Abstract
While aromatase inhibitors (AIs) have been known to cause minor elevations in liver enzymes, severe hepatotoxicity is rare. To the best of our knowledge, this is the first reported case of Letrozole-induced hepatitis with autoimmune features. A 70-year-old female with estrogen positive, invasive ductal carcinoma of the breast, presented with jaundice 3 months after starting letrozole. Hepatic transaminases were markedly elevated and her ANA and anti-smooth muscle antibody was positive. Liver biopsy featured drug-induced hepatitis. After stopping letrozole, liver tests trended back to normal within 3 weeks. She scored 9 for Roussel-Uclaf Causality Assessment Method (RUCAM). Over the last 10 years, there have been reported cases of drug-induced hepatitis secondary to AIs. We anticipate that there will be more widespread use of AIs based on recommendations from the TEXT, SOFT and extended AI trials. Therefore, physicians must be aware of this rare but life-threatening complication.
INTRODUCTION
Prior to 2007, more than 30 000 patients with breast cancer have been treated with aromatase inhibitors (AIs) with good liver safety [1]. Approximately, 185 000 new cases of invasive breast cancer are diagnosed yearly, and at least half are eligible for therapy with AI [2]. AI are used as first-line adjuvant hormonal therapy in postmenopausal women with hormone-receptor positive breast cancer [3]. Over the past 10 years, there have been reported cases of hepatitis and autoimmune diseases resulting from AI use [4, 5]. We report a rare case of letrozole-induced hepatitis with autoimmune features. Health professionals ought to be aware of the hepatotoxic effect of this agent, because there is likely to be extended use of AI in postmenopausal women [6], and increased use in premenopausal women with breast cancer [7].
CASE
A 70-year-old female with estrogen receptor positive, invasive ductal carcinoma of the breast (T1aN0M0), was initially managed with lumpectomy and radiation, and subsequently started on Letrozole. Her other comorbidities included hypertension which was managed on atenolol 50 mg once daily, and chronic urinary tract infections (UTIs), for which she was on Macrobid since more than a year. She had no history of alcohol or illicit drug use. After starting letrozole, she was seen in clinic at 2 weeks and then at 3 months. At her 3-month visit, her exam was unremarkable other than icterus. Laboratory evaluation showed AST 2030 (14–33 IU/l); ALT 2323 (10–42IU/l); albumin 2.9 (3.8–4.9 g/dl); direct bilirubin 7.3 (0–0.2 mg/dl); indirect bilirubin 2.6 mg/dl; total bilirubin 10.3 (0.2–1.0 mg/dl); alkaline phosphatase 298 (35–104 IU/l); PT 14.5; INR 1.1; aPTT 33. Further workup revealed a negative Hepatitis profile, but positive autoimmune workup. ANA was positive with 1:160 speckled pattern, alpha 1 antitrypsin 231 (90–200 mg/dl), antimitochondrial antibody 22.5 (0–20 units), anti-smooth muscle antibody 36 (0–19 units).
She was hospitalized to expedite workup in view of severe hepatic impairment. Liver biopsy showed hepatocellular injury with portal tract and lobular inflammation with cholestasis consistent with drug-induced hepatitis (Figs 1 and 2). After withdrawal of the drug, she clinically improved over the following three weeks and her liver tests trending back to normal. Thereafter, she was monitored in the outpatient clinic every 4 weeks for 2 months, and remained asymptomatic with normal liver tests. Her subsequent follow-up intervals were gradually extended.
Figure 1:
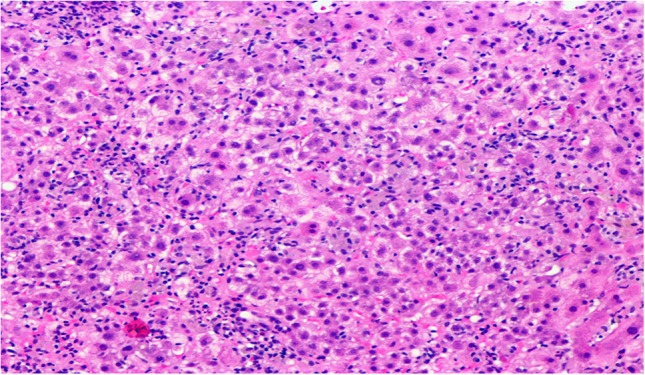
Liver tissue with apoptotic bodies, lobular mixed inflammation and clusters of ceroid cells.
Figure 2:
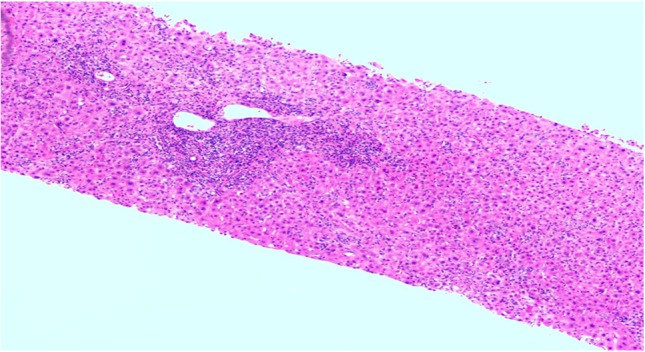
Portal triads show mild bile ductular proliferation with mixed inflammation predominantly neutrophils, in addition to lymphocytes, eosinophils and fewer plasma cells.
DISCUSSION
Approximately, 185 000 new cases of invasive breast cancer are diagnosed yearly, and at least half of these women are both postmenopausal and eligible for adjuvant therapy with AI [2]. Letrozole is a nonsteroidal inhibitor of aromatase, which effectively blocks estrogen synthesis in postmenopausal women [8]. It is used as therapy for estrogen receptor positive breast cancer, usually after resection and after failure of tamoxifen [8]. It was approved for use in postmenopausal women with estrogen receptor positive breast cancer in the USA in 1997 [8].
While AI have been reported to cause minor elevation in liver enzymes, severe hepatotoxicity is rarely reported [3]. Liver injury is believed to be due to metabolic and immune-mediated damage, superimposed on individual susceptibility [4]. From this class of medication anastrozole and exemestane have been reported to cause drug-induced hepatitis [1, 3]. Anastrozole has been specifically reported in cases of drug- induced autoimmune hepatitis [4].
Letrozole on the other hand, have been reported to cause elevated liver enzymes in up to 1% of women. These elevations are usually mild, asymptomatic, self-limited and rarely require dose modification [8]. It is metabolized in the liver by the cytochrome P450 system and is a strong inhibitor of CYP 2A6 and to a lesser extent CYP 2C19 [8]. Thus, liver injury from letrozole might arise as a result of a toxic or immunogenic metabolite [8]. There have been no published instances of clinically apparent liver injury associated with long term letrozole therapy as of 2017 according to the US National Library of Medicine [2]. Furthermore, there have been no cases of severe jaundice, acute liver failure, chronic hepatitis or vanishing bile duct syndrome attributed to letrozole use [8]. To the best of our knowledge, this is the first case of drug- induced autoimmune hepatitis secondary to letrozole.
The risk of recurrence of hormone-receptor–positive breast cancer continues indefinitely [6]. Reduction in that risk of recurrence has been achieved with tamoxifen, AIs or a combination of the two. In the postmenopausal group, AI therapy was previously recommended up to five years, however it is now recommended up to 10 years based on the extended AI trial [6]. The TEXT and SOFT trials showed that exemestane, is significantly more effective than tamoxifen, in premenopausal women [7]. We expect there to be more widespread use of AI.
Review of the English literature reports four cases of AI induced hepatitis (Table 1). All patients were admitted to hospital. Three of the four patients were exposed to anastrozole. All patients’ onset of hepatitis occurred within 6 months, however hepatitis occurred within 3 weeks for two of the four patients. All had improvement in liver tests after withdrawal of the drug, however, one patient required a tapered course of steroids due to features of autoimmune hepatitis. One patient was treated with tamoxifen after resolution of her hepatitis which she tolerated well.
Table 1:
Literature review of characteristics of patients with hepatitis due to AIs
| Reference | Age | Interval between onset of hepatitis and drug use | Drug | Dose | Inpatient admission | Antibodies positive | Outcome |
|---|---|---|---|---|---|---|---|
| de la Cruz et al. [1] | 58 | 3 weeks | anastrozole | 1 mg/day | Yes | Improved liver tests after 1 month however patient died from another cause | |
| Inno et al [10] | 70 | 4 months | anastrozole | 1 mg/day | Yes | ANA | Normal liver tests after one month, then switched to tamoxifen which was well tolerated. |
| Bao et al. [3] | 47 | 3 weeks | exemestane | 25 mg/day | Yes | Liver tests normalized after 4 months | |
| Islam et al. [10] | 66 | 6 months | anastrozole | Yes | ANA, anti-smooth muscle antibody | Initial improvement after 3 months, 12 months later treated with tapered steroids for asymptomatic acute liver derangement with elevated IgG |
Diagnosis of autoimmune hepatitis is confirmed by liver biopsy [4]. Overall, the time lapse between drug exposure and the onset of hepatitis, the age of the patient, exclusion of other non-drug-related causes, improvement achieved after drug withdrawal and letrozole being reported as a potential cause of drug-induced liver disease in the product label, allow our case to achieve a score of 9 according the Roussel-Uclaf Causality Assessment Method (RUCAM) [9].
The goal of treatment is withdrawal of the drug. Patients generally do well with improvement in liver function.
CONCLUSION
AI is an extremely rare cause of hepatitis. It can present in association with autoimmune features. In the modern era, where breast cancer management is rapidly evolving, and AI use is becoming more widespread, with many patients expected to be on these medications for up to 10 years, physicians must be aware of this rare but life-threatening complication. After starting AI therapy, it may be important to monitor these patients closely during the first 6 months when drug-induced hepatitis is most common, followed by the minimum requirement of six month follow-up.
CONFLICT OF INTEREST STATEMENT
The authors have no areas of conflict to declare.
FUNDING
The authors have not received funding.
PATIENT CONSENT
Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial board of this journal.
AUTHORS' CONTRIBUTION
The idea for reporting this case was that of B.G., K.S. and L.Z. Further intellectual content and editing was done by all authors. All authors saw, edited and approved the final contents. K.S. assumes responsibility for the integrity of the contents.
REFERENCES
- 1. De la Cruz L, Romero-Vazquez J, Jiménez-Sáenz M, Padron JR, Herrerias-Gutierrez JM. Severe acute hepatitis in a patient treated with anastrozole. Lancet 2007;369:23–4. [DOI] [PubMed] [Google Scholar]
- 2. Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract 2007;61:2051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bao T, Fetting J, Mumford L, Zorzi J, Shahverdi K, Jeter S, et al. Severe prolonged cholestatic hepatitis caused by exemestane. Breast Cancer Res Treat 2010;121:789–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Islam MS, Wright G, Tanner P, Lucas R. A case of anastrazole-related drug-induced autoimmune hepatitis. Clin J Gastroenterol 2014;7:414–7. [DOI] [PubMed] [Google Scholar]
- 5. Zarkavelis G, Kollas A, Kampletsas E, Vasiliou V, Kaltsonoudis E, Drosos A, et al. Aromatase inhibitors induced autoimmune disorders in patients with breast cancer: a review. J Adv Res 2016;7:719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209–19. doi:10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Francis PA, Regan MM, Fleming GF, Láng I, Ciruelos E, Bellet M, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 2015;372:436–46. doi:10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. https://livertox.nih.gov/Letrozole.htm. Accessed 25 June 2017.
- 9. Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci 2016;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inno A, Basso M, Vecchio FM, Marsico VA, Cerchiaro E, D’Argento E, et al. Anastrozole-related acute hepatitis with autoimmune features: a case report. BMC Gastroenterol 2011;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]


