Abstract
Background and Aims In Utricularia nelumbifolia, the nuclei of placental nutritive tissue possess unusually shaped projections not known to occur in any other flowering plant. The main aim of the study was to document the morphology and ultrastructure of these unusual nuclei. In addition, the literature was searched to find examples of nuclear tubular projections in other plant groups, and the nuclei of closely related species of Utricularia (i.e. sects Iperua, Orchidioides, Foliosa and Utricularia) were examined.
Methods To visualize the complexity of the nuclear structures, transmission electron microscopy (TEM) was used, and 3-D ultrastructural reconstructions were made using the serial block face scanning electron microscopy (SBEM) technique. The nuclei of 11 Utricularia species, i.e. U. nelumbifolia, U. reniformis, U. cornigera, U. nephrophylla (sect. Iperua), U. asplundii, U. alpina, U. quelchii (sect. Orchidioides), U. longifolia (sect. Foliosa), U. intermedia, U. minor and U. gibba (sect. Utricularia) were examined.
Key Results Of the 11 Utricularia species examined, the spindle-like tubular projections (approx. 5 μm long) emanating from resident nuclei located in placental nutritive tissues were observed only in U. nelumbifolia. These tubular nuclear extensions contained chromatin distributed along hexagonally shaped tubules. The apices of the projections extended into the cell plasma membrane, and in many cases also made contact at the two opposing cellular poles, and with plasmodesmata via a short cisterna of the cortical endoplasmic reticulum. Images from the SBEM provide some evidence that the nuclear projections are making contact with those of neighbouring cells.
Conclusions The term chromatubules (chromatin-filled tubules) for the nuclear projections of U. nelumbifolia placental tissue was proposed here. Due to the apparent association with the plasma membrane and plasmodesmata, it was also speculated that chromatubules are involved in nucleus–cell–cell communication. However, further experimental evidence is required before any functional hypothesis can be entertained.
Keywords: Chromatubules, nucleus, plant nuclear shape, plasmodesmata, placental nutritive tissue, micropylar embryo sac haustorium, micropylar endosperm haustorium, SBEM, ultrastructure
INTRODUCTION
Utricularia (Lentibulariaceae, Lamiales) are carnivorous plants that possess suction traps for trapping small organisms. The genus contains many habitat-specific forms including aquatic, terrestrial, epiphytic, lithophytic and rheophytic species (Taylor, 1989). Some highly specialized species, such as U. nelumbifolia, are aquatic epiphytes that inhabit Bromeliad tanks (Taylor, 1989; Płachno and Świątek, 2010). Although Utricularia inflorescences share a typical angiosperm floral bauplan, their rootless vegetative bauplan is quite divergent (Rutishauser, 2016).
Genome size is variable in Utricularia, with a tendency for genome miniaturization; the smallest genomes were recorded in U. sect. Vesiculina (U. purpurea, 79 Mbp), U. sect. Foliosa (U. longifolia, 97 Mbp) and U. sect. Utricularia (U. floridana, 100 Mbp; U. gibba, 103 Mbp) (Veleba et al., 2014). Utricularia and sister genus Genlisea exhibit the fastest rates of nucleotide substitution known within angiosperms (Jobson and Albert, 2002; Müller et al., 2004; Wicke et al., 2013), with an observed high rate of gene turnover linked to the evolution of a compact genome (Carretero-Pauletet et al., 2015a, b).
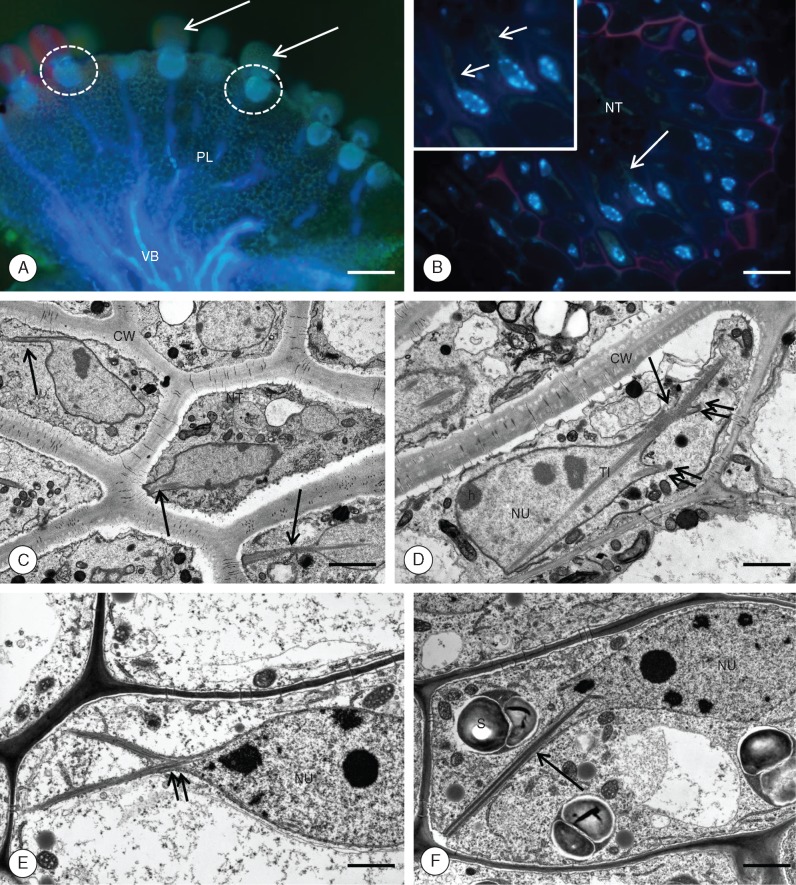
In most angiosperms, the female gametophyte remains in the ovule surrounded by sporophytic tissue. However, in many Utricularia species, the female gametophyte grows outside the ovule where it is partially exposed and able to make contact with sporophytic tissue (i.e. placenta). The haustorial component of the female gametophyte penetrates the placenta and makes contact with a specialized spherically shaped cluster of cells called ‘nutritive tissue’ (Fig. 1A). This tissue differentiates in close proximity to the ovule base during the early stages of female gametophytic development (Khan, 1954; Płachno and Świątek, 2008, 2012; Płachno, 2011).
Fig. 1.
Utricularia nelumbifolia. The localization and general structure of the placental nutritive tissue. (A, B). The general morphology and histology of U. nelumbifolia placenta and nutritive tissue. (A) PL, placenta; VB, vascular bundles; arrows, ovules; encircled, nutritive tissue. Whole mounted preparation stained with DAPI. Fluorescence microscopy. Scale bar = 180 μm. (B) NT, nutritive tissue, the arrow marks the nuclear projection. Inset: a fragment of nutritive tissue at higher magnification; arrows point to nuclear projections. Histocryl semi-thin section stained with DAPI. Fluorescence microscopy. Scale bar = 20 μm. (C, D) Ultrastructure of nutritive tissue cells (NT). CW, cell walls; NU, nucleus; h, chromocentre; arrows, nuclear projections; double arrows, nuclear projection branching; TI, tubule-like inclusions. Material prepared for the SBEM method, transmission electron microscopy (TEM). Scale bars = 2·75 μm in (C) and 1·80 μm in (D). (E) Nutritive cell ultrastructure after fixing for microtubules. Note bifurcating nuclear projection. NU, nucleus. TEM. Scale bar = 1·5 μm. (F) Nutritive cell ultrastructure after fixation in modified Karnovsky fixative. The arrow points to the nuclear projection; NU, nucleus; S, starch grain. TEM. Scale bar = 1·4 μm.
Nuclei of the placenta nutritive tissue in Utricularia nelumbifolia have spindle-like tubular projections (unknown among other flowering plants), and the aim of our study was to document in detail their morphology and ultrastructure. We examined whether or not the projections occur in plants from different genera and in other Utricularia species (sects Iperua, Orchidioides, Foliosa and Utricularia). We also examined nuclei of vegetative tissue for the presence of the projections, and performed the search across several growing seasons.
Finally, we used serial block face scanning electron microscopy (SBEM) to constructe a three-dimensional model of the placental nutritive cells that allowed visualization of the number of projections per nucleus, and whether or not the projections extend into, and interacted with, neighbouring cell nuclei.
MATERIALS AND METHODS
Plant material
Inflorescences and vegetative tissue (phylloclades) of Utricularia nelumbifolia Gardner (sect. Iperua) were obtained from two sources: the greenhouse collection at the Botanic Garden of the Jagiellonian University in Kraków, Poland and Liberec Botanical Garden, Czech Republic. Material was collected four times over a period of 10 years. Flowers of other species: U. reniformis, U. cornigera, U. nephrophylla (sect. Iperua), U. asplundii, U. alpina, U. quelchii (sect. Orchidioides), U. longifolia (sect. Foliosa), U. intermedia, U. minor and U. gibba (sect. Utricularia) were collected from the Liberec Botanical Garden, Czech Republic, Prague Botanical Garden, Czech Republic, Botanic Garden of the Jagiellonian University in Kraków and from a locality in southern Poland (the Jeleniak–Mikuliny Nature Reserve, Lubliniec). Material from the Nature Reserve was collected under a permit from the Polish Ministry of the Environment (Płachno and Świątek, 2011).
Electron microscopy
Placentas with ovules (from buds of different sizes from mature flowers), and other tissues (parts of corolla, phylloclades) were fixed in 2·5 % formaldehyde and 2·5 % glutaraldehyde in a 0·05 m cacodylate buffer (pH 7·0) for 2 d. The material was post-fixed in 1 % OsO4 in a cacodylate buffer for 24 h at approx. 4 °C, rinsed in the same buffer, treated with 1 % uranyl acetate in distilled water for 1 h, dehydrated with acetone and embedded in an Epoxy Embedding Medium Kit (Fluka). Semi-thin sections were stained with methylene blue and examined using an Olympus BX60 microscope. Ultrathin sections were cut on a Leica ultracut UCT ultramicrotome. After contrasting with uranyl acetate and lead citrate, the sections were examined using a Hitachi H500 electron microscope at 75 kV.
A second type of fixation for TEM was used to check whether the morphology of the nuclei was independent of the fixation methodology. Thus a part of the material for ultrastructural studies was fixed in a mixture of 4 % formaldehyde (freshly prepared from paraformaldehyde) and 0·25 % glutaraldehyde in a piperazine buffer (fixing as for microtubules). The material was post-fixed in 1 % OsO4 in a cacodylate buffer for 24 h at approx. 4 °C, rinsed in the same buffer, treated with 1 % uranyl acetate in distilled water for 1 h, dehydrated with acetone and embedded in an Epoxy Embedding Medium Kit (Fluka).
Serial block face scanning electron microscopy (SBEM)
Tissue fixation and preparation was adopted from Deerinck et al. (2010) with some modifications. Utricularia nelumbifolia placentas were fixed in 2·5 % formaldehyde and 2·5 % glutaraldehyde in a 0·05 m cacodylate buffer (pH 7·0) for 2 d. After fixation, tissue was washed three times for 15 min with the same buffer. After washing, samples were post-fixed with 3 % potassium ferrocyanide in 0·3 m cacodylate buffer mixed with an equal volume of 4 % aqueous solution of osmium tetroxide for 1 h. Tissue was then washed three times for 5 min in ddH2O and incubated in 1 % solution of thiocarbohydrazide (Ted Pella) for 20 min at 60 °C. After that, samples were washed three times for 5 min in ddH2O and placed in 2 % aqueous osmium tetroxide for 30 min, then tissue was washed again three times for 5 min in ddH2O and incubated overnight in 1 % aqueous uranyl acetate in 4 °C. The samples were then rinsed three times for 5 min in ddH2O and put into a freshly prepared Walton’s lead aspartate for 30 min at 60 °C, washed five times for 3 min in ddH2O and dehydrated for 10 min in each of 30, 50, 70 and 96 % ethanol solution, then placed in anhydrous 100 % ethanol three times for 20 min, a 1:1 solution of acetone and ethanol for 15 min and twice for 15 min in 100 % acetone. After dehydration, samples were placed in a mixture of 25 % Epoxy Embedding Medium (Sigma, St. Louis, MO, USA) in acetone for 24 h, then in 50 % epoxy resin in acetone for 24 h and in 75 % resin in acetone for another 24 h and, after saturation, samples were left overnight for evaporation of acetone, then embedded between two layers of Aclar (EMS) and left for polymerization.
Polymerized samples were cut out and glued with cyanoacrylate on aluminium specimen pins (Gatan, Pleasanton, CA, USA) and mounted in the Gatan 3View system installed in a Zeiss Sigma scanning electron microscope. The serial block face images were then collected, section thickness was 60 nm and pixel size of collected images was 60 × 60 nm. The size of the imaged area was 32·3 × 32·3 μm which provided an image resolution of 5500 × 5500 pixels. The number of collected images was 700; however, after data analysis, only 204 images were selected for further processing, which provided 12·24 μm of overall analysed sample thickness. Because of the large volume of data, images for analysis were resized from the original resolution of 5500 × 5500 pixels to 1000 × 1000 pixels, with this operation performed using Fiji Software (Schindelin et al., 2012). Resized images were manually segmented, and 3-D models created in Imaris software (Bitplane Inc.).
Visualization of nuclei using epifluorescence microscopy
The placentas of U. nelumbifolia were fixed in 4 % formaldehyde (for 30–40 min), washed in phosphate-buffered saline (PBS), dehydrated in a graded ethanol series, and then infiltrated and embedded in Histocryl resin (London Resin Company Ltd, Basingstoke, Hampshire, UK). The Histocryl sections (1 μm) were stained with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg mL–1). Whole mounted preparations and Histocryl sections were examined using an Olympus BX60 epifluorescence microscope equipped with the appropriate filters.
RESULTS
Of the 11 examined species of Utricularia, nuclei with spindle-like tubular projections (chromatubules) were only observed in the placental cells of U. nelumbifolia. Nuclei from all other examined cell types contained typical proteinaceous paracrystalline inclusions.
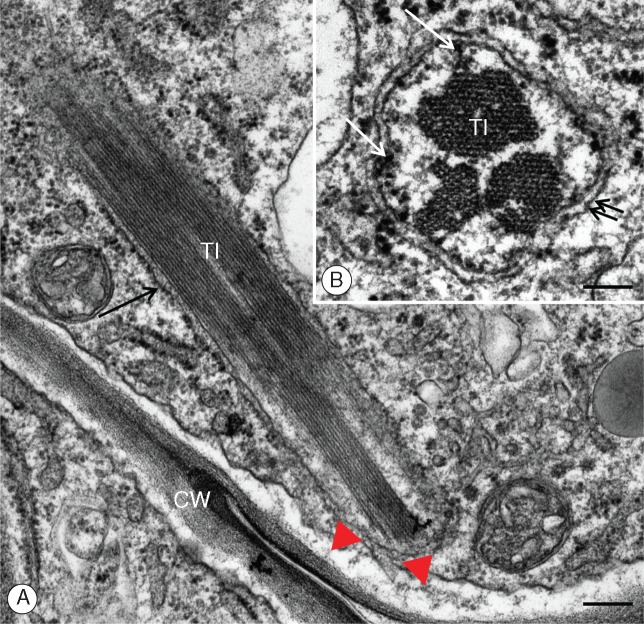
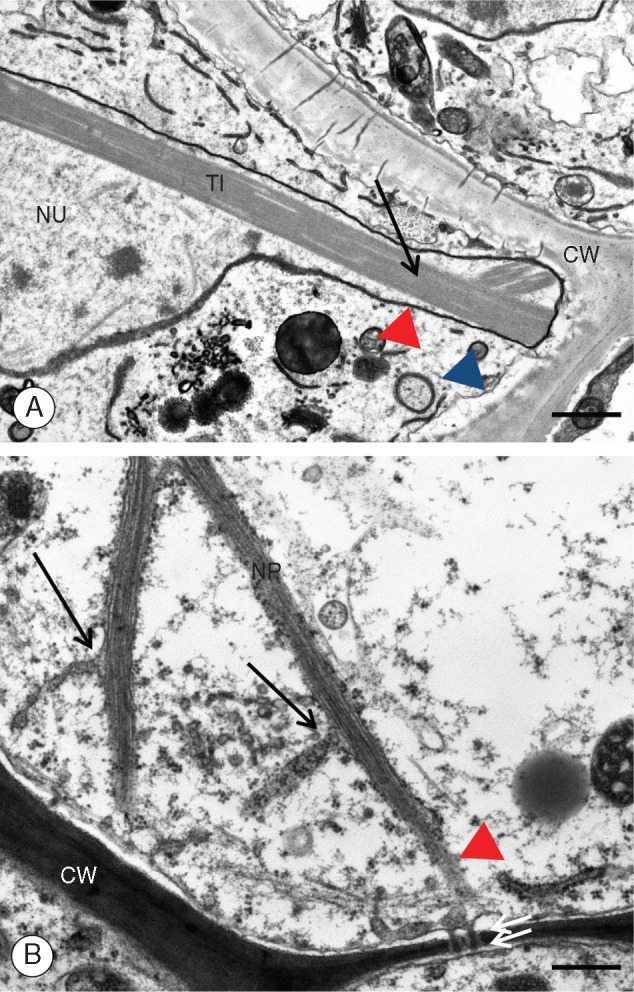
The nuclei of the placental nutritive tissue of U. nelumbifolia possess long chromatubules often extending to the cell membrane (Figs 1–5). TEM analysis revealed that chromatubules are a continuation of the inner and outer membranes of the nuclear envelope and are either simple or bifurcating structures (Figs 1C–E, 2B and 3A, B). They are usually approx. 5 μm in length, and vary in width from 0·31 to 1·48 μm near the base, and from 0·25 to 1·16 μm near the apical tip (Table 1). The apices extend towards the cell periphery (Figs 1C, F and 2A), often forming direct contact with the plasma membranes (Figs 1C, F, 2A, B, 4B and 5A, B). TEM analysis revealed that chromatubules contain electron-dense material adjoining the inner nuclear envelope (Fig. 3B), with this material here interpreted as chromatin. The striking feature of each chromatubule is the presence of proteinaceous tubule-like inclusions forming orderly structures quite unlike the randomly arranged analogous structures located in the central regions of nuclei (Figs 1C–F. 2A, B and 3A, B). We confirmed the organized structure of these inclusions using transversal sectioning, which revealed a bundled array of hexagonal shaped lamellar tubules (Fig. 3B). These tubule-like inclusions seem to modify the shape of the chromatubule (Figs 1A–F and 2A). Ultrastructural analysis showed that the nuclear envelope of the chromatubules may be in direct contact with the plasma membrane, with contact sites observed numerous times (Figs 1C, F, 2A and 5A, B), in positions where plasmodesmata are situated (Fig. 5A, B). Sometimes we observed the endoplasmic reticulum extending from the outer nuclear envelope of the chromatubules towards plasmodesmata (Fig. 2B). Regardless of the fixation methods used (such as modified Karnovsky fixative, fixing for microtubules, and highly membrane-contrasting chemicals used for the SBEM methodology), the morphological and ultrastructural properties of nuclear tissue nuclei were broadly the same (Fig. 1C–F).
Fig. 2.
Utricularia nelumbifolia. Ultrastructure of the placental nutritive tissue cells with emphasis on the ultrastructure of the nuclei. (A) A fragment of nuclear projection. CW, cell wall; NU, nucleus; TI, tubule-like inclusions; red arrowhead, nuclear envelope; blue arrowhead, cell membrane. Material prepared for the SBEM method, TEM. Scale bar = 0·85 μm. (B) A magnified fragment of the nutritive cell nucleus shown in Fig. 1E. CW, cell wall; NP, nuclear projection; arrows show continuity between the outer nuclear membrane and the endoplasmic reticulum; double arrows, plasmodesmata; the red arrowhead marks the continuity of the outer nuclear membrane with the endoplasmic reticulum and plasmodesmata. Tissue fixed as for microtubules. TEM. Scale bar = 0·3 μm.
Fig. 3.
Utricularia nelumbifolia. Ultrastructure of the placental nutritive tissue cells with emphasis on the ultrastructure of nuclear projections. (A) The ultrastructure of the projection apex. CW, cell wall; TI, tubule-like inclusions; arrows marks the outer nuclear membrane decorated with ribosomes; red arrows point to the contact area between the nuclear envelope and the cell membrane. Modified Karnovsky fixative. TEM. Scale bar = 0·18 μm. (B) A cross-section through the nuclear projection. TI, tubule-like inclusions; long white arrows point to chromatin associated with the nuclear envelope; double arrows mark the outer nuclear membrane. Modified Karnovsky fixative. TEM. Scale bar = 0·1 μm.
Fig. 4.
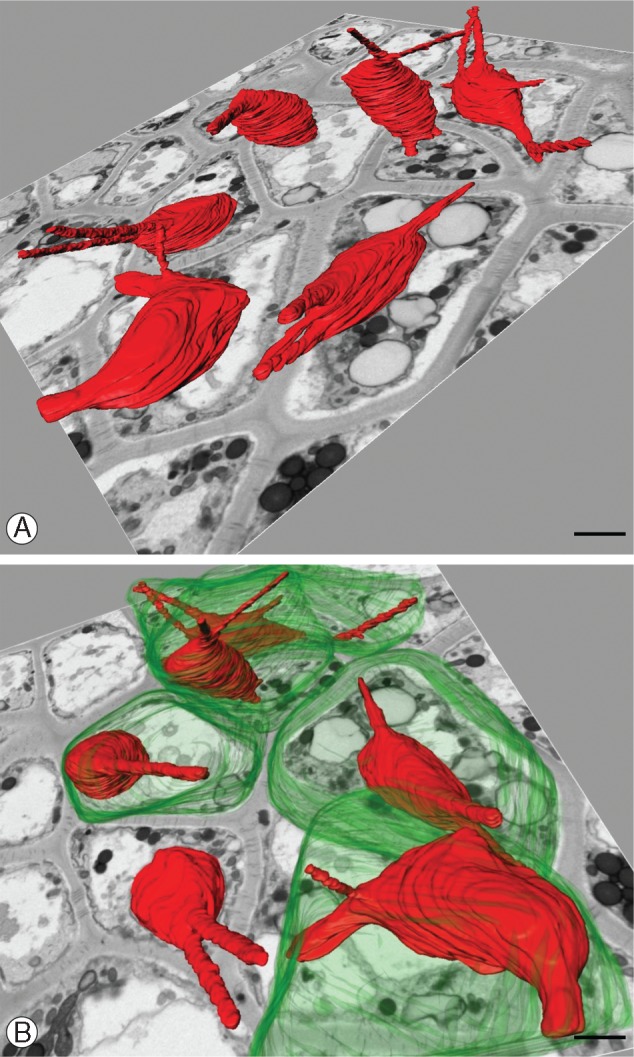
Utricularia nelumbifolia. Three-dimensional reconstruction of a selected fragment of the nutritive tissue. (A) Nuclei are in red – note that there are no specific patterns in spatial orientation of the nuclear projections. Background – the single microphotography showing the ultrastructure of the nutritive tissue. Scale bar = 2 μm. (B) A fragment of the reconstruction shown in (A) rotated and magnified. Cell membranes are in green. Scale bar = 3 μm.
Fig. 5.
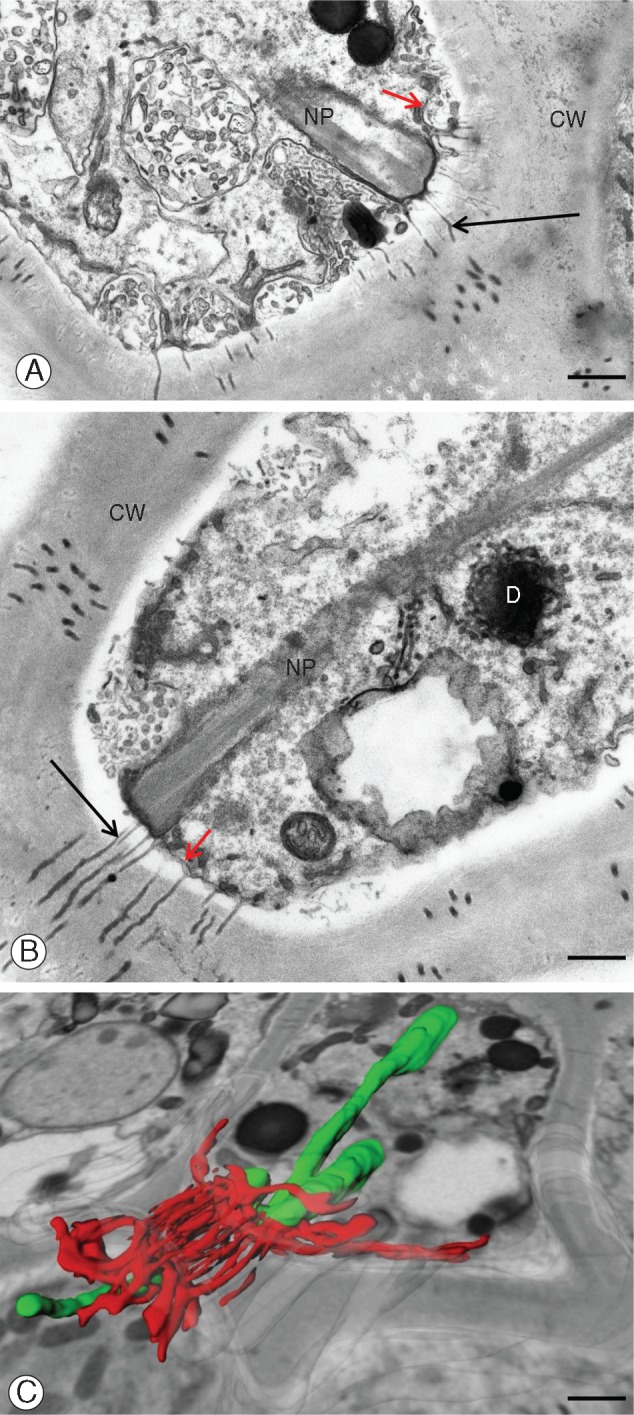
Utricularia nelumbifolia. Cell–cell contact via plasmodesmata in placental nutritive tissue: the contact points between nuclear projections and cell membrane. (A, B) Ultrastructural details. CW, cell wall; D, dictyosome; NP, nuclear projection; black arrows mark plasmodesmata; red arrows point to the cell membrane. Tissue prepared for the SBEM method. Scale bars in (A) and (B) = 0·55 μm. (C) Three-dimensional reconstruction showing the association of nuclear projections (in green) with the endoplasmic reticulum and plasmodesmata (both shown in red). Background: a part of a single microphotograph showing the cell ultrastructure. Scale bar = 1 μm.
Table 1.
Chromatuble length and width
| No. of projections | Length (µm) | Width at the base (µm) | Width of the tip (µm) |
|---|---|---|---|
| 1 | 2·0 | 0·31 | 0·27 |
| 2 | 1·04 | 0·45 | |
| 3 | 4·8 | 1·5 | 1·16 |
| 4 | 4·5 | 0·75 | 0·25 |
| 5 | 5·61 | 0·77 | 0·84 |
| 6 | 1·92 | 1·48 | 0·71 |
| 7 | 3·83 | 0·58 | 0·25 |
To better characterize the number and shape of the chromatubules and determine the spatial relationship between chromatubules from neighbouring cells, we made ultrastructural 3-D reconstructions of placenta tissue fragments using SBEM methodology. Our reconstructions (Fig. 4A, B) revealed that each nucleus may possess up to five chromatubules. The orientation of these nuclear extensions is not specific, i.e. they seem to be arranged randomly in different directions (Fig. 4A, B). In some instances, the apices of chromatubules from neighbouring cells were observed seemingly to conjugate, separated only by the plasma membrane and cell wall (Figs 4B and 5C). It is interesting that both TEM and SBEM analysis showed that the region in which the chromatubules make contact with the cell periphery is usually rich in plasmodesmata (Fig. 5A–C).
DISCUSSION
The generalized image of the plant nucleus as a uniform, more or less rounded organelle, is correct for the majority of plant tissue types. However, nuclei of specialized cells are known to possess characteristic grooves and invaginations (Collings et al., 2000), especially in those involved in secretory or nutritional functions (e.g. endosperm or suspensor haustoria), or due to physiological hypertrophy (Nagl, 1992). Nuclear grooves and invaginations increase the surface area of the nuclear envelope – the site of exchange between the nucleus and cytoplasm.
In Utricularia aurea, irregularly shaped giant nuclei, from the endosperm haustoria and endosperm–placental syncytia, have been described and illustrated by Khan (1954). Following on from Khan (1954), Płachno and Świątek (2012) examined two giant nuclei from tissue of the endosperm–placental syncytium equalling the ‘micropylar endosperm haustorium’ in Khan’s paper 1954 in U. intermedia, describing it as being lobed and surrounded by a 3-D microtubule ‘cage’. Within each endosperm–placental syncytium, Płachno and Świątek (2012) observed two giant nuclei from the endosperm haustorium and many small sized nuclei that originated from the placental nutritive cells.
The first report of syncytium formation in Utricularia was by Merz (1897), who observed two differenly shaped large haustorium nuclei that were often lobed or indented.
Merz (1897) had already determined that Torenia (Linderniaceae) had a haustorial embryo sac for food supply that was derived from the surrounding sporophytic tissue (i.e. the funiculus rather than the placenta). As mentioned above, in Utricularia the female gametophyte grows outside of the ovule where it is partially exposed and able to make contact with other sporophytic tissue for supply of nutrients. In contrast to Torenia, Utricularia possesses an extra-embryonic sac in which the central cell develops externally, while the egg apparatus remains in the ovule (Płachno, 2011). In Utricularia, the embryo sac haustorium formed by the central cell is the first stage (after fertilization) in the development of the micropylar endosperm haustorium and the placenta–endosperm syncytium (thus far only known in insects and Utricularia).
In other families of the order Lamiales, there have been observed aggressive endosperm haustoria (Johri et al., 1992). Examples include branching micropylar haustorium with amoeboid-like nuclei in the parasitic Orobanche cernua (Orobanchaceae) (Tiagi, 1951; Wardlaw, 1955), and the giant hypertrophied nuclei, described from the endosperm haustorium in Pedicularis sylvatica (Orobanchaceae; Berg, 1954) and Rhinanthus serotinus (Schrophulariaceae; Świerczyńska et al., 2013).
Based on previous evidence, it seems clear that there is a tendency for irregularly shaped giant nuclei inside their micropylar endosperm haustoria within the order Lamiales. However, nuclei of the placental nutritive cells (e.g. U. nelumbifolia) differ from the above-mentioned nuclei of the endosperm haustoria in that they maintain a regular cell shape (as compared with amoeboid-shaped endosperm nuclei) and size (i.e. not hypertrophied).
Large haustorium nuclei do not have projections with internal hexagonally shaped tubules (Płachno and Świątek, 2012; Płachno et al., 2013). The cytological differentiation of endosperm haustoria has been linked to polyploidization (D’Amato, 1989; Nagl, 1992), and nuclei from haustoria possess characters indicative of polyploidization (e.g. giant size, polytenic chromosomes and abundant nucleoli). In contrast to the above, placental nutritive cells in U. nelumbifolia have none of these characters, and the literature is devoid of any observations of similar shaped nuclei.
Although endosperm–placental syncytia have been described for Utricularia species from sect. Utricularia (Płachno and Świątek, 2012), the development of endosperm in U. nelumbifolia (sect. Iperua) has not yet been described.
The shape of the nuclei can also be altered by pathological conditions, especially in unstable cytological tissues. One such example involves heterochromatin-filled surface ‘spikes’ that were recorded in long-term callus cells of Allium fistulosum L., interpreted by Joachimiak and Ilnicki (2003) as a means for heterochromatin elimination from the nucleus.
It may be the case that chromatubles are of viral origin, and there are examples of virally induced membrane tubule formation (Laporte et al., 2003). However, our examination included U. nelumbifolia accessions that were derived from two different sources, making viral infection of both specimens unlikely. In addition, we did not observe any pathological changes in the source specimens, and therefore it is likely that the chromatubles are a stable characteristic of the species.
In all examined members of the family Lentibulariaceae, proteinaceous paracrystalline inclusions in nuclei are present (e.g. Thomas and Gouranton, 1979; Fineran and Lee, 1980; Płachno et al., 2016). However, the inclusions are observed in the central part of nuclei in all cases except U. nelumbifolia in which they are also present in the chromatubules; and it seems that the inclusions have modified the shape of the nucleus.
Stromules (stroma-filled tubules) are small structures extending from the surface of plastids that superficially resemble chromatubules, with the most obvious differences involving the presence of stroma rather than chromatin and the lack of proteinaceous tubule-like inclusions in stromules (Gunning, 2005; Hanson and Sattarzadeh, 2011).
Chromatubles potentially connect cell poles, and are possibly interacting with the plasma membrane and plasmodesmata, and neighbouring cells. The observed consistent structure and positioning of the chromatubules make it unlikely that they are a random phenomenon, invoking the possibility that they are involved in nucleus–cell–cell communication, thereby constituting a novel functional process for the plant nucleus.
It may be of significance that chromatubles are positioned in the region of the female gametophyte that makes contact with the non-ovular sporophytic tissue. Although this suggests communication between two generations (i.e. sporophyte– gametophyte), the lack of chromatubles in the sporophytic tissue implies that this is unlikely.
Our ultrastructural observations show some evidence that the desmotubules of adjacent plasmodesma are forming a continuous connection with the envelope of the chromatubules. Thus, it is conceivable that macromolecules such as mRNA, proteins and transcription factors are being transported not via the cytoplasm but rather via a direct route from the nucleus to the desmotubule.
Another possible function of the chromatubules involves anchoring and stabilization of the nucleus within the cell. Such a function for ramified, amoeba-like nuclei was suggested in some animal cells, e.g. specialized nurse cells in some insect ovaries (Żelazowska and Biliński, 2001). However, in this case, during late oogenesis there is a phase of rapid transport of nurse cell cytoplasm via wide cytoplasmic channels (termed intercellular bridges or ring canals) towards growing oocytes; long nuclear extensions seem to prevent nuclei from physically blocking the intercellular bridges (Żelazowska and Biliński, 2001).
The germ cells of animals are often interconnected via wide (<10–15 μm) intercellular bridges. Intercellular bridges are stabilized contractile rings that are able to open and close at specific times, and through which flow cytoplasm, organelles, Golgi complexes and centrioles. Macromolecules such as mRNA (maternal information) can also pass through the bridges, and they are therefore analogous to cytoplasmic canals formed during cytomixis in higher plants (Heslop-Harrison, 1966; Guo and Zheng, 2004). Even so, such direct cytoplasm transfer between plant cells has not been observed thus far.
Conclusions
We here propose the name chromatubules, i.e. chromatin-filled tubules for nuclear projections of U. nelumbifolia placental nuclei (analogously to stromules of plastids). Our discovery provides far more questions than answers: are chromatubules functional in any sense, and if so are they involved in the mediation and trafficking of macromolecules between cells and tissues? Are they the result of overexpression of a gene/genes involved in proteinaceous tubule-like inclusions formation? An integrated approach, using an in vivo experimental system and green fluorescent protein (GFP)-marked molecules (e.g. ribonucleoprotein complexes) for the detection of intracellular and cell–cell transport, should help to answer these questions.
ACKNOWLEDGEMENTS
The authors would like to express their sincere thanks to Dr Miroslav Studnička (director of Liberec Botanical Garden, Czech Republic) and Dr Vlastik Rybka (Prague Botanical Garden, Czech Republic) for providing plant material for investigation. We also thank horticulturist Lucyna Kurleto, for her conscientious care of the living collection of carnivorous plants located at the Botanical Garden of Jagiellonian University in Kraków.
LITERATURE CITED
- Berg RY. 1954. Development and dispersal of the seed of Pedicularis silvatica. Nytt Magasin for Botanikk 2: 1–60. [Google Scholar]
- Carretero-Paulet L, Chang T-H, Librado P, et al. 2015a. Genome-wide analysis of adaptive molecular evolution in the carnivorous plant Utricularia gibba. Genome Biology and Evolution 7: 444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero-Paulet L, Librado P, Chang T-H, et al. 2015b. High gene family turnover rates and gene space adaptation in the compact genome of the carnivorous plant Utricularia gibba. Molecular Biology and Evolution 32: 1284–1295. [DOI] [PubMed] [Google Scholar]
- Collings DA, Carter CN, Rink JC, Scott AC, Wyatt SE, Allen NS.. 2000. Plant nuclei can contain extensive grooves and invaginations. The Plant Cell 12: 2425–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato F. 1989. Polyploidy in cell differentiation. Caryologia 42: 183–211. [Google Scholar]
- Deerinck TJ, Bushong EA, Thor A, Ellisman MH.. 2010. NCMIR methods for 3D EM: a new protocol for preparation of biological specimens for serial block face scanning electron microscopy http://ncmir.ucsd.edu/sbfsem-protocol.pdf
- Fineran BA, Lee MSL.. 1980. Organization of mature external glands on the trap and other organs of the bladderwort Utricularia monanthos. Protoplasma 103: 17–34. [Google Scholar]
- Gunning BE. 2005. Plastid stromules: video microscopy of their outgrowth, retraction, tensioning, anchoring, branching, bridging, and tip shedding. Protoplasma 225: 33–42. [DOI] [PubMed] [Google Scholar]
- Guo G-Q, Zheng G-C.. 2004. Hypotheses for the functions of intercellular bridges in male germ cell development and its cellular mechanisms. Journal of Theoretical Biology 229: 139–146. [DOI] [PubMed] [Google Scholar]
- Hanson M, R, Sattarzadeh A.. 2011. Stromules: recent insights into a long neglected feature of plastid morphology and function. Plant Physiology 155: 1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison J. 1966. Cytoplasmic connexions between angiosperm meiocytes. Annals of Botany 30: 221–230. [Google Scholar]
- Joachimiak A, Ilnicki T.. 2003. Nuclear morphology, polyploidy, and chromatin elimination in tissue culture of Allium fistulosum. Acta Societatis Botanicorum Poloniae 72: 11–17. [Google Scholar]
- Jobson RW, Albert VA.. 2002. Molecular rates parallel diversification contrasts between carnivorous plant sister lineages. Cladistics 18: 127–136. [DOI] [PubMed] [Google Scholar]
- Johri BM,, Ambegaokar KB, Srivastava PS.. 1992. Comparative embryology of angiosperms, 2 Bde., XXV + 1221 S., 362 Abb., 32 Tab. Berlin: Springer-Verlag. [Google Scholar]
- Khan R. 1954. A contribution to the embryology of Utricularia flexuosa Vahl. Phytomorphology 4: 80–117. [Google Scholar]
- Laporte C, Vetter G, Loudes AM, et al. 2003. Involvement of the secretory pathway and the cytoskeleton in intracellular targeting and tubule assembly of Grapevine fan leaf virus movement protein in tobacco BY-2 cells. The Plant Cell 15: 2058–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz M. 1897. Untersuchungen über die Samenentwickelung der Utricularien (Studies on the seed development of Utricularia spp.). Flora (Allgemeine Botanische Zeitung) 84: 69–87. [Google Scholar]
- Müller K, Borsch T, Legendre L, Porembski S, Theisen I, Barthlott W.. 2004. Evolution of carnivory in Lentibulariaceae and the Lamiales. Plant Biology 6: 477–490. [DOI] [PubMed] [Google Scholar]
- Nagl W. 1992. The polytenic endosperm haustorium of Rhinanthus minor (Scrophulariaceae) functional ultrastructure. Canadian Journal of Botany 70: 1997–2004. [Google Scholar]
- Płachno BJ. 2011. Female germ unit in Genlisea and Utricularia, with remarks about the evolution of the extra-ovular female gametophyte in members of Lentibulariaceae. Protoplasma 248: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Świątek P. 2008. Cytoarchitecture of Utricularia nutritive tissue. Protoplasma 234: 25–32. [DOI] [PubMed] [Google Scholar]
- Płachno BJ, Świątek P.. 2010. Unusual embryo structure in viviparous Utricularia nelumbifolia, with remarks on embryo evolution in genus Utricularia. Protoplasma 239: 69–80. [DOI] [PubMed] [Google Scholar]
- Płachno BJ, Świątek P.. 2011. Actin cytoskeleton in the extra-ovular embryo sac of Utricularia nelumbifolia (Lentibulariaceae). Protoplasma 249: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Świątek P.. 2012. Syncytia in plants: cell fusion in endosperm–placental syncytium formation in Utricularia (Lentibulariaceae). Protoplasma 248: 425–435. [DOI] [PubMed] [Google Scholar]
- Płachno BJ, Świątek P, Sas-Nowosielska H, Kozieradzka-Kiszkurno M.. 2013. Organisation of the endosperm and endosperm–placenta syncytia in bladderworts (Utricularia, Lentibulariaceae) with emphasis on the microtubule arrangement. Protoplasma 250: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczyńska M, Świątek P, Davies K.. 2016. Floral micro-morpholgy of the Australian carnivorous bladderwort Utricularia dunlopii, a putative pseudocopulatory species. Protoplasma 253: 1463–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser R. 2016. Evolution of unusual morphologies in Lentibulariaceae (bladderworts and allies) and Podostemaceae (river-weeds): a pictorial report at the interface of developmental biology and morphological diversification. Annals of Botany 117: 811–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, et al. 2012. ‘Fiji: an open-source platform for biological-image analysis’. Nature Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Świerczyńska J, Kozieradzka-Kiszkurno M, Bohdanowicz J.. 2013. Rhinanthus serotinus (Schönheit) Oborny (Scrophulariaceae): immunohistochemical and ultrastructural studies of endosperm chalazal haustorium development. Protoplasma 250: 1369–1380 [DOI] [PubMed] [Google Scholar]
- Taylor P. 1989. The genus Utricularia: a taxonomic monograph. London: HMSO. [Google Scholar]
- Tiagi B. 1951. Studies in the family Orobanchaceae. III. A contribution to the embryology of Orobanche cernua and O. aegyptiatica. Phytomorphology 1: 158–169. [Google Scholar]
- Thomas D, Gouranton J.. 1979. Ultrastructural and autoradiographic study of the intranuclear inclusions of Pinguicula lusitanica L. Planta 145: 89–93. [DOI] [PubMed] [Google Scholar]
- Veleba A, Bures P, Adamec L, Smarda P, Lipnerová I, Horová L.. 2014. Genome size and genomic GC content evolution in the miniature genome sized family Lentibulariaceae. New Phytologist 203: 22–28. [DOI] [PubMed] [Google Scholar]
- Wardlaw CW. 1955. Embryogenesis in plants. London: Methuen; New York: John Wiley. [Google Scholar]
- Wicke S, Schäferhoff B, dePamphilis CW, Müller KF.. 2013. Disproportional plastome-wide increase of substitution rates and relaxed purifying selection in genes of carnivorous Lentibulariaceae. Molecular Biology and Evolution 31: 529–545. [DOI] [PubMed] [Google Scholar]
- Żelazowska M, Biliński SM.. 2001. Ultrastructure and function of nurse cells in phthirapterans. Possible function of ramified nurse cell nuclei in the cytoplasm transfer. Arthropod Structure and Development 30: 135–143. [DOI] [PubMed] [Google Scholar]