Progesterone, testosterone, 17β-estradiol, cortisol, corticosterone, aldosterone, thyroxine and tri-iodothyronine are detectable in baleen of eight whale species. The presence of a broad array of hormones in baleen of multiple species indicates that baleen hormone analysis may be widely applicable for studies of reproduction, stress and metabolism of the large whales.
Keywords: Baleen, cetaceans, hormones, marine mammals, reproduction, stress
Abstract
Recent studies have demonstrated that some hormones are present in baleen powder from bowhead (Balaena mysticetus) and North Atlantic right (Eubalaena glacialis) whales. To test the potential generalizability of this technique for studies of stress and reproduction in large whales, we sought to determine whether all major classes of steroid and thyroid hormones are detectable in baleen, and whether these hormones are detectable in other mysticetes. Powdered baleen samples were recovered from single specimens of North Atlantic right, bowhead, blue (Balaenoptera [B.]musculus), sei (B. borealis), minke (B. acutorostrata), fin (B. physalus), humpback (Megaptera novaeangliae) and gray (Eschrichtius robustus) whales. Hormones were extracted with a methanol vortex method, after which we tested all species with commercial enzyme immunoassays (EIAs, Arbor Assays) for progesterone, testosterone, 17β-estradiol, cortisol, corticosterone, aldosterone, thyroxine and tri-iodothyronine, representing a wide array of steroid and thyroid hormones of interest for whale physiology research. In total, 64 parallelism tests (8 species × 8 hormones) were evaluated to verify good binding affinity of the assay antibodies to hormones in baleen. We also tested assay accuracy, although available sample volume limited this test to progesterone, testosterone and cortisol. All tested hormones were detectable in baleen powder of all species, and all assays passed parallelism and accuracy tests. Although only single individuals were tested, the consistent detectability of all hormones in all species indicates that baleen hormone analysis is likely applicable to a broad range of mysticetes, and that the EIA kits tested here perform well with baleen extract. Quantification of hormones in baleen may be a suitable technique with which to explore questions that have historically been difficult to address in large whales, including pregnancy and inter-calving interval, age of sexual maturation, timing and duration of seasonal reproductive cycles, adrenal physiology and metabolic rate.
Introduction
Large whales are of interest to conservation biologists due to their key ecological role, unique biological traits (e.g. large body size, long lifespan) and continued low population numbers relative to historic norms (Magera et al., 2013; Atkinson et al., 2015; Thomas and Reeves, 2015). Despite the cessation of commercial whaling, many whale populations remain threatened or endangered, and most are subject to a wide variety of anthropogenic impacts (Magera et al., 2013; Thomas and Reeves, 2015). Unfortunately, management and recovery efforts have been hampered by the fact that many basic aspects of cetacean life histories remain poorly understood, largely due to the logistic complications of locating and sampling free-swimming whales at sea (Hunt et al., 2013; Atkinson et al., 2015). Perhaps most problematic from a physiological perspective, there is still no proven method for live-capture of large whales, nor for collection of blood samples from free-swimming whales. Without traditional physiological analyses of plasma samples, many physiological features of the large whales still remain unknown or only roughly estimated, including such essential information as gestation length, inter-calving interval, reproductive rate, reproductive cycling (e.g. potential seasonal estrous cycles in females and/or seasonal testosterone cycles in males), and physiological responses to environmental stressors (both anthropogenic and natural).
Endocrinological analysis of alternative (non-plasma) sample types offers a potential avenue forward. In terrestrial taxa, conservation biologists now routinely employ endocrine techniques for non-plasma sample types that are more easily garnered than blood, including such sample types as faeces, hair and feather (Schwarzenberger, 2007; Sheriff et al. 2011; Terwissen et al., 2014; Dettmer et al., 2015; Romero and Fairhurst, 2016). Such approaches often focus on the steroid hormones and to a lesser degree the thyroid hormones; steroid and thyroid hormones are highly conserved across vertebrates (i.e. unlike many peptide hormones; Miller, 2005; Kawauchi and Sower, 2006), do not degrade rapidly, and are known to be deposited in a wide assortment of body tissues (Bentley, 1998; Amaral, 2010). This suite of hormones is also ideally suited for addressing questions of conservation interest. The gonadal steroids play key roles in reproduction (progestagens and estrogens for females, and androgens for males), while the adrenal steroids are critical for coordination of the vertebrate response to stress (particularly the glucocorticoids cortisol and corticosterone) as well as osmotic regulation (e.g. the mineralocorticoid aldosterone) (Bentley, 1998). Thyroid hormones, generally thought to be drivers of metabolic rate in the mammals (Hulbert, 2000), have received less attention from conservation biologists than the steroids, but are proving to be increasingly useful for wildlife physiology studies as indices of nutritional state and activity (Eales, 1988; Ayres et al., 2012; Joly et al., 2015; Wilsterman et al., 2015). Thyroid hormone studies on wildlife usually focus either on tri-iodothyronine (T3), the active hormone in tissues, and/or its immediate precursor thyroxine (T4), the major circulating pro-hormone (Bentley, 1998).
Significant progress has been made at recovering and quantifying some of the hormones described above from alternative tissue types in cetaceans, particularly faecal samples, respiratory vapour (‘blow’), and biopsy dart samples of skin and blubber (Rolland et al., 2005; Hunt et al., 2006, 2014a; Kellar et al., 2006; reviewed in Hunt et al., 2013; De Mello and de Oliveira, 2016) and most recently earwax plugs (Trumble et al., 2013). For example, progestagens in faeces, blow and blubber are significantly elevated in pregnant females, while faecal glucocorticoids and mineralocorticoids have been shown to correlate with exposure to environmental stressors such as chronic ocean noise and fishing gear entanglement (Rolland et al., 2005; Hunt et al., 2006; Hogg et al., 2009; Kellar et al., 2013; Burgess et al., 2017). However, despite these encouraging advances, sample collection rate is low for all of these sample types. Especially, it is rare to obtain repeated samples from the same individual whale over time (Hunt et al., 2013). Without repeated sampling from individuals across time, it remains difficult to study physiological aspects of the long reproductive cycles of cetaceans as well as long-term phenomena such as responses to chronic stress.
Baleen has recently been recognized as a tissue type that may contain a longitudinal time series of endocrine history covering multiple years (Hunt et al., 2014b, 2016). In mysticete whales, vertical strips or ‘plates’ of baleen are suspended in parallel along the right and left sides of the whale’s mouth, serving as a filter-feeding apparatus (St. Aubin et al., 1984). Stable isotope (SI) data indicate that each baleen plate grows continuously and slowly from a highly vascularized root region in the upper jaw, wearing away steadily at the distal (lower) tip (Mitani et al., 2006; Lubetkin et al., 2008, 2012; Lysiak et al., 2008; Ryan et al., 2013). The number of years of baleen growth represented by a given baleen plate can often be determined with high accuracy via counting of annual cycles in SI ratios along the length of the baleen plate, since SI ratios often change seasonally in baleen as these migratory species alternate between summer prey and winter prey (Mitani et al., 2006; Lubetkin et al., 2008, 2012; Lysiak et al., 2008; Ryan et al., 2013). Our recent studies using these techniques (hormonal analyses combined with SI analyses) have demonstrated that progesterone and cortisol are detectable in baleen of bowhead whales (Balaena mysticetus, ‘bowhead’) and North Atlantic right whales (Eubalaena glacialis, ‘right’), and that peaks in both hormones occur in regions of baleen known (from the SI data and resulting baleen growth rate estimates) to have grown during documented pregnancies (Hunt et al., 2014b, 2016). Thus, baleen quite likely contains a longitudinal endocrine history that spans the time period of growth of the baleen plate, which can be a decade or more for the species with longest baleen, bowheads and rights. Even in species with shorter baleen, a single baleen plate encompasses at least a full year of growth, ~1.3–1.4 years in the gray whale (Eschrichtius robustus, ‘gray’; Caraveo-Patiño et al., 2007) and minke whale (Balaenoptera acutorostrata, ‘minke’; Mitani et al., 2006) and usually multiple years in the humpback whale (Megaptera novaeangliae, ‘humpback’); finback whale (B. physalus, ‘fin’) and sei whale (B. borealis, ‘sei’; Bentaleb et al., 2011; Ryan et al., 2013; Eisenmann et al., 2016) (no estimates are yet available for blue whale, B. musculus, ‘blue’). These growth durations are sufficiently long to encompass complete pregnancies, inter-calving intervals in most species, and potentially long-term responses to a variety of environmental stressors, data that would otherwise require collection of many dozens of samples. For example, a single baleen plate from an adult right whale contains a continuous time series of endocrine information that spans 9–10 years (Hunt et al., 2016, 2017), perhaps comparable to a collection of weekly or monthly plasma samples collected repeatedly from the same individual over an entire decade. Two additional advantages of baleen are, first, that it is a dry and long-lasting tissue that holds steroid hormones in stable condition for at least one decade (Hunt et al., 2016); second, considerable archives of baleen samples exist in stranding archives and in natural history museums that may enable retrospective study of historic populations.
Hormonal analysis of baleen therefore has the potential to reveal both annual and multi-year patterns in physiology from both modern and historic specimens. However, to date only two hormones have been investigated (progesterone and cortisol; Hunt et al., 2014b, 2016) and in only two species, right and bowhead, which are closely related (Marx and Fordyce, 2015). It is unknown whether hormones are also present in baleen of other cetacean taxa, and it is also unclear whether other hormones may be present as well—particularly testosterone, 17β-estradiol, corticosterone, aldosterone and thyroid hormones—and, specifically, whether they might be detectable with commercially available immunoassay kits (the most widespread and lowest-cost endocrine technique presently available). If any of these hormones prove detectable with immunoassays, at least two types of validations will be necessary before the technique can enter widespread use (Palme, 2005; Goymann, 2012; Kersey and Dehnhard, 2014). First, laboratory validations (the focus of this study) must be performed to verify that the assays can accurately quantify the desired hormones, i.e. even in the presence of baleen extract. Second, physiological validations (also known as ‘biological validations’) will also be necessary for each hormone and each species, to verify that baleen hormone concentrations do in fact correspond to physiological state of the animal (e.g. demonstration that baleen of known-pregnant whales contains elevated progesterone and elevated glucocorticoids, as shown for bowheads and rights in Hunt et al., 2014b, 2016, 2017). Note that a third class of validations, pharmacological validations (infusions of hormones such as ACTH, hormone receptor blockers, radiolabelled hormones, etc.), are typically not possible in large whales (Hunt et al., 2013) but, fortunately, thorough attention to laboratory validations and physiological validations can demonstrate whether the assay in question can accurately identify animals of the desired physiological state.
As a first step in investigating whether baleen hormone technique is of widespread applicability to baleen whales, we performed laboratory validations for eight hormones in baleen (physiological validations are being addressed in parallel studies, to be published separately). Initial laboratory validations of immunoassays for any novel sample type from a given species commonly include a parallelism test (assay of serially diluted sample alongside hormone standards) and an accuracy test (assay of a set of hormone standards that have been spiked with pooled sample), both typically performed initially on a single specimen from the species in question (Grotjan and Keel, 1996). A parallelism test confirms that the assay antibody exhibits good binding affinity to the desired hormone in that sample type from that species; the accuracy test verifies that the assay is able to distinguish high from low concentrations with acceptable mathematical accuracy (Grotjan and Keel, 1996). Therefore, as a first step in investigating whether baleen hormone technique is of widespread applicability to baleen whales, we tested baleen of eight mysticete species (right, bowhead, blue, sei, minke, fin, humpback and gray), representing a broad range of mysticete taxa, for parallelism in commercial enzyme immunoassay (EIA) kits for progesterone, testosterone, 17β-estradiol, cortisol, corticosterone, aldosterone and the thyroid hormones T3 and T4, representing all major classes of vertebrate steroid and thyroid hormones. Where sample volume permitted, we tested accuracy as well. Our investigation includes all known genera of mysticete whales except Caperea, the pygmy right whale (Marx and Fordyce, 2015). Our goals were to determine (i) which species of mysticetes have detectable hormones in baleen, (ii) which (if any) of the eight hormones are detectable and (iii) whether commercial EIAs perform well with baleen extract (i.e. good parallelism and accuracy).
Methods
Baleen samples
Since this study required semi-destructive sampling of rare specimens, and initial parallelism validations can be accomplished with single individuals, sample sizes in this pilot trial were restricted to one individual per species and one baleen plate per individual. One baleen plate each from single specimens of right, bowhead, blue, sei, minke, fin, humpback and gray whales were selected for study from baleen archived in previous years by US marine mammal stranding networks on the east and west coasts (Table 1). For blue, fin and gray whales, a baleen plate from a second individual whale was later studied to further inspect parallelism results for cortisol; in these cases the individuals are distinguished as #1 and #2, e.g. blue whale #1 and blue whale #2. All plates had been stored at room temperature for multiple years, in some cases decades, between date of specimen collection and hormone assays in 2016–17 (Table 1). Since assay validations require pulverization of a relatively large area of baleen (i.e. in order to provide ample extract for testing), less-valuable specimens were selected for these initial validations; therefore, several specimens do not have associated necropsy data and some are of unknown sex and unknown age-at-death (Table 1). Note that sex of the whale, age at death, reproductive state, cause of death, years in storage of the baleen, and potentially storage conditions may all affect hormone concentrations in baleen (see, e.g. Hunt et al., 2014b, 2016, 2017), but this was not a concern in this study given that our primary focus was simply whether hormones are present and detectable.
Table 1:
Baleen specimens used for testing hormone assay parallelism and accuracy
| Species | Short id | Carcass accession code | Holding institutiona | Sex | Collection year | Other information |
|---|---|---|---|---|---|---|
| Eubalaena glacialis | Right | VMSM2004-1004 | WHOI | Female | 2004 | Eg #1004 ‘Stumpy,’ adult female, died due to shipstrike |
| Balaena mysticetus | Bowhead | n/a | NAU | Unk | Before 1991 | Collected in legal native subsistence hunt; historic educational specimen |
| Balaenoptera musculus | Blue #1 | MMASYBM9812 | NBWM/WHOI | Male | 1998 | ‘KOBO,’ subadult, 2002cm length, died due to shipstrike; museum display specimen |
| Balaenoptera musculus | Blue #2 | HMSC151101Bm | OSU | Male | 2015 | Necropsy specimen; adult, 2127 cm length |
| Balaenoptera borealis | Sei | COA150609Bb | COA | Female | 2015 | Necropsy specimen from marine mammal stranding network |
| Balaenoptera acutorostrata | Minke | COA140717Ba | COA | Female | 2014 | Necropsy specimen from marine mammal stranding network |
| Balaenoptera physalus | Fin #1 | COA830916Bp | COA | Unk | 1983 | Necropsy specimen from marine mammal stranding network |
| Balaenoptera physalus | Fin #2 | HMSC090306Bp | OSU | Male | 2009 | Subadult, 1678 cm length |
| Megaptera novaeangliae | Humpback | COA150611Mn | CCS | Female | 2015 | ‘Spinnaker,’ subadult, entangled in fishing gear |
| Eschrichtius robustus | Gray #1 | HMSCNAU3 | HMSC | Unk | Unk | Necropsy specimen from marine mammal stranding network |
| Eschrichtius robustus | Gray #2 | HMSC03C2 | OSU | Female | 2003 | Calf, entangled in fishing gear |
aCCS = Center for Coastal Studies, Provincetown, MA; COA = Allied Whale, College of the Atlantic, Bar Harbor, ME; HMSC = Hatfield Marine Science Center, Oregon State University, Newport, OR; NAU = Northern Arizona University, Flagstaff, AZ; NBWM = New Bedford Whaling Museum, New Bedford, MA; WHOI = Woods Hole Oceanographic Institution, Woods Hole, MA.
Baleen pulverization and hormone extraction
Once in the laboratory, plates were cleaned of surface dust by wiping three times with Kimwipes (Sigma-Aldrich, St. Louis, MO, USA) wetted with 70% isopropyl alcohol, and allowed to air-dry. A single 4 cm × 2 cm region at the base of each plate (i.e. growth zone, newest baleen) was then pulverized with a hand-held Dremel rotary tool (Model 395 Type 5) fitted with a flexible extension and a tungsten-carbide ball tip, with the drill speed set at 2. Baleen powder was collected on a weigh paper below the plate and mixed with a metal spatula. Due to the large volumes of baleen extract needed for validations, five separate 100 mg subsamples of the well-mixed baleen powder were then weighed on a digital scale to the nearest 0.1 mg, with each aliquot transferred to a 16 × 100 mm borosilicate glass tube. Considerable care was taken to avoid potential cross-contamination between specimens; all drilling occurred in a fume hood or biological safety cabinet, with comprehensive cleaning between samples (eight washes of 70% ethanol of all tools, the tungsten-carbide drilling tip, the digital scale and the work surface, with multiple changes of gloves).
Hormones were extracted by mixing each 100 mg aliquot of weighed powder with 6.00 mL of 100% high-performance liquid chromatography (HPLC)-grade methanol, vortexing 2 h, and centrifuging for 15 min at 3000 g. This is an adaptation of our previously published protocol (Hunt et al. 2014b, 2016) with the volume of methanol increased from 4.0 to 6.0 mL (per 100 mg of baleen powder) to improve extraction efficiency. Supernatant (containing hormones) was pipetted to a separate tube and dried down in a ThermoSavant SpeedVac SPD121P (Thermo Fisher Scientific, Waltham, MA, USA) under vacuum at 35°C for 5 h. Dried samples were reconstituted in 500 uL of EIA assay buffer (buffer ‘X065,’ Arbor Assays, Ann Arbor, MI, USA), sonicated 1 min, and vortexed 5 min. The 500 uL reconstitution volume was selected following initial studies in bowhead and right whale (Hunt et al. 2014b, 2016, 2017), in which this volume was found to concentrate samples enough for acceptable detectability of at least two hormones (progesterone and cortisol), while still retaining good assay accuracy and sufficient volume for multiple immunoassays. However, we emphasize that the ideal extraction and reconstitution protocol for baleen powder has not yet been definitively determined, and that further optimization of these methods may be possible. For each species, the five separate 500 uL extracts (originally from the same large powdered sample) were combined, vortexed 15 s to equalize hormone concentrations, and transferred to a cryovial for storage at −80°C. This is termed the ‘1:1’ (full-strength) extract and was used for all assays. The 1:1 extracts from each species were then serially diluted by progressive halving in EIA assay buffer, producing a total of eight dilutions: 1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64 and 1:128. All eight dilutions from all eight species were then assayed in duplicate in all eight immunoassays. Hormone assays occurred within 3 months of extraction.
In a few cases where hormone content proved to be so low or so high that only the non-linear ‘shoulder’ of the parallelism curve was recovered, additional dilutions were created that were either more dilute (e.g. 1:256, 1:512, etc.) or more concentrated (e.g. ‘2×’, twice as concentrated as 1:1), as appropriate. All dilutions were then re-assayed together in a subsequent assay, in order to recover the central linear portion of the binding curve for more accurate comparison to the slope of the standard curve.
Hormone assays
All assays were colorimetric EIAs from the same manufacturer (Arbor Assays, Ann Arbor, MI). A single manufacturer was selected so that baleen extracts could be reconstituted into a single assay buffer that would be suitable for all assays; this avoids the need to repeatedly dry down extracts for reconstitution in separate buffers, minimizes associated pipetting losses, and hence minimizes the mg of baleen powder required from the rare specimens. The specific EIA kit manufacturer was selected based on previous successful use of certain kits (progesterone, cortisol) with bowhead and right whale baleen extract (Hunt et al. 2014b, 2016, 2017); additionally, several of this manufacturer’s assay kits are specifically designed for use with non-plasma sample types of wildlife. The manufacturer’s protocols were followed exactly for the progesterone, testosterone, 17β-estradiol and corticosterone EIAs. Other EIA protocols were slightly modified as follows: cortisol, standards brought up in the X065 buffer (based on technical advice from the manufacturer); aldosterone, ‘overnight’ format used but with 50ul of standards and samples; T4, ‘urine’ format used but with 50 uL of standards and samples; T3, 50uL used of standards and samples. All assays followed standard QA/QC including assay in duplicate of all non-specific binding wells, blanks (zero dose), standards and all samples, with results averaged accordingly; re-assay of any sample with coefficient of variation (CV) > 10% between wells; and exclusion of any single standard with >10% CV from the standard curve. In cases where two or more standards had CV > 10% (this was rare), the entire assay was re-run. See Table 2 for additional assay details including catalog numbers, antibody sources, manufacturer’s reported inter- and intra-assay precision (aka ‘assay variation’), sensitivity and cross-reactivities.
Table 2:
Details of immunoassays used to test hormone detectability in baleen powder. All assays were colorimetric enzyme immunoassay kits from Arbor Assays (Ann Arbor, MI, USA). Detection limit, intra-assay precision and inter-assay precision are from manufacturer’s protocols, except for detection limit for aldosterone and T4 assays, in which due to protocol alterations the detection limit was conservatively estimated as one-half the dose of the lowest standard
| Hormone (assay catalog #) | Source of antibody | Standard range (pg/mL), # standards | Detection limit (pg/mL) | Intra-assay precisiona (%) | Inter-assay precisiona (%) | Cross-reactivities |
|---|---|---|---|---|---|---|
| Progesterone (K025) | Mouse monoclonal | 50–3200 | 52.9 | 3.9 | 5.7 | Hydroxyprogesteronesb: 3α, 172%; 3β, 188%; 11α, 2.7%; 11β, 147%. Pregnenolone 5.9%, other steroids <0.1% |
| n = 7 | ||||||
| Testosterone (K032) | Rabbit polyclonal | 41–10 000 | 30.6 | 10.9 | 9.3 | Dihydrotestosterone 56.8%, androstenedione 0.27%, other steroids <0.1% |
| n = 7 | ||||||
| 17β-Estradiol (K030) | Rabbit polyclonal | 39–10 000 | 26.5 | 5.1 | 8.4 | Estrone 0.73%, other steroids <0.1% |
| n = 5 | ||||||
| Cortisol (K003) | Mouse monoclonal | 100–3200 | 45.4 | 8.8 | 8.1 | Dexamethasone 18.8%, prednisolone 7.8%, corticosterone 1.2%, cortisone 1.2%, other steroids <0.1% |
| n = 6 | ||||||
| Corticosterone (K014) | Sheep polyclonal | 78–10 000 | 16.9 | 5.2 | 7.9 | Desoxycorticosterone 12.3%, tetrahydrocorticosterone 0.76%, aldosterone 0.62%, cortisol 0.38%, progesterone 0.24%, dexamethasone 0.12%, other steroids <0.1% |
| n = 8 | ||||||
| Aldosterone (K052) | Sheep polyclonal | 3.9–4000 | 1.9 | 6.9 | 19.5 | All tested steroids <0.1% |
| n = 6 | ||||||
| Tri-iodothyronine, T3 (K056) | Sheep polyclonal | 78–5000 | 46.6 | 6.3 | 13.4 | T4, 0.88%, reverse T3 < 0.1% |
| n = 7 | ||||||
| Thyroxine, T4 (K050) | Mouse monoclonal | 62.5–4000 | 31.3 | 3.0 | 7.1 | Reverse T3 89.0%, T3 5.23% |
| n = 7 |
aAverage of three (most assays) or four (corticosterone assay) coefficients of variation reported by the assay manufacturer for various concentrations of the relevant hormone.
bProgesterone assay uses the ‘CL425’ antibody widely used to detect hydroxylated progesterone metabolites common in mammalian faeces (Graham et al., 2001). Cross-reactivities are defined in reference to progesterone.
Parallelism was tested with assay of eight serial dilutions of baleen extract for each species, assayed alongside known-dose hormone standards. Due to microplate size limitations (12 columns, of which 2 are devoted to the standard curve), right, blue, sei, minke and fin whales were typically run in one assay, followed by bowhead, humpback, gray and a new standard curve in a separate assay, and follow-up assays for tests of additional dilutions or additional individual whales (e.g. blue #2, fin #2, gray #2). For simplicity, only the first assay’s standard curve is displayed on figures, but note that statistical testing compared each species’ data to the standard curve run in the same assay. Bowhead and right whale baleen had previously been tested for progesterone and cortisol parallelism (Hunt et al. 2014b, 2016, 2017); these previously published data are displayed on figures and tables, and are reanalyzed here with multiple-comparisons corrections, for ease of comparison to new data from other species.
Accuracy tests require a much greater volume of sample than parallelism tests, and therefore accuracy could not be evaluated for all hormones due to limited sample volume from these rare specimens. Thus, we prioritized accuracy testing for progesterone and testosterone, due to their importance in male and female reproductive assessment, respectively, and cortisol, due to its key role in the adrenal stress response. Cortisol was chosen for accuracy testing rather than corticosterone due to the assumption that cetaceans are ‘cortisol-dominant’, i.e. cortisol is thought to be the major circulating glucocorticoid in cetacean plasma (reviewed in St. Aubin, 2001; Atkinson et al., 2015). In most cases, dilutions of 1:16 were tested for progesterone and testosterone, and 1:2 for cortisol; for a few species, stronger dilutions were tested. These dilutions were selected by consulting parallelism data for a dilution that fell between 60 and 80% percent-bound (percentage of antibody bound to labelled hormone), a region of the parallelism binding curve that typically has good mathematical accuracy while requiring a minimal volume of sample. For each accuracy test, a full standard curve, non-specific binding wells and blank (zero dose) were spiked in duplicate with an equal volume of appropriately diluted baleen extract, and the spiked standards were then assayed alongside a second standard curve that was spiked only with buffer.
Statistical analysis
Parallelism results were graphed as percentage of antibody bound vs. log[relative dose], with the strongest dilution for each species assigned a nominal value and each subsequent dilution assigned a relative dose of 1/2 that of the previous dilution. The resulting binding curves were assessed with an F test for difference of slope, with the linear portion of each species’ binding curve compared to the standard curve that had been assayed in the same assay (Grotjan and Keel, 1996; Ezan and Grassi, 2000). A Bonferroni multiple-comparisons correction was used for each group of eight species tested for a certain hormone, i.e. the significance threshold for each hormone was set at α= 0.05/8 = 0.00625. (Note that in parallelism testing, the desired result is lack of significance, and thus Type II errors are a greater concern than Type I errors; more stringent multiple comparisons tests were not employed so as to limit risk of Type II errors.) Accuracy results were graphed as observed dose vs. known standard dose and were assessed using linear regression, with acceptable accuracy defined as r2 > 0.95 and slope within the range of 0.7–1.3 (ideal slope = 1.0) (Grotjan and Keel, 1996; Ezan and Grassi, 2000).
Results
Detectability and parallelism
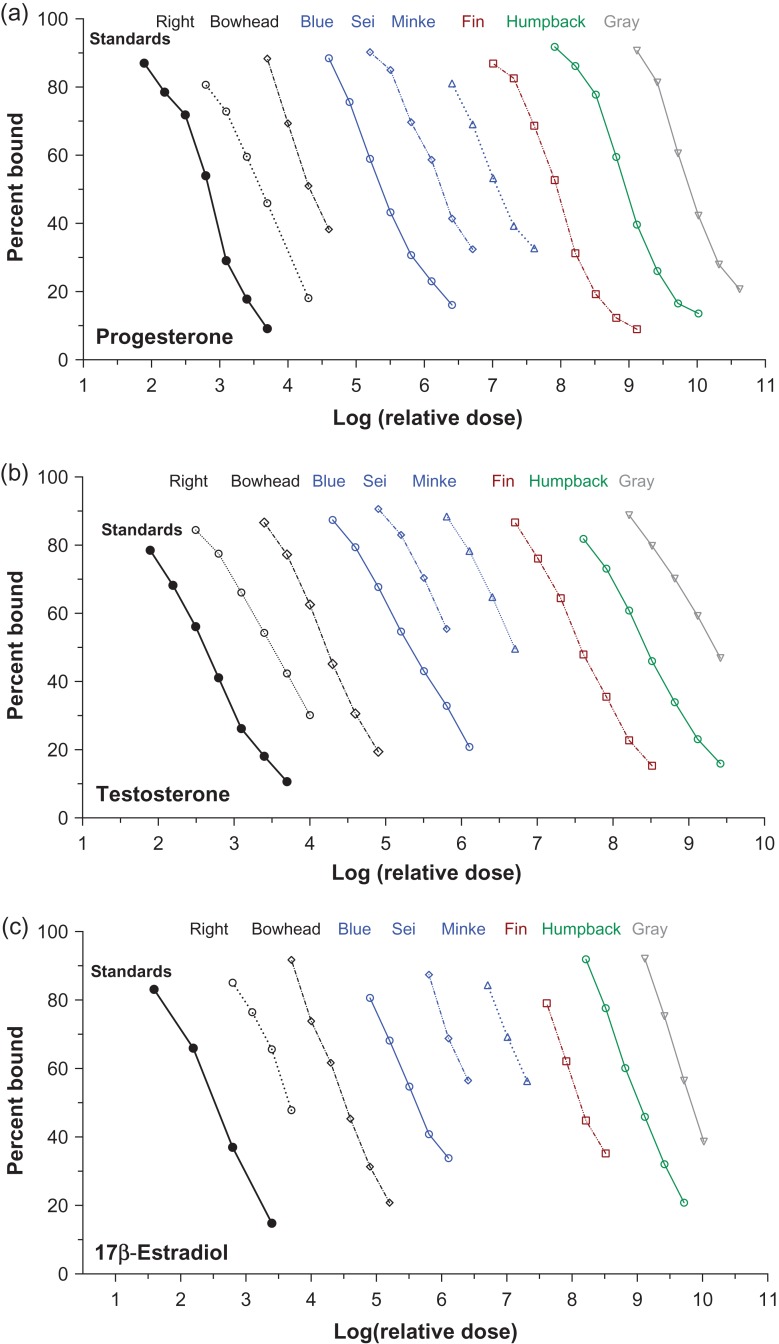
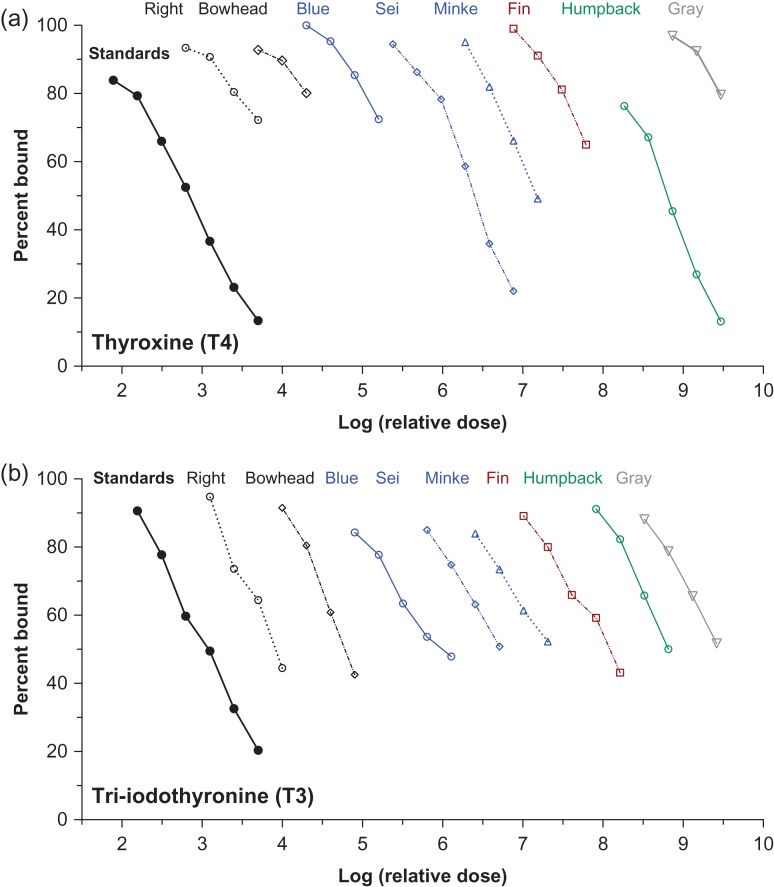
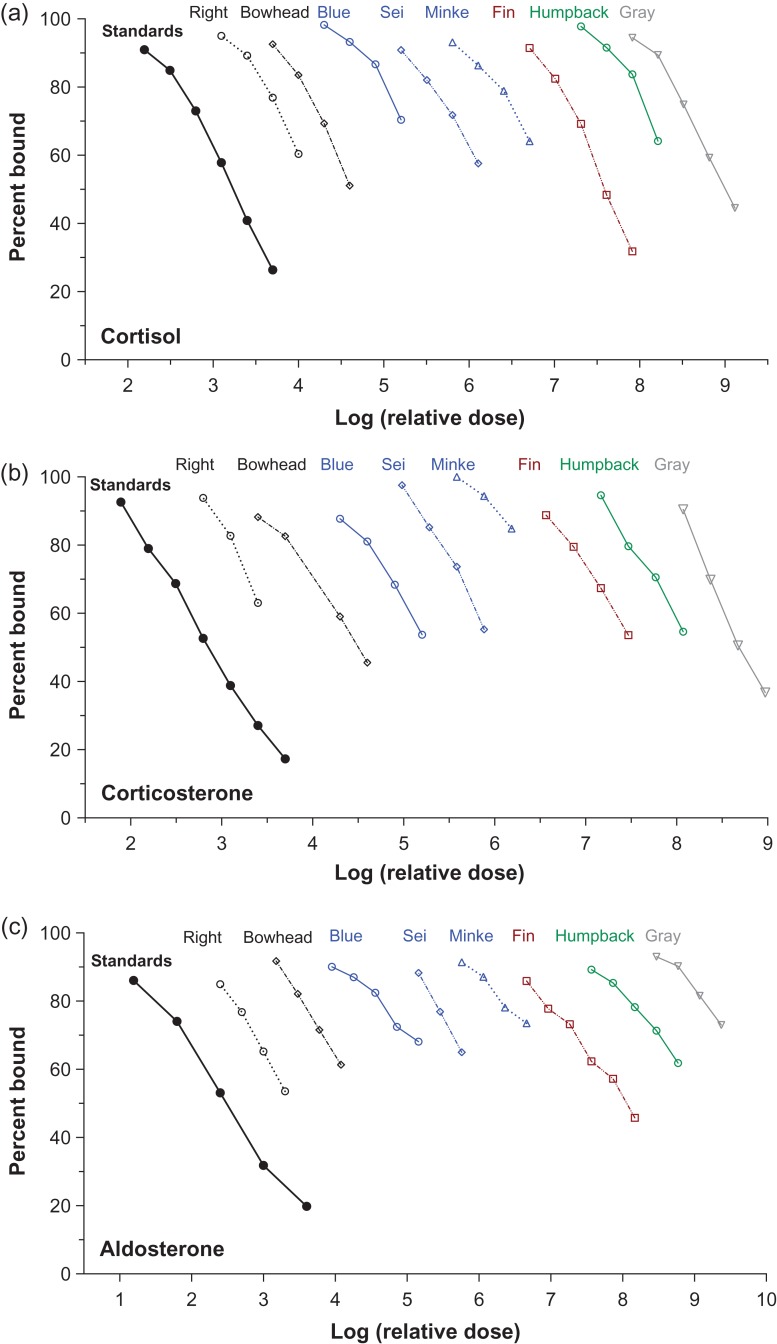
Immunoreactive hormones were detectable in baleen powder of all species tested, in all eight hormone assays. There were no significant differences in slope between the linear portions of the binding curves of serially diluted baleen extract as compared to pure hormone standards run in the same assay (Figs 1–3, Table 3; note that x-axes of Figs 1–3 show relative dose, not actual dose).
Figure 1:
Parallelism graphs for the reproductive steroids progesterone (top), testosterone (middle) and 17β-estradiol (bottom) tested with serially diluted baleen extracts of eight whale species. Similar colours indicate closely related taxa, i.e. right (North Atlantic right whale) and bowhead are closely related, and blue, sei and minke whales are closely related (fin whale is not grouped with other Balaenoptera following recent phylogenetic analyses, e.g. Marx and Fordyce, 2015). Dilutions with no detectable hormone are not shown
Figure 3:
Parallelism graphs for the thyroid hormones thyroxine (T4) and tri-iodothyronine (T3) tested with serially diluted baleen extracts of eight whale species. Similar colours indicate closely related species; right = North Atlantic right whale. Dilutions with no detectable hormone are not shown
Table 3:
Results of individual F-tests for parallelism for baleen extract of eight mysticete whales in eight enzyme immunoassays, comparing slopes of the linear portions of the standard curve and the serial dilution of baleen extract for each species. Variable degrees of freedom are due to some dilutions having undetectable hormone content for some species. All P values are > 0.00625, the Bonferroni-corrected alpha value for each hormone. ‘Right’ = North Atlantic right whale; T3 = tri-iodothyronine; T4 = thyroxine
| Species | Progesterone | 17β-Estradiol | Testosterone | Cortisol | Corticosterone | Aldosterone | T3 | T4 |
|---|---|---|---|---|---|---|---|---|
| Righta | F1,8 = 0.3856 | F1,5 = 0.6831 | F1,9 = 1.521 | F1,6 = 3.682 | F1,6 = 2.624 | F1,5 = 2.051 | F1,7 = 0.1184 | F1,6 = 0.5985 |
| P = 0.3856 | P = 0.4462 | P = 0.3073 | P = 0.1034 | P = 0.1564 | P = 0.2115 | P = 0.7409 | P = 0.4685 | |
| Bowheada | F1,7 = 1.184 | F1,6 = 1.898 | F1,10 = 2.198 | F1,6 = 0.2496 | F1,9 = 2.707 | F1,4 = 0.33553 | F1,7 = 0.1443 | F1,3 = 2.501 |
| P = 0.3126 | P = 0.2174 | P = 0.1690 | P = 0.6352 | P = 0.1343 | P = 0.5936 | P = 0.7153 | P = 0.2119 | |
| Blue | F1,8 = 2.141 | F1,6 = 1.343 | F1,10 = 0.9145 | F1,5 = 0.4851 | F1,7 = 1.684 | F1,4 = 4.317 | F1,7 = 2.074 | F1,4 = 5.751 |
| P = 0.1816 | P = 0.2906 | P = 0.3615 | P = 0.5172 | P = 0.2355 | P = 0.1063 | P = 0.1930 | P = 0.0745 | |
| Sei | F1,8 = 1.928 | F1,5 = 0.9750 | F1,7 = 1.071 | F1,5 = 3.718 | F1,6 = 2.148 | F1,3 = 1.707 | F1,6 = 0.0228 | F1,8 = 3.293 |
| P = 0.2024 | P = 0.3688 | P = 0.7530 | P = 0.1117 | P = 0.1931 | P = 0.2825 | P = 0.8849 | P = 0.1071 | |
| Minke | F1,7 = 0.9518 | F1,5 = 1.045 | F1,7 = 0.2176 | F1,5 = 0.7135 | F1,5 = 1.630 | F1,3 = 1.519 | F1,6 = 0.3154 | F1,5 = 0.5093 |
| P = 0.3618 | P = 0.3536 | P = 0.6551 | P = 0.4368 | P = 0.2577 | P = 0.3055 | P = 0.5947 | P = 0.1709 | |
| Fin | F1,10 = 1.186 | F1,5 = 3.962 | P1,10 = 0.0488 | F1,6 = 5.015 | F1,7 = 1.607 | F1,7 = 1.575 | F1,7 = 0.0835 | F1,4 = 2.158 |
| P = 0.3016 | P = 0.1032 | P = 0.8296 | P = 0.0664 | P = 0.2455 | P = 0.2497 | P = 0.7809 | P = 0.2157 | |
| Humpback | F1,10 = 1.210 | F1,6 = 2.826 | F1,10 = 0.8866 | F1,6 = 5.345 | F1,7 = 0.0069 | F1,4 = 2.005 | F1,7 = 0.8685 | F1,5 = 0.1192 |
| P = 0.2970 | P = 0.1438 | P = 0.3686 | P = 0.0601 | P = 0.9359 | P = 0.2297 | P = 0.3824 | P = 0.7440 | |
| Gray | F1,8 = 0.0118 | F1,4 = 20.3 | F1,7 = 0.2623 | F1,7 = 0.2437 | F1,8 = 2.726 | F1,3 = 0.1849 | F1,6 = 0.1006 | F1,6 = 2.089 |
| P = 0.9163 | P = 0.0108 | P = 0.6243 | P = 0.6366 | P = 0.1373 | P = 0.6962 | P = 0.7619 | P = 0.1985 |
aRight and bowhead results for progesterone and cortisol are from data previously published in Hunt et al. (2014b, 2016) and are presented here for comparison.
In some cases hormone concentration was quite low, such that only the uppermost part of the binding curve was recovered (i.e. sometimes only two or three of the most concentrated dilutions had detectable hormone), particularly for cortisol, aldosterone and T4. In most such cases parallelism was still testable, but cortisol especially tended to have low concentration in baleen extract and initially showed imperfect parallelism for blue whale #1, fin whale #1 and gray whale #1 (data not shown). These three individual whales had extremely low baleen cortisol concentrations and thus it is likely that only the non-linear ‘shoulder’ portion of the upper part of the binding curve was recovered. Upon testing baleen from a second whale for these three species (blue whale #2, fin whale #2 and gray whale #2), in all three cases the second whale had higher baleen cortisol content, the linear portion of the curve was recovered, and parallelism was good (these three individuals’ data are shown in Fig. 2 and in the cortisol column in Table 3).
Figure 2:
Parallelism graphs for the adrenal steroids cortisol (top), corticosterone (middle) and aldosterone (bottom) tested with serially diluted baleen extracts of eight whale species. Similar colours indicate closely related species; right = North Atlantic right whale. Dilutions with no detectable hormone are not shown
Assay accuracy was acceptable for all species for the three assays tested (progesterone, testosterone and cortisol), with linear relationships of observed vs. expected concentration and a slope within the desired range of 0.7–1.3 (Table 4). The progesterone assay tended to have slightly ‘shallow’ accuracy, i.e. slope of ~0.8 rather than the ideal of 1.0, but accuracy was nonetheless acceptable (straight line that consistently distinguished low from high concentrations).
Table 4:
Results of accuracy tests (‘matrix effect’ tests) for progesterone, testosterone and cortisol assays tested with baleen extract of eight mysticete whale species. Slope of observed dose in standards spiked with baleen extracted vs. unspiked standards is shown, along with coefficient of determination (r2) of the linear regression line. A slope close to 1.0 (acceptable range = 0.7–1.3) and r2 close to 1.00 indicates that the assay can quantify hormone in the presence of baleen extract with acceptable mathematical accuracy. Each assay was tested with the dilution shown in parentheses (where 1:1 = extract produced from 100 mg baleen powder vortexed with 6.0 mL methanol, dried, and brought up in 0.5 mL assay buffer). ‘Right’ = North Atlantic right whale.
| Progesterone | Testosterone | Cortisol | |
|---|---|---|---|
| Righta | Slope = 0.7360 | Slope = 0.9268 | Slope = 1.060 |
| r2 = 0.9968 | r2 = 0.9999 | r2 = 0.9906 | |
| (1:4) | (1:16) | (1:1) | |
| Bowheada | Slope = 0.7194 | Slope = 1.046 | Slope = 1.013 |
| r2 = 0.9989 | r2 = 0.9942 | r2 = 0.9998 | |
| (1:4) | (1:16) | (1:1) | |
| Blue | Slope = 0.8969 | Slope = 1.067 | Slope = 1.036 |
| r2 = 0.9975 | r2 = 0.9963 | r2 = 0.9999 | |
| (1:16) | (1:16) | (1:4) | |
| Sei | Slope = 0.7953 | Slope = 1.017 | Slope = 1.025 |
| r2 = 0.9860 | r2 = 0.9998 | r2 = 0.9997 | |
| (1:16) | (1:16) | (1:4) | |
| Minke | Slope = 0.8534 | Slope = 1.017 | Slope = 1.010 |
| r2 = 0.9991 | r2 = 0.9939 | r2 = 0.9928 | |
| (1:16) | (1:16) | (1:4) | |
| Fin | Slope = 0.7927 | Slope = 1.026 | Slope = 1.037 |
| r2 = 0.9854 | r2 = 0.9980 | r2 = 0.9993 | |
| (1:16) | (1:16) | (1:4) | |
| Humpback | Slope = 0.8819 | Slope = 1.122 | Slope = 1.121 |
| r2 = 0.9991 | r2 = 0.9995 | r2 = 0.9988 | |
| (1:16) | (1:16) | (1:4) | |
| Gray | Slope = 0.9280 | Slope = 0.9241 | Slope = 0.968 |
| r2 = 0.9986 | r2 = 0.9999 | r2 = 0.9984 | |
| (1:16) | (1:4) | (1:2) |
aRight and bowhead results for progesterone and cortisol are from data previously published in Hunt et al. (2014b, 2016) and are presented here for comparison.
Certain hormones exhibited differences in relative concentration that appeared consistent across species. Corticosterone:cortisol ratio was > 1.0 (i.e. more corticosterone than cortisol) for all individuals tested except the minke whale. Corticosterone concentration was occasionally as much as two times higher (gray whale) or three times higher (bowhead whale) than cortisol. Among the reproductive hormones, estradiol tended to be present at lower concentration than either progesterone or testosterone. For thyroid hormones, T3 concentrations were similar across all individual whales (across species) but T4 was highly variable, with high concentrations in the humpback and sei whale baleen, and low concentrations in other individuals.
Discussion
Immunoreactive steroid and thyroid hormones were easily detectable in baleen extracts from eight mysticete whale species tested with commercially available immunoassay kits, and parallelism and accuracy results were consistently good. The validation results indicate that the specific EIAs tested here perform well with baleen extract, with good binding affinity (as indicated by good parallelism) and accurate discrimination of high from low concentrations despite potential matrix effects (i.e. due to baleen extract). The good validation results are notable given that baleen extracts were not filtered and not purified via column chromatography or other means. In fact, baleen extracts at the stronger dilutions of 1:1, 1:2 and 1:4 were visibly cloudy, with the 1:1 ‘full-strength’ extract often being nearly opaque, yet parallelism results indicate that the assays performed well even at these stronger concentrations. It is striking that parallelism was so consistently good across 64 separate parallelism tests; our interpretation is that all steroid and thyroid hormones are routinely deposited in whale baleen. Even so, we recommend that future studies employ HPLC, mass spectrometry or other methods to verify the chemical identity of immunoreactive substances that the EIA antibodies are detecting.
Generally, the assay sensitivity and hormone concentrations that we observed indicate that it should be feasible to use EIAs to assay for a full panel of hormones from a relatively small mass of baleen powder. Most EIAs require 100 uL of sample for testing in duplicate (e.g. 50 uL per well × 2 wells), but diluted extract can be used for certain assays (particularly the reproductive steroids). Our present extraction method generates 500 uL of extract from a 100 mg powdered baleen sample. If the hormone concentrations seen in these individual whales are typical, we calculate it would be possible to assay all eight hormones with a total of ~250 uL of such extract. This compares favourably to volumes needed for mass spectrometry, which can detect a full panel of steroids in a single run but often requires a larger total volume of sample. Continued optimization of extraction protocol along with testing of additional assay dilutions may enable further reduction of the mass of baleen powder needed from rare specimens.
Of the eight hormones, progesterone and testosterone were consistently present at highest concentration and were easily detectable in baleen extracts of all species studied. Estradiol was easily detectable in all samples as well, though at slightly lower concentrations. The adrenal steroids and thyroid hormones were generally present at lower concentrations than the reproductive steroids and in some cases were barely detectable (particularly cortisol, aldosterone and T4). All of these patterns are consistent with relative concentrations typically seen in mammalian plasma (K. Hunt, pers. obs.); estradiol tends to circulate at lower concentration than progestogens and androgens, and adrenal and thyroid hormones also are often low in plasma and/or in peripheral tissues (e.g., T4, is converted to T3 by peripheral tissues, resulting in low concentrations of T4 in many tissues; Bentley, 1998).
In most individual whales, baleen corticosterone was markedly higher in concentration than baleen cortisol, sometimes 2-fold or 3-fold higher. Similar patterns have recently been reported in right whale baleen, in which baleen corticosterone concentrations are routinely ~4× higher than baleen cortisol (Hunt et al., 2017). Historically, cortisol has been assumed to be the more abundant of the two glucocorticoids in mysticete plasma (reviewed in St Aubin, 2001; Atkinson et al., 2015). However, plasma glucocorticoids have only been assessed in mysticete whales in a few cases, all involving acute stress situations (stranding or hunting; e.g. Kjeld, 2001, Rolland et al., 2017). It is therefore unclear which of the two glucocorticoids may normally be more abundant in plasma and, importantly, which of the two may be more useful to study for questions regarding stress physiology. From a sample-mass perspective, assay of the more abundant glucocorticoid—corticosterone, it seems—would require a smaller mass of baleen powder, since the extract could be routinely diluted for assay, as described above. This issue is of some importance given high interest in assessing stress of historic populations from which only small subsamples may be available (e.g. small amounts of baleen powder left over from stable-isotope analyses). However, since there are some indications that cortisol and corticosterone may respond differently to acute vs. chronic stress in mammals (Koren et al., 2012), it may be most informative to study both glucocorticoids where possible.
In sum, our findings indicate that commercially available immunoassays appear to be a viable technique for analysis of hormone concentrations in baleen extracts from a wide variety of mysticete whale species. We caution that our results represent very small sample sizes, in most cases single samples from single individuals. However, initial assay validations of parallelism and accuracy usually are generalizable within a given species as long as the same extraction method and same assay kit is used, i.e. with the same antibody. Our results may only apply to the particular extraction method used here (methanol extraction, with reconstitution into appropriate assay buffer), and may also be limited to the specific commercial assay kits tested. Previous experience has shown that different assay kits, even if marketed as detecting the same hormone, typically employ different antibodies that may have radically differing cross-reactivities to other hormones and may also exhibit idiosyncratic reactions to non-plasma sample matrices (K. Hunt, pers. obs.). We therefore advise that validation tests be repeated if different commercial EIA kits are used or if extraction protocol is altered.
Overall, the good performance of commercial immunoassay kits for multiple steroid and thyroid hormones across a wide range of mysticete taxa bodes well for the potential utility of the baleen-hormone technique for investigating large whale physiology for conservation and management purposes, particularly given available historic archives of baleen. The range of hormones in baleen includes the major reproductive steroids of both males and females, two major adrenal steroids (cortisol, corticosterone) that are intimately involved in the mammalian stress response, a mineralocorticoid (aldosterone) involved both in stress responses and in osmotic regulation, and the two major thyroid hormones. Baleen hormone analysis therefore may have the potential to enable retrospective analysis of multi-year patterns of whale physiology that have until now been very difficult to address, potentially including pregnancy and inter-calving intervals, estrous cycles of females, testosterone cycles of males, age of sexual maturity in both sexes, metabolic rate and long-term responses to acute and chronic stress. We emphasize that many validations remain to be performed, such as mass spectrometry and HPLC to verify hormone chemical identity, stable-isotope studies to assess variability in baleen growth rate within and across species as well as across seasons, tests of variability of hormone deposition within and across baleen plates, further optimization of extraction protocols, and, importantly, physiological validations—verification that patterns in baleen hormones down the length of full baleen plates correlate with known physiological histories of individual whales. Once these further tests are performed, baleen hormone analyses may be a valuable new addition to the available toolkit for studies of physiology in the large whales.
Acknowledgements
We are very grateful to the many individuals and institutions who have assisted with stranding responses, necropsy and sample archiving over the years, including the National Marine Fisheries Service of the U.S. National Oceanographic and Atmospheric Administration, the Virginia Aquarium, the Cape Cod Stranding Network, the New England Aquarium, the International Fund for Animal Welfare, all other cooperating stranding network members, Scott Landry, Jim Rice and Jenn Tackaberry. We are also grateful to Tad Theimer (Northern Arizona University) and Jordan Berson (New Bedford Whaling Museum) for access to rare historic specimens, as well as Danielle Dillon (Northern Arizona University) and Russell Hart (Arbor Assays) for invaluable laboratory assistance and advice. Baleen was collected under NOAA stranding authorizations to the Woods Hole Oceanographic Institution, Allied Whale/College of the Atlantic, the Center for Coastal Studies, the New England Aquarium and Oregon State University; endangered species were studied under National Marine Fisheries Service ESA Section 10 Permits #1557-03 and #15672; baleen sampling in 2016 and 2017 occurred under a NOAA marine mammal parts authorization to K. Hunt.
Funding
This work was supported by (1) the Center for Bioengineering Innovation at Northern Arizona University and (2) the New England Aquarium.
References
- Amaral RS. (2010) Use of alternative matrices to monitor steroid hormones in aquatic mammals: a review. Aquatic Mamm 36:162–171. [Google Scholar]
- Atkinson S, Crocker D, Houser D, Mashburn K (2015) Stress physiology in marine mammals: how well do they fit the terrestrial model? J Comp Physiol B 185:463–486. [DOI] [PubMed] [Google Scholar]
- Ayres KL, Booth RK, Hempelmann JA, Koski KL, Emmons CK, Baird RW, Balcomb-Bartok K, Hanson MB, Ford MJ, Wasser SK (2012) Distinguishing the impacts of inadequate prey and vessel traffic on an endangered killer whale (Orcinus orca) population. PLoS One 7:e36842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentaleb I, Martin C, Vrac M, Mate B, Mayzaud P, Siret D, De Stephanis R, Guinet C (2011) Foraging ecology of Mediterranean fin whales in a changing environment elucidated by satellite tracking and baleen plate stable isotopes. Mar Ecol Prog Ser 438:285–302. [Google Scholar]
- Bentley P. (1998) Comparative Vertebrate Endocrinology. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Burgess E, Hunt K, Rolland R, Kraus S (2017) Adrenal responses of large whales: integrating fecal aldosterone as a complementary biomarker. Gen Comp Endocrinol 252:103–110. DOI10.1016/j.ygcen.2017.07.026. [DOI] [PubMed] [Google Scholar]
- Caraveo-Patiño J, Hobson KA, Soto LA (2007) Feeding ecology of gray whales inferred from stable-carbon and nitrogen isotopic analysis of baleen plates. Hydrobiologia 586:17–25. [Google Scholar]
- De Mello DMD, De Oliveira CA (2016) Biological matrices for sampling free-ranging cetaceans and the implications of their use for reproductive endocrine monitoring. Mamm Rev. DOI:10.1111/mam.12055. [Google Scholar]
- Dettmer AM, Rosenberg KL, Suomi SJ, Meyer JS, Novak MA (2015) Associations between parity, hair hormone profiles during pregnancy and lactation, and infant development in Rhesus monkeys (Macaca mulatta). PLoS One. DOI:10.1371/journal.pone.0131692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eales JG. (1988) The influence of nutritional state on thyroid function in various vertebrates. Am Zool 28:351–362. [Google Scholar]
- Eisenmann P, Fry B, Holyoake C, Coughran D, Nicol S, Nash S (2016) Isotopic evidence of a wide spectrum of feeding strategies in Southern Hemisphere humpback whale baleen records. PLoS One. DOI:10.137/journal.pone.0156698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezan E, Grassi J (2000) Optimization In Gosling JP (ed). Immunoassays: A Practical Approach. Oxford University Press, Oxford, UK: 187–210. [Google Scholar]
- Goymann W. (2012) On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol Evol 3:757–765. [Google Scholar]
- Graham L, Schwarzenberger F, Mostl E, Galama W, Savage A (2001) A versatile enzyme immunoassay for the determination of progestogens in feces and serum. Zoo Biol 20:227–236. [Google Scholar]
- Grotjan HE, Keel BA (1996) Data interpretation and quality control In Diamandis EP, Christopoulos TK (eds). Immunoassay. Academic Press, San Diego, CA: pp. 51–95. [Google Scholar]
- Hogg CJ, Rogers TL, Shorter A, Barton K, Miller PJO, Nowacek D (2009) Determination of steroid hormones in whale blow: it is possible. Mar Mamm Sci 25:605–618. [Google Scholar]
- Hunt KE, Lysiak NS, Moore MJ, Rolland RM (2016) Longitudinal progesterone profiles from baleen of female North Atlantic right whales (Eubalaena glacialis) match recent calving history. Conserv Physiol 4:cow014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KE, Lysiak NS, Moore MJ, Rolland RM (2017) Multi-year longitudinal profiles of cortisol and corticosterone recovered from baleen of North Atlantic right whales (Eubalaena glacialis). Gen Comp Endocrinol. DOI:10.1016/j.ygcen.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Hunt KE, Moore MJ, Rolland RM, Kellar NM, Hall AJ, Kershaw J, Raverty SA, Davis CE, Yeates LC, Fauquier DA (2013) Overcoming the challenges of studying conservation physiology in large whales: a review of available methods. Conserv Physiol 1:cot006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KE, Rolland RM, Kraus SD (2014. a) Detection of steroid and thyroid hormones via immunoassay of North Atlantic right whale (Eubalaena glacialis) respiratory vapor. Mar Mamm Sci 30:796–809. [Google Scholar]
- Hunt KE, Rolland RM, Kraus SD, Wasser SK (2006) Analysis of fecal glucocorticoids in the North Atlantic right whale (Eubalaena glacialis). Gen Comp Endocrinol 148:260–272. [DOI] [PubMed] [Google Scholar]
- Hunt KE, Stimmelmayr R, George C, Hanns C, Suydam R, Brower H, Rolland RM (2014. b) Baleen hormones: a novel tool for retrospective assessment of stress and reproduction in bowhead whales (Balaena mysticetus). Conserv Physiol 2:cou030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ. (2000) Thyroid hormones and their effects: a new perspective. Biol Rev 75:519–631. [DOI] [PubMed] [Google Scholar]
- Joly K, Wasser SK, Booth RK (2015) Non-invasive assessment of the interrelationships of diet, pregnancy rate, group composition, and physiological and nutritional stress of barren-ground caribou in late winter. PLoS One 10:e0127586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi H, Sower SA (2006) The dawn and evolution of hormones in the adenohypophysis. Gen Comp Endocrinol 148:3–14. [DOI] [PubMed] [Google Scholar]
- Kellar NM, Keliher J, Trego ML, Catelani KN, Hanns C, George JC, Rosa C (2013) Variation of bowhead whale progesterone and concentrations across demographic groups and sample matrices. Endang Sp Res 22:61–72. [Google Scholar]
- Kellar NM, Trego ML, Marks CI, Dizon AE (2006) Determining pregnancy from blubber in three species of delphinids. Mar Mamm Sci 22:1–16. [Google Scholar]
- Kersey DC, Dehnhard M (2014) The use of noninvasive and minimally invasive methods in endocrinology for threatened mammalian species conservation. Gen Comp Endocrinol 203:296–306. [DOI] [PubMed] [Google Scholar]
- Kjeld M. (2001) Concentrations of electrolytes, hormones, and other constituents in fresh postmortem blood and urine of fin whales (Balaenoptera physalus). Can J Zool 79:438–446. [Google Scholar]
- Koren L, Nakagawa S, Burke T, Soma KK, Wynne-Edwards KE, Geffen E (2012) Non-breeding feather concentrations of testosterone, corticosterone and cortisol are associated with subsequent survival in wild house sparrows. Proc R Soc B Biol Sci 279:1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubetkin S, Zeh J, George J (2012) Statistical modeling of baleen and body length at age in bowhead whales (Balaena mysticetus). Can J Zool 90:915–931. [Google Scholar]
- Lubetkin SC, Zeh JE, Rosa C, George JC (2008) Age estimation for young bowhead whales (Balaena mysticetus) using annual baleen growth increments. Can J Zool 86:525–538. [Google Scholar]
- Lysiak NS, Moore MJ, Knowlton AR, Valiela I (2008) Interpreting a long-term stable isotope record serived from North Atlantic right whale baleen: implications for ecosystem-level changes in the Gulf of Maine? Proceedings of the 2008 Ocean Sciences Meeting, March 2–7, Orlando, Florida.
- Magera AM, Flemming JEM, Kaschner K, Christensen LB, Lotze HK (2013) Recovery trends in marine mammal populations. PLoS One. 10.1371/journal.pone.0077908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx F, Fordyce R (2015) Baleen boom and bust: a synthesis of mysticete phylogeny, diversity and disparity. R Soc Open Sci 2:140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RP. (2005) GnRHs and GnRH receptors. Anim Reprod Sci 88:5–28. [DOI] [PubMed] [Google Scholar]
- Mitani Y, Bando T, Takai N, Sakamoto W (2006) Patterns of stable carbon and nitrogen isotopes in the baleen of common minke whale Balaenoptera acutorostrata from the western North Pacific. Fisheries Sci 72:69–76. [Google Scholar]
- Palme R. (2005) Measuring fecal steroids: guidelines for practical application. Annals NY Acad Sci 1046:75–80. DOI:10.1196/annals.1343.007. [DOI] [PubMed] [Google Scholar]
- Rolland RM, Hunt KE, Kraus SD, Wasser SK (2005) Assessing reproductive status of right whales (Eubalaena glacialis) using fecal hormone metabolites. Gen Comp Endocrinol 142:308–317. [DOI] [PubMed] [Google Scholar]
- Rolland RM, McLellan WA, Moore MJ, Harms CA, Hunt KE (2017) Fecal glucocorticoids and anthropogenic injury and mortality in North Atlantic right whales (Eubalaena glacialis). Endanger Species Res. 10.3354/esr00866. [Google Scholar]
- Romero LM, Fairhurst GD (2016) Measuring corticosterone in feathers: strengths, limitations, and suggestions for the future. Comp Biochem Physiol A Molec Integ Physiol 202:112–122. [DOI] [PubMed] [Google Scholar]
- Ryan C, McHugh B, Truman C, Sabin R, Deaville R, Harrod C, Berrow S, O’Connor I (2013) Stable isotope analysis of baleen reveals resource partitioning among sympatric rorquals and population structure in fin whales. Mar Ecol Prog Ser 479:251–261. [Google Scholar]
- Schwarzenberger F. (2007) The many uses of noninvasive faecal steroid monitoring in zoo and wildlife species. Int Zoo Yearbook 41:52–74. [Google Scholar]
- Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166:869–887. [DOI] [PubMed] [Google Scholar]
- St. Aubin D. (2001) Endocrinology In Dierauf L, Gulland FMD (eds). CRC Handbook of Marine Mammal Medicine, Ed 2 CRC Press, Boca Raton, FL. [Google Scholar]
- St. Aubin DJ, Stinson RH, Geraci JR (1984) Aspects of the structure and composition of baleen, and some possible effects of exposure to petroleum hydrocarbons. Can J Zool 62:193–198. [Google Scholar]
- Terwissen CV, Mastromonaco GF, Murray DL (2014) Enzyme immunoassays as a method for quantifying hair reproductive hormones in two felid species. Cons Physiol 2:cou044 DOI:10.1093/con-phys/cou044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Reeves R (2015) Status of the world’s baleen whales. Mar Mamm Sci 32:682–734. [Google Scholar]
- Trumble S, Robinson E, Berman-Kowalewski M, Potter C, Usenko S (2013) Blue whale earplug reveals lifetime contaminant exposure and hormone profiles. Proc Natl Acad Sci 110:16922–16926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsterman K, Buck C, Barnes B, Williams C (2015) Energy regulation in context: free-living female Arctic ground squirrels modulate the relationship between thyroid hormones and activity among life history stages. Horm Behav 75:111–119. [DOI] [PubMed] [Google Scholar]