Abstract
Vitamin D deficiency is a common issue, particularly in obese populations, and is tested by assessing serum 25(OH)D concentrations. This study aimed to identify factors that contribute to the vitamin D status in fifty morbidly obese individuals recruited prior to bariatric surgery. Data collected included serum 25(OH)D concentrations, dietary and supplement intake of vitamin D, sun exposure measures, skin colour via spectrophotometry, and genotype analysis of several single nucleotide polymorphisms in the vitamin D metabolism pathway. Results showed a significant correlation between serum 25(OH)D concentrations and age, and serum 25(OH)D and ITAC score (natural skin colour). Natural skin colour accounted for 13.5% of variation in serum 25(OH)D, with every 10° increase in ITAC score (i.e., lighter skin) leading to a 9 nmol/L decrease in serum 25(OH)D. Multiple linear regression using age, ITAC score, and average UV index in the three months prior to testing, significantly predicted serum 25(OH)D concentrations (R2 = 29.7%). Single nucleotide polymorphisms for all vitamin D genes tested, showed lower serum 25(OH)D for those with the rare genotype compared to the common genotype; this was most pronounced for fok1 and rs4588, where those with the rare genotype were insufficient (<50 nmol/L), and those with the common genotype were sufficient (≥50 nmol/L). Assessing vitamin D status in individuals with morbid obesity requires testing of 25(OH)D, but potential risk factors for this population include natural skin colour and age.
Keywords: vitamin D, morbid obesity, sun exposure, skin colour, biomarkers, micronutrients
1. Introduction
Vitamin D refers to a group of fat-soluble secosteroids that act as a hormone in the body. There are five forms of vitamin D, of which vitamin D2 and vitamin D3 are physiologically important. Classical physiological roles for vitamin D include calcium homeostasis and bone metabolism [1], but in recent years, a more varied role for vitamin D has been identified [2,3]. The majority of vitamin D3 is produced endogenously in the skin from dehydro-cholesterol after exposure to ultraviolet B (UVB) rays. Vitamin D2 and vitamin D3 are also found in supplements and some food sources. Vitamin D is transported in the blood, attached to a binding protein, and is metabolised in the liver to 25-hydroxyvitamin D (25(OH)D), and in the kidneys to 1α,25-dihydroxyvitamin D (1,25(OH)2D). The majority of the active form of vitamin D, 1,25(OH)2D, is produced in the kidneys, although almost all tissues in the body have the ability to produce it [4]. 1,25(OH)2D has both genomic and non-genomic effects, through either a nuclear or membrane receptor [5,6,7,8].
Assessing an individual’s vitamin D status is a difficult task; currently, serum 25(OH)D concentration is used as a biomarker for vitamin D status. There are many definitions for sufficiency. The Endocrine Society define deficiency as <50 nmol/L (<20 ng/mL), and insufficiency as 52.5–72.5 nmol/L (21–29 ng/mL) [9]. In Australia, serum 25(OH)D concentrations ≥50 nmol/L are considered sufficient for the general population, with graded concentrations of insufficiency; mild (49–30 nmol/L), moderate (29–12.5 nmol/L), and severe (<12.5 nmol/L) [10]. Higher concentrations are recommended for specific sub-groups, e.g., >60 nmol/L for falls prevention in the elderly [11], and >82.5 nmol/L for reducing colorectal cancer risk [2]. In Australia, deficiency affects around 6% of the population in summer and around 49% of the population in winter [12]. In the USA, around 32% of the population are classified as deficient [13]. There are many issues to consider when assessing a persons’ vitamin D status, including age, gender, physical activity levels, sun exposure, skin colour, diet, and supplement intake. The influence of individual genetics of the person assayed may also affect their status. Single nucleotide polymorphisms (SNPs) in the genes that encode for the vitamin D receptor (VDR) [14,15,16] and vitamin D binding protein (DBP) [17,18] have the potential to influence the activity of 1,25(OH)2D.
The majority of vitamin D comes from endogenous production that requires exposure of the skin to UVB rays from sunlight. In assessing a person’s vitamin D status, information regarding sun exposure can help identify those at risk of deficiency. Several questionnaires have been developed to assess sun exposure using a combination of questions about clothing, time spent outdoors, sunscreen use and skin colour [19,20]. Those with darker skin colour appear to require long periods of UV exposure to reach sufficient serum 25(OH)D concentrations [21]. Conversely, those with very light skin are also at risk due to increased sun protective behaviours [22]. Previously, measures of natural and tanned skin colour using spectrophotometry have identified associations with vitamin D status [23]. It has been postulated that tanned skin colour is an important determinant of 25(OH)D status [23]. This suggests that the natural skin colour of a person is not as important as the amount of sun exposure they receive when assessing vitamin D status.
Vitamin D deficiency is very common in obese populations, including bariatric patients [24,25]. There is an inverse correlation between obesity and low vitamin D status, but it is not clear whether vitamin D is a cause or a consequence of obesity. Strengthening the link between the two are the associations between vitamin D deficiency and many of the co-morbidities associated with obesity [26,27,28,29]. There are several theories on the link between vitamin D deficiency and obesity. One of these is volumetric dilution of 25(OH)D through the greater tissue mass of obese individuals, thereby limiting the 25(OH)D in the blood and indicating a lower vitamin D status. Reduced sun exposure, sun protective behaviours and covering of skin could also impact endogenous vitamin D production [24,30]. Both liver and kidney disease are common in obese populations and can impair metabolism of vitamin D to 25(OH)D and then the hormonally-active form—1,25(OH)2D [28,31]. Rare alleles for SNPs in the VDR and DBP have been associated with higher body weight and body mass index (BMI), and lower vitamin D status [32,33,34,35], suggesting a potential genetic link between body weight and vitamin D status.
As vitamin D deficiency is an important and common issue for obese individuals, we aimed to investigate the relationship between several factors, including BMI, sun exposure, and skin colour, with vitamin D status in a group of morbidly obese individuals. The aim of this paper is to identify factors beyond the standard 25(OH)D measurement that may aid in assessing vitamin D status of morbidly obese individuals.
2. Materials and Methods
2.1. Participants
Participants were recruited as part of a study into vitamin D supplementation post bariatric surgery at the Wesley Hospital, Brisbane, Australia. The data presented here are the pre-surgical information collected. Inclusion criteria included: age ≥18 years, and accepted for bariatric surgery by surgical team. The surgical team use the AACE/TOS/ASMBS Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient to assess patient suitability for surgery [36]. Exclusion criteria included: pregnancy, age <18 years, taking medications that affected vitamin D levels, vitamin D supplement use in the last three months, or having liver or kidney disease. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Human Research Ethics Committee of the University of Queensland (#2015000446) and Uniting Care Human Research Ethics Committee (#1502).
2.2. Study Design
In this cross-sectional study, participants were recruited at the time of their initial consultation with the bariatric surgeon. Participant characteristics were collected from study enrolment forms and clinical records. Data collected included pre-surgery 25(OH)D status, age, gender, BMI, season of vitamin D testing, sun exposure behaviours, and assessment of skin colour using spectrophotometry.
2.3. Data Collection
2.3.1. Anthropometry
Weight (kg) and height (m) were measured to the nearest 0.1 kg and 1 cm. Weight and height were measured using digital column scales (SECA 769, Chino, CA, USA). BMI was calculated using weight (kg)/height (m)2.
2.3.2. Biochemistry
As part of standard care, the surgical team requested the following biochemical parameters from serum: 25(OH)D, parathyroid hormone, calcium, iron studies (iron, ferritin, transferrin), full blood count (red blood cells, white blood cells, platelets), and liver function tests (aspartate aminotransferase, alkaline phosphatase, alanine aminotransferase, albumin, bilirubin). Participants used one of two pathology laboratories available throughout Queensland. Participants’ biochemistry results were collected, where available, pre-surgery, 3 months, 6 months, and 12 months post-surgery. Parathyroid hormone was measured by immunoassay (Centaur XP; Siemens, Tarrytown, NY, USA or Cobas 8000 E602; Roche Diagnostics, Mannheim, Germany). Calcium was measured by immunoassay (Architect I2000sr; Abbott, Abbott Park, IL, USA or Advia 2400; Siemens, Tarrytown, NY, USA). Iron studies were measured by immunoassay (Architect I2000sr; Abbott, Abbott Park, IL, USA or Advia 2400; Siemens, Tarrytown, NY, USA). Full blood counts were measured by XN-10 Hematology Analyser (Sysmex, Kobe, Japan). Liver function tests were measured using immunoassay (Advia 2400; Siemens, Tarrytown, NY, USA).
Vitamin D was measured with automated chemiluminescent competitive immunoassay (Liaison XL; DiaSorin, Stillwater, MN, USA or ADVIA Centaur XP; Siemens, Tarrytown, NY, USA). The Liaison XL measurement range is 10–375 nmol/L (4–150 ng/mL). It is reported to demonstrate equimolar cross-reactivity with 25(OH)D3 (100%) and 25(OH)D2 (104%), and cross reactivity of <1% with 3-epi-25(OH)D3 [37]. Precision analysis for Liaison XL have been reported between 12.6 and 10.8% [38]. The Centaur XP measurement range is 10.5–375 nmol/L (4.2–150 ng/mL). It is reported to demonstrate equimolar cross-reactivity with 25(OH)D3 (100.7%) and 25(OH)D2 (104.5%), and cross-reactivity of 1.1% with 3-epi-25(OH)D3 [39]. Precision analysis for Centaur XP have been reported between 4.2 and 11.9% [38]. Both laboratories use the Royal College of Pathologists of Australasia Quality Assurance Program for vitamin D, and one uses the Vitamin D External Quality Assessment Scheme. Vitamin D status was defined using the following ranges: sufficient ≥50 nmol/L, mildly insufficient 49–25 nmol/L, moderately insufficient 24–12.5 nmol/L, and severely insufficient <12.5 nmol/L [10].
2.3.3. Sun Exposure
Participants completed a questionnaire on sun exposure and clothing worn on either workdays or non-workdays in the last three months, based on a previously validated survey [40]. From this data, an average sun exposure time per day was calculated (min/day). Using a modified rule of nines method for estimating percentage of Body Surface Area (%BSA) in individuals with obesity [41], an average daily %BSA exposed to the sun was calculated from information on clothing worn each day.
2.3.4. Skin Colour
Skin colour measurements were taken using a Spectrophotometer CM-2600/D (Konica Minolta, Tokyo, Japan). This instrument measures skin reflectance of light within the wavelength range of 360 nm to 740 nm. The data is reported using the Commission Internationale de L’Eclairage L*a*b* system. Where L* indicates the lightness or brightness of the skin [42]. Readings were taken on the inner arm (natural skin colour) and the outer forearm (tanned skin colour) using the specular component included (SCI) results for L*a*b. Individual typology angles were calculated using the following formula: ITA = (ArcTangent ((L − 50)/b)) × 180/π [43]. Skin colour was then classified using the ITA into the following groups: very light > 55 > light > 41 > intermediate > 28 > tanned > −10 > brown > −30 > dark [43]. ITA calculations were used to create a measure of tan by subtracting the natural skin colour score (ITAC) from the tanned skin colour score (ITAF), i.e., the difference in ITA score between natural and tanned skin.
2.3.5. UV Index
The average UV index in the three months prior to vitamin D testing was recorded for each participant using the data from the Australian Radiation Protection and Nuclear Safety Agency (http://www.arpansa.gov.au). Average UV Index in the three months prior to testing was used as it can take 2–5 months for serum 25(OH)D concentrations to plateau, and it is suggested to not retest for three months [10].
2.3.6. Dietary Vitamin D Intake
Participants completed a diet questionnaire based on a previously validated food frequency questionnaire [44]. Serve sizes were based on the Australia Guide to Healthy Eating [45]. Vitamin D3 equivalents per serve were calculated based on the NUTTAB 2011-12 Vitamin D food database [46]. The NUTTAB 2011-12 Vitamin D database determined vitamin D3, 25(OH)D3, vitamin D2, and 25(OH)D2 content using normal phase high-performance liquid chromatography, with ultraviolet detection, on an extract of saponified samples of each food. Vitamin D equivalents were calculated with a factor that takes into account the potentially higher bioavailability of the 25-hydroxy forms of vitamin D. Dietary vitamin D equivalents intake for each participant was calculated for foods containing vitamin D, by calculating the vitamin D equivalents per serve, and multiplying by the minimum number of serves per week indicated by the participant’s response (see Table 1).
Table 1.
Vitamin D equivalents calculations by response option.
| Vitamin D Source | Serve Size | 0–1 Serves Per Week (μg/week) | 1 to 4 Serves Per Week (μg/week) | 5+ Serves Per Week (μg/week) |
|---|---|---|---|---|
| Beef | 65 g cooked | 0.0 | 0.3 | 1.3 |
| Canned fish, tuna | 85 g | 0.0 | 2.0 | 10.2 |
| Mushrooms | 75 g | 0.0 | 1.7 | 8.6 |
| Eggs, whole | 120 g | 0.0 | 2.5 | 12.6 |
| Milk, whole | 250 mL | 0.0 | 0.3 | 1.5 |
| Salmon | 100 g | 0.0 | 20.0 | 100.0 |
| Diary blend spread | 10 g | 0.0 | 1.0 | 5.0 |
Based on source: NUTTAB 2010 (Food Standards Australia New Zealand); The University of New South Wales; Professor Heather Greenfield and co-workers at the University of New South Wales; Tables of composition of Australian Aboriginal Foods (J Brand-Miller, KW James and PMA Maggiore).
2.3.7. Single Nucleotide Polymorphisms
DNA was extracted from whole blood samples from 45 participants using QIAamp DNA Blood Mini kit (#51104, Qiagen, Hilden, Germany). Five SNPs were genotyped using a MassARRAY System (Agena Bioscience, San Diego, CA, USA), conducted by the Australian Genomics Research Facility, The University of Queensland, Brisbane, Australia. Participants were identified as either common homozygous, heterozygous, or rare homozygous for each SNP (see Table 2). Global minor allele frequency data was sourced from 1000 Genomes [47].
Table 2.
Single nucleotide polymorphisms of interest.
| SNP | Gene | MAF | Common Homozygous | Heterozygous | Rare Homozygous |
|---|---|---|---|---|---|
| Rs1544410 (bsm1) | VDR | 0.2959 | GG | GA | AA |
| Rs2228570 (fok1) | VDR | 0.3285 | CC | TC | TT |
| Rs731236 (taq1) | VDR | 0.2766 | TT | TC | CC |
| Rs4588 | DBP | 0.2079 | CC | CA | AA |
| Rs7041 | DBP | 0.3816 | GG | GT | TT |
MAF minor allele frequency.
2.4. Statistical Analysis
Statistical analysis was conducted using SPSS 24 (IBM Corp. Released 2015. IBM SPSS Statistics for Macintosh, Version 24.0. Armonk, NY, USA: IBM Corp.). Variables were assessed for normality and transformed where possible. Pearson correlation and Spearman Rank Correlation were used where appropriate. Linear and multiple regression models were used to determine the effect of independent variables on serum 25(OH)D. One-way ANCOVA was used to determine significant differences between mean serum 25(OH)D concentrations while accounting for covariates, and Bonferroni multiple comparisons test was used to identify significant differences between groups. Allelic frequencies were tested against Hardy–Weinburg equilibrium. Significance was set at p < 0.05.
3. Results
3.1. Characteristics
See Table 3 for participant characteristics. Fifty participants were recruited (80% female), the age range was 23 to 61 years, 70% were Obese Class III (>40 kg/m2), 58% were vitamin D sufficient (>50 nmol/L), 35% were vitamin D insufficient (<50 nmol/L), and serum 25(OH)D concentrations ranged from 21 to 103 nmol/L with a normal distribution. The majority (83%) had very light/light constitutive skin colour, and 74% had intermediate/tanned facultative skin colour. Sun exposure times ranged from 0 to 309 min/day, and body surface area exposed to the sun ranged from 0 to 52.5%. Skin colour measurements were not conducted on three participants due to equipment malfunction. Average sun exposure time, body surface exposure and dietary vitamin D intake were not reported in all participants, due to missing or inaccurate data reported in the sun and diet questionnaires.
Table 3.
Participant characteristics.
| n | Mean (SD) | 95% CI | |
|---|---|---|---|
| Weight (kg) | 50 | 126.7 (24.4) | 119.8–133.7 |
| BMI (kg/m2) | 50 | 43.9 (7.3) | 41.8–46.0 |
| Plasma 25(OH)D (nmol/L) | 48 | 56.8 (20.3) | 50.9–62.7 |
| Natural skin colour (ITAC score) | 47 | 50.2 (8.2) | 47.8–52.6 |
| Tanned skin colour (ITAF score) | 47 | 26.6 (11.7) | 23.1–30.0 |
| Degree of tan (ITAC–ITAF) | 47 | 23.6 (8.2) | 21.2–26.0 |
| Average sun exposure (min/day) | 42 | 65.4 (64.9) | 45.2–85.7 |
| Average BSA exposed (%) | 41 | 17.6 (14.9) | 12.9–22.3 |
| Dietary Vitamin D (ug/day) | 40 | 1.9 (1.4) | 1.4–2.4 |
BMI body mass index, BSA body surface area, 25(OH)D 25-hydroxyvitamin D, SD standard deviation.
3.2. Correlation Analysis
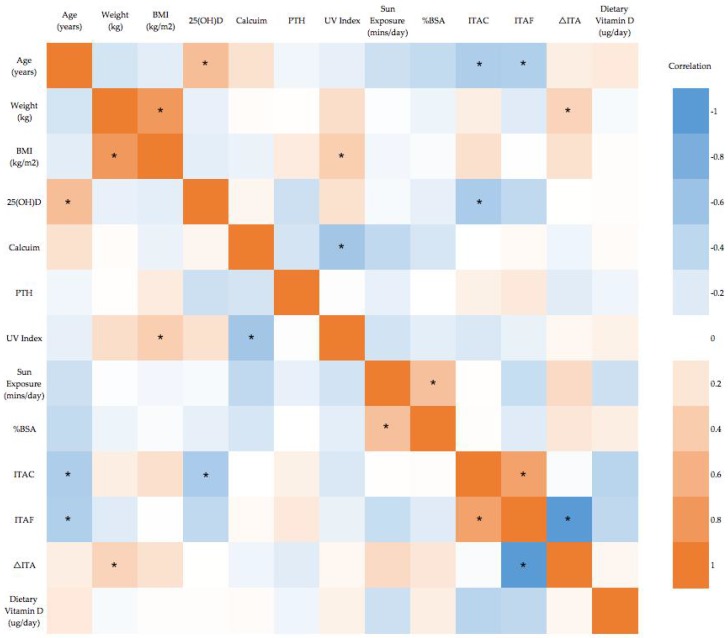
Pearson’s or Spearman’s rank correlations were run between all variables (Figure 1). Significant correlations with serum 25(OH)D were found for age and natural skin colour (ITAC). Correlation between serum 25(OH)D and tanned skin colour trended toward significance (p = 0.074).
Figure 1.
Correlation heat map for all variables. Blue indicates strong negative correlation and Orange indicates strong positive correlation. * p < 0.05. BMI body mass index, PTH parathyroid hormone, UV ultraviolet, BSA body surface area, ITA individual typology angle.
3.3. Weight and BMI
No significant correlation was found between weight or BMI, and serum 25(OH)D concentrations. A one-way ANOVA was conducted to determine if serum 25(OH)D concentrations were different between weight or BMI quartiles. There was no significant difference in serum 25(OH)D concentration between weight quartiles, F(3, 44) = 0.305, p = 0.822, or BMI quartiles, F(3, 44) = 1.041, p = 0.384 (Table 4).
Table 4.
Serum 25(OH)D concentrations by weight and BMI quartile. Serum 25(OH)D is presented as mean ± standard deviation.
| Quartile | Weight (kg) | Serum 25(OH)D (nmol/L) | BMI (kg/m2) | Serum 25(OH)D (nmol/L) |
|---|---|---|---|---|
| 1 | ≤107 kg | 59.5 ± 21.5 | ≤39 | 58.9 ± 22.4 |
| 2 | 108–123 kg | 59.8 ± 18.3 | 40–42.6 | 64.1 ± 22.3 |
| 3 | 124–141 kg | 54.2 ± 23.7 | 42.7–46.8 | 50.5 ± 18.2 |
| 4 | ≥142 kg | 53.7 ± 18.1 | ≥42.6 | 53.8 ± 17.5 |
3.4. Dietary Vitamin D
Dietary intake of vitamin D is predicted to contribute 5–10% of total vitamin D intake in Australian populations [10]. The Recommended Daily Intakes for vitamin D are 5 μg/day for those aged 19–50 years, and 10 μg for those aged 51–70 years. Average maximum dietary intake prior to surgery was calculated as 1.9 μg/day for this group of individuals, with a range of 0 to 4.7 μg/day. The average Australia adult is estimated to obtain 1.2 to 2.6 μg/day [48] showing this population is within the normal range for Australian adults. There was no significant correlation between dietary vitamin D and serum 25(OH)D concentrations (Figure 1).
3.5. Skin Colour
There was a significant correlation between natural skin colour (ITAC) and serum 25(OH)D concentrations, a trend towards a significant correlation between tanned skin colour (ITAC) (p = 0.074) and serum 25(OH)D concentrations, and no significant correlation between degree of tan (ΔITA) and serum 25(OH)D concentrations (Figure 1). ITAC was significantly correlated with age, (r = −0.315, p = 0.015) and ITAF (r = 0.716, p < 0.01). ITAF was significantly correlated with age (r = −0.340, p = 0.019), and change in ITA (r = 0.719, p < 0.01).
Linear regression analysis was completed to assess the relationship between natural skin colour (ITAC) and serum 25(OH)D concentrations. Natural skin colour accounted for 13.5% of the variation in serum 25(OH)D concentrations, (adj. R2 = 11%). Natural skin colour significantly predicted serum 25(OH)D concentrations, F(1, 43) = 6.711, p = .013. For each 10° increase of ITAC score (i.e., lighter natural skin colour), serum 25(OH)D concentrations decrease by 9 nmol/L. Serum 25(OH)D concentrations were predicted using this model for each skin colour group (Table 5).
Table 5.
Predicted serum 25(OH)D concentrations from linear regression model for ITAC score.
| ITAC Score | Serum 25(OH)D Range (nmol/L) |
|---|---|
| Very light | <53 |
| Light | 54–66 |
| Intermediate | 67–78 |
| Tanned | 79–95 |
| Brown | 96–132 |
| Dark | >133 |
Linear regression analysis was completed to assess the relationship between tanned skin colour (ITAF) and serum 25(OH)D concentrations. Tanned skin colour accounted for 7.2% of the variation in serum 25(OH)D concentrations, (adj. R2 = 5.1%). Tanned skin colour was trending toward significance with serum 25(OH)D concentrations, F(1, 43) = 3.344, p = 0.074. For each 10° decrease in ITAF score (i.e., darker tan) serum 25(OH)D concentrations increase by 5 nmol/L. Serum 25(OH)D concentrations were predicted using this model for each skin colour group (Table 6).
Table 6.
Predicted serum 25(OH)D concentrations from linear regression model for ITAF score.
| ITAF Score | Serum 25(OH)D Range (nmol/L) |
|---|---|
| Very light | <44 |
| Light | 45–51 |
| Intermediate | 52–57 |
| Tanned | 58–66 |
| Brown | 67–85 |
| Dark | >85 |
3.6. Sun Exposure
Sun exposure was measured through the sun exposure questionnaire, where participants reported the times spent in direct sunlight, and clothing worn at that time. From this information, an average minutes of sun exposure per day and the %BSA exposed to the sun were calculated. There was no correlation between %BSA or sun exposure minutes and serum 25(OH)D concentrations (Figure 1). Sun exposure minutes and %BSA were significantly positively correlated (r = 0.486, p = 0.001), suggesting those who spent more time in the sun had more skin exposed.
3.7. Single Nucleotide Polymorphisms
No statistically significant differences in mean serum 25(OH)D concentration were found for any SNP genotype (Table 7); however, lower serum 25(OH)D concentrations were found in the rare genotype compared to the common genotype for all vitamin D SNPs (bsm1, taq1, fok1, rs4588, rs7041). Combinations of two rare genotypes were also examined for any differences in serum 25(OH)D concentrations, weight, or BMI. No significant differences in serum 25(OH)D, weight or BMI were found between those with or without both rare genotypes for bsm1 and taq1 (n = 8), or rs4588 and rs7041 (n = 14), or fok1 and rs4588 (n = 10).
Table 7.
Minor allele frequencies for each SNP, and mean serum 25(OH)D concentration, weight, and BMI by genotype for SNPs of interest. p values calculated from one-way ANOVAs.
| SNP | MAF | Genotype | n | Serum 25(OH)D (nmol/L) | p Value | Weight (kg) | p Value | BMI (kg/m2) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Rs1544410 (bsm1) | GG | 18 | 57.8 (22.9) | 0.820 | 118.4 (23.9) | 0.340 | 42.5 (7.5) | 0.426 a | |
| GA | 17 | 54.2 (17.1) | 130.7 (27.8) | 45.5 (8.8) | |||||
| 0.38 | AA b | 8 | 53.4 (19.0) | 126.0 (18.1) | 44.9 (6.2) | ||||
| Rs2228570 (fok1) | CC | 18 | 59.5 (20.6) | 0.238 | 123.4 (31.5) | 0.585 a | 44.0 (9.7) | 0.342 a | |
| CT | 19 | 55.6 (20.1) | 124.1 (18.4) | 43.7 (6.8) | |||||
| 0.36 | TT b | 6 | 43.7 (11.1) | 130.5 (22.7) | 45.8 (3.6) | ||||
| Rs731236 (taq1) | TT | 19 | 57.3 (22.4) | 0.878 | 117.7 (23.4) | 0.213 | 42.2 (7.4) | 0.238 a | |
| TC | 16 | 54.7 (17.6) | 132.4 (27.8) | 46.0 (8.7) | |||||
| 0.37 | CC b | 8 | 53.4 (19.0) | 126.0 (18.1) | 44.9 (6.2) | ||||
| Rs4588 | CC | 23 | 56.9 (20.4) | 0.210 | 121.0 (25.7) | 0.117 a | 44.0 (7.5) | 0.440 a | |
| CA | 16 | 57.8 (19.0) | 125.4 (24.8) | 42.9 (7.7) | |||||
| 0.28 | AA b | 4 | 39.0 (12.7) | 142.7 (8.7) | 49.4 (9.8) | ||||
| Rs7041 | GG | 14 | 59.6 (20.9) | 0.601 | 123.4 (31.6) | 0.275 a | 45.7 (8.7) | 0.237 a | |
| GT | 23 | 54.4 (19.1) | 122.6 (21.1) | 42.0 (6.6) | |||||
| 0.41 | TT b | 6 | 50.7 (20.6) | 135.5 (20.5) | 48.3 (8.5) |
a Kruskal–Wallis H test; b homozygous risk genotype, SNP single nucleotide polymorphism, MAF minor allele frequency, BMI body mass index.
3.8. Multiple Regression Model
A multiple regression model was developed to identify the contributions of independent variables to serum 25(OH)D concentrations. Variables were chosen based on their univariate relationship with serum 25(OH)D concentrations, and effect sizes ≥ intermediate. Independent variables included in the model were age, ITAC, and UV Index. The multiple regression model statistically significantly predicted serum 25(OH)D concentrations, F(3, 41) = 7.202, p = 0.001, adj. R2 = 29.7%, intermediate effect size [49] (Table 8). Age was the only variable that significantly predicted serum 25(OH)D, showing each year increase in age is associated with an increase of 0.9 nmol/L serum 25(OH)D.
Table 8.
Multiple regression coefficients.
| Model | B | SEB | β | p Value |
|---|---|---|---|---|
| Constant | 19.588 | 28.908 | 0.502 | |
| Age | 0.932 | 0.273 | 0.467 | 0.001* |
| ITAC | −0.444 | 0.346 | −0.176 | 0.206 |
| UV Index | 3.528 | 1.965 | 0.232 | 0.080 |
* p < 0.05, B unstandardized coefficient, SEB standard error of unstandardized coefficient, β beta, ITA individual typology angle, UV ultraviolet.
4. Discussion
Determinants of vitamin D status in morbidly obese individuals were examined, and factors considered were dietary vitamin D intake, BMI, skin colour, and sun exposure. The key findings were (i) natural skin colour accounted for 13.5% of the variation in serum 25(OH)D concentrations; (ii) there was a significant positive association between age and serum 25(OH)D; (iii) weight and BMI were not significantly associated with serum 25(OH)D concentrations; (iv) there was no relationship between sun exposure time, or amount of skin exposed, with 25(OH)D concentrations in this group.
Natural skin colour (ITAC score) accounted for 13.5% of the variation in serum 25(OH)D concentrations. Results showed that as natural skin colour becomes darker, serum 25(OH)D concentrations increased, suggesting those with darker natural skin colour have higher 25(OH)D concentrations. ITAC score was also used to predict maximal mean serum 25(OH)D concentrations for each skin colour category. It is well documented that those with darker natural skin colour have lower serum 25(OH)D concentrations [21,50]. As this study had only recruited participants with an intermediate skin colour or lighter, it is possible the increase in 25(OH)D concentration with increasing natural skin colour was due to sun protective behaviours from those with lighter skin. For similar reasons, the predictive model became unreliable when dealing with those in the tanned and brown skin colour categories. Although when comparing changes in ITA score i.e., degree of tanning, sun exposure times, and %BSA, there was no correlation between any of these measures and natural skin colour, potentially suggesting no differences in sun protective behaviours regardless of natural skin colour.
A trend towards significance was seen between tanned skin colour (ITAF score) and serum 25(OH)D concentrations. Tanned skin colour accounted for 7.2% of the variation in serum 25(OH)D concentrations in this group. As ITAF score increased (i.e., darker tan), 25(OH)D concentrations increased, suggesting that those with a darker tan (up to intermediate) will have higher 25(OH)D concentrations. Previous research has shown that tanned skin colour is a significant predictor of 25(OH)D [23]. This result came from a larger group but with similar proportions of participants in each skin colour group. Our results confirm those of Rockell et al. [23], showing that for each 10° decrease in ITAF score (i.e., darker tan) serum 25(OH)D concentrations increase by 5 nmol/L [23].
The Australian Health Survey (2011–12) showed similar rates of deficiency between genders, and an increase in vitamin D status with age, concurrent with increases in supplement use [12]. Population data in Australia show varying effects of age on serum 25(OH)D, with some showing an increase with age [12,51] and others a decrease with age [19]. There was a significant positive association between age and serum 25(OH)D concentrations in our study. Further investigation of the variables showed that age positively correlated with natural skin colour and tanned skin colour, suggesting that the older participants had darker natural and tanned skin colour. There was no relationship between age and degree of tan, or %BSA exposed to the sun, or sun exposure time.
Weight and BMI were not significantly associated with serum 25(OH)D concentrations. There are several meta-analyses that have shown a significant decrease in serum 25(OH)D concentrations with increasing weight or BMI, although not all included morbidly obese individuals [52,53,54]. There are several studies in morbidly obese individuals that found a significant [55,56,57,58,59,60,61] or borderline significant [62,63] inverse relationship between BMI and 25(OH)D concentrations, with small to large effect sizes. It is possible that once a certain BMI threshold is reached, the dilution effects of obesity on vitamin D status plateau, and the effect becomes minimal, hence the trend of lower serum 25(OH)D in this study, but the lack of a significant difference. Sun exposure, or lack of it, appears to have a major influence on vitamin D status in Australian obese populations. A study into determinants of serum 25(OH)D in Australian Adults reported that the amount of skin exposed to the sun was the single largest contributor to serum 25(OH)D concentrations, followed by location, season, and personal UV radiation exposure [19]. BMI only explained 4% of the variance in their population, which include a wide range of BMI [19].
As the majority of vitamin D3 is produced endogenously in the skin, it is logical to expect that a relationship would exist between sun exposure times, the %BSA exposed, and vitamin D status. There was no relationship between sun exposure times or %BSA, with serum 25(OH)D concentrations in our study. Previous research into determinants of vitamin D status in Australian adults found a significant association between vitamin D status and time spent outdoors (rs = 0.16, p < 0.0001), and vitamin D status and the percentage of clothing cover (rs = −0.50, p < 0.001), in a population with a wide range of BMI, including individuals with obesity [19]. There are a few possible reasons why no relationship was identified between these measures and vitamin D status in our study. This study reports behaviours of a specific group of morbidly obese individuals, and so represented only their sun exposure practices, whereas the AusD study included a wide range of BMI and a larger sample size. The data provided on sun exposure times and body surface area exposed was self-reported data, and may not have been accurate. There was a trend toward a positive correlation between change in ITA (degree of tan) and time in the sun. This would be expected, as longer sun exposure would generally lead to an increase in tan, providing some evidence that this measure of sun exposure is accurate. Vitamin D is stored in adipose tissue [64], and there is some evidence that muscle may store 25(OH)D [65], thereby reducing the amount of 25(OH)D available in the circulation for measurement. This may impact the ability to correlate vitamin D status with any measures of skin colour, or sun exposure, especially in those with high amounts of adipose tissue or large muscle mass, as seen in this group of morbidly obese individuals.
Three SNPs for the VDR and two for the DBP were analysed in this group. No significant differences in serum 25(OH)D concentrations were seen between genotypes of any gene. Although all homozygous rare genotypes had lower mean serum 25(OH)D concentrations than the common homozygous genotype. From a clinical perspective, VDR fok1 and DBP rs4588 both indicated insufficient vitamin D status for those with homozygous rare genotypes compared to homozygous common genotypes. Rare alleles for all four of the main variants for the VDR gene have been associated with lower serum 25(OH)D concentration and vitamin D deficiency in a range of populations [15,16,17,66,67,68]. This suggests a possible method of personalised nutrition in this population, by considering specialised treatments for the patients with a genetic predisposition for lower serum 25(OH)D, based on the SNP genotype, particularly for fok1 and rs4588. This information could contribute to the overall assessment of vitamin D status, and identify those that may achieve more benefit from supplementation.
The VDR bsm1 rare allele has been associated with higher weight, BMI [32,33], and lower percentage of excess weight loss in bariatric patients [69]. The taq1 rare allele has also been associated with higher BMI and weight [34]. The fok1 rare allele has not been associated with weight and BMI previously [70,71,72]. In this group of morbidly obese individuals, there was no statistically significant difference in weight, BMI or %EWL, between rare or common genotypes for bsm1, taq1, and fok1. Of clinical significance was the difference in mean serum 25(OH)D concentrations between common homozygous and rare homozygous individuals for fok1 and rs4588, where the common homozygous genotype was vitamin D sufficient, and the rare homozygous genotype was vitamin D deficient.
Both DBP SNP common alleles have been associated with higher BMI in females, but not males [35]. We found no significant differences in weight or BMI between the genotypes for both DBP SNPs. The difference in results could be related to differences in sample populations, this study only included patients with BMI > 30, whereas the previous study included a wide range of BMI (16.93–57.21 kg/m2) [35]. It is important to note that the SNP analysis from this study was under powered, and so results must be considered with caution. Particularly those that conflict with results from adequately powered studies. Post hoc power analysis using G*Power [73] indicated that power ranged from 6 to 63% for these analyses, and required sample sizes from 69 to 957 for 80% power.
There are several limitations to this study. Serum 25(OH)D concentrations were measured using chemiluminescent competitive immunoassays, on two different platforms. The gold standard in measuring 25(OH)D concentration is liquid chromatography with tandem mass spectrometry. Issues with measuring 25(OH)D arise from the ability of these assays to release 25(OH)D from its binding protein or other carriers like albumin, the hydrophobic properties of 25(OH)D, and the differing antibody specificities to the metabolites [9]. Although both pathology laboratories reported using quality assurance programs, both platforms have potential issues with under or over recovering metabolites, negative biases, and large deviations at lower concentrations of 25(OH)D (~50 nmo/L) [38]. This highlights an issue for clinicians in interpreting results for the 25(OH)D assay. Recruitment did not occur over a full year, this led to lower numbers of participants recruited post summer and autumn. This influences the results of differences in serum 25(OH)D between seasons, and potentially, the range of tanned skin colours. Using a small range of BMI has also minimised the effects of weight and BMI on serum 25(OH)D; use of a wider range, including normal, overweight, and obese individuals may have shown a more pronounced effect. A similar issue is found with the skin colour assessments, as individuals recruited only had natural skin colour from very light to intermediate. This limits the ability of results to be applied to those with darker natural skin colour. Our sample size was also under powered for SNP analysis, and so results should be considered with caution.
5. Conclusions
In this group of morbidly obese individuals lighter natural skin colour and younger age are potential risk factors for vitamin D insufficiency. It appears sun exposure time, the amount of skin exposed to the sun, weight and BMI, do not influence vitamin D status in this population. The vitamin D pathway SNPs investigated showed no statistically significant effects on vitamin D status, although clinically significant differences were found for VDR fok1 and DBP rs4588. Future research into the determinants of vitamin D status using a larger group of morbidly obese individuals could provide further information on the genetic susceptibility to vitamin D deficiency in this at-risk group. A group with a wider range of natural skin colours could also help to develop an understanding of the contribution of skin colour and tanned skin colour to vitamin D status.
Acknowledgments
This research was supported by The University of Queensland. Open access was funded by PhD funds. The authors would like to acknowledge the valuable support from the Wesley Medical Research (Project No. 2015-03).
Author Contributions
C.F.D. conceived and designed the study, collected and analysed data, and contributed to manuscript preparation. B.D.R. collected data, and contributed to manuscript preparation. I.M. collected data, and contributed to manuscript preparation. S.R. collected data, and contributed to manuscript preparation. J.B.P. conceived and designed the study, and contributed to manuscript preparation. J.D.B. conceived and designed the study, and contributed to manuscript preparation. O.R.L.W. conceived and designed the study, and contributed to manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Anderson P.H., Turner A.G., Morris H.A. Vitamin D actions to regulate calcium and skeletal homeostasis. Clin. Biochem. 2012;45:880–886. doi: 10.1016/j.clinbiochem.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Gorham E.D., Holick M.F., Garland C.F., Garland F.C., Grant W.B., Mohr S.B., Lipkin M., Newmark H.L., Giovannucci E., Wei M. Optimal vitamin D status for colorectal cancer prevention: A quantitative meta analysis. Am. J. Prev. Med. 2007;32:210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Szodoray P., Zeher M., Bodolay E., Nakken B., Gaal J., Jonsson R., Szegedi A., Zold E., Szegedi G., Brun J.G., et al. The complex role of vitamin D in autoimmune diseases. Scand. J. Immunol. 2008;68:261–270. doi: 10.1111/j.1365-3083.2008.02127.x. [DOI] [PubMed] [Google Scholar]
- 4.Bikle D.D. Extra renal synthesis of 1,25-dihydroxyvitamin D and its health implications. Clin. Rev. Bone Min. Metab. 2009;7:114–125. doi: 10.1007/s12018-009-9033-y. [DOI] [Google Scholar]
- 5.Yamamoto Y., Yoshizawa T., Fukuda T., Shirode-Fukuda Y., Yu T., Sekine K., Sato T., Kawano H., Aihara K.-I., Nakamichi Y., et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology. 2013;154:1008–1020. doi: 10.1210/en.2012-1542. [DOI] [PubMed] [Google Scholar]
- 6.Balesaria S., Sangha S., Julian R.F.W. Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:1193–1197. doi: 10.1152/ajpgi.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kriebitzsch C., Verlinden L., Eelen G., Tan B.K., Van Camp M., Bouillon R., Verstuyf A. The impact of 1,25(OH)2D3 and its structural analogs on gene expression in cancer cells—A microarray approach. Anticancer Res. 2009;29:3471–3483. [PubMed] [Google Scholar]
- 8.Norman A.W., Okamura W.H., Bishop J.E., Henry H.L. Update on biological actions of 1α,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D3. Mol. Cell. Endocrinol. 2002;197:1–13. doi: 10.1016/S0303-7207(02)00273-3. [DOI] [PubMed] [Google Scholar]
- 9.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M., Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 10.Nowson C.A., McGrath J.J., Ebeling P.R., Haikerwal A., Daly R.M., Sanders K.M., Seibel M.J., Mason R.S. Vitamin D and health in adults in Australia and New Zealand: A position statement. Med. J. Aust. 2012;196:686–687. doi: 10.5694/mja11.10301. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari H.A., Henschkowski J., Dawson-Hughes B., Staehelin H.B., Orav J.E., Stuck A.E., Theiler R., Wong J.B., Egli A., Kiel D.P. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. Br. Med. J. 2009;339:843–846. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Australian Bureau of Statistics . Australian Health Survey: First Results 2011–12. Australian Bureau of Statistics; Canberra, Australia: 2012. [Google Scholar]
- 13.Looker A.C., Johnson C.L., Lacher D.A., Pfeiffer C.M., Schleicher R.L., Sempos C.T. Vitamin D status: United States, 2001–2006. NCHS Data Brief. 2011;59:1–8. [PubMed] [Google Scholar]
- 14.Santos B.R., Mascarenhas L.P.G., Satler F., Boguszewski M.C.S., Spritzer P.M. Vitamin D deficiency in girls from South Brazil: A cross-sectional study on prevalence and association with vitamin D receptor gene variants. BMC Pediatr. 2012;12 doi: 10.1186/1471-2431-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitanaka S., Isojima T., Takaki M., Numakura C., Hayasaka K., Igarashi T. Association of vitamin D-related gene polymorphisms with manifestation of vitamin D deficiency in children. Endocr. J. 2012;59:1007–1014. doi: 10.1507/endocrj.EJ12-0143. [DOI] [PubMed] [Google Scholar]
- 16.Bora G., Ozkan B., Dayangaç-Erden D., Erdem-Yurter H., Coşkun T. Vitamin D receptor gene polymorphisms in Turkish children with vitamin D deficient rickets. Turk. J. Pediatr. 2008;50:30–33. [PubMed] [Google Scholar]
- 17.Santos B.R., Mascarenhas L.P.G., Boguszewski M.C.S., Spritzer P.M. Variations in the Vitamin D-binding protein (DBP) gene are related to lower 25-hydroxyvitamin D levels in healthy girls: A cross-sectional study. Horm. Res. Paediatr. 2013;79:162–168. doi: 10.1159/000348847. [DOI] [PubMed] [Google Scholar]
- 18.Cheung C.L., Lau K.S., Sham P.C., Tan K.C., Kung A.W. Genetic variant in vitamin D binding protein is associated with serum 25-hydroxyvitamin D and vitamin D insufficiency in southern Chinese. J. Hum. Genet. 2013;58:749–751. doi: 10.1038/jhg.2013.84. [DOI] [PubMed] [Google Scholar]
- 19.Kimlin M.G., Lucas R.M., Harrison S.L., van der Mei I., Armstrong B.K., Whiteman D.C., Kricker A., Nowak M., Brodie A.M., Sun J. The Contributions of solar ultraviolet radiation exposure and other determinants to serum 25-hydroxyvitamin D concentrations in Australian adults: The AusD study. Am. J. Epidemiol. 2014;179:864–874. doi: 10.1093/aje/kwt446. [DOI] [PubMed] [Google Scholar]
- 20.Yu C.-L., Li Y., Freedman D.M., Fears T.R., Kwok R., Chodick G., Alexander B., Kimlin M.G., Kricker A., Armstrong B.K., et al. Assessment of lifetime cumulative sun exposure using a self-administered questionnaire: Reliability of two approaches. Cancer Epidemiol. Biomark. Prev. 2009;18:464–471. doi: 10.1158/1055-9965.EPI-08-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall L.M., Kimlin M.G., Aronov P.A., Hammock B.D., Slusser J.R., Woodhouse L.R., Stephensen C.B. Vitamin D intake needed to maintain target serum 25-hydroxyvitamin D concentrations in participants with low sun exposure and dark skin pigmentation is substantially higher than current recommendations. J. Nutr. 2010;140:542–550. doi: 10.3945/jn.109.115253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malvy D.J.M., Guinot C., Preziosi P., Galan P., Chapuy M.C., Maamer M., Arnaud S., Meunier P.J., Hercberg S., Tschachler E. Relationship between vitamin D status and skin phototype in general adult population. Photochem. Photobiol. 2000;71:466–469. doi: 10.1562/0031-8655(2000)071<0466:RBVDSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Rockell J.E.P., Skeaff C.M., Williams S.M., Green T.J. Association between quantitative measures of skin color and plasma 25-hydroxyvitamin D. Osteoporos. Int. 2008;19:1639–1642. doi: 10.1007/s00198-008-0620-4. [DOI] [PubMed] [Google Scholar]
- 24.Wortsman J., Matsuoka L.Y., Chen T.C., Lu Z., Holick M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 25.Blum M., Dallal G.E., Dawson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J. Am. Coll. Nutr. 2008;27:274–279. doi: 10.1080/07315724.2008.10719700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calle E.E., Thun M.J. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 27.Apovian C.M., Gokce N. Obesity and cardiovascular disease. Circulation. 2012;125:1178–1182. doi: 10.1161/CIRCULATIONAHA.111.022541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eliades M., Hernaez R., Spyrou E., Agrawal N., Lazo M., Brancati F.L., Potter J.J., Koteish A.A., Clark J.M., Guallar E. Meta-analysis: Vitamin D and non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013;38:246–254. doi: 10.1111/apt.12377. [DOI] [PubMed] [Google Scholar]
- 29.Holick M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004;80:1678–1688. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 30.Norval M., Wulf H.C. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br. J. Dermatol. 2009;161:732–736. doi: 10.1111/j.1365-2133.2009.09332.x. [DOI] [PubMed] [Google Scholar]
- 31.González E.A., Sachdeva A., Oliver D.A., Martin K.J. Vitamin D insufficiency and deficiency in chronic kidney disease. Am. J. Nephrol. 2004;24:503–510. doi: 10.1159/000081023. [DOI] [PubMed] [Google Scholar]
- 32.Nasser M.A.-D., Franca R.G., Omar S.A.-A., Majed S.A., Khalid M.A., Hossam M.D., Cristina A., Andrea S.C., Irma S., Abdul Khader M., et al. Vitamin D receptor gene polymorphisms are associated with obesity and inflammosome activity. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0102141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye W.Z., Reis A.F., Dubois-Laforgue D., Bellanné-Chantelot C., Timsit J., Velho G. Vitamin D receptor gene polymorphisms are associated with obesity in type 2 diabetic subjects with early age of onset. Eur. J. Endocrinol. 2001;145:181–186. doi: 10.1530/eje.0.1450181. [DOI] [PubMed] [Google Scholar]
- 34.Vasilopoulos Y., Sarafidou T., Kotsa K., Papadimitriou M., Goutzelas Y., Stamatis C., Bagiatis V., Tsekmekidou X., Yovos J.G., Mamuris Z. VDR TaqI is associated with obesity in the Greek population. Gene. 2013;512:237–239. doi: 10.1016/j.gene.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 35.Almesri N., Das N.S., Ali M.E., Gumaa K., Giha H.A. Independent associations of polymorphisms in vitamin D binding protein (GC) and vitamin D receptor (VDR) genes with obesity and plasma 25OHD3 levels demonstrate sex dimorphism. Appl. Physiol. Nutr. Metab. 2016;41:345. doi: 10.1139/apnm-2015-0284. [DOI] [PubMed] [Google Scholar]
- 36.Mechanick J.I., Youdim A., Jones D.B., Garvey W.T., Hurley D.L., McMahon M.M., Heinberg L.J., Kushner R., Adams T.D., Shikora S. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21:S1–S27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiaSorin . DiaSorin; Stillwater, MN, USA: 2012. [(accessed on 27 September 2017)]. LIAISON 25 OH Vitamin D TOTAL Assay [directional insert] Available online: http://www.diasorin.com/en/liaisonr-25-oh-vitamin-d-total-assay. [Google Scholar]
- 38.Freeman J., Wilson K., Spears R., Shalhoub V., Sibley P. Performance evaluation of four 25-hydroxyvitamin D assays to measure 25-hydroxyvitamin D2. Clin. Biochem. 2015;48:1097–1104. doi: 10.1016/j.clinbiochem.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Siemens Healthineers . Siemens Healthcare Diagnostics Inc.; Tarrytown, NY, USA: 2013. [(accessed on 27 September 2017)]. ADVIA Centaur Vitamin D Total (VitD) Assay [directional insert 10699279_EN Rev. A, 2013–07] Available online: https://www.healthcare.siemens.com/laboratory-diagnostics/assays-by-diseases-conditions/bone-metabolism-assays/advia-centaur-vitamin-d-total-assay. [Google Scholar]
- 40.Vu L.H., Whiteman D.C., van der Pols J.C., Kimlin M.G., Neale R.E. Serum Vitamin D Levels in Office Workers in a Subtropical Climate. Photochem. Photobiol. 2011;87:714–720. doi: 10.1111/j.1751-1097.2011.00899.x. [DOI] [PubMed] [Google Scholar]
- 41.Neaman K.C., Andres L.A., McClure A.M., Burton M.E., Kemmeter P.R., Ford R.D. A new method for estimation of involved bsas for obese and normal-weight patients with burn injury. J. Burn Care Res. 2011;32:421–428. doi: 10.1097/BCR.0b013e318217f8c6. [DOI] [PubMed] [Google Scholar]
- 42.Taylor S., Westerhof W., Im S., Lim J. Noninvasive techniques for the evaluation of skin color. J. Am. Acad. Dermatol. 2006;54:S282–S290. doi: 10.1016/j.jaad.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 43.Chardon A., Cretois I., Hourseau C. Skin colour typology and suntanning pathways. Int. J. Cosmet. Sci. 1991;13:191–208. doi: 10.1111/j.1467-2494.1991.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 44.Collins C.E., Boggess M.M., Watson J.F., Guest M., Duncanson K., Pezdirc K., Rollo M., Hutchesson M.J., Burrows T.L. Reproducibility and comparative validity of a food frequency questionnaire for Australian adults. Clin. Nutr. 2014;33:906–914. doi: 10.1016/j.clnu.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 45.National Health and Medical Research Council . Australian Dietary Guidelines. National Health and Medical Research Council; Canberra, Australia: 2013. [Google Scholar]
- 46.Food Standards Australia New Zealand . NUTTAB 2010—Australian Food Composition Tables. Food Standards Australia New Zealand; Canberra, Australia: 2010. [Google Scholar]
- 47.The 1000 Genomes Project Consortium A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nowson C.A., Margerison C. Vitamin D intake and vitamin D status of Australians. Med. J. Aust. 2002;177:149–152. doi: 10.5694/j.1326-5377.2002.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 49.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. L. Erlbaum Associates; Hillsdale, NJ, USA: 1988. [Google Scholar]
- 50.Libon F., Cavalier E., Nikkels A.F. Skin color is relevant to vitamin D synthesis. Dermatology. 2013;227:250–254. doi: 10.1159/000354750. [DOI] [PubMed] [Google Scholar]
- 51.Gill T.K., Hill C.L., Shanahan E.M., Taylor A.W., Appleton S.L., Grant J.F., Shi Z., Dal Grande E., Price K., Adams R.J. Vitamin D levels in an Australian population. BMC Public Health. 2014;14:1001. doi: 10.1186/1471-2458-14-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pereira-Santos M., Costa P.R.F., Assis A.M.O., Santos C.A.S.T., Santos D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015;16:341–349. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 53.Yao Y., Zhu L., He L., Duan Y., Liang W., Nie Z., Jin Y., Wu X.L., Fang Y. A meta-analysis of the relationship between vitamin D deficiency and obesity. Int. J. Clin. Exp. Med. 2015;8:14977–14984. [PMC free article] [PubMed] [Google Scholar]
- 54.Saneei P., Salehi-Abargouei A., Esmaillzadeh A. Serum 25-hydroxy vitamin D levels in relation to body mass index: A systematic review and meta-analysis. Obes. Rev. 2013;14:393–404. doi: 10.1111/obr.12016. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen V.T., Li X., Elli E.F., Ayloo S.M., Castellanos K.J., Fantuzzi G., Freels S., Braunschweig C.L. Vitamin D, inflammation, and relations to insulin resistance in premenopausal women with morbid obesity. Obesity. 2015;23:1591–1597. doi: 10.1002/oby.21131. [DOI] [PubMed] [Google Scholar]
- 56.Boonchaya-Anant P., Holick M., Apovian C. Serum 25-Hydroxyvitamin D Levels and Metabolic Health Status in Extremely Obese Individuals. Obesity. 2014;22:2539–2543. doi: 10.1002/oby.20877. [DOI] [PubMed] [Google Scholar]
- 57.Censani M., Stein E.M., Shane E., Oberfield S.E., McMahon D.J., Lerner S., Fennoy I. Vitamin D Deficiency Is Prevalent in Morbidly Obese Adolescents Prior to Bariatric Surgery. ISRN Obesity. 2013;2013 doi: 10.1155/2013/284516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fish E., Beverstein G., Olson D., Reinhardt S., Garren M., Gould J. Vitamin D Status of Morbidly Obese Bariatric Surgery Patients. J. Surg. Res. 2010;164:198–202. doi: 10.1016/j.jss.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 59.Mahlay N.F., Verka L.G., Thomsen K., Merugu S., Salomone M. Vitamin D Status Before Roux-en-Y and Efficacy of Prophylactic and Therapeutic Doses of Vitamin D in Patients After Roux-en-Y Gastric Bypass Surgery. Obes. Surg. 2009;19:590–594. doi: 10.1007/s11695-008-9698-1. [DOI] [PubMed] [Google Scholar]
- 60.Aasheim E.T., Hofsø D., Hjelmesæth J., Birkeland K.I., Bøhmer T. Vitamin status in morbidly obese patients: A cross-sectional study. Am. J. Clin. Nutr. 2008;87:362–369. doi: 10.1093/ajcn/87.2.362. [DOI] [PubMed] [Google Scholar]
- 61.Ybarra J., Sanchez-Hernandez J., Gich I., De Leiva A., Rius X., Rodriguez-Espinosa J., Perez A. Unchanged hypovitarninosis D and secondary hyperparathyroidism in morbid obesity after bariatric surgery. Obes. Surg. 2005;15:330–335. doi: 10.1381/0960892053576758. [DOI] [PubMed] [Google Scholar]
- 62.Grace C., Vincent R., Aylwin S.J. High prevalence of vitamin D insufficiency in a United Kingdom urban morbidly obese population: Implications for testing and treatment. Surg. Obes. Relat. Dis. 2014;10:355–360. doi: 10.1016/j.soard.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 63.Goldner W.S., Stoner J.A., Thompson J., Taylor K., Larson L., Erickson J., McBride C. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: A comparison with non-obese controls. Obes. Surg. 2008;18:145–150. doi: 10.1007/s11695-007-9315-8. [DOI] [PubMed] [Google Scholar]
- 64.Mawer E.B., Backhouse J., Holman C.A., Lumb G.A., Stanbury S.W. The distribution and storage of vitamin D and its metabolites in human tissues. Clin. Sci. 1972;43:413–431. doi: 10.1042/cs0430413. [DOI] [PubMed] [Google Scholar]
- 65.Abboud M., Puglisi D.A., Davies B.N., Rybchyn M., Whitehead N.P., Brock K.E., Cole L., Gordon-Thomson C., Fraser D.R., Mason R.S. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology. 2013;154:3022. doi: 10.1210/en.2012-2245. [DOI] [PubMed] [Google Scholar]
- 66.Vupputuri M.R., Goswami R., Gupta N., Ray D., Tandon N., Kumar N. Prevalence and functional significance of 25-hydroxyvitamin D deficiency and vitamin D receptor gene polymorphisms in Asian Indians. Am. J. Clin. Nutr. 2006;83:1411–1419. doi: 10.1093/ajcn/83.6.1411. [DOI] [PubMed] [Google Scholar]
- 67.Mao S., Huang S. Vitamin D receptor gene polymorphisms and the risk of rickets among Asians: A meta-analysis. Arch. Dis. Child. 2014;99:232–238. doi: 10.1136/archdischild-2013-304379. [DOI] [PubMed] [Google Scholar]
- 68.Al-Daghri N.M., Al-Attas O.S., Alkharfy K.M., Khan N., Mohammed A.K., Vinodson B., Ansari M.G.A., Alenad A., Alokail M.S. Association of VDR-gene variants with factors related to the metabolic syndrome, type 2 diabetes and vitamin D deficiency. Gene. 2014;542:129–133. doi: 10.1016/j.gene.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 69.Alexandrou A., Armeni E., Kaparos G., Rizos D., Tsoka E., Deligeoroglou E., Creatsa M., Augoulea A., Diamantis T., Lambrinoudaki I. Bsm1 vitamin D receptor polymorphism and calcium homeostasis following bariatric surgery. J. Investig. Surg. 2015;28:8–17. doi: 10.3109/08941939.2014.943857. [DOI] [PubMed] [Google Scholar]
- 70.Gu J., Xiao W., He J., Zhang H., Hu W., Hu Y., Li M., Liu Y., Fu W., Yu J. Association between VDR and ESR1 gene polymorphisms with bone and obesity phenotypes in Chinese male nuclear families—Association between VDR and ESR1 gene polymorphisms with bone and obesity phenotypes in Chinese male nuclear families. Acta Pharmacol. Sin. 2009;30:1634–1642. doi: 10.1038/aps.2009.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ochs-Balcom H.M., Chennamaneni R., Millen A.E., Shields P.G., Marian C., Trevisan M., Freudenheim J.L. Vitamin D receptor gene polymorphisms are associated with adiposity phenotypes. Am. J. Clin. Nutr. 2011;93:5–10. doi: 10.3945/ajcn.2010.29986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Filus A. Relationship between vitamin D receptor BsmI and FokI polymorphisms and anthropometric and biochemical parameters describing metabolic syndrome. The Aging Male. 2008;11:134–139. doi: 10.1080/13685530802273426. [DOI] [PubMed] [Google Scholar]
- 73.Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]