Abstract
Background and Aims
Ophiocaryon is a lesser known genus in Sabiaceae. This study examines flowers of six Ophiocaryon species in comparison with Meliosmaalba, to identify taxonomically informative characters for understanding relationships within the family Sabiaceae, to imply previously unknown pollination mechanisms of Ophiocaryon, and to contribute to the placement of Sabiaceae within the early-diverging eudicots.
Methods
Floral morphology and anatomy of six Ophiocaryon species and M. alba were studied and described using scanning electron microscopy, clearing techniques and resin sectioning.
Key Results
Novel characters of Ophiocaryon were identified, e.g. conical cells on petals, different kinds of orbicules in anthers, stomata on nectary appendage tips and ovary, two distinct surface patterns on stamens and ovary, tanniferous cell layers in the ovary wall, and acorn-shaped unitegmic ovules with very short integuments. Comparison of floral characters between Ophiocaryon and Meliosma found that the calyx, corolla, androecium and gynoecium of Ophiocaryon resemble an undeveloped state of the latter taxon, reflecting a paedomorphic regression of the flower of Ophiocaryon. The flower morphology and anatomy of Ophiocaryon was compared with its putative sister species M. alba, but no clear shared derived characters could be detected. Moreover, the findings of scent, presence of conical cells on petals and a nectary suggest flowers are pollinated by small insects with a secondary pollen presentation on the cupula of fertile stamens.
Conclusions
We found that Ophiocaryon may be derived from ancestors that were similar to extant Meliosma in their flower structure and pollination mechanism. However, the lack of shared derived characters between Ophiocaryon and its phylogenetic sister group M. alba is puzzling and requires further investigations on the diversity of the latter species.
Keywords: Basal eudicots, floral anatomy, Meliosma alba, nectary, Ophiocaryon, paedomorphosis, Sabiaceae, secondary pollen presentation
INTRODUCTION
Ophiocaryon is a small genus of Sabiaceae distributed in South America (Aymard and Daly, 2006). The first scientific record of the genus was made by Endlicher (1841) based on R. Schomburgk’s 1840 illustration of plants from British Guyana, which later became the type of the genus, O. paradoxum (Schomburgk, 1845). The common name ‘snake nut tree’ is based on the coiled embryo, which resembles a snake’s head and tail (Schomburgk, 1840;Aymard and Daly, 2006). About a decade after the first description, Bentham (1859) described a novel monotypic genus, Phoxanthus, with P. heterophyllum as a single species, from specimens collected in Brazil without realizing the similarity with Ophiocaryon. This classification was also accepted by Warburg (1895), who provided information on other characters that could delimit these two genera, such as differences in petal shape and embryo structure. Later, Urban (1895, 1900) lumped Phoxanthus into Ophiocaryon and this treatment has been accepted ever since. The recent revision of the genus by Barneby (1972) has described four new species of Ophiocaryon and subdivided the recognized seven species into two series, an Ophiocaryon group having rounded petals and a Phoxanthus group with lanceolate petals. This classification reflects a compromise between Warburg’s and Urban’s treatments. The latest study in connection with the Flora of Venezuelan Guyana project has updated the present number of species of Ophiocaryon to nine (Aymard and Daly, 2006). However, besides these taxonomic investigations, there are no other studies dealing with the mutual relationships between species of Ophiocaryon or the relationships between this genus and the rest of the family.
Ophiocaryon belongs to the family Sabiaceae with two other genera, Sabia and Meliosma. All three genera share pentamerous flowers with a differentiated perianth, superposed sepals, petals and stamens, disporangiate monothecate stamens and two fused carpels (Bentham and Hooker, 1862;Urban, 1900;Kubitzki, 2007). Sabia has actinomorphic flowers with five fertile stamens, while Meliosma and Ophiocaryon share monosymmetrical flowers with two fertile and three sterile stamens. Several taxonomists recognized a closer relationship between Ophiocaryon and Meliosma (Planchon, 1855;Dahlgren, 1980;Takhtajan, 1997;Heywood et al., 2007), which was confirmed by a recent phylogenetic study of Sabiaceae based on molecular data (Zúñiga, 2015). These results indicate that Ophiocaryon is embedded within Meliosma as part of a basal clade with M. alba. A close relationship of Ophiocaryon and M. alba has never been revealed before.
The historical position of Sabiaceae on the tree of life is complicated. Before molecular classifications, most authors linked Sabiaceae with Rutales or Sapindales (Bentham and Hooker, 1862;Hutchinson, 1973;Dahlgren, 1980;Thorne, 1992;Takhtajan, 1997), while some authors pointed out a relationship with Ranunculales (Bentham and Hooker, 1862;Cronquist, 1996). Many papers with a novel research approach of molecular phylogenetic reconstruction firmly support the position of the Sabiaceae in the early-diverging eudicots close to Ranunculales (e.g. Hoot et al., 1999;Savolainen et al., 2000a, b; Soltis et al., 2000, 2011.Kim et al., 2004;Worberg et al., 2007) or Proteales (e.g. Chase et al., 1993;Barniske et al., 2012;Sun et al., 2016). Recently published molecular classifications have included Sabiaceae in the Proteales based on the strong association of the two groups [APG IV (Angiosperm Phylogeny Group, 2016)].
The pentamerous perianth in Sabiaceae is interesting since it is an unusual feature in the basal eudicot grade, which is predominantly dimerous and trimerous (Endress, 2010). The origin of pentamery is likely to be associated with the more closely related groups of Sabiaceae, viz. Ranunculales and Proteales. Previous anatomical and developmental observations in Sabia and Meliosma discussed three hypothetical origins, i.e. from a continuous spiral, a dimerous or a trimerous origin (Ronse De Craene et al., 2015b). However, there are no morphological studies involving the lesser known third genus of Sabiaceae, Ophiocaryon, and its floral morphology remains largely unexplored.
The objective of this study was to investigate the floral morphology and anatomy of selected species of Ophiocaryon and compare the obtained information with the two other genera in Sabiaceae previously studied (Wanntorp and Ronse De Craene, 2007;Ronse De Craene and Wanntorp, 2008;Ronse De Craene et al., 2015a, b) to uncover potentially important features that may support the relationships within the family and among other early-diverging eudicots.
MATERIALS AND METHODS
Samples from six out of nine species of Ophiocaryon, viz. O. duckei, O. heterophyllum, O. klugii, O. paradoxum, O. maguirei and O. manausense, together with M. alba, were used in this study in the form of spirit material or dried herbarium specimens (Table 1). Fresh materials were fixed in an FAA solution (90 % ethanol at 70 %, 5 % acetic acid, 5 % formaldehyde at 40 %) and then transferred and stored in 70 % ethanol. Dried herbarium specimens were soaked in a solution of 6:1 10 % Aerosol-OT aqueous solution/acetone following the protocol of Ayensu (1967), then stored in 70 % ethanol. For morphological observations, fully or nearly fully opened mature flowers were dissected under a light microscope (Zeiss Stemi SV6), dehydrated in an ethanol–acetone series, critical-point dried using CO2 in a K850 critical-point dryer (Quorum Technologies), coated with platinum in an Emitech K575X Sputter Coater, and examined with an LEO Supra 55VP scanning electron microscope. For anatomical investigation, materials were dehydrated through an ethanol infiltration medium series, embedded in Technovit resin and sectioned with a Leica RM2235 rotary microtome at 5–10 μm thickness. Sections produced from the microtome were put on slides, stained with toluidine blue, observed under a light microscope (Zeiss Axioskop) and photographed with an AxioCam MRc5 (Zeiss). For tissue clearing, flower materials were transferred into 10 % sodium hydroxide (NaOH) solution for 2 d or until the tissue became clear, rinsed with distilled water, stained with aqueous safranin, observed under a dissecting microscope (Zeiss Stemi 2000-C) and photographed with an AxioCam MRc 5 (Zeiss).
Table 1.
Origin of species of Ophiocaryon and Meliosma used in this study
| Species | Codes | Collector with Number | Place of collection/ origin |
|---|---|---|---|
| O. heterophyllum (Benth.) Urb. | 2K 7/180*, 6F 5/169* | Honorio and Saavedra 160, 162 | IIAP-CIJH, Loreto, Peru |
| O. duckei Barneby** | K000601639 | Ducke 1611 | RBG, Kew/Brazil |
| O. klugii Barneby** | K000601629 | Klug 2706 | RBG, Kew/Peru |
| O. paradoxum R.H. Schomb.** | K000601630, K000601631 | Jenman 2410, Jenman s.n. | RBG, Kew/Guyana |
| O. maguirei Barneby** | K000601634 | Maguire 32144 | RBG, Kew |
| O. manausense (W.A. Rodigues) Barneby** | – | Ribeiro 931 | RBG, Kew |
| M. alba (Schitdl.) Walp. | 19734015A* (under the synonym M. beaniana Rehder and E.H. Wilson) | Wilson A154 | RBGE/China |
IIAP-CIJH, Instituto de Investigaciones de la Amazonía Peruana, Jenaro Herrera Research Centre; RBG, Kew, Royal Botanic Garden Kew; RBGE, Royal Botanic Garden Edinburgh.
*Tree code 1.
**Herbarium specimens.
RESULTS
Morphology
A floral morphological investigation was carried out in six species of Ophiocaryon, i.e. O. duckei, O. heterophyllum, O. manausense, O. maguirei, O. klugii and O. paradoxum. The description is mainly based on O. heterophyllum, for which pickled material was available, with some additional information from the other species since the basic morphology of all six species is similar. The observation of specimens shows that flowers are clustered in highly condensed plagiotropic and multi-flowered branched thyrsoid inflorescences (Fig. 1A). Lateral branches are clearly cymose with upper flowers developing earlier (Fig. 1B). The rachis is covered with multicellular trichomes. Each flower is on a short pedicel subtended by a bract. Bracts have a fimbriate margin covered with multicellular trichomes.
Fig. 1.
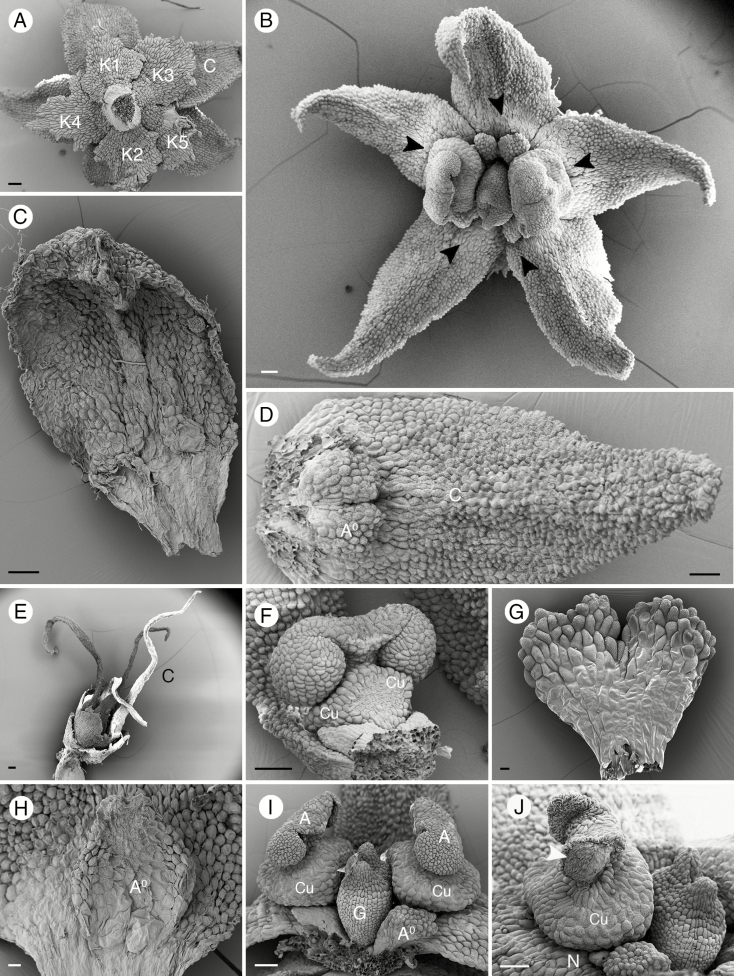
Inflorescence and mature flowers of a representative species of Ophiocaryon (O. heterophyllum). (A) Compound leaves and inflorescence. (B) Partial inflorescence with blooming flowers. (C) Mature flower close to anthesis. Note that the two inner petals are smaller than the three outer petals (arrowheads point to smaller petals). (D) Mature flower at anthesis. Scale bars: (A) = 1 cm; (B–D) = 500 μm.
Most of the species included in this study have a pentamerous perianth (Figs 1B, (C, D and 2A, B) except O. duckei, which is tetramerous (possibly dimerous?). The perianth is obviously bipartite with a calyx and corolla series (Fig. 2A). The calyx consists of four or five sepals (Fig. 2A). Sepals have an ovate shape with a lobed margin, which is sometimes fimbriate with multicellular trichomes (Fig. 2A). The calyx is membranous, occasionally with a white margin (O. duckei and O. paradoxum). Prophylls were not observed in any of the studied species. However, the two outer sepals of O. heterophyllum are slightly smaller than the inner three sepals (Fig. 2A). Sepals are arranged in a 2/5 (quincuncial) pattern (Fig. 2A), except in tetramerous O. duckei, which has a decussate arrangement. In all species, the position of each petal is opposite to a sepal, although those opposite the inner sepals are slightly off the median line (Fig. 2A). The arrangement of petals is the same as that of sepals, with a quincuncial arrangement in pentamerous species (Figs 1C and 2A, B) and a decussate arrangement in tetramerous species. In O. heterophyllum, the two inner petals are smaller than the outer three (Figs 1C and 2B). The corolla shape is variable among the four species, as described by Barneby (1972). Ophiocaryon paradoxum, O. duckei and O. maguirei, which belong to the Ophiocaryon series, have ovate or obovate petals with an obtuse tip (Fig. 2C). The difference between them is that O. paradoxum and O. maguirei have a pentamerous perianth while O. duckei has a tetramerous perianth. In the species of the Phoxanthus series, O. heterophyllum has lanceolate petals with an acute apex (Figs 1B, C, D and 2B, D), while in O. klugii the petals have a linear–lanceolate shape (Fig. 2E). We could not describe the petal shape of O. manausense because the specimen lacked petals, but it was assigned in a previous description (Barneby, 1972) to the Phoxanthus series from its lanceolate petals with an acute apex. Flowers of Ophiocaryon are weakly monosymmetrical, with the symmetry line running obliquely relative to the axis (Fig. 1D).
Fig. 2.
Floral morphological structures of Ophiocaryon. (A, B, D, F, G, I, J) O. heterophyllum; (C) O. duckei; (E) O. klugii. (H) O. paradoxum. (A) Superposed calyx and corolla, with two outermost sepals (K1, 2) smaller than the three inner sepals (K3–5). (B) A fully anthetic flower. Note arrangement of stamens opposite inner petals and small staminodes opposite outer petals (arrowheads). (C) A petal with ovate shape and obtuse apex. (D) A petal with ovate shape and acuminate apex, attached to a bilobed staminode. (E) A flower with elongated oval-shaped petal with acuminate apex. (F) A short swollen stamen attached to a petal with disporangiate, monothecal anther; note the rough upper surface and smooth lower surface. (G) A bilobed obcordate staminode with rough upper surface and smooth lower surface. (H) An ovate staminode with two small lobes at the apex. (I) Two stamens on lateral sides of the ovary; note cup-shaped structure under the anther (Cu). (J) Curling up of the anther wall after release of the pollen grains (arrowhead). A, stamen; A0, staminode; K, calyx; C, corolla; Cu, cupula; G, pistil. Scale bars: (A–F, I, J) = 100 μm; (G) = 20 μm; (H) = 30 μm.
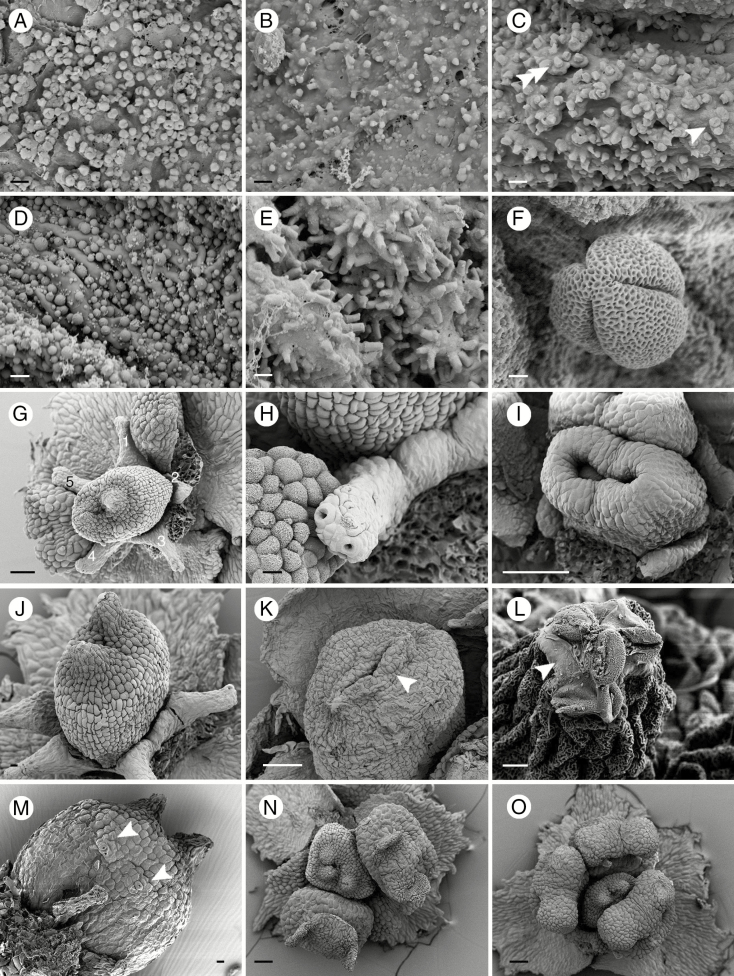
In all species, the androecium consists of two fertile stamens and two or three staminodes (Fig. 2B). The two fertile stamens are located on the lateral side of the ovary opposite the smaller petals (Fig. 2B). The filaments are short and swollen on the upper part, jointly forming a cup-shaped structure (cupula) with the lower part of the connective (Fig. 2F, I). The epidermal cells in the upper part of the stamens have a striate cuticle, while the lower part is smooth (Fig. 2F). The three staminodes of Ophiocaryon are flat with an obcordate shape and have a bilobed apex (Fig. 2G), with the exception of O. paradoxum, which has ovate staminodes with two small lobes (Fig. 2H). Staminodes also have a rough upper surface and smooth lower surface, similar to fertile stamens (Fig. 2G, H). The base of the filaments is clasped by the petal base and appears postgenitally fused (Fig. 2F); fusion appears more strongly between the petal and staminode (Fig. 2D, H). In the anther, there are two pollen sacs with one slit each (bisporangiate dithecal) attached to the thick connective on the abaxial side (dorsifixed) (Fig. 2F, I). Anthers of both fertile stamens are bent towards the ovary (Fig. 2I). The slit opening the pollen sacs creates an upward movement of the anther walls to disperse pollen grains to the sides of the anther (latrose dehiscence) (Fig. 2J). Orbicules, i.e. sporopollenin structures located on the adaxial surface of the microsporangial wall of the anthers (Verstraete et al., 2014), were observed in five Ophiocaryon species, i.e. O. duckei, O. heterophyllum, O. klugii, O. maguirei and O. paradoxum, and appear to be present in all observed species. The orbicules of O. paradoxum are oval-shaped with a central depression (Fig. 3A), whilst O. heterophyllum has orbicules with a polygonal shape without any depression (Fig. 3B). In O. duckei, the orbicules are dimorphic, mainly with a polygonal shape, but some are oval-shaped with a central depression (Fig. 3C). Ophiocaryon klugii has smooth spherical-shaped orbicules (Fig. 3D) and O. maguirei has rod-shaped orbicules (Fig. 3E). Pollen grains are oval-shaped with three grooves and with a reticulate surface (Fig. 3F; tricolporate; see the Anatomy section).
Fig. 3.
Details of anthers and floral structures of Ophiocaryon. (A, F) O. paradoxum; (B, G, N, O) O. heterophyllum; (C) O. duckei; (D) O. klugii; (E, L) O. maguirei; (K, M) O. manausense. (A) Oval-shaped orbicules with central compression situated on the inner anther wall. (B) Polygonal-shaped orbicules. (C) Mixture of oval-shaped orbicules with central compression (arrowhead) and polygonal shaped orbicules (double arrowhead). (D) Spherical-shaped orbicules. (E) Rod-shaped orbicules. (F) A pollen grain with three grooves and reticulate ektexine. (G) Narrow nectar disc with five appendages surrounding the ovary. (H) A nectary appendage with two stomata. (I) A young ovary comprising two horseshoe-shaped carpels that fuse congenitally at the base. (J) Ovary with rough-celled style and stigmatic area surface and smooth lower surface. (K) Ovary with horizontally appressed style (arrow). (L) Tip of stigma with pollen grains glued by exudate (arrow). (M) Stomata on ovary wall (arrows). (N) A flower with three-carpellate ovary. (O) A flower with three fertile stamens and two staminodes. Scale bars: (A–E) = 1 μm; (F) = 2 μm; (G, I, L, N, O) = 100 μm; (H, J, K) = 20 μm; (M) = 10 μm.
Within the androecium there is a narrow nectary as a ring surrounding the ovary base (Fig. 3G). Five appendages emerging from the nectar ring are observed in O. heterophyllum (Fig. 3G), O. paradoxum and O. klugii (occasionally six). In other species, appendages are also present but their exact number and morphology could not be determined due to their poor condition caused by drying. Two or more stomata are present on the tip of each nectary appendage (Fig. 3H).
In the centre of the flower there is a sessile ovary. It usually consists of two fused carpels with two short diverging styles and weakly developed stigmatic tissue (Fig. 3G, J). In O. manausense the styles are longer than in other species. Figure 3I represents a young stage showing two distinct carpel primordia that are initiated before being raised by a congenitally fused base. The mature ovary is syncarpous by extensive basal growth. The synascidiate zone of the ovary contains two superposed ovules per carpel and the symplicate zone is very short. In O. maguirei the styles are horizontally pressed against the top of the ovary (Fig. 3K). The upper part of the ovary is usually not completely fused (Fig. 3G, K). We often found pollen grains accumulating on the tips of the styles and glued with a secreted substance (Fig. 3L). The upper part of the ovary inclusive of the stigmatic area has a rough surface while the lower part is smooth (Fig. 3G, J). In some samples we found stomata on the ovary surface (O. maguirei, O. manausense and O. paradoxum) (Fig. 3M).
There are several anomalous variations in the number of floral parts of Ophiocaryon. For example, we observed the presence of four (di- or tetramerous flowers) or six petals (tri- or hexamerous), a three-carpellate ovary (Fig. 3N) and three fertile stamens (Fig. 3O) in flowers of O. heterophyllum.
Anatomy
Four species of Ophiocaryon, i.e. O. duckei, O. heterophyllum, O. klugii and O. paradoxum, were included in the anatomical study. The basic anatomy of all four species is very similar. Therefore, we base the description on O. heterophyllum with some extra details from other species.
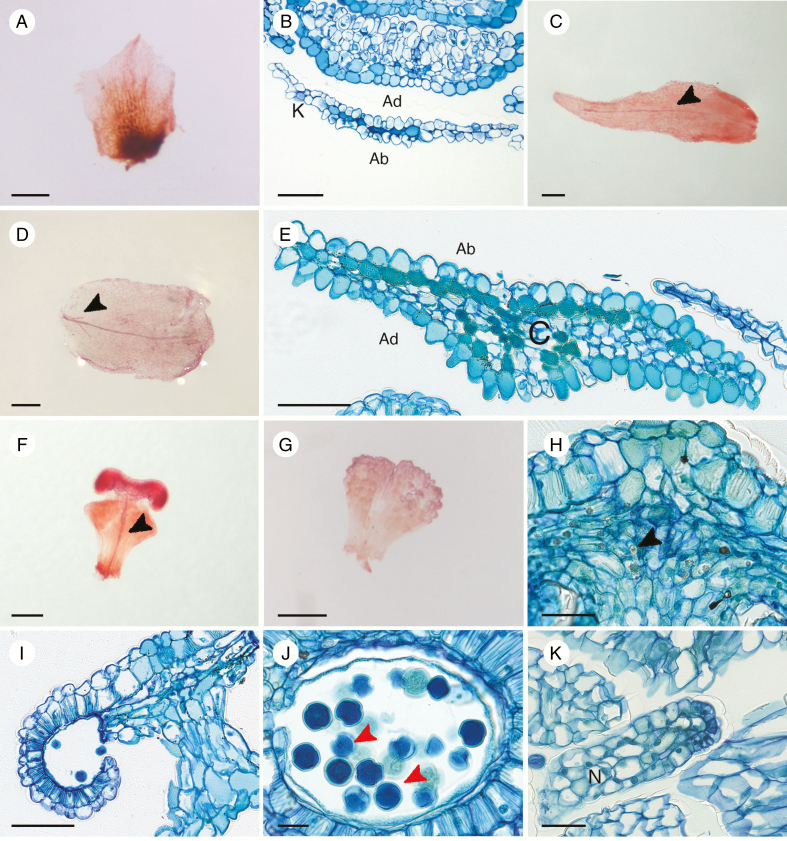
The anatomical investigation of the perianth of all species found that sepals have no vascular trace (Fig. 4A, B), while most species have one vascular bundle in the petals (Fig. 4C). Cells are flat on both the abaxial and the adaxial surface of sepals, with some evidence of conical cells (Fig. 4B). In O. duckei, the vascular bundle in the petal branches at the base into two short lateral traces and one long middle trace (Fig. 4D). Conical cells are mainly observed on the adaxial side of petals of all four species in this study (Fig. 4E), with some evidence of weakly developed conical cells on the abaxial side of O. duckei, O. heterophyllum and O. paradoxum petals (Fig. 4E). Damage from herbarium drying in O. klugii prevented us observing the presence of conical cells on the abaxial side. In all investigated species, darkly stained cells were found forming a layer under the lower epidermis or scattered within the petals (Fig. 4E).
Fig. 4.
Floral anatomical structures of Ophiocaryon. (A–C, E, F, H–K) O. heterophyllum; (D) O. duckei; (G) O. paradoxum. (A) A cleared sepal without vascular bundle. (B) Transverse section showing a superposed sepal and petal; note the thin sepal anatomy without any vein. (C) A cleared petal with one vascular bundle (arrowhead). (D) A cleared petal with one main vein branching into two short veins at the base (arrowhead). (E) Transverse section of a petal showing tanniferous cells on the abaxial side and scattered around the mesophyll; conical cells are present mainly on the adaxial side of the petal. (F) A fertile stamen with a single vein (arrowhead). (G) A non-vascularized staminode. (H) Longitudinal section of a fertile stamen with calcium oxalate crystals within the connective tissue (arrowhead). (I) Longitudinal section of a fertile stamen showing the break-up of the anther wall, releasing pollen grains. Three layers of the anther wall are still visible, i.e. epidermis, endothecium and middle layer. (J) Longitudinal section of anther showing the presence of endoapertures of pollen grains (arrowheads), which indicate the type of Ophiocaryon pollen as tricolporate; endothecium, middle layer and tapetum are visible. (K) A non-vascularized nectary appendage composed of secretory cells. Ab, abaxial side; Ad, adaxial side; C, corolla; K, calyx; N, nectary. Scale bars: (A, C, D, F, G) = 200 μm; (B, E, I) = 100 μm; (H, K) = 50 μm; (J) = 20 μm.
In all species, the fertile stamen has one vascular bundle running from the base through the filament to the connective tissue (Fig. 4F). There is usually no vascular bundle present in the staminodes (Fig. 4G). There are several calcium oxalate crystals containing cells in the connective tissue (Fig. 4H). The anther wall consists of four layers, i.e. a single epidermal layer, an endothecium with cells processing fibrous thickenings, a thin middle layer and a thin tapetum (Fig. 4I, J). When dehiscing, the anther wall breaks open via slits (Fig. 4I); next the wall bends upward, releasing pollen grains. Pollen grains are tricolporate (Fig. 4J). The nectar ring surrounding the ovary consists of small secretory cells and there are no vascular bundles present (Fig. 4K).
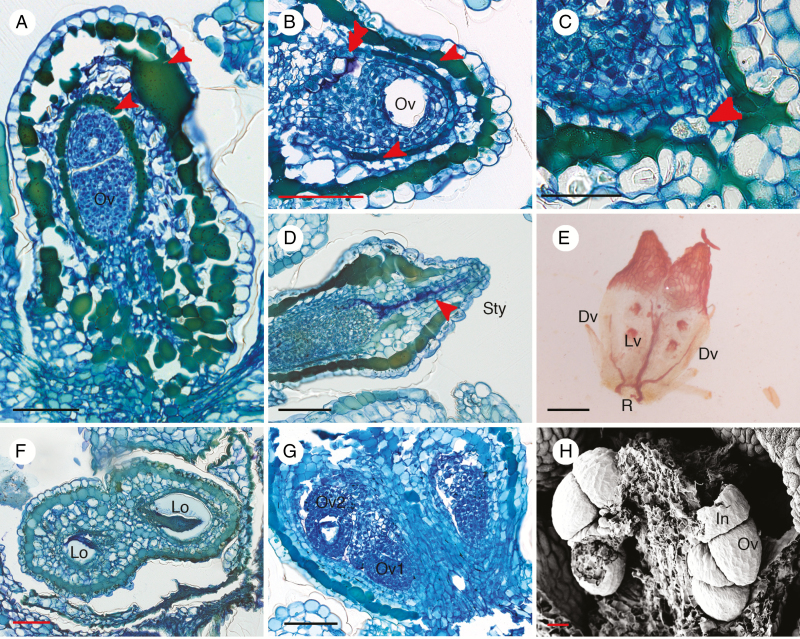
The anatomical description of the gynoecium is based on O. heterophyllum since herbarium specimens from other species were too damaged. Sectioning of the ovary showed that there are two layers of tanniferous cells in the ovary wall; one layer is situated under the outer epidermis and another is situated immediately below the inner epidermis (Fig. 5A, B). The inner layer is usually narrower than the outer one (Fig. 5A, B). Moreover, cells containing calcium oxalate are present in the basal part and walls of the ovary (Fig. 5C). Inside the style, there is secretion from cells along the stylar canal (Fig. 5D). There is a vascular girdle at the base of the ovary (Fig. 5E). Two vascular bundles run on the ventral side and each branch higher up into Y-shaped short bundles running in the upper part of the ovary (Fig. 5E). The remaining two unbranched vascular bundles supply the ovary on the dorsal sides of the carpels until they fade out on the upper part of the ovary (Fig. 5E). Therefore, there is no vascular bundle extending into the style (Fig. 5E). Transverse sections showed that the two carpels are fused at the margin, producing a syncarpous gynoecium with two locules (Fig. 5F). In each locule there are two superposed ovules (Fig. 5F, G) occupying the narrow cavity within the ovary. Generally, one of the ovules is larger than the other (Fig. 5G). The placentation is axile with alternating ovule attachment (Fig. 5F, G). This indicates that two ovules are initiated on the same plane on different sides of the carpel margin, but overlap and become superposed by lack of space. Ovules are hemi-anatropous and are attached to the placenta via a short funiculus (Fig. 5G, H). The ovules are crassinucellar with a single reduced integument (unitegmic), making the young ovules look like acorns (Fig. 5G, H). Cells lining the stylar canal produce a secretion that also envelops the ovule tip (Fig. 5D). There is no micropyle since the single integument is reduced and does not cover the nucellus.
Fig. 5.
Anatomical structures of Ophiocaryon gynoecium. (A–D, G, H) O. heterophyllum; (E) O. paradoxum; (F) O. duckei. (A) Longitudinal section of ovary presents two layers of tanniferous cells, in the hypodermis and endodermis (arrowheads); two superposed ovules are visible inside a locule. (B) Transverse section of ovary showing an ovule inside a locule; there are two layers of tanniferous tissue in the ovary wall (arrowheads); note purple intralocular hairs (double arrowhead). (C) Presence of calcium oxalate crystal at the base of the ovary (arrowhead). (D) Longitudinal section of young ovary with secretion visible in the stylar canal (arrowhead). (E) Cleared ovary showing vascular bundles forming a ring (R) at the base and branching into two Y-shaped lateral veins (Lv) and two dorsal veins (Dv); no vascular bundles reach into the style. (F) Transverse section of an ovary consisting of two carpels with axile placentation; ovules are alternately attached on different sides of the locule. (G) Two superposed ovules are present in each locule. (H) Scanning electron microscopic image of young ovules demonstrating that integuments of ovules are reduced and swollen. Dv, dorsal vein; In, integument; Lv, lateral vein; Lo, locule; Ov, ovule; R, vascular ring; Sty, style. Scale bars: (A–D, F, G) = 100 μm; (E) = 200 μm; (H) = 30 μm.
Floral structure of Meliosma alba
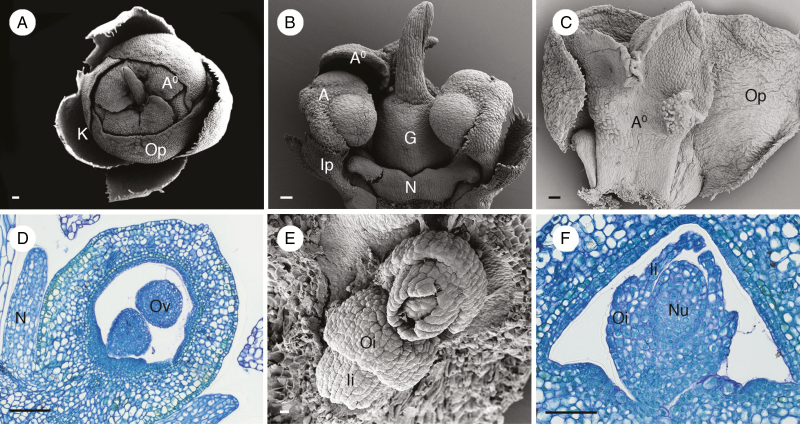
Flowers of the Asian lineage M. alba were examined. We found that its flower morphology is similar to other species of Meliosma previously studied (Wanntorp and Ronse De Craene, 2007;Ronse De Craene and Wanntorp, 2008). The perianth of M. alba is bipartite with differentiated sepals and petals (Fig. 6A). The calyx consists of four or five sepals while the corolla consists of five petals (Fig. 6A), as previously reported in some species of Meliosma (Wanntorp and Ronse De Craene, 2007). Petals of M. alba are arranged in a quincuncial aestivation with three big outer petals and two small inner petals (Fig. 6A–C). Inside the corolla there are two fertile stamens and three staminodes located opposite to the inner petals and outer petals respectively (Fig. 6A–C). Three staminodes form a dome covering the anthers of the fertile stamens and gynoecium (Fig. 6A). There is a nectary ring surrounding the base of the ovary with appendages alternating with the members of the androecium (Fig. 6B, D). At the centre of the flower there is an ovary comprising two carpels with two long, closely appressed and twisted styles (Fig. 6B). The ovary wall contains two layers of slightly dark-stained cells in the outer and inner hypodermis (Fig. 6D). Ovules are crassinucellar and bitegmic (Fig. 6E, F). The outer integument is two or three cells thick and is shorter in length compared with the inner integument, which is two cells thick (Fig. 6E, F).
Fig. 6.
Floral morphology and anatomy of Meliosma alba. (A) Top view of nearly open flower showing four sepals (K), three outer petals (Op) and a dome formed by three staminodes (A0). (B) Lateral view of flower showing two fertile stamens (A) opposite two small inner petals (Ip) on lateral sides of the two-carpellate ovary (G) and one of the three staminodes (A0) (two were removed). A well-developed nectar disc (N) with appendages is visible at the base of the ovary. (C) Adaxial view of an outer petal (Op) and a corresponding staminode (A0). (D) Longitudinal section of a young ovary showing two ovules within one locule; the ovary wall has two faint tanniferous tissue layers in the outer and inner hypodermis. (E) View of two superposed ovules within a locule; note the two integuments, the outer (Oi) reduced compared with the inner (Ii). (F) Transverse section showing crassinucellate ovule with two integuments; the outer integument is reduced while the inner integument is well developed and forms a micropyle. A, stamen; A0, staminode; K, calyx; G, ovary; Ii, inner integument; Ip, inner petal; N, nectary; Nu, nucellus; Op, outer petal; Oi, outer integument; Ov, ovule. Scale bars: (A–C, F) = 100 μm; (D) = 200 μm; (E) = 20 μm.
DISCUSSION
Comparison of floral characters with other Sabiaceae
Flowers of Ophiocaryon show a distinctive bipartite perianth separated into calyx and corolla. In the calyx there are five sepals arranged with imbricate quincuncial (in pentamerous species) or decussate (in tetramerous species) aestivation. Observations in O. heterophyllum showed that the two outer sepals are slightly smaller in size than the three inner sepals (Figs 1C and 2A), which was reported by previous studies (Urban, 1895;Aymard and Daly, 2006). From previous floral examinations in Meliosma, there is no agreement about describing the two outermost perianth parts either as prophylls (Endress, 2010) or sepals (Wanntorp and Ronse De Craene, 2007). From the present study, there is no significant difference in general appearance between the two outer and three inner sepals in Ophiocaryon. Therefore, these two outermost perianth parts should be interpreted as sepals. The results further agree with previous reports that there are clearly no prophylls in Ophiocaryon (Urban, 1895;Aymard and Daly, 2006;Kubitzki, 2007).
The five petals of Ophiocaryon look similar at anthesis. However, observations in young flowers have shown that the two inner petals are smaller than the other petals and are curved, covering the two opposing fertile stamens. This dimorphic character bears resemblance to the petals of Meliosma, which show an even greater dimorphism with much smaller petals opposite the fertile stamens (Ronse De Craene and Wanntorp, 2008), emphasizing the close relationship between Meliosma and Ophiocaryon. In addition, anatomical and morphological examination of Ophiocaryon petals found that there are conical cells with rough surface present mainly on the adaxial side. This feature is also found in the genus Sabia but not in Meliosma (Ronse De Craene et al., 2015b). The presence of conical cells on petals is usually associated with the pollination process (Whitney et al., 2011).
The Ophiocaryon androecium consists of two fertile stamens and three staminodes. Fertile stamens are short and swollen, or described as cuneiform-shaped (Schomburgk, 1845), which is unique for the Sabiaceae. The top of the filament below the anther shows an expansion into a cup-shaped structure (cupula) (Figs 2F, I, J and 4F). Urban (1895) suggested that the cupula is developed from the upper part of the filament and the lower part of the connective with no distinct boundary. Results of this study found that the upper part of the stamen has a rough surface different from the smooth lower part (Figs 2F, I, J and 4F). In the cupula there is a transition from darkly stained cells (connective) to lightly stained cells (filament) (Fig. 4F), which is congruent with the interpretation of Urban. Compared with species of Meliosma (Ronse De Craene and Wanntorp, 2008), the cupula is weakly developed. Alternating with the fertile stamens there are three staminodes, which are also weakly developed. Staminodes also show a transition from a rough upper surface to a smooth lower surface, similar to fertile stamens (Fig. 2G). There is no cupula formation in the staminodes, which suggests that they have no function associated with pollen presentation.
The nectary of Ophiocaryon is a low ring-shaped structure surrounding the base of the gynoecium. It consists anatomically of numerous densely staining cells without any vascular bundles (Fig. 4K). Previous descriptions stated that the nectar disc in Ophiocaryon has five appendages (Bentham and Hooker, 1862;Urban, 1900;Aymard and Cuello, 2005;Kubitzki, 2007), which were also observed in this study (Fig. 3G). Moreover, each appendage alternates with the members of the androecium (stamens and staminodes) and bears stomata at the tip (Fig. 3H). This feature was not noticed from previous descriptions since it is a minute structure. A similar nectar disc with appendages bearing apical stomata was described in the other two genera as well (Ronse De Craene and Wanntorp, 2008;Ronse De Craene et al., 2015b). However, contrary to Sabia and Meliosma, the lower part of the nectary is weakly developed, while the appendages may be variously expanded.
The ovary of Ophiocaryon is found to consist of two congenitally fused carpels (Fig. 3G, I–K), comparable to the previous report of Meliosma (Wanntorp and Ronse De Craene, 2007). The two carpels are not completely united in the upper part, leading to diverging styles (Fig. 3G, I, K), similar to Sabia but contrary to Meliosma with long appressed styles (Baillon, 1874;van de Water, 1980;Ronse De Craene et al., 2015b). However, in Sabia fusion of carpels appears to occur mainly postgenitally (Ronse De Craene et al., 2015b). Observations with scanning electron microscopy and tissue clearing found that the cell surface of the upper part of the ovary is rough while the lower part is smooth (Figs 3G, J and 5E). This result is the first record of this character in Ophiocaryon and appears to be unique within Sabiaceae. Most of the Sabia species investigated have a smooth ovary surface, except S. japonica, which has unicellular trichomes at the dorsal side of the carpels (Ronse De Craene et al., 2015b). Similar trichomes were observed in Meliosma as well (Ronse de Craene and Wanntorp, 2008). It is possible that the different surface texture in Ophiocaryon is caused by a difference in the pressure of other parts of the flower, which is more limited in the upper part of the ovary. Staminodes and stamens similarly have a lower smooth surface and an upper rough surface. The rough surface of the ovary including the stigmatic area, as well as the petals and androecium, may assist in the pollination process since the rough surface texture allows a better grip for pollinating insects (stamen, Figs 2I, J and 4F; ovary, Figs 3G, J and 5E). Observations in O. paradoxum, O. maguirei and O. manausense showed stomata on the surface of the ovary (Fig. 3M), which is unique within Sabiaceae since it has never been observed in Sabia and Meliosma before. However, this feature is also reported for the ovary surface in the majority of Ranunculales and Proteales (Endress and Igersheim, 1999).
Observation of O. heterophyllum demonstrated that there are two superposed ovules with axile placentation occupying all the space within one locule (Fig. 5F, G), similar to Sabia and Meliosma (Endress and Igersheim, 1999;Ronse De Craene and Wanntorp, 2008;Ronse De Craene et al., 2015b). Ovules have a crassinucellate nucellus and hemi-anatropous shape (Fig. 5G, H). Small numbers of intralocular hairs were observed in the present study (Fig. 5B), similar to previous reports in Sabia and Meliosma (Ronse De Craene and Wanntorp, 2008;Ronse De Craene et al., 2015b). Moreover, Ophiocaryon ovules have a weakly developed integument (unitegmy) and its connection to the swollen funiculus makes it look comparable to an acorn in a young developmental stage (Fig. 5G, H). This feature is unique to Ophiocaryon and to our knowledge has never been reported before. The development of the integument in other Sabiaceae is variable. Previous examinations of ovules found that unitegmy is present in Sabia and some Meliosma (Mauritzon, 1936;Raju, 1952;Endress and Igersheim, 1999;Ronse De Craene and Wanntorp, 2008;Ronse De Craene et al., 2015b), while bitegmic ovules with a reduced outer layer were observed in some Meliosma (Ronse De Craene and Wanntorp, 2008), including M. alba, which is the phylogenetic sister group of Ophiocaryon (Fig. 6A–F). Therefore, it is suggested that the single integument in Ophiocaryon represents the inner one.
Pollination biology of Ophiocaryon and Sabiaceae
The pollination biology of Ophiocaryon has not been mentioned in any previous studies. A plausible biotic pollen dispersal mechanism can be speculated from the present morphological and anatomical investigation. The presence of a ring-like nectary surrounding the pistil and the conical cells on petals suggest pollination by animals, such as insects small enough to be attracted by these small flowers. Different functional interpretations of conical cells on petals have been presented before, such as light reflection, temperature increase, scent formation or a foothold for pollinators (e.g. Endress, 1994;Whitney et al., 2011). The presence of scent in Ophiocaryon was reported in several specimens by different collectors (O. heterophyllum, E. Honorio 159, 160; O. maguirei, B. Maguire 32144).
The fertile stamens of Ophiocaryon bear a cupula, a cup-shaped structure located under the anther, which may have a function as a secondary pollen presentation area (Fig. 2F, I, J). The proposed function of this structure is supported by the dehiscence of pollen sacs, which release pollen grains to the side of the stamen (latrorse dehiscence) right on top of the cupula. Pollen will potentially be collected by insects that try to access the nectar produced by the nectary appendages, which are located between the stamens (Fig. 2J). This possible secondary pollen presentation of the cupula is similar to the connective disc in some Meliosma (Ronse De Craene and Wanntorp, 2008). However, the pollination system in Meliosma is different due to its explosive mechanism, whereby the anther is released from a staminodial dome (Ronse De Craene and Wanntorp, 2008), while the staminodes of Ophiocaryon do not have any specializations. The sister group of Ophiocaryon, M. alba, shares the explosive pollen release mechanism, as illustrated by J. D. Zúñiga (https://www.youtube.com/watch?v=nOrwIbRAo54&feature=youtu.be, accessed 24 May 2017). However, some species of Meliosma apparently do not have the explosive mechanism, and the pollen lying on the cupula is picked up by visiting insects (van Beusekom, 1971; Ronse De Craene and Wanntorp, 2008).
Intrageneric relationships
At present, four Ophiocaryon species are recognized as belonging to the Ophiocaryon series while another five species are in the Phoxanthus series (Aymard and Daly, 2006). However, apart from these taxonomical works, no other research has examined the legitimacy of the relationships within the genus. A recent molecular phylogeny including three Ophiocaryon species, two from the Phoxanthus series (O. heterophyllum and O. klugii) and one from the Ophiocaryon series (O. maguirei) (Zúñiga, 2015), cannot prove the legitimacy of series classification since it represents an unbalanced taxon sampling between the two series. The present study found that petals are still a good character to distinguish each series and even species in some specimens. Ophiocaryon klugii resembles O. heterophyllum closely in morphology. Ophiocaryon duckei and O. paradoxum of the Ophiocaryon series share the white-margined sepals and occasionally tetramery. However, observations of the orbicules found that they show variation in shape and size (Table 2). Our observations found that orbicules of O. duckei (Ophiocaryon series) are dimorphic, similar to both O. heterophyllum (Phoxanthus series) and O. paradoxum (Fig. 3C). Orbicules of O. maguirei are rod-shaped and distinct from those of two other Ophiocaryon series species, i.e. O. paradoxum and O. duckei (Fig. 3E). Moreover, orbicules of O. heterophyllum (Fig. 3B) and O. klugii (Fig. 3D) are different, although they are assigned to the same Phoxanthus series. Perhaps the appearance of orbicules in Ophiocaryon may be variable and not sufficiently informative for an intrageneric classification, but it is a valuable character for interspecific identification. Orbicules have been observed in Meliosma and Sabia (P. Thaowetsuwan and L. Ronse De Craene, pers. obs.), but no comparative study of different species has been carried out. Tetramerous (possibly dimerous) flowers are found in some Ophiocaryon species, e.g. O. duckei, O. barnebyanum and occasionally O. paradoxum (Table 2). This feature is distinct from other Ophiocaryon and is rare within Sabiaceae. Ronse De Craene et al. (2015a) observed the occasional presence of dimerous flowers in Sabia japonica and interpreted this as a further reduction from pentamerous flowers. Future molecular research in Sabiaceae should include more Ophiocaryon species to test the legitimacy of previous intrageneric classification.
Table 2.
Comparison of morphological characters of Ophiocaryon species used in the present study
| Floral character | Phoxanthus series | Ophiocaryon series | ||||
|---|---|---|---|---|---|---|
| O. heterophyllum | O. klugii | O. manausense | O. duckei | O. paradoxum | O. maguirei | |
| Merism | 5 | 5 | 52 | 41 | 5(4)1 | 5 |
| Symmetry | Monosymmetrical | Monosymmetrical | Monosymmetrical2 | Disymmetrical | Monosymmetrical | Monosymmetrical |
| Perianth aestivation | Quincuncial | Quincuncial | Quincuncial2 | Decussate | Quincuncial | Quincuncial |
| Petal shape | Lanceolate | Linear–lanceolate | Lanceolate2 | Ovate | Ovate | Ovate |
| Petal apex | acuminate | acuminate | acuminate2 | obtuse | obtuse | obtuse |
| Number of stamens/ staminodes | 2/3 | 2/3 | 2/3 | 2/2 | 2/3 | 2/3 |
| Staminode appearance | Obcordate, 2 lobes | Obcordate, 2 lobes | Obcordate, 2 lobes | Obcordate, 2 lobes | Ovate, 2 small lobes | Obcordate, 2 lobes |
| Orbicule shape | polygonal | spherical | ?3 | Polygonal + oval with central compression | Oval with central compression | Rod-shaped |
| Nectary disc appendages | + | + | + | + | + | + |
| Stomata on ovary surface | +/– | – | + | – | + | + |
| Style | Erect, short | Erect, short | Erect, long | Erect, short | Erect, short | Horizontal, short |
| Tanniferous layers in ovary wall | + | + | ?3 | + | + | ?3 |
| Number of integuments | 1 | ?4 | ?4 | ?4 | ?4 | ?4 |
1Could be interpreted as dimerous.
2The character could not be observed in the present study. Data from Rodrigues (1964) and Barneby (1972).
3Sample not available or excluded from the study.
4Damage from herbarium preparation prevented observation in the present study.
Relationship within Sabiaceae with special emphasis on Meliosma alba
The relationship of the three genera within Sabiaceae has been mostly discussed in terms of morphology in the past (Bentham and Hooker, 1862;Warburg, 1895;Urban, 1900;Kubitzki, 2007). Ophiocaryon is found to be more closely related to Meliosma than Sabia, as supported by several characters, e.g. habit, petiole feature, inflorescence form, floral symmetry, androecium components and nectary and fruit structure (reviewed in Heywood et al., 2007;Table 3). Moreover, there is a previous report showing that the wood anatomy of Meliosma is more similar to Ophiocaryon than to Sabia (Carlquist et al., 1993). Some authors even classified Meliosma and Ophiocaryon as a separate family (Meliosmaceae) from Sabia (Dahlgren, 1980;Takhtajan, 1997;Heywood et al., 2007). Interestingly, there was a suggestion to merge the two genera on the basis of morphological similarity (Planchon, 1855). A recently published phylogenetic study including all three genera of Sabiaceae has firmly supported the close relationship of Meliosma and Ophiocaryon, especially M. alba (Zúñiga, 2015). In the present study, several floral characters have been identified that are shared between Ophiocaryon and Meliosma. The petal dimorphism is shared between Ophiocaryon and Meliosma but not with Sabia. The perianth of Sabia is polysymmetrical while it is monosymmetrical in Meliosma owing to the two inner reduced petals and the presence of staminodes opposite the outer petals (Fig. 6A–C). In Ophiocaryon the perianth looks polysymmetrical in the fully open flower. However, the two inner petals are slightly smaller compared with the outer petals (Figs 1C and 2B). The difference is more obvious in flowers that are not fully anthetic, where two inner petals are wrapped on top of the stamens making the perianth look monosymmetrical (Fig. 1C). Meliosma and Ophiocaryon also share a similar oblique monosymmetry (Wanntorp and Ronse De Craene, 2007;Fig. 1D). The arrangement of the perianth parts is likewise different in each genus. Sabia has a perianth with a whorled phyllotaxy (Ronse De Craene et al., 2015a), while Meliosma and Ophiocaryon have a perianth with a spiral arrangement due to the different size of the petals (Wanntorp and Ronse De Craene, 2007;Fig. 2B). Moreover, basal fusion of petals with stamens is present in Meliosma and Ophiocaryon (Ronse De Craene and Wanntorp, 2008;Fig. 2D, F, H), while it is clasped but not fused in Sabia (Ronse De Craene et al., 2015b). The androecium of Ophiocaryon comprises two fertile stamens and three staminodes, resembling Meliosma (Figs 2B and 6A), while all five stamens are fertile in Sabia. The morphology and function of the filament is also different. Sabia has broad and straight or incurved filaments without specific function (Ronse De Craene et al., 2015b), while in Meliosma and Ophiocaryon filaments have a special structure that possibly has a role as a secondary pollen presentation area (Ronse De Craene and Wanntorp, 2008;Fig. 2F, I, J). In addition, the anatomical examination of the gynoecium in the present study reveals strong similarities between Ophiocaryon and Meliosma. The ovary wall of Ophiocaryon has two layers of tanniferous cells in the outer and inner hypodermal positions (Fig. 5A, B). This feature is also present in some Meliosma species, e.g. M. dillenifolia ssp. cuneifolia, M. pinnata, M. veitchiorum (Ronse De Craene and Wanntorp, 2008) and M. alba (Fig. 6F), but not in Sabia (Ronse De Craene et al., 2015b). Endress and Igersheim (1999) stated that tanniferous tissue is present in Sabiaceae and Proteales carpels but did not mention the exact position of this tissue in the carpel. Carpels in Sabia are mainly postgenitally fused (Ronse De Craene et al., 2015a), while fusion in Meliosma and Ophiocaryon is mainly the result of common basal growth (congenital fusion: Wanntorp and Ronse De Craene, 2007;Fig. 3G, I, J). Furthermore, styles of both Ophiocaryon and Meliosma are not supplied by vascular bundles (Ronse De Craene and Wanntorp, 2008;Fig. 5E), contrary to Sabia, with a vascularized style (Ronse De Craene et al., 2015b).
Table 3.
Comparison of morphological characters of the three genera of Sabiaceae: Sabia, Meliosma, including M. alba, and Ophiocaryon
| Character | Sabia | Meliosma | Meliosma alba (Asian lineage) | Ophiocaryon |
|---|---|---|---|---|
| Habit | Liana or scandent shrub | Shrub, tree | Tree | Tree |
| Merism | 5 | 5 | 5 | 5 or 4 |
| Symmetry | Polysymmetrical | Monosymmetrical | Monosymmetrical | Weakly mono- or disymmetrical |
| Perianth aestivation | Quincuncial | Quincuncial | Quincuncial | Quincuncial |
| Dimorphism of petals | No | Strong | Strong | Weak |
| Fusion of petal and stamen | – | + | + | +/– |
| Number of stamens/staminodes | 5/0 | 2/3 | 2/3 | 2/3 or 2/2 |
| Secondary pollen presentation structure | Absent or on unspecialized filament | Platform on filament | Platform on filament | Cup-shaped (cupula) |
| Staminodial dome | – | + | + | – |
| Nectary disc appendage | 5 or none | 5 or none | 5 | 5 |
| Pollen explosion mechanism | – | +/– | –? | – |
| Tanniferous layer in ovary wall | – | +/–? | + | + |
| Calcium oxalate crystals in anthers | + | + | + | + |
| Ovary fusion | Postgenital | Congenital | Congenital | Congenital |
| Style vascularised | + | – | – | – |
| Number of ovule integuments | 1 | 1 or 2 | 2 | 1 |
| Fruit | Schizocarpa | Drupea,b | Drupea | Drupec |
Based on data from Ronse De Craene and Wanntorp (2008) and Ronse De Craene et al. (2015b) and this study, with additional data from aLixiu and Brach (2007), bvan Beusekom (1971) and cAymard and Cuello (2005).
The recent phylogenetic tree revealed that one species of Meliosma, M. alba, forms a clade with Ophiocaryon separated from other Meliosma species (Zúñiga, 2015). Zúñiga (2015) also suggested that M. alba may be preferably allocated to another new genus rather than merged with Ophiocaryon. Meliosma alba has a disjunct distribution, with Asian and North American populations that appear to be similar morphologically and genetically (van Beusekom, 1971; Zúñiga, 2015). A previous revision of Old World Meliosma suggested that M. alba, together with M. veitchiorum, morphologically belongs to a primitive group (subgenus Kingsboroughia, section Kingsboroughia) that lacks the explosive pollen distribution mechanism (van Beusekom, 1971). However, the basal position of the latter species is not supported by the molecular phylogeny (Zúñiga, 2015). We could not observe the explosive mechanism in Asian M. alba, which is fully absent in Ophiocaryon. However, explosive pollen dispersal was observed in the North American M. alba. Moreover, a previous wood anatomical study in Sabiaceae found that M. alba shares a similar growth ring structure with O. paradoxum and some other Meliosma, viz. M. myriantha, M. kirkii and M. parviflora (Carlquist et al., 1993). However, investigations of M. alba in the present study could not find any floral character that separates it from other Meliosma or links it firmly with Ophiocaryon (Table 3). The lack of clearly connecting characters in this study may be because the sample of M. alba used came from the Asian lineage. Further investigations in M. alba from the North American lineage may reveal some informative features that wait to be discovered. Therefore, we suggest keeping M. alba in the genus Meliosma until clearer evidence is discovered.
Possible paedomorphic origin of Ophiocaryon
The recent Sabiaceae phylogenetic tree revealed that Ophiocaryon is embedded within Meliosma as sister group of M. alba. Since there are a number of similar characters between the two genera, it is possible that Ophiocaryon may be a derived form of Meliosma. Paedomorphosis is the phenomenon where morphology of one particular taxon resembles an embryonic or juvenile form of closely related taxa, which can be the result of earlier termination of ontogeny (progenesis) or a deceleration of the development rate (neoteny) (Box and Glover, 2010). Comparison of several homologous characters between Ophiocaryon and Meliosma at anthesis found that Ophiocaryon has simplified floral characters that resemble the juvenile state of Meliosma flowers. In the calyx of Ophiocaryon no vascular bundles were found (Fig. 4A, B), while there is one present in Meliosma (Ronse De Craene and Wanntorp, 2008). Petals of Ophiocaryon usually contain one main vascular bundle with possibly little branching at the base in some species (Fig. 4C, D), while in Meliosma three outer petals have one main vein at the base that branches into five to seven veins in the upper part and two inner petals have a Y-shaped branching vein (P. Thaowetsuwan and L. Ronse De Craene, pers. obs.). Fertile stamens of Ophiocaryon also have a cupula (Fig. 2F, I, J), but it is far less developed than in Meliosma, where it can be used to distinguish among species (Ronse De Craene and Wanntorp, 2008). Since staminodes of Ophiocaryon and Meliosma are very different in form and function, it can be suggested that the scale-like shape of staminodes in Ophiocaryon resembles that of the undeveloped staminodes of Meliosma. Comparison of the nectary also shows that a large disc is developed in Sabia and Meliosma, while in Ophiocaryon the disc resembles a narrow ledge and is almost restricted to the appendages. This study also found that the gynoecium of Ophiocaryon usually does not close completely (Fig. 3G, K), contrary to the completely closed carpels in Meliosma (Ronse De Craene and Wanntorp, 2008). In Ophiocaryon, the stigmatic area is found to be weakly developed compared with the well-developed elongated stigmata in Meliosma (Ronse De Craene and Wanntorp, 2008). Furthermore, observations in O. heterophyllum found that Ophiocaryon has acorn-shaped unitegmic ovules with a swollen funiculus. Earlier reports support the reduction of one integument in Sabiaceae, resulting in loss of the outer integument in some Meliosma (Ronse De Craene and Wanntorp, 2008;Fig. 6E, F) and a single integument in Sabia and some Meliosma (Ronse De Craene and Wanntorp, 2008;Ronse De Craene et al., 2015b). Observations of a reduced outer integument in Meliosma (Ronse De Craene and Wanntorp, 2008;Fig. 6E, F) may help evaluate the scenario of integument reduction in Sabiaceae from bitegmic to unitegmic. However, the recent Sabiaceae phylogeny found that Ophiocaryon is in the same clade as M. alba, which has bitegmic ovules (Fig. 6E, F). It is not known whether specimens of American origin have two integuments. The M. alba population in Mexico may be polymorphic in the number of integument layers. Future embryological studies should be carried out in the Neotropical M. alba.
In summary, based on anatomical and morphological evidence, it can be stated that Ophiocaryon flowers represent a clear example of paedomorphic evolution from Meliosma-like ancestral flowers. However, the comparative timing of development between Meliosma and Ophiocaryon cannot be obtained from the results of this study; therefore, we cannot estimate whether progenesis or neoteny is responsible for the occurrence of paedomorphosis in Ophiocaryon.
CONCLUSIONS
The present study has clarified the floral structure of Ophiocaryon and reported several new and unique characters, e.g. a weakly zygomorphic pentamerous or disymmetrical tetramerous perianth, two short and swollen fertile stamens bearing two pollen sacs over a cup-shaped structure (cupula), three or two scale-like staminodes, an intrastaminal nectar disc with five appendages, a bicarpellate ovary with a smooth lower surface and rough upper surface, and unitegmic ovules with a swollen funiculus resembling an acorn. Orbicules are present but variable, which may help to clarify some systematic and biological aspects of this lesser-known genus of Sabiaceae. We also found that the floral structure of Ophiocaryon is strongly reminiscent of an undeveloped flower form (paedomorphosis) of Meliosma with a far lower degree of dimorphism, which supports the closer relationship of both taxa that has been pointed out in the previous phylogenetic study of Zúñiga (2015). However, further investigations of Neotropical M. alba specimens are needed to reveal their common characters with Ophiocaryon. Further combinations of morphological, taxonomical, molecular and ecological data will be required to shed light on the biology of Ophiocaryon, the least-known genus of Sabiaceae.
ACKNOWLEDGEMENTS
We thank the Royal Botanic Gardens, Kew, for permission to sample herbarium material for this study, especially Elizabeth Woodgyer for arranging the collection and transfer of materials. Technical assistance with light microscopy and scanning electron microscopy from Frieda Christie is acknowledged. We thank Nattapol Krjaisit and Subhorn Khonthapagdee for providing a literature reference. We thank Dr Doug Daly for sending some herbarium material of Ophiocaryon. We also thank our colleague Nidsen Saavedra and Euridice Honorio from the Jenaro Herrera Research Centre in Peru for obtaining the flower material and research permits. The Royal Botanic Garden Edinburgh is supported by the Scottish Government’s Rural and Environment Science and Analytical Services Division. This paper is part of the Master’s degree dissertation of the first author, funded by the Development and Promotion of Science and Technology talents project (DPST) Scholarship, Royal Thai Government.
LITERATURE CITED
- Angiosperm Phylogeny Group.. 2016. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Ayensu ES. 1967. Aerosol OT solution – an effective softener of herbarium specimens for anatomical study. Stain Technology 42: 155–156. [DOI] [PubMed] [Google Scholar]
- Aymard GAC, Cuello NLA. 2005. Sabiaceae. In: Berry EP, Yatskievych K, Holst BK eds. Flora of the Venezuelan Guyana. St Louis: Missouri Botanical Garden, 39–43. [Google Scholar]
- Aymard GAC, Daly DC. 2006. Two new species of Ophiocaryon (Sabiaceae) from South America. Brittonia 58: 270–276. [Google Scholar]
- Baillon H. 1874. Série des Sabia. Histoire des plantes V. Paris: Hachette. [Google Scholar]
- Barneby RC. 1972. Meliosmaceae – Ophiocaryon. Memoirs of the New York Botanical Garden 23: 114–120. [Google Scholar]
- Barniske A, Borsch T, Mueller K et al. . 2012. Phylogenetics of early branching eudicots: comparing phylogenetic signal across plastid introns, spacers, and genes. Journal of Systematics and Evolution 50: 85–108. [Google Scholar]
- Bentham G. 1859. On Brachynema and Phoxanthus, two new genera of Brazilian plants. Transactions of the Linnean Society of London 22: 125–127. [Google Scholar]
- Bentham G, Hooker JD. 1862. Genera plantarum, Vol. I, Part I. London: Lovell Reeve. [Google Scholar]
- van Beusekom CF. 1971. Revision of Meliosma (Sabiaceae), section Lorenzanea excepted, living and fossil, geography and phylogeny. Blumea 19: 355–529. [Google Scholar]
- Box MS, Glover BJ. 2010. A plant developmentalist’s guide to paedomorphosis: reintroducing a classic concept to a new generation. Trends in Plant Science 15: 241–246. [DOI] [PubMed] [Google Scholar]
- Carlquist S, Morrell PL, Manchester SR. 1993. Wood anatomy of Sabiaceae (s.l.); ecological and systematic implications. Aliso 13: 521–549. [Google Scholar]
- Chase MW, Soltis DE, Olmstead RG, Morgan D, 1993. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden 80: 528–548, 550–580. [Google Scholar]
- Cronquist A. 1996. An integrated system of classification of flowering plants, 2nd edn New York: Columbia University Press. [Google Scholar]
- Dahlgren RMT. 1980. A revised system of classification of the angiosperms. Botanical Journal of the Linnean Society 80: 91–124. [Google Scholar]
- Endlicher SFL. 1841. Genera plantarum secundum ordines naturales disposita, Supplementum I. Vindobonae: Apud fr. Beck, Universitatis Bibliopolam. [Google Scholar]
- Endress PK. 1994. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press. [Google Scholar]
- Endress PK. 2010. Flower structure and trends of evolution in eudicots and their major subclades. Annals of the Missouri Botanical Garden 97: 541–583. [Google Scholar]
- Endress PK, Igersheim A. 1999. Gynoecium diversity and systematics of the basal eudicots. Botanical Journal of the Linnean Society 130: 305–393. [Google Scholar]
- Heywood VH, Brumitt K, Culham A, Seberg O. 2007. Flowering plant families of the world. Ontario: Firefly Books. [Google Scholar]
- Hoot SB, Magallon S, Crane PR. 1999. Phylogeny of basal eudicots based on three molecular data sets: atpB, rbcL, and 18S nuclear ribosomal DNA sequences. Annals of the Missouri Botanical Garden 86: 1–32. [Google Scholar]
- Hutchinson J. 1973. The families of flowering plants: arranged according to a new system based on their probable phylogeny, 3rd edn Oxford: Clarendon Press. [Google Scholar]
- Kim S, Soltis D, Soltis P, Zanis M, Suh Y. 2004. Phylogenetic relationships among early-diverging eudicots based on four genes: were the eudicots ancestrally woody?Molecular Phylogenetics and Evolution 31: 16–30. [DOI] [PubMed] [Google Scholar]
- Kubitzki K. 2007. Sabiaceae. In: Kubitzki K, ed. The families and genera of vascular plants IX. Berlin: Springer, 413–417. [Google Scholar]
- Lixiu G, Brach AR. 2007. Sabiaceae. In: Wu ZY, Raven PH, Hong DY, eds. Flora of China, Vol. 12 (Hippocastanaceae through Theaceae). Beijing: Science Press; St Louis: Missouri Botanical Garden Press, 25–42. [Google Scholar]
- Mauritzon J. 1936. Zur Embryologie und systematischen Abgrenzung der Reihen Terebinthales und Celastrales. Botaniska Notiser 1936: 161–212. [Google Scholar]
- Nandi OI, Chase MW, Endress PK. 1998. A combined cladistic analysis of angiosperms using rbcL and non-molecular data sets. Annals of the Missouri Botanical Garden 85: 137–214. [Google Scholar]
- Planchon JE. 1855. Affinités et synonymie de quelques genres nouveaux. Annales des Sciences Naturelles 4: 295–296. [Google Scholar]
- Raju MVS. 1952. Embryology of Sabiaceae. Current Science 21: 107–108. [Google Scholar]
- Rodrigues WA. 1964. Uma nova Sabiácea na Amazônia. I.N.P.A. Serie Botânica 17: 1–7. [Google Scholar]
- Ronse De Craene LP, Wanntorp L. 2008. Morphology and anatomy of the flower of Meliosma (Sabiaceae): implications for pollination biology. Plant Systematics and Evolution 271: 79–91. [Google Scholar]
- Ronse De Craene LP, Quandt D, Wanntorp L. 2015a. Floral development of Sabia (Sabiaceae): Evidence for the derivation of pentamery from a trimerous ancestry. American Journal of Botany 102: 336–349. [DOI] [PubMed] [Google Scholar]
- Ronse De Craene LP, Quandt D, Wanntorp L. 2015b. Flower morphology and anatomy of Sabia (Sabiaceae): structural basis of an advanced pollination system among basal eudicots. Plant Systematics and Evolution 301: 1543–1553. [Google Scholar]
- Savolainen V, Chase MW, Hoot SB. 2000a. Phylogenetics of flowering plants based on combined analysis of plastid atpB and rbcL gene sequences. Systematic Biology 49: 306–362. [DOI] [PubMed] [Google Scholar]
- Savolainen V, Fay MF, Albach DC, et al. . 2000b. Phylogeny of the eudicots: a nearly complete familial analysis based on rbcL gene sequences. Kew bulletin 55: 257–309. [Google Scholar]
- Schomburgk RH. 1840. Description of the snake-nut tree of Guiana. Annals of Natural History 5: 202–204. [Google Scholar]
- Schomburgk RH. 1845. A description of Ophiocaryon paradoxum, on the snake nut tree of Guiana. London Journal of Botany 4: 375–378. [Google Scholar]
- Soltis DE, Soltis P, Chase M, et al. . 2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society 133: 381–461. [Google Scholar]
- Soltis DE, Smith SA, Cellinese N, et al. . 2011. Angiosperm phylogeny: 17 genes, 640 taxa. American Journal of Botany 98: 704–730. [DOI] [PubMed] [Google Scholar]
- Sun Y, Moore MJ, Zhang S, et al. . 2016. Phylogenomic and structural analyses of 18 complete plastomes across nearly all families of early-diverging eudicots, including an angiosperm-wide analysis of IR gene content evolution. Molecular Phylogenetics and Evolution 96: 93–101. [DOI] [PubMed] [Google Scholar]
- Takhtajan AL. 1997. Diversity and classification of flowering plants. New York: Columbia University Press. [Google Scholar]
- Thorne RF. 1992. Classification and geography of the flowering plants. Botanical Review 58: 225–327. [Google Scholar]
- Urban I. 1895. Ueber die Sabiaceengattung Meliosma.Berichte der Deutschen Botanischen Gesellschaft 13: 211–222. [Google Scholar]
- Urban I. 1900. Sabiaceae. Symbolae Antillanae seu Fundamenta Florae Indiae Occidentalis, 499–503. [Google Scholar]
- Verstraete B, Moon H, Smets E, Huysmans S. 2014. Orbicules in flowering plants: a phylogenetic perspective on their form and function. Botanical Review 80: 107–134. [Google Scholar]
- Wanntorp L, Ronse De Craene LP. 2007. Flower development of Meliosma (Sabiaceae): evidence for multiple origins of pentamery in the eudicots. American Journal of Botany 94: 1828–1836. [DOI] [PubMed] [Google Scholar]
- Warburg O. 1895. Sabiaceae. In: Engler A, Prantl K eds. Die natürlichen Pflanzenfamilien, Vol. III. Leipzig: Wilhelm Engelman, 367–374. [Google Scholar]
- van de Water TPM. 1980. A taxonomic revision of the genus Sabia (Sabiaceae). Blumea 26: 1–64. [Google Scholar]
- Whitney HM, Bennett VK, Dorling M, et al. . 2011. Why do so many petals have conical epidermal cells?Annals of Botany 108: 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worberg A, Quandt D, Barniske AM, et al. . 2007. Phylogeny of basal eudicots: insights from non-coding and rapidly evolving DNA. Organisms Diversity & Evolution 7: 55–77. [Google Scholar]
- Zúñiga JD. 2015. Phylogenetics of Sabiaceae with emphasis on Meliosma based on nuclear and chloroplast data. Systematic Botany 40: 761–775. [Google Scholar]