA degradation, to benefit their own gene expression. In this way, the host transcription machinery is deprived of targets and the virus can redirect the machinery to its own viral expression. As a beneficial side-effect to the virus, lowering host transcription will also reduce the effectiveness of the immune system. Therefore, during infection with many human viruses, the accumulation of viral proteins is accompanied by a progressive global reduction in the production of host proteins, a phenomenon that has been termed “host shutoff”. Viral factors that can induce host shutoff include severe acute respiratory syndrome (SARS) coronavirus nsp1 [128,129], herpes simplex virus 1 (HSV-1) vhs [130,131], vaccinia encoded decapping nucleases D9 and D10 [132,133,134], and two recently described viral endonucleases: Kaposi’s sarcoma-associated herpesvirus (KSHV) SOX [135,136] and Influenza A Virus (IAV) PA-X protein [137].
Like all influenza viruses, IAV is a segmented negative strand RNA virus. The PA-X protein-encoding gene overlaps with the open reading frame (ORF) for the PA FluPol subunit [138]. Ribosomal frameshifting on a rare CGU codon results in a fusion protein with the 191-aa N-terminal mRNA endonuclease domain of PA and an alternative C-terminal X domain of either 41 or 61 aa [139,140]. Consequently, PA-X lacks the CTD of PA responsible for its interaction with the PB1 subunit of FluPol, and was found to share sequence similarity with the above described viral host shutoff proteins that trigger RNA degradation.
KSHV is a γ-herpesvirus that causes tumors of endothelial cells and B cells. During lytic infection with KSHV, global degradation of host RNA is triggered by a virally encoded protein named shutoff and exonuclease (SOX) [135,141,142]. SOX cleaves most cellular mRNAs at random positions [136,143,144]. Although the α-herpesvirus vhs and γ-herpesvirus SOX proteins are not orthologs, they have a similar mechanism of action [144]; both are RNA endonucleases that cut host mRNAs into fragments. The initial cleavage is followed by degradation of the RNA body by the cellular 5′-3′ exonuclease XrnI and potentially other enzymes [136,143]. This mechanism is shared by other viruses with shutoff-RNases like PA-X for IAV and nsp1 for SARS [137,144,145]. Moreover, all known viral host shutoff-RNases, degrade mRNAs transcribed by the cellular RNAPII complex and spare ncRNAs transcribed by RNAPI and RNAPIII [137,143,144].
Notably, viral mRNA transcription escapes from decay-induced repression, and this escape also relies on XrnI. However, the mechanisms that allow viral mRNAs, unlike cellular mRNAs, to escape from XrnI cleavage are unknown.
11. Perspective
A complex interplay between viruses and the host transcription machinery that is mediated by nascent RNA has recently become increasingly apparent. Here, we explored a few of the myriad pathways by which viruses (i) use their own nascent RNA—in cis or in trans—as a scaffold to recruit host transcription factors; (ii) use host-capped RNAs as primers for RNA synthesis; (iii) use termination of nascent RNA synthesis to create sub-genomic mRNAs, and (iv) and down-regulate host nascent RNA production for their own benefit.
Viral RNA synthesis pathways are promising targets for antiviral development because these mechanisms are essential for virus replication. However, in order to identify additional weak spots in viral infection programs, it is important to understand how viruses regulate their gene expression and to disentangle the complex ways in which these pathways are intertwined with host transcription. Many antivirals display adverse side-effects because the viral target molecule and process was incompletely understood. The recently described new insights into the roles of nascent RNA in viral gene regulation are important steps in the path to new, more effective antivirals with fewer side-effects.
Acknowledgments
We thank Michael Bellecourt, Tatiana Mishanina and Indroneil Ghosh for helpful comments on the manuscript. This research was partially supported by NIH grant GM38660.
Author Contributions
All authors wrote, read, and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.McLaughlin R.N., Malik H.S. Genetic conflicts: The usual suspects and beyond. J. Exp. Biol. 2017;220:6–17. doi: 10.1242/jeb.148148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emerman M., Malik H.S. Paleovirology—Modern consequences of ancient viruses. PLoS Biol. 2010;8:e1000301. doi: 10.1371/journal.pbio.1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco-Melo D., Gifford R.J., Bieniasz P.D. Co-option of an endogenous retrovirus envelope for host defense in hominid ancestors. Elife. 2017;6:e22519. doi: 10.7554/eLife.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer L.M., Balaji S., Koonin E.V., Aravind L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res. 2006;117:156–184. doi: 10.1016/j.virusres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Wei H., Zhou M.M. Viral-encoded enzymes that target host chromatin functions. Biochim. Biophys. Acta. 2010;1799:296–301. doi: 10.1016/j.bbagrm.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harwig A., Das A.T., Berkhout B. Retroviral micrornas. Curr. Opin. Virol. 2014;7:47–54. doi: 10.1016/j.coviro.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Soliman M., Srikrishna G., Balagopal A. Mechanisms of HIV-1 control. Curr. HIV/AIDS Rep. 2017;14:101–109. doi: 10.1007/s11904-017-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Y.E., Steitz J.A. Virus meets host microrna: The destroyer, the booster, the hijacker. Mol. Cell. Biol. 2014;34:3780–3787. doi: 10.1128/MCB.00871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jolma A., Yan J., Whitington T., Toivonen J., Nitta K.R., Rastas P., Morgunova E., Enge M., Taipale M., Wei G., et al. DNA-binding specificities of human transcription factors. Cell. 2013;152:327–339. doi: 10.1016/j.cell.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Jensen S., Thomsen A.R. Sensing of RNA viruses: A review of innate immune receptors involved in recognizing RNA virus invasion. J. Virol. 2012;86:2900–2910. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinn J., Guttman M. Rna function. RNA and dynamic nuclear organization. Science. 2014;345:1240–1241. doi: 10.1126/science.1252966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cramer P., Armache K.J., Baumli S., Benkert S., Brueckner F., Buchen C., Damsma G.E., Dengl S., Geiger S.R., Jasiak A.J., et al. Structure of eukaryotic RNA polymerases. Annu. Rev. Biophys. 2008;37:337–352. doi: 10.1146/annurev.biophys.37.032807.130008. [DOI] [PubMed] [Google Scholar]
- 13.Egloff S., Murphy S. Cracking the RNA polymerase II CTD code. Trends Genet. 2008;24:280–288. doi: 10.1016/j.tig.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Hsin J.P., Xiang K., Manley J.L. Function and control of RNA polymerase II C-terminal domain phosphorylation in vertebrate transcription and RNA processing. Mol. Cell. Biol. 2014;34:2488–2498. doi: 10.1128/MCB.00181-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reich E., Franklin R.M., Shatkin A.J., Tatum E.L. Effect of actinomycin D on cellular nucleic acid synthesis and virus production. Science. 1961;134:556–557. doi: 10.1126/science.134.3478.556. [DOI] [PubMed] [Google Scholar]
- 16.Koonin E.V., Gorbalenya A.E., Chumakov K.M. Tentative identification of RNA-dependent RNA polymerases of dsRNA viruses and their relationship to positive strand RNA viral polymerases. FEBS Lett. 1989;252:42–46. doi: 10.1016/0014-5793(89)80886-5. [DOI] [PubMed] [Google Scholar]
- 17.Zanotto P.M., Gibbs M.J., Gould E.A., Holmes E.C. A reevaluation of the higher taxonomy of viruses based on RNA polymerases. J. Virol. 1996;70:6083–6096. doi: 10.1128/jvi.70.9.6083-6096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dulin D., Vilfan I.D., Berghuis B.A., Hage S., Bamford D.H., Poranen M.M., Depken M., Dekker N.H. Elongation-competent pauses govern the fidelity of a viral RNA-dependent RNA polymerase. Cell Rep. 2015;10:983–992. doi: 10.1016/j.celrep.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Jin Z., Leveque V., Ma H., Johnson K.A., Klumpp K. Assembly, purification, and pre-steady-state kinetic analysis of active RNA-dependent RNA polymerase elongation complex. J. Biol. Chem. 2012;287:10674–10683. doi: 10.1074/jbc.M111.325530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao C.C., Singh P., Ecker D.J. De novo initiation of viral RNA-dependent RNA synthesis. Virology. 2001;287:251–260. doi: 10.1006/viro.2001.1039. [DOI] [PubMed] [Google Scholar]
- 21.Sainsbury S., Bernecky C., Cramer P. Structural basis of transcription initiation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2015;16:129–143. doi: 10.1038/nrm3952. [DOI] [PubMed] [Google Scholar]
- 22.Grunberg S., Hahn S. Structural insights into transcription initiation by RNA polymerase II. Trends Biochem. Sci. 2013;38:603–611. doi: 10.1016/j.tibs.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z., Roeder R.G. Structure and function of a human transcription factor TFIIIB subunit that is evolutionarily conserved and contains both TFIIB- and high-mobility-group protein 2-related domains. Proc. Natl. Acad. Sci. USA. 1995;92:7026–7030. doi: 10.1073/pnas.92.15.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pugh B.F. Control of gene expression through regulation of the TATA-binding protein. Gene. 2000;255:1–14. doi: 10.1016/S0378-1119(00)00288-2. [DOI] [PubMed] [Google Scholar]
- 25.Sikorski T.W., Buratowski S. The basal initiation machinery: Beyond the general transcription factors. Curr. Opin. Cell Biol. 2009;21:344–351. doi: 10.1016/j.ceb.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dynlacht B.D., Hoey T., Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991;66:563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- 27.Thomas M.C., Chiang C.M. The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 2006;41:105–178. doi: 10.1080/10409230600648736. [DOI] [PubMed] [Google Scholar]
- 28.Murakami K., Elmlund H., Kalisman N., Bushnell D.A., Adams C.M., Azubel M., Elmlund D., Levi-Kalisman Y., Liu X., Gibbons B.J., et al. Architecture of an RNA polymerase II transcription pre-initiation complex. Science. 2013;342:1238724. doi: 10.1126/science.1238724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giardina C., Lis J.T. DNA melting on yeast RNA polymerase II promoters. Science. 1993;261:759–762. doi: 10.1126/science.8342041. [DOI] [PubMed] [Google Scholar]
- 30.Plaschka C., Hantsche M., Dienemann C., Burzinski C., Plitzko J., Cramer P. Transcription initiation complex structures elucidate DNA opening. Nature. 2016;533:353–358. doi: 10.1038/nature17990. [DOI] [PubMed] [Google Scholar]
- 31.Kwak H., Lis J.T. Control of transcriptional elongation. Annu. Rev. Genet. 2013;47:483–508. doi: 10.1146/annurev-genet-110711-155440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., O’Brien K.L., Roca A., Wright P.F., Bruce N., et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremaglio C.Z., Noton S.L., Deflube L.R., Fearns R. Respiratory syncytial virus polymerase can initiate transcription from position 3 of the leader promoter. J. Virol. 2013;87:3196–3207. doi: 10.1128/JVI.02862-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farsetta D.L., Chandran K., Nibert M.L. Transcriptional activities of reovirus RNA polymerase in recoated cores. Initiation and elongation are regulated by separate mechanisms. J. Biol. Chem. 2000;275:39693–39701. doi: 10.1074/jbc.M004562200. [DOI] [PubMed] [Google Scholar]
- 35.Morin B., Rahmeh A.A., Whelan S.P. Mechanism of RNA synthesis initiation by the vesicular stomatitis virus polymerase. EMBO J. 2012;31:1320–1329. doi: 10.1038/emboj.2011.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong W., Ferrari E., Lesburg C.A., Maag D., Ghosh S.K., Cameron C.E., Lau J.Y., Hong Z. Template/primer requirements and single nucleotide incorporation by hepatitis C virus nonstructural protein 5B polymerase. J. Virol. 2000;74:9134–9143. doi: 10.1128/JVI.74.19.9134-9143.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noton S.L., Fearns R. Initiation and regulation of paramyxovirus transcription and replication. Virology. 2015;479–480:545–554. doi: 10.1016/j.virol.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo L., Fearns R., Collins P.L. Analysis of the gene start and gene end signals of human respiratory syncytial virus: Quasi-templated initiation at position 1 of the encoded mRNA. J. Virol. 1997;71:4944–4953. doi: 10.1128/jvi.71.7.4944-4953.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selisko B., Potisopon S., Agred R., Priet S., Varlet I., Thillier Y., Sallamand C., Debart F., Vasseur J.J., Canard B. Molecular basis for nucleotide conservation at the ends of the dengue virus genome. PLoS Pathog. 2012;8:e1002912. doi: 10.1371/journal.ppat.1002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap T.L., Xu T., Chen Y.L., Malet H., Egloff M.P., Canard B., Vasudevan S.G., Lescar J. Crystal structure of the dengue virus RNA-dependent RNA polymerase catalytic domain at 1.85-angstrom resolution. J. Virol. 2007;81:4753–4765. doi: 10.1128/JVI.02283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noton S.L., Deflube L.R., Tremaglio C.Z., Fearns R. The respiratory syncytial virus polymerase has multiple RNA synthesis activities at the promoter. PLoS Pathog. 2012;8:e1002980. doi: 10.1371/journal.ppat.1002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whelan S.P., Barr J.N., Wertz G.W. Transcription and replication of nonsegmented negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 2004;283:61–119. doi: 10.1007/978-3-662-06099-5_3. [DOI] [PubMed] [Google Scholar]
- 43.Dong H., Fink K., Zust R., Lim S.P., Qin C.F., Shi P.Y. Flavivirus RNA methylation. J. Gen. Virol. 2014;95:763–778. doi: 10.1099/vir.0.062208-0. [DOI] [PubMed] [Google Scholar]
- 44.Saeedi B.J., Geiss B.J. Regulation of flavivirus RNA synthesis and capping. Wiley Interdiscip. Rev. RNA. 2013;4:723–735. doi: 10.1002/wrna.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decroly E., Ferron F., Lescar J., Canard B. Conventional and unconventional mechanisms for capping viral mRNA. Nat. Rev. Microbiol. 2011;10:51–65. doi: 10.1038/nrmicro2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartelma G., Padmanabhan R. Expression, purification, and characterization of the RNA 5′-triphosphatase activity of dengue virus type 2 nonstructural protein 3. Virology. 2002;299:122–132. doi: 10.1006/viro.2002.1504. [DOI] [PubMed] [Google Scholar]
- 47.Issur M., Geiss B.J., Bougie I., Picard-Jean F., Despins S., Mayette J., Hobdey S.E., Bisaillon M. The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA. 2009;15:2340–2350. doi: 10.1261/rna.1609709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray D., Shah A., Tilgner M., Guo Y., Zhao Y., Dong H., Deas T.S., Zhou Y., Li H., Shi P.Y. West Nile virus 5′-cap structure is formed by sequential guanine N-7 and ribose 2′-O methylations by nonstructural protein 5. J. Virol. 2006;80:8362–8370. doi: 10.1128/JVI.00814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Selisko B., Wang C., Harris E., Canard B. Regulation of flavivirus RNA synthesis and replication. Curr. Opin. Virol. 2014;9:74–83. doi: 10.1016/j.coviro.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poch O., Blumberg B.M., Bougueleret L., Tordo N. Sequence comparison of five polymerases (l proteins) of unsegmented negative-strand RNA viruses: Theoretical assignment of functional domains. J. Gen. Virol. 1990;71:1153–1162. doi: 10.1099/0022-1317-71-5-1153. [DOI] [PubMed] [Google Scholar]
- 51.Blondot M.L., Dubosclard V., Fix J., Lassoued S., Aumont-Nicaise M., Bontems F., Eleouet J.F., Sizun C. Structure and functional analysis of the RNA- and viral phosphoprotein-binding domain of respiratory syncytial virus M2-1 protein. PLoS Pathog. 2012;8:e1002734. doi: 10.1371/journal.ppat.1002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran T.L., Castagne N., Dubosclard V., Noinville S., Koch E., Moudjou M., Henry C., Bernard J., Yeo R.P., Eleouet J.F. The respiratory syncytial virus M2-1 protein forms tetramers and interacts with RNA and p in a competitive manner. J. Virol. 2009;83:6363–6374. doi: 10.1128/JVI.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tawar R.G., Duquerroy S., Vonrhein C., Varela P.F., Damier-Piolle L., Castagne N., MacLellan K., Bedouelle H., Bricogne G., Bhella D., et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science. 2009;326:1279–1283. doi: 10.1126/science.1177634. [DOI] [PubMed] [Google Scholar]
- 54.Ogino T., Banerjee A.K. Unconventional mechanism of mRNA capping by the RNA-dependent RNA polymerase of vesicular stomatitis virus. Mol. Cell. 2007;25:85–97. doi: 10.1016/j.molcel.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Te Velthuis A.J., Fodor E. Influenza virus RNA polymerase: Insights into the mechanisms of viral RNA synthesis. Nat. Rev. Microbiol. 2016;14:479–493. doi: 10.1038/nrmicro.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reich S., Guilligay D., Pflug A., Malet H., Berger I., Crepin T., Hart D., Lunardi T., Nanao M., Ruigrok R.W., et al. Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature. 2014;516:361–366. doi: 10.1038/nature14009. [DOI] [PubMed] [Google Scholar]
- 57.Pflug A., Guilligay D., Reich S., Cusack S. Structure of influenza a polymerase bound to the viral RNA promoter. Nature. 2014;516:355–360. doi: 10.1038/nature14008. [DOI] [PubMed] [Google Scholar]
- 58.Hengrung N., El Omari K., Serna Martin I., Vreede F.T., Cusack S., Rambo R.P., Vonrhein C., Bricogne G., Stuart D.I., Grimes J.M., et al. Crystal structure of the RNA-dependent RNA polymerase from influenza C virus. Nature. 2015;527:114–117. doi: 10.1038/nature15525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robb N.C., Te Velthuis A.J., Wieneke R., Tampe R., Cordes T., Fodor E., Kapanidis A.N. Single-molecule FRET reveals the pre-initiation and initiation conformations of influenza virus promoter RNA. Nucleic Acids Res. 2016;44:10304–10315. doi: 10.1093/nar/gkw884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reich S., Guilligay D., Cusack S. An in vitro fluorescence based study of initiation of RNA synthesis by influenza B polymerase. Nucleic Acids Res. 2017;45:3353–3368. doi: 10.1093/nar/gkx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez-Alonso M., Hengrung N., Fodor E. RNA-free and ribonucleoprotein-associated influenza virus polymerases directly bind the serine-5-phosphorylated carboxyl-terminal domain of host RNA polymerase II. J. Virol. 2016;90:6014–6021. doi: 10.1128/JVI.00494-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu W., Gallagher G.R., Dai W., Liu P., Li R., Trombly M.I., Gammon D.B., Mello C.C., Wang J.P., Finberg R.W. Influenza A virus preferentially snatches noncoding RNA caps. RNA. 2015;21:2067–2075. doi: 10.1261/rna.054221.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koppstein D., Ashour J., Bartel D.P. Sequencing the cap-snatching repertoire of H1N1 influenza provides insight into the mechanism of viral transcription initiation. Nucleic Acids Res. 2015;43:5052–5064. doi: 10.1093/nar/gkv333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sikora D., Rocheleau L., Brown E.G., Pelchat M. Deep sequencing reveals the eight facets of the influenza A/HongKong/1/1968 (H3N2) virus cap-snatching process. Sci. Rep. 2014;4:6181. doi: 10.1038/srep06181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pritlove D.C., Poon L.L., Fodor E., Sharps J., Brownlee G.G. Polyadenylation of influenza virus mRNA transcribed in vitro from model virion RNA templates: Requirement for 5′ conserved sequences. J. Virol. 1998;72:1280–1286. doi: 10.1128/jvi.72.2.1280-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu Y., Nemeroff M., Krug R.M. The influenza virus NS1 protein binds to a specific region in human U6 snRNA and inhibits U6-U2 and U6-U4 snRNA interactions during splicing. RNA. 1995;1:304–316. [PMC free article] [PubMed] [Google Scholar]
- 67.Nojima T., Gomes T., Grosso A.R., Kimura H., Dye M.J., Dhir S., Carmo-Fonseca M., Proudfoot N.J. Mammalian net-seq reveals genome-wide nascent transcription coupled to RNA processing. Cell. 2015;161:526–540. doi: 10.1016/j.cell.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henriques T., Gilchrist D.A., Nechaev S., Bern M., Muse G.W., Burkholder A., Fargo D.C., Adelman K. Stable pausing by RNA polymerase II provides an opportunity to target and integrate regulatory signals. Mol. Cell. 2013;52:517–528. doi: 10.1016/j.molcel.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adelman K., Lis J.T. Promoter-proximal pausing of RNA polymerase II: Emerging roles in metazoans. Nat. Rev. Genet. 2012;13:720–731. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wagschal A., Rousset E., Basavarajaiah P., Contreras X., Harwig A., Laurent-Chabalier S., Nakamura M., Chen X., Zhang K., Meziane O., et al. Microprocessor, SETX, XRN2, and RRP6 co-operate to induce premature termination of transcription by RNAPII. Cell. 2012;150:1147–1157. doi: 10.1016/j.cell.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen V.T., Kiss T., Michels A.A., Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/Cyclin T complexes. Nature. 2001;414:322–325. doi: 10.1038/35104581. [DOI] [PubMed] [Google Scholar]
- 72.Tsai M.C., Manor O., Wan Y., Mosammaparast N., Wang J.K., Lan F., Shi Y., Segal E., Chang H.Y. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Z., Zhu Q., Luo K., Zhou Q. The 7SK small nuclear RNA inhibits the CDK9/Cyclin T1 kinase to control transcription. Nature. 2001;414:317–322. doi: 10.1038/35104575. [DOI] [PubMed] [Google Scholar]
- 74.Zhao J., Sun B.K., Erwin J.A., Song J.J., Lee J.T. Polycomb proteins targeted by a short repeat RNA to the mouse x chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee N., Moss W.N., Yario T.A., Steitz J.A. EBV noncoding RNA binds nascent RNA to drive host PAX5 to viral DNA. Cell. 2015;160:607–618. doi: 10.1016/j.cell.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berkhout B., Silverman R.H., Jeang K.T. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell. 1989;59:273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- 77.Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 78.Liu J., Perkins N.D., Schmid R.M., Nabel G.J. Specific NF-κB subunits act in concert with Tat to stimulate human immunodeficiency virus type 1 transcription. J. Virol. 1992;66:3883–3887. doi: 10.1128/jvi.66.6.3883-3887.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kinoshita S., Chen B.K., Kaneshima H., Nolan G.P. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/S0092-8674(00)81630-X. [DOI] [PubMed] [Google Scholar]
- 80.Berkhout B., Jeang K.T. Functional roles for the TATA promoter and enhancers in basal and Tat-induced expression of the human immunodeficiency virus type 1 long terminal repeat. J. Virol. 1992;66:139–149. doi: 10.1128/jvi.66.1.139-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Opijnen T., Jeeninga R.E., Boerlijst M.C., Pollakis G.P., Zetterberg V., Salminen M., Berkhout B. Human immunodeficiency virus type 1 subtypes have a distinct long terminal repeat that determines the replication rate in a host-cell-specific manner. J. Virol. 2004;78:3675–3683. doi: 10.1128/JVI.78.7.3675-3683.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeeninga R.E., Hoogenkamp M., Armand-Ugon M., de Baar M., Verhoef K., Berkhout B. Functional differences between the long terminal repeat transcriptional promoters of human immunodeficiency virus type 1 subtypes a through g. J. Virol. 2000;74:3740–3751. doi: 10.1128/JVI.74.8.3740-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Das A.T., Harwig A., Berkhout B. The HIV-1 Tat protein has a versatile role in activating viral transcription. J. Virol. 2011;85:9506–9516. doi: 10.1128/JVI.00650-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones K.A., Kadonaga J.T., Luciw P.A., Tjian R. Activation of the aids retrovirus promoter by the cellular transcription factor, SP1. Science. 1986;232:755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- 85.Zenzie-Gregory B., Sheridan P., Jones K.A., Smale S.T. HIV-1 core promoter lacks a simple initiator element but contains a bipartite activator at the transcription start site. J. Biol. Chem. 1993;268:15823–15832. [PubMed] [Google Scholar]
- 86.Luse D.S. Promoter clearance by RNA polymerase II. Biochim. Biophys. Acta. 2013;1829:63–68. doi: 10.1016/j.bbagrm.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kao S.Y., Calman A.F., Luciw P.A., Peterlin B.M. Anti-termination of transcription within the long terminal repeat of HIV-1 by Tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 88.Palangat M., Meier T.I., Keene R.G., Landick R. Transcriptional pausing at +62 of the HIV-1 nascent RNA modulates formation of the TAR RNA structure. Mol. Cell. 1998;1:1033–1042. doi: 10.1016/S1097-2765(00)80103-3. [DOI] [PubMed] [Google Scholar]
- 89.Ping Y.H., Rana T.M. DSIF and NELF interact with RNA polymerase II elongation complex and HIV-1 Tat stimulates P-TEFb-mediated phosphorylation of RNA polymerase II and DSIF during transcription elongation. J. Biol. Chem. 2001;276:12951–12958. doi: 10.1074/jbc.M006130200. [DOI] [PubMed] [Google Scholar]
- 90.Natarajan M., Schiralli Lester G.M., Lee C., Missra A., Wasserman G.A., Steffen M., Gilmour D.S., Henderson A.J. Negative elongation factor (NELF) coordinates RNA polymerase II pausing, premature termination, and chromatin remodeling to regulate HIV transcription. J. Biol. Chem. 2013;288:25995–26003. doi: 10.1074/jbc.M113.496489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palangat M., Landick R. Roles of RNA:DNA hybrid stability, RNA structure, and active site conformation in pausing by human RNA polymerase II. J. Mol. Biol. 2001;311:265–282. doi: 10.1006/jmbi.2001.4842. [DOI] [PubMed] [Google Scholar]
- 92.Larson M.H., Mooney R.A., Peters J.M., Windgassen T., Nayak D., Gross C.A., Block S.M., Greenleaf W.J., Landick R., Weissman J.S. A pause sequence enriched at translation start sites drives transcription dynamics in vivo. Science. 2014;344:1042–1047. doi: 10.1126/science.1251871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Komissarova N., Kashlev M. RNA polymerase switches between inactivated and activated states by translocating back and forth along the DNA and the RNA. J. Biol. Chem. 1997;272:15329–15338. doi: 10.1074/jbc.272.24.15329. [DOI] [PubMed] [Google Scholar]
- 94.Das A.T., Harwig A., Vrolijk M.M., Berkhout B. The TAR hairpin of human immunodeficiency virus type 1 can be deleted when not required for Tat-mediated activation of transcription. J. Virol. 2007;81:7742–7748. doi: 10.1128/JVI.00392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.D′Orso I., Frankel A.D. RNA-mediated displacement of an inhibitory SNRNP complex activates transcription elongation. Nat. Struct. Mol. Biol. 2010;17:815–821. doi: 10.1038/nsmb.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yik J.H., Chen R., Pezda A.C., Samford C.S., Zhou Q. A human immunodeficiency virus type 1 Tat -like arginine-rich RNA-binding domain is essential for HEXIM1 to inhibit RNA polymerase II transcription through 7SK snRNA-mediated inactivation of P-TEFb. Mol. Cell. Biol. 2004;24:5094–5105. doi: 10.1128/MCB.24.12.5094-5105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muniz L., Egloff S., Ughy B., Jady B.E., Kiss T. Controlling cellular P-TEFb activity by the HIV-1 transcriptional transactivator Tat. PLoS Pathog. 2010;6:e1001152. doi: 10.1371/journal.ppat.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schulte A., Czudnochowski N., Barboric M., Schonichen A., Blazek D., Peterlin B.M., Geyer M. Identification of a Cyclin T-binding domain in HEXIM1 and biochemical analysis of its binding competition with HIV-1 Tat. J. Biol. Chem. 2005;280:24968–24977. doi: 10.1074/jbc.M501431200. [DOI] [PubMed] [Google Scholar]
- 99.Michels A.A., Nguyen V.T., Fraldi A., Labas V., Edwards M., Bonnet F., Lania L., Bensaude O. MAQ1 and 7SK RNA interact with CDK9/Cyclin T complexes in a transcription-dependent manner. Mol. Cell. Biol. 2003;23:4859–4869. doi: 10.1128/MCB.23.14.4859-4869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barboric M., Yik J.H., Czudnochowski N., Yang Z., Chen R., Contreras X., Geyer M., Matija Peterlin B., Zhou Q. Tat competes with HEXIM1 to increase the active pool of P-TEFb for HIV-1 transcription. Nucleic Acids Res. 2007;35:2003–2012. doi: 10.1093/nar/gkm063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cho W.K., Jang M.K., Huang K., Pise-Masison C.A., Brady J.N. Human T-lymphotropic virus type 1 TAX protein complexes with P-TEFb and competes for BRD4 and 7SK SNRNP/HEXIM1 binding. J. Virol. 2010;84:12801–12809. doi: 10.1128/JVI.00943-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou M., Lu H., Park H., Wilson-Chiru J., Linton R., Brady J.N. Tax interacts with P-TEFb in a novel manner to stimulate human T-lymphotropic virus type 1 transcription. J. Virol. 2006;80:4781–4791. doi: 10.1128/JVI.80.10.4781-4791.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Young L.S., Rickinson A.B. Epstein-barr virus: 40 years on. Nat. Rev. Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 104.Delecluse H.J., Feederle R., O′Sullivan B., Taniere P. Epstein barr virus-associated tumours: An update for the attention of the working pathologist. J. Clin. Pathol. 2007;60:1358–1364. doi: 10.1136/jcp.2006.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tycowski K.T., Guo Y.E., Lee N., Moss W.N., Vallery T.K., Xie M., Steitz J.A. Viral noncoding RNAs: More surprises. Genes Dev. 2015;29:567–584. doi: 10.1101/gad.259077.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moss W.N., Steitz J.A. Genome-wide analyses of Epstein-Barr virus reveal conserved RNA structures and a novel stable intronic sequence RNA. BMC Genom. 2013;14:543. doi: 10.1186/1471-2164-14-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fok V., Friend K., Steitz J.A. Epstein-Barr virus noncoding RNAs are confined to the nucleus, whereas their partner, the human La protein, undergoes nucleocytoplasmic shuttling. J. Cell Biol. 2006;173:319–325. doi: 10.1083/jcb.200601026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gulley M.L., Tang W. Laboratory assays for Epstein-Barr virus-related disease. J. Mol. Diagn. 2008;10:279–292. doi: 10.2353/jmoldx.2008.080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lindahl T., Adams A., Bjursell G., Bornkamm G.W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J. Mol. Biol. 1976;102:511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- 110.Arvey A., Tempera I., Tsai K., Chen H.S., Tikhmyanova N., Klichinsky M., Leslie C., Lieberman P.M. An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe. 2012;12:233–245. doi: 10.1016/j.chom.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee N., Steitz J.A. Noncoding RNA-guided recruitment of transcription factors: A prevalent but undocumented mechanism? Bioessays. 2015;37:936–941. doi: 10.1002/bies.201500060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee N., Yario T.A., Gao J.S., Steitz J.A. EBV noncoding RNA EBER2 interacts with host RNA-binding proteins to regulate viral gene expression. Proc. Natl. Acad. Sci. USA. 2016;113:3221–3226. doi: 10.1073/pnas.1601773113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burroughs A.M., Ando Y., de Hoon M.J., Tomaru Y., Nishibu T., Ukekawa R., Funakoshi T., Kurokawa T., Suzuki H., Hayashizaki Y., et al. A comprehensive survey of 3′ animal miRNA modification events and a possible role for 3′ adenylation in modulating miRNA targeting effectiveness. Genome Res. 2010;20:1398–1410. doi: 10.1101/gr.106054.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richard P., Manley J.L. Transcription termination by nuclear RNA polymerases. Genes Dev. 2009;23:1247–1269. doi: 10.1101/gad.1792809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wahle E., Keller W. The biochemistry of polyadenylation. Trends Biochem. Sci. 1996;21:247–250. doi: 10.1016/S0968-0004(96)10030-X. [DOI] [PubMed] [Google Scholar]
- 116.Norbury C.J. Cytoplasmic RNA: A case of the tail wagging the dog. Nat. Rev. Mol. Cell Biol. 2013;14:643–653. doi: 10.1038/nrm3645. [DOI] [PubMed] [Google Scholar]
- 117.Eliseeva I.A., Lyabin D.N., Ovchinnikov L.P. Poly(A)-binding proteins: Structure, domain organization, and activity regulation. Biochemistry. 2013;78:1377–1391. doi: 10.1134/S0006297913130014. [DOI] [PubMed] [Google Scholar]
- 118.Lim J., Ha M., Chang H., Kwon S.C., Simanshu D.K., Patel D.J., Kim V.N. Uridylation by TUT4 and TUT7 marks mRNA for degradation. Cell. 2014;159:1365–1376. doi: 10.1016/j.cell.2014.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gebhard L.G., Filomatori C.V., Gamarnik A.V. Functional RNA elements in the dengue virus genome. Viruses. 2011;3:1739–1756. doi: 10.3390/v3091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khromykh A.A., Meka H., Guyatt K.J., Westaway E.G. Essential role of cyclization sequences in flavivirus RNA replication. J. Virol. 2001;75:6719–6728. doi: 10.1128/JVI.75.14.6719-6728.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chizhikov V., Patton J.T. A four-nucleotide translation enhancer in the 3′-terminal consensus sequence of the nonpolyadenylated mRNAs of rotavirus. RNA. 2000;6:814–825. doi: 10.1017/S1355838200992264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo L., Allen E.M., Miller W.A. Base-pairing between untranslated regions facilitates translation of uncapped, nonpolyadenylated viral RNA. Mol. Cell. 2001;7:1103–1109. doi: 10.1016/S1097-2765(01)00252-0. [DOI] [PubMed] [Google Scholar]
- 123.Li Y., Yamane D., Masaki T., Lemon S.M. The yin and yang of hepatitis C: Synthesis and decay of hepatitis C virus RNA. Nat. Rev. Microbiol. 2015;13:544–558. doi: 10.1038/nrmicro3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 125.Kincaid R.P., Burke J.M., Sullivan C.S. RNA virus microRNA that mimics a B-cell oncomiR. Proc. Natl. Acad. Sci. USA. 2012;109:3077–3082. doi: 10.1073/pnas.1116107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Whisnant A.W., Kehl T., Bao Q., Materniak M., Kuzmak J., Lochelt M., Cullen B.R. Identification of novel, highly expressed retroviral microRNAs in cells infected by bovine foamy virus. J. Virol. 2014;88:4679–4686. doi: 10.1128/JVI.03587-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harwig A., Jongejan A., van Kampen A.H., Berkhout B., Das A.T. Tat-dependent production of an HIV-1 TAR-encoded miRNA-like small RNA. Nucleic Acids Res. 2016;44:4340–4353. doi: 10.1093/nar/gkw167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kamitani W., Narayanan K., Huang C., Lokugamage K., Ikegami T., Ito N., Kubo H., Makino S. Severe acute respiratory syndrome coronavirus NSP1 protein suppresses host gene expression by promoting host mRNA degradation. Proc. Natl. Acad. Sci. USA. 2006;103:12885–12890. doi: 10.1073/pnas.0603144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kamitani W., Huang C., Narayanan K., Lokugamage K.G., Makino S. A two-pronged strategy to suppress host protein synthesis by SARS coronavirus NSP1 protein. Nat. Struct. Mol. Biol. 2009;16:1134–1140. doi: 10.1038/nsmb.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kwong A.D., Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA. 1987;84:1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Read G.S., Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of α (immediate early) viral polypeptides. J. Virol. 1983;46:498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parrish S., Moss B. Characterization of a second vaccinia virus mRNA-decapping enzyme conserved in poxviruses. J. Virol. 2007;81:12973–12978. doi: 10.1128/JVI.01668-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parrish S., Resch W., Moss B. Vaccinia virus D10 protein has mRNA decapping activity, providing a mechanism for control of host and viral gene expression. Proc. Natl. Acad. Sci. USA. 2007;104:2139–2144. doi: 10.1073/pnas.0611685104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parrish S., Moss B. Characterization of a vaccinia virus mutant with a deletion of the D10R gene encoding a putative negative regulator of gene expression. J. Virol. 2006;80:553–561. doi: 10.1128/JVI.80.2.553-561.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Glaunsinger B., Ganem D. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol. Cell. 2004;13:713–723. doi: 10.1016/S1097-2765(04)00091-7. [DOI] [PubMed] [Google Scholar]
- 136.Abernathy E., Gilbertson S., Alla R., Glaunsinger B. Viral nucleases induce an mRNA degradation-transcription feedback loop in mammalian cells. Cell Host Microbe. 2015;18:243–253. doi: 10.1016/j.chom.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khaperskyy D.A., Schmaling S., Larkins-Ford J., McCormick C., Gaglia M.M. Selective degradation of host RNA polymerase II transcripts by influenza A virus PA-X host shutoff protein. PLoS Pathog. 2016;12:e1005427. doi: 10.1371/journal.ppat.1005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jagger B.W., Wise H.M., Kash J.C., Walters K.A., Wills N.M., Xiao Y.L., Dunfee R.L., Schwartzman L.M., Ozinsky A., Bell G.L., et al. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012;337:199–204. doi: 10.1126/science.1222213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Firth A.E., Jagger B.W., Wise H.M., Nelson C.C., Parsawar K., Wills N.M., Napthine S., Taubenberger J.K., Digard P., Atkins J.F. Ribosomal frameshifting used in influenza A virus expression occurs within the sequence UCC_UUU_CGU and is in the +1 direction. Open Biol. 2012;2:120109. doi: 10.1098/rsob.120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shi M., Jagger B.W., Wise H.M., Digard P., Holmes E.C., Taubenberger J.K. Evolutionary conservation of the PA-X open reading frame in segment 3 of influenza A virus. J. Virol. 2012;86:12411–12413. doi: 10.1128/JVI.01677-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Covarrubias S., Richner J.M., Clyde K., Lee Y.J., Glaunsinger B.A. Host shutoff is a conserved phenotype of γ herpesvirus infection and is orchestrated exclusively from the cytoplasm. J. Virol. 2009;83:9554–9566. doi: 10.1128/JVI.01051-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bagneris C., Briggs L.C., Savva R., Ebrahimi B., Barrett T.E. Crystal structure of a KSHV-SOX-DNA complex: Insights into the molecular mechanisms underlying DNase activity and host shutoff. Nucleic Acids Res. 2011;39:5744–5756. doi: 10.1093/nar/gkr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Covarrubias S., Gaglia M.M., Kumar G.R., Wong W., Jackson A.O., Glaunsinger B.A. Coordinated destruction of cellular messages in translation complexes by the γ herpesvirus host shutoff factor and the mammalian exonuclease XRN1. PLoS Pathog. 2011;7:e1002339. doi: 10.1371/journal.ppat.1002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gaglia M.M., Covarrubias S., Wong W., Glaunsinger B.A. A common strategy for host RNA degradation by divergent viruses. J. Virol. 2012;86:9527–9530. doi: 10.1128/JVI.01230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bavagnoli L., Cucuzza S., Campanini G., Rovida F., Paolucci S., Baldanti F., Maga G. The novel influenza a virus protein PA-X and its naturally deleted variant show different enzymatic properties in comparison to the viral endonuclease PA. Nucleic Acids Res. 2015;43:9405–9417. doi: 10.1093/nar/gkv926. [DOI] [PMC free article] [PubMed] [Google Scholar]