Abstract
Paragonimiasis is an important food-borne parasitic zoonosis caused by trematodes of genus Paragonimus. We report case series of paragonimiasis with common symptoms of cough with blood tinged sputum, shortness of breath, chest pain with occasional fever, eosinophilia and radiological findings mimicking pulmonary tuberculosis and had taken anti-tubercular drug despite all investigation negative for tuberculosis without improvement. They all had common history of consumption of raw/undercooked crab. There is a local belief in remote villages of Nepal that eating raw crab helps in healing bone fracture and cure jaundice. Microscopic examination of sputum sample revealed the ova of Paragonimus species. All patients were treated with praziquantel and got improved. Pulmonary paragonimiasis is endemic in Southeast Asia including Nepal. So, it has to be differentiated from pulmonary tuberculosis in the patient with symptoms of cough, chest pain and hemoptysis with eosinophilia and having history of consumption of raw/undercooked crabs or crayfish.
INTRODUCTION
Paragonimiasis is a parasitic infestation caused by genus Paragonimus which mimicks pulmonary tuberculosis and requires differentiation from the same in tuberculosis endemic country [1–4]. Paragonimiasis has been recognized as an important cause of pulmonary disease worldwide especially in Asia, West-Central Africa, and Central and South America [3]. On initial presentation, the signs and symptoms most commonly mimic pulmonary tuberculosis or lung cancer with haemoptysis, pleural effusion and peripheral blood eosinophilia [3]. In the areas where people eat undercooked crab/crayfish, this disease should be considered as the differential diagnosis to avoid anti-tubercular treatment for non-tubercular conditions [3, 4]. We report case series of paragonimiasis with common history of consumption of raw/undercooked crab. There is a local belief in remote villages of Nepal that eating raw crab helps in healing bone fracture and cure jaundice. They take crab as traditional medicine to cure many diseases and had consumed raw (minced) crab due to these beliefs.
CASE PRESENTATION
Case 1
A 25-year-old female from Syanja district was admitted in our hospital with shortness of breath. She had complain of chest pain, cough with production of blood tinged sputum. Her CT scan showed small pneumothorax and peripheral blood smear revealed eosinophilia (41%). She had the history of consumption of raw crab for rapid healing of her fractured bone.
Case 2
Second was a 32-year-old male from Gulmi district who had suffered since 2 years, done all investigation including bone marrow examination, visited different hospitals of Nepal from Gulmi–Palpa–Butwal–Bharatpur and finally to Tribhuvan University Teaching Hospital (TUTH) at Kathmandu. He had history of consumption of raw (minced) crab for the cure of his jaundice and he said his jaundice was improved by eating raw crab. After few months, he developed chest pain and cough with production of blood tinged sputum for which he was investigated and diagnosed clinically as pulmonary tuberculosis with polyserositis hypereosnophilia syndrome [eosinophilia (36%)]. He had taken anti-tubercular drug despite all investigations negative for tuberculosis due his sign and symptoms. His bone marrow examination revealed hypercellular bone marrow with eosinophilia.
Case 3
A 10-year-old child, son of our second case also had history of consumption of raw (minced) crab for the cure of his jaundice as advised by his grandmother who presented with complain of cough with blood tinged sputum, shortness of breath, chest pain and occasional fever. His chest X-ray revealed pleural and pericardial effusion (Fig. 1) mimicking pulmonary tuberculosis and was on anti-tubercular drug despite all investigations negative for tuberculosis for 5 months without improvement. Peripheral blood smear examination revealed eosinophilia (46%).
Figure 1:

chest X-ray of case 3 showing pericardial effusion and left sided pleural effusion
Case 4
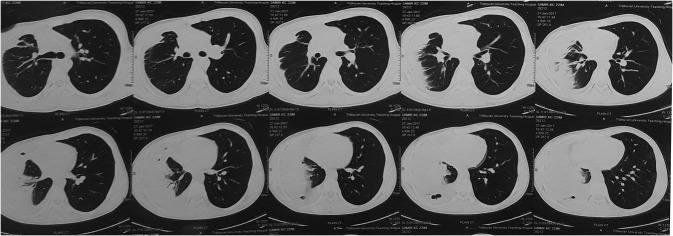
A 22-year-old male presented with hemoptysis and chest pain with history of consumption of undercooked crab. His chest x-ray revealed homogenous opacity in the right middle and lower lobe (Fig. 2). CT scan revealed partial collapse with consolidation of right middle lobe with free fluid in right pleural cavity and enhancing thickening of pleura suggestive of empyema thoracic (Fig. 3). He was evaluated for tuberculosis and lung cancer. Peripheral blood smears examination revealed eosinophilia (71%) and cytopathology smear examination of pleural fluid showed few mesothelial cells, macrophages with numerous neutrophils and eosinophil.
Figure 2:

chest X-ray of case 4 showing right middle and lower lobe opacity
Figure 3:
CT scan of case 4
INVESTIGATION AND OUTCOMES
On the background of history of consumption of raw crab, clinical symptoms and eosinophilia, microscopic examination of sputum sample in all cases were done which revealed oval, yellowish-brown eggs with a flattened operculum resting on shoulders (Figs 4–7) with the measurement of 75–85 μm in length by 45–55 μm in width (Fig. 8). Six and three consecutive sputum sample were examined in cases 3 and 4, respectively, to detect the characteristic ova of Paragonimus species whereas first sample was enough for the detection of the ova of the parasite in cases 1 and 2. The size of the detected ova was measured using cell sensation software version 1.12 for DP73 camera installed to the Olympus BX53 microscope used for the microscopy. On the basis of characteristic egg morphology and its measurement, ova of Paragonimus species was diagnosed. Diagnosis was further confirmed by Centres for Disease Control and Prevention (CDC), Atlanta Georgia, USA as ova of Paragonimus species. All patient were treated with praziquantel 25 mg per kg of body weight three times daily for three days and they got improved. Only first and third cases required another course of praziquantel. Follow up examination after one month revealed resolution of their symptoms, radiological findings and normal eosinophil count with no ova of Paragonimus species in their sputum sample.
Figure 4:

ova of Paragonimus species with Charcot Leyden crystal seen in case 1
Fagure 7:
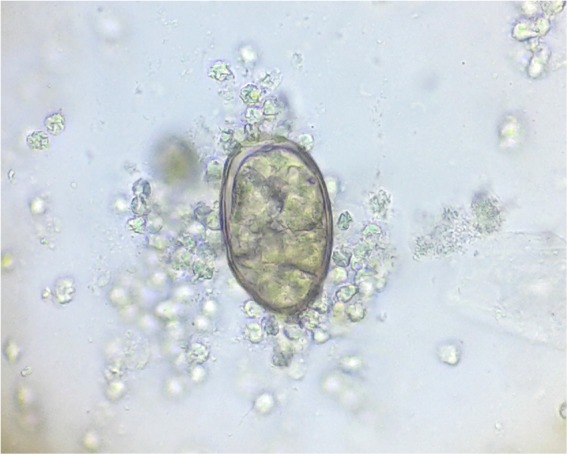
ova of Paragonimus species seen in case 4
Figure 8:

measurement of the ova (77 μm by 50 μm) using cell sensation software version 1.12 for DP73 camera installed to the Olympus BX53 microscope used for the microscopy
Figure 5:

ova of Paragonimus species seen in case 2
Figure 6:

ova of Paragonimus species seen in case 3
DISCUSSION
Paragonimiasis is an important food-borne parasitic zoonosis caused by one or more of the trematode species of the genus Paragonimus [1, 2]. Paragonimiasis is a disease which is frequently misdiagnosed as pulmonary tuberculosis usually when patient present with hemoptysis especially in the endemic region where both disease coexist [3, 4]. It is estimated that 22.8 million people worldwide are at a risk of paragonimiasis, with 195 million people in China [4]. Most cases of paragonimiasis occur throughout eastern (China, Japan, Philippines, South Korea and Taiwan), southeastern (Laos, Thailand and Vietnam), and southwestern (China) Asia and are caused by Paragonimus westermani [5].
The parasites utilize two intermediate hosts and a definitive hosts like wild mammals and humans to complete its life cycle. First intermediate hosts are the fresh water molluscan species and the second intermediate hosts are the fresh water crab species [6]. Life cycle begins with the production and passage of fertilized, operculate eggs from sexually competent adult trematodes that reside within the lungs of definitive mammalian host. The eggs are expectorated and either expelled or swallowed and passed in the faeces. The eggs in fresh or brackish water eventually hatch and release a ciliated miracidium which invades the first intermediate host (snail) [7]. Parasite develops in snail to form cercaria which leaves the snail and invade the second intermediate host (crustaceans). Cercaria develops into infective stage called metacercaria [4, 5]. Definitive hosts like humans get infected by consumption of raw, undercooked, or alcohol-pickled fresh water crabs or crayfish harbouring the viable metacercaria (infective stage) of Paragonimus species [5]. In definite hosts, the metacercaria excyst in the duodenum and migrate to the lungs to mature into adult worms that produce eggs. The unembronated eggs erode the bronchial wall and lead to cough and sputum production laden with eggs or if swallowed pass through stool [8, 9]. Thus, the life cycle continues.
A definitive diagnosis of paragonimiasis can be made by finding characteristic golden brown, ellipsoidal or oval operculated Paragonimus ova in the clinical specimens such as sputum, aspirated fluids and faeces by microscopy but it is difficult to make a diagnosis of paragonimiasis by microscopy. Although, the presence of ova in expectorated sputum is specific, the sensitivity of this test is low (28–38%) and repeated sputum sample examinations may increase the sensitivity of the test [4, 10]. Stool examination is also insensitive and the ova are not usually found in pleural fluid [4, 11]. Serological testing for antiparagonimus antibody by enzyme-linked immunosorbent assay (ELISA) has a sensitivity of 100% and a specificity of 91–100% [12]. It is a useful test for establishing the diagnosis of paragonimaisis. However, it is not available in Nepal [4]. Eosinophilia in peripheral blood smear is a supporting evidence [6]. Parziquantel at a dose of 75 mg/kg/day for 3 days is the drug of choice for paragonimiasis. However, another course of praziquantel is required in patients with unsatisfactory responses, persistent symptoms, or pulmonary involvement [13]. Clinically, paragonimiasis may be broadly classified into pulmonary, extra-pulmonary and pleuropulmonary forms [7]. Pulmonary paragonimiasis is the commonest clinical form of paragonimiasis occurring in 76–90% of cases [14]. Major clinical symptoms include chest pain, difficult breathing and coughing up rusty brown or blood-stained sputum or recurrent haemoptysis which mimics the pulmonary tuberculosis. So, pulmonary paragonimiasis has to be differentiated from pulmonary tuberculosis.
CONCLUSION
Pulmonary paragonimiasis is endemic in Southeast Asia including Nepal. However, it is not frequently reported from Nepal. In most of the cases, it is misdiagnosed as pulmonary tuberculosis and leads to long term anti-tubercular treatment while paragonimiasis could be treated by 3 days course of praziquantel. So patient with symptoms of cough, chest pain and hemoptysis with eosinophilia should be ruled out for paragonimiasis by examine the clinical samples directly under the microscope or by serological testing of antiparagonimus antibody by ELISA.
ACKNOWLEDGEMENTS
We would like to thanks Prof. Jeevan Bahadur Sherchand, Prof. Bharat Mani Pokharel, Prof. Basista Rijal, Prof. Keshab Parajuli, Assistant Professor Niranjan Prasad Shah, Hari Prasad Kattel, Assistant Professor Dr Sangita Sharma, Dr Mahesh Adhikari, Dr Neha Shrestha, Dr Sanjit Sah, Dr Ranjana Sah and Dr Rupendra Thapa for their constant support and guidance.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest.
FUNDING
Not applicable.
ETHICAL APPROVAL
There is no need for ethical approval for a case series according to the local ethical guidelines.
CONSENT
Written informed consent was taken from the individual patients and from the guardian in case 3 to publish this case series and related photographic evidence.
GUARANTOR
Dr Ranjit Sah.
REFERENCES
- 1. Kalhan S, Sharma P, Sharma S, Kakria N, Dudani S, Gupta A. Paragonimus westermani infection in lung: a confounding diagnostic entity. Lung India 2015;32:265–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lane MA, Barsanti MC, Santos CA, Yeung M, Lubner SJ, Weil GJ. Human paragonimiasis in North America following ingestion of Raw Crayfish. Clin Infect Dis 2009;49:e55–61. [DOI] [PubMed] [Google Scholar]
- 3. Prasad KJ, Basu A, Khana S, Wattal C. Pulmonary paragonimiasis mimicking tuberculosis. JAPI 2015;63:82–3. [PubMed] [Google Scholar]
- 4. Sah R, Khadka S, Sherchand JB, Parajuli K, Shah NP, Mishra SK, et al. Paragonimiasis: first autochthonous case report from Nepal. J Inst Med 2016;38:134–6. [Google Scholar]
- 5. Diaz JH. Paragonimiasis acquired in the United States: native and nonnative species. Clin Microbiol Rev 2013;26:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh TS, Sugiyama H, Rangsiruji A. Paragonimus & paragonimiasis in India. Indian J Med Res 2012;136:192–204. [PMC free article] [PubMed] [Google Scholar]
- 7. Procop GW. North American Paragonimiasis (caused by Paragonimus kellicotti) in the context of global paragonimiasis. Clin Microbiol Rev 2009;22:415–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DPDx Laboratory Identification of Parasitic Disease of Public Health Concern. Centre for Disease Control and Prevention (CDC), Paragonimiasis https://www.cdc.gov/dpdx/paragonimiasis/index.html.
- 9. Chatterjee KD. 13th edition parasitology (protozoology and helminthology), 2009.
- 10. Shim YS, Cho SY, Han YC. Pulmonary Paragonimiasis: a Korean perspective. Semi Respir Med 1991;12:35–45. [Google Scholar]
- 11. Shih YC, Ch’En YH, Chang YC. Paragonimiasis of central nervous system: observation on 76 cases. Chin Med J 1958;77:10–9. [PubMed] [Google Scholar]
- 12. Narain K, Devi KR, Mahanta J. Development of enzyme-linked immunosorbent assay for serodiagnosis of human paragonimiasis. Indian J Med Res 2005;121:739–46. [PubMed] [Google Scholar]
- 13. Gong Z, Miao R, Shu M, Zhu Y, Wen Y, Guo Q, et al. Paragonimiasis in Children in Southwest China: a retrospective case reports review from 2005 to 2016. Medicine (Baltimore) 2017;96:e7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh TS, Mutum SS, Razaque MA. Pulmonary paragonimiasis: clinical features, diagnosis and treatment of 39 cases in Manipur. Trans R Soc Trop Med Hyg 1986;80:967–71. [DOI] [PubMed] [Google Scholar]