Abstract
Objective
The aim of this study was to assess whether aural stimulation with ointment containing capsaicin improves swallowing function in elderly patients with dysphagia.
Study design
A randomized, placebo-controlled, double-blind, comparative study.
Settings
Secondary hospital.
Patients and methods
Twenty elderly dysphagic patients with a history of cerebrovascular disorder or Parkinson’s disease were randomly divided into two groups: 10 receiving aural stimulation with 0.025% capsaicin ointment and 10 stimulated with placebo. The ointments were applied to the external auditory canal with a cotton swab. Then, swallowing of a bolus of blue-dyed water was recorded using transnasal videoendoscopy, and the swallowing function was evaluated according to both endoscopic swallowing scoring and Sensory-Motor-Reflex-Clearance (SMRC) scale.
Results
The sum of endoscopic swallowing scores was significantly decreased 30 and 60 min after a single administration in patients treated with capsaicin, but not with placebo. Reflex score, but not Sensory, Motion and Clearance scores, of the SMRC scale was significantly increased 5, 30 and 60 min after single administration in patients treated with capsaicin, but not with placebo. No patient showed signs of adverse effects.
Conclusion
As capsaicin is an agonist of the transient receptor potential vanilloid 1 (TRPV1), these findings suggest that improvement of the swallowing function, especially glottal closure and cough reflexes, in elderly dysphagic patients was due to TRPV1-mediated aural stimulation of vagal Arnold’s nerve with capsaicin, but not with a nonspecific mechanical stimulation with a cotton swab.
Keywords: dysphagia, capsaicin, cough reflex, videoendoscopy, SMRC scale, external auditory canal
Introduction
Dysphagia affects elderly and neurological patients, increasing the risk for aspiration pneumonia.1 Patients with dysphagia show difficulty in moving a bolus safely from the oral cavity to the esophagus, which allows it to penetrate into the airways. Thus, dysphagia can cause severe complications such as aspiration pneumonia, respiratory infection and malnutrition or death.
There is evidence that dysphagia is related to oropharyngolaryngeal sensory deficits, and that the therapeutic sensory stimuli on the oropharynx compensate for the loss of oropharyngolaryngeal sensitivity in elderly dysphagic patients.2 In fact, it was reported that vagal sensory stimulation in the pharynx with a capsaicin pastille improves the delay in swallowing function in elderly patients with dysphagia.3 However, oral administration of capsaicin pastille has the risk of causing aspiration in these patients. Accordingly, we then proposed the hypothesis that ectopic stimulation of the Arnold’s branch of the vagus in the external auditory canal with capsaicin also improved swallowing function. In our previous study, we indeed showed that aural stimulation with capsaicin ointment improved swallowing function in elderly patients with dysphagia, suggesting that aural stimulation with capsaicin ointment may be a safer therapy for dysphagia.4
Capsaicin is the main component of red pepper. It is an agonist of transient receptor potential vanilloid 1 (TRPV1)5 and activates TRPV1 in peripheral sensory C-fibers. As a subset of neurons in the vagal nodose ganglion expresses TRPV1,6 our previous study suggested that ointment containing capsaicin activates TRPV1 in vagal Arnold’s nerve of the external auditory canal, resulting in improvement of the swallowing function.4 However, we cannot exclude the possibility that a nonspecific mechanical stimulation of the external auditory canal with a cotton swab rather than the TRPV1-mediated stimulation with capsaicin improves swallowing function in dysphagic patients. To exclude this possibility, we designed a randomized, placebo-controlled, double-blind, comparative study to compare the effects of ointment containing capsaicin on swallowing function to those of placebo in elderly patients with dysphagia.
Patients and methods
Study design
This study was a double-blind, randomized controlled, comparative study. It was conducted at Kochi National Hospital from July 2014 to March 2015. It was approved by the Committees for Medical Ethics of Kochi National Hospital and Tokushima University Hospital, and a written informed consent was obtained from each patient prior to the study. The Clinical Trial registration number of this study is UMIN000013443.
Subjects
Twenty elderly patients with dysphagia were enrolled in this study. The sample size was calculated based on the following parameters: α=0.05, β=0.20, power =80%, estimated difference =1.5 and SD=1.0. The calculation determined that the minimal size for each group was eight patients. Accordingly, 20 patients were enrolled and divided into two groups: 10 patients in the capsaicin group and 10 in the placebo group. Their baseline clinical characteristics are shown in Table 1. Patients complained of choking upon swallowing water or food, but had no obstructive lesion in the pharyngolarynx as confirmed by endoscopy. They suffered from an old cerebrovascular disorder or Parkinson’s disease, and no patients had an active ear disease such as otitis externa or myringitis.
Table 1.
Patient characteristics
| Charasteristic | Capsaicin (n=10) |
Placebo (n=10) |
P-value |
|---|---|---|---|
| Age, yearsa | 80.4±9.5 | 80.1±5.9 | NS* |
| Sex: M/F, n | 10/0 | 9/1 | NS** |
| Pathology: Ce/Pa, n | 9/1 | 8/2 | NS** |
| Duration after onset, monthsb | 15.5 | 20.0 | NS* |
| ESSa,c | 5.3±1.4 | 5.4±1.4 | NS* |
| SMRC scalea | |||
| Sensory score | 2.5±0.5 | 2.8±0.4 | NS* |
| Motion score | 1.9±0.3 | 1.8±0.4 | NS* |
| Reflex score | 1.5±0.5 | 1.6±0.5 | NS* |
| Clearance score | 2.0±0 | 1.7±0.5 | NS* |
Notes:
Mean ± SD,
median,
ESS, total score of endoscopic swallowing scoring.
Mann–Whitney U test,
Fisher’s exact test.
Abbreviations: Ce, cerebrovascular disease; NS, not significant; Pa, Parkinson’s disease; SMRC, Sensory–Motor–Reflex–Clearance.
Intervention
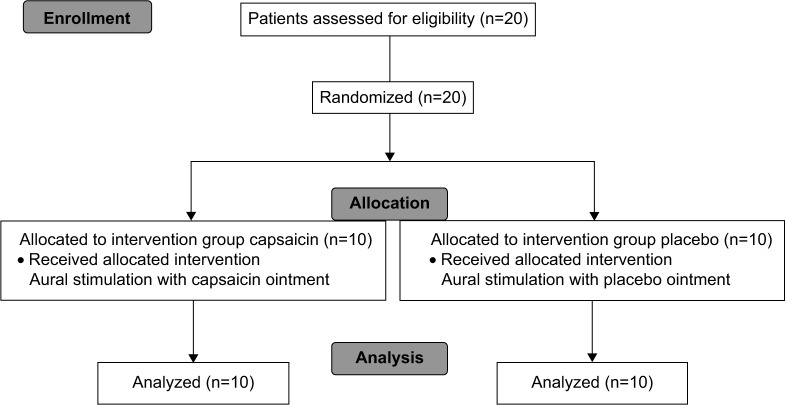
The patients had a baseline videoendoscopic evaluation of the swallowing function using both endoscopic swallowing scoring and Sensory–Motor–Reflex–Clearance (SMRC) scale. They were then divided into two groups by computerized randomization: a capsaicin group (group C) and a placebo group (group P). Their swallowing function was evaluated by transnasal videoendoscopy 5, 30 and 60 min after a single application of 0.5 g of 0.025% capsaicin or placebo ointment to the right external auditory canal with a cotton swab under an otoscope (Figure 1). The randomization and supply of ointment were performed by a blinded pharmacist who was not involved in the study. Throughout the study, the patients were observed to evaluate the occurrence of any adverse effects such as otalgia or headache.
Figure 1.
Patient flowchart.
Capsaicin ointment and placebo
Based on the Japanese Pharmacopoeia (16th edition) published by the Ministry of Health, Labor and Welfare of Japan, 0.025% capsaicin ointment was prepared according to the protocol of Japanese Drug Preparation of Hospital Pharmacy (4th edition) as follows: 25 mg of capsaicin (Sigma-Aldrich Co., St Louis, MO, USA) was dissolved in 500 μL of 100% ethanol (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and the solution was then mixed with 100 g hydrophilic ointment. Placebo was only hydrophilic ointment of the same color and viscosity as the capsaicin ointment.
Videoendoscopy
The standard protocol of videoendoscopic evaluation of swallowing proposed by the Oto-Rhino-Laryngological Society of Japan was used.7 Accordingly, patients were seated facing an otolaryngologist. Water was dyed with blue food coloring for ease of visualization and given to the patient in a bolus of 3 mL. Swallowing of the blue-dyed water was recorded by the video rhinolaryngoscope system with a flexible fiber optic endoscope of 3.1 mm diameter (VNL-100S®; Pentax, Tokyo, Japan). The video images of swallowing were evaluated using both endoscopic swallowing scoring and SMRC scale by another otolaryngologist blinded to clinical data and independent from the examiner.
Evaluation of the swallowing function
Endoscopic swallowing scoring was used to evaluate the total swallowing function with videoendoscopy (Table 2).8,9 The endoscopic swallowing scoring consists of four swallowing components: 1) salivary pooling in vallecula and pyriform sinuses, 2) the response of glottal closure reflex induced by touching the epiglottis with endoscope, 3) location of the bolus at the time of swallowing onset assessed by endoscopic whiteout and 4) the extent of pharyngeal clearance after swallowing of blue-dyed water. Each item was scored on a scale of 0–3 as shown in Table 2, and the sum of each score was used as an index of total swallowing function. Clinically, the full score is 12, and a score of more than 7 indicates a serious risk for aspiration, while a score of 7 or less during endoscopic swallowing evaluation with test jelly reliably predicts the ability to eat pureed diets.9
Table 2.
Endoscopic swallowing scoring
| 1. Salivary pooling in vallecula and pyriform sinuses |
| 0: No pooling |
| 1: Pooling at only vallecula |
| 2: Pooling in vallecula and pyriform sinuses and no penetration into the larynx |
| 3: Pooling in vallecula and pyriform sinuses and penetration into the larynx |
| 2. The response of glottal closure reflex induced by touching epiglottis with endoscope |
| 0: Marked reflex by one touch |
| 1: Slow and/or weak reflex by one touch |
| 2: Reflex by two or three touches |
| 3: No reflex despite three touches |
| 3. The location of the bolus at the time of swallow onset assessed by endoscopic whiteout |
| 0: Pharyngeal |
| 1: Vallecula |
| 2: Pyriform sinuses |
| 3: No swallowing |
| 4. The extent of pharyngeal clearance after swallowing of blue-dyed water |
| 0: No residues |
| 1: Pharyngeal residues remain, but are absent after swallowing is attempted two or three times |
| 2: Pharyngeal residues remain, but do not penetrate into larynx |
| 3: Pharyngeal residues remain and penetrate into larynx |
The swallowing function consists of four subfunctions: sensory initiation of swallowing reflex, motion to hold a bolus in the oral cavity and induce laryngeal elevation, glottal closure and cough reflexes and pharyngeal clearance of a bolus into the esophagus.10,11 Accordingly, we used the SMRC scale to evaluate the four subfunctions of the swallowing function with videoendoscopy separately: 1) Sensory: initiation of swallowing reflex as assessed by endoscopic whiteout; 2) Motion: holding bolus in the oral cavity and inducing laryngeal elevation according to instructions; 3) Reflex: glottal closure and cough reflexes induced by touching epiglottis or arytenoids with endoscope and 4) Clearance: pharyngeal clearance of the bolus after swallowing (Table 3).12 Each function except Motion was scored on a scale of 0–3, and Motion was scored on a scale of 0–2 as shown in Table 3. SMRC scale scores were used as an index of each swallowing subfunction. Clinically, Score 0 of any function indicates no oral feeding because of the high risk for aspiration. Score 1 or 2 of any function (1 of Motion) indicates the need for intervention such as changing the form of food and swallowing training. In our recent study, we also showed a significant correlation between cough reflex sensitivity to citric acid in the cough reflex test and Reflex score with videoendoscopy in elderly patients with dysphagia (manuscript in preparation).
Table 3.
SMRC scale
| 1. Sensory: Initiation of swallowing reflex as assessed by endoscopic whiteout |
| 3: Bolus at vallecula |
| 2: Bolus at pyriform sinuses |
| 1: Bolus retention at bottom of pyriform sinuses |
| 0: No swallowing initiation |
| 2. Motion: holding bolus in oral cavity and inducing laryngeal elevation according to instructions |
| 2: Both holding bolus in oral cavity and inducing laryngeal elevation |
| 1: Either holding bolus in oral cavity or inducing laryngeal elevation |
| 0: Neither of them |
| 3. Reflex: glottal closure and cough reflexes induced by touching epiglottis or arytenoids with endoscope |
| 3: Brisk cough reflex |
| 2: Brisk glottal closure reflex with weak or delayed cough reflex |
| 1: Weak or delayed glottal closure and cough reflexes |
| 0: Neither of them |
| 4. Clearance: pharyngeal clearance of bolus after swallowing |
| 3: No bolus and saliva residues after swallowing once or twice |
| 2: No bolus residue after swallowing once or twice, but saliva residue |
| 1: No bolus residue after swallowing three times or more |
| 0: Bolus residue even after swallowing three times or more |
Abbreviation: SMRC, Sensory–Motor–Reflex–Clearance.
Statistical analysis
The primary outcome was improvement of the sum of endoscopic swallowing scores and each score of SMRC scale in patients with dysphagia after aural stimulation with capsaicin ointment or placebo. Comparison of baseline clinical characteristics between groups C and P was carried out by Mann–Whitney U test and Fisher’s exact test. Changes in endoscopic swallowing scoring and SMRC scale after stimulation with capsaicin ointment or placebo were analyzed by Friedman test and Shirley–Williams post hoc test, and P≤0.05 was considered statistically significant. Statistical analysis was done using Microsoft® Excel 2010 Japanese version (Microsoft Japan, Tokyo, Japan) and Statcel version 3 (OMS Publishing Inc., Saitama, Japan).
Results
Baseline clinical characteristics of each group are shown in Table 1. There were no statistical differences in age, sex, pathology, duration after onset, total score of endoscopic swallowing scoring or SMRC scale between groups C and P.
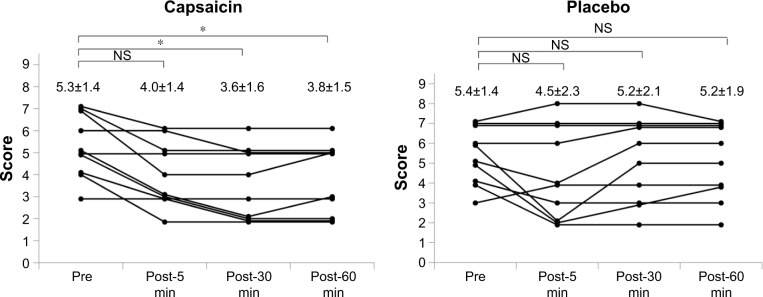
Changes in the sum of endoscopic swallowing scores after single aural administration of capsaicin ointment or placebo are shown in Figure 2. The endoscopic swallowing scores of group C were significantly decreased 30 and 60 min after single aural administration of capsaicin ointment (P=0.05). But the endoscopic swallowing scores of group P were unchanged after single aural administration of placebo. Mean changes in endoscopic swallowing scores from baseline (pre) to post-5, -30 and -60 min in groups C and P are shown in Figure 3. The decrease in endoscopic swallowing scores of group C was significantly greater than that of group P at post-30 and -60 min (P<0.05).
Figure 2.
Changes in endoscopic swallowing scores after single aural administration in capsaicin and placebo groups.
Notes: *P=0.003 in Friedman test; P=0.05 with Shirley–Williams post hoc test. Data shown as mean ± SD. n=10.
Abbreviation: NS, not significant.
Figure 3.
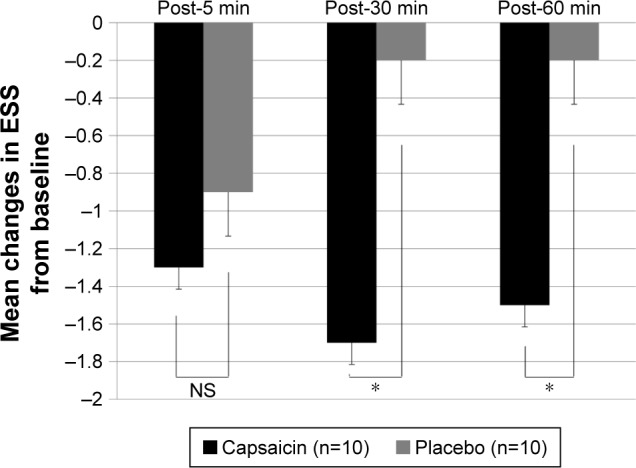
Mean changes in endoscopic swallowing scores from baseline (pre) to post-5, -30 and -60 min in capsaicin (black) and placebo (gray) groups.
Note: *P<0.05 in Mann–Whitney U test.
Abbreviations: NS, not significant; ESS, endoscopic swallowing scoring.
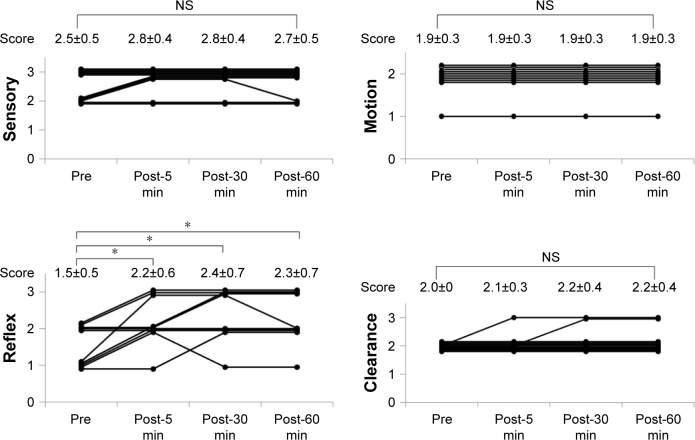
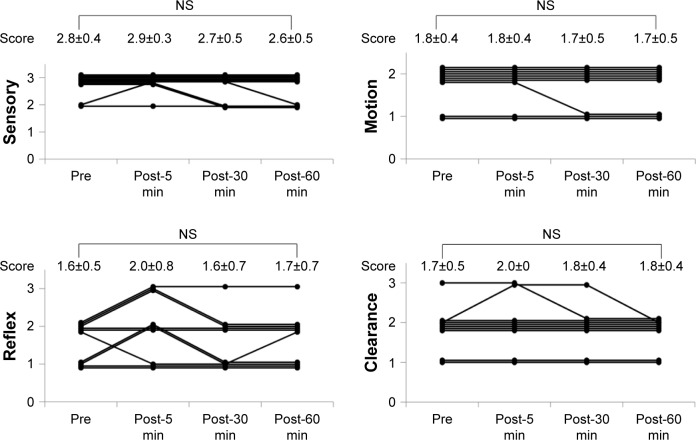
Sensory, Motion, Reflex and Clearance scores of SMRC scale after single aural administration of capsaicin ointment or placebo are shown in Figures 4 and 5. Reflex scores, but not Sensory, Motion and Clearance scores, of group C were significantly increased 5, 30 and 60 min after single aural administration of capsaicin ointment (P=0.05). However, any scores of SMRC scale in group P were unchanged after a single aural administration with placebo. Mean changes in Reflex scores from baseline (pre) to post-5, -30 and -60 min in groups C and P are shown in Figure 6. The increase of the Reflex score in group C was significantly higher than that of group P at post-30 and -60 min (P<0.05). No adverse effects including otalgia, headache, otitis externa and myringitis were induced in any patient up to 120 min after administration.
Figure 4.
Changes in SMRC scale after single aural administration in capsaicin group.
Notes: *P=0.003 in Friedman test; P=0.05 with Shirley–Williams post hoc test. Data shown as mean ± SD, n=10.
Abbreviations: NS, not significant; SMRC, Sensory–Motor–Reflex–Clearance.
Figure 5.
Changes in SMRC scale after single aural administration in placebo group.
Note: Data shown as mean ± SD, n=10.
Abbreviations: NS, not significant; SMRC, Sensory–Motor–Reflex–Clearance.
Figure 6.
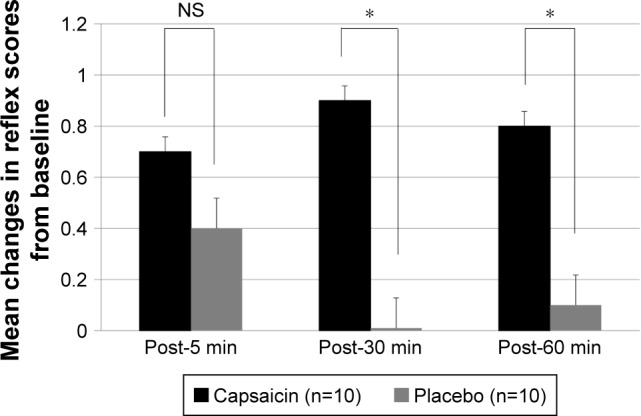
Mean changes in reflex scores from baseline (pre) to post-5, -30 and -60 min in capsaicin (black) and placebo (gray) groups.
Note: *P<0.05 in Mann–Whitney U test.
Abbreviation: NS, not significant.
Discussion
In this study, the sum of endoscopic swallowing scores was improved up to 60 min after the stimulation of the external auditory canal with 0.025% capsaicin ointment in elderly patients with dysphagia. Moreover, this study demonstrates that among the four subfunctions of swallowing function, only the Reflex score of glottal closure and cough reflexes was improved by stimulation with capsaicin ointment. These effects were not observed in the placebo group, indicating that it was not a nonspecific mechanical stimulation, but the capsaicin ointment applied to the external auditory canal that improved their swallowing function, especially glottal closure and cough reflexes. Furthermore, nonresponders in group C suggest individual differences in the effects of capsaicin ointment. But the nonspecific mechanical stimulation of cotton swab might improve the laryngeal reflex in some responders in group P.
Accordingly, it is suggested that capsaicin-TRPV1-mediated stimulation of the Arnold’s branch of the vagus in the external auditory canal improved swallowing function in these elderly dysphagic patients. Indeed, the auricular branch of the vagus, the Arnold’s nerve, is distributed to the external auditory canal, and its stimulation triggers cough reflex.13 This is frequently encountered by otolaryngologists in the management of the external auditory canal, such as ear syringing.14 As the stimulation of sensory C-fiber branches of the vagus in the laryngotracheal mucosa with capsaicin induces cough reflex,15 it is suggested that stimulation of Arnold’s nerve, a branch of the vagus distributed to the external auditory canal, with capsaicin improved glottal closure and cough reflexes in dysphagic patients. On the other hand, it was reported that oral administration of a pastille containing capsaicin improved the delay in swallowing function in patients with dysphagia.3 Oral administration of capsaicin stimulates the sensory C-fibers of the trigeminal, glossopharyngeal and vagal nerves in the oral and pharyngolaryngeal mucosa, but aural stimulation with capsaicin stimulates only the Arnold’s branch of the vagus. It is, therefore, suggested that oral administration of capsaicin improves the delay in swallowing function, while aural stimulation improves only the Reflex score of glottal closure and cough reflexes, but not Sensory, Motion and Clearance scores in dysphagic patients.
Glottal closure and cough reflexes are important airway protective mechanisms against aspiration. It was shown that sensitivity of the cough reflex was reduced in patients with aspiration pneumonia.16 Angiotensin-converting enzyme inhibitors have been shown to reduce the prevalence of aspiration pneumonia in post-stroke patients, because angiotensin-converting enzyme inhibitor induces cough.17–19 This study demonstrated that the aural stimulation with capsaicin improved glottal closure and cough reflexes in elderly patients with dysphagia, and future studies are necessary to investigate if the aural stimulation with capsaicin could have similar application to prevent aspiration pneumonia. Chronic exposure to high-dose capsaicin causes long-term functional impairment of sensory fibers due to desensitization of TRPV1 and depletion of neuropeptides such as substance P (capsaicin defunctionalization).20 However, in our previous study, repeated alternative application with ointment containing 0.025% capsaicin to each external auditory canal once a day for 7 days still improved swallowing function in patients with dysphagia.4 Therefore, once-a-day alternative application to each external auditory canal with low-dose capsaicin may be a useful method to prevent capsaicin defunctionalization, although it is still uncertain how long the effects of single application with capsaicin ointment last. Furthermore, multicenter studies are required to explore whether repeated aural stimulation with 0.025% capsaicin ointment in such a way to prevent capsaicin defunctionalization really reduces the incidence of aspiration pneumonia in patients with dysphagia.
There are some limitations to this study. There was a preponderance of males. The endoscopic swallowing scoring and SMRC scale are subjective evaluations.21 Further studies are needed to objectively evaluate the swallowing function and cough reflex using videofluoroscopy22 and cough reflex test.23 Aural stimulation with capsaicin ointment is effective in patients with nonobstructive, but not obstructive dysphagia.
In conclusion, this study demonstrated that the topical application of an ointment containing 0.025% capsaicin to the external auditory canal improves swallowing function, especially glottal closure and cough reflexes, in elderly patients with dysphagia. Therefore, repeated aural stimulation with capsaicin ointment may be a safe and promising intervention to prevent aspiration pneumonia in elderly dysphagic patients due to improvement of glottal closure and cough reflexes via Arnold’s ear-cough reflex.
Acknowledgments
The authors thank Professor Bukasa Kalubi for his critical reading of the manuscript. The abstract of this paper was presented at the 2017 DRS Annual Meeting as an oral presentation with interim findings. The oral presentation’s abstract was published in “Oral Abstracts” in Dysphagia.
Footnotes
Author contributions
EK designed the study, collected data and wrote a part of the manuscript. OJ designed the study and analyzed data. SN collected data. HO analyzed, interpreted data and wrote a part of the manuscript. IK interpreted data. NT supervised the study and corrected the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Baijens LW, Clavé P, Cras P, et al. European Society for Swallowing Disorders – European Union Geriatric Medicine Society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin Interv Aging. 2016;11:1403–1428. doi: 10.2147/CIA.S107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Berdugo D, Rofes L, Casamitjana JF, Padron A, Quer M, Clave P. Oropharyngeal and laryngeal sensory innervation in the pathophysiology of swallowing disorders and sensory stimulation treatments. Ann N Y Acad Sci. 2016;1380(1):104–120. doi: 10.1111/nyas.13150. [DOI] [PubMed] [Google Scholar]
- 3.Ebihara T, Takahashi H, Ebihara S, et al. Capsaicin troche for swallowing dysfunction in older people. J Am Geriatr Soc. 2005;53(5):824–828. doi: 10.1111/j.1532-5415.2005.53261.x. [DOI] [PubMed] [Google Scholar]
- 4.Kondo E, Jinnouchi O, Ohnishi H, et al. Effects of aural stimulation with capsaicin ointment on swallowing function in elderly patients with non-obstructive dysphagia. Clin Interv Aging. 2014;9:1661–1667. doi: 10.2147/CIA.S67602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev. 2009;60(1):267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51(2):159–212. [PubMed] [Google Scholar]
- 7.Committee on Dysphagia of the Oto-Rhino-Laryngological Society of Japan: Practice Guidelines for Dysphagia. Kyoto: Kanehara Shuppan; 2008. [Google Scholar]
- 8.Hyodo M, Nishikubo K, Hirose K. New scoring proposed for endoscopic evaluation and clinical significance. Nippon Jibiinkoka Gakkai Kaiho. 2010;113(8):670–678. doi: 10.3950/jibiinkoka.113.670. Japanese. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto T, Horiuchi A, Makino T, Kajiyama M, Tanaka N, Hyodo M. Determination of the cut-off score of an endoscopic scoring method to predict whether elderly patients with dysphagia can eat pureed diets. World J Gastrointest Endosc. 2016;8(6):288–294. doi: 10.4253/wjge.v8.i6.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langmore SE. Endoscopic evaluation of oral and phraryngeal phases of swallowing. GI Motility Online [serial on the Internet] 2006. May, [Accessed March 30, 2017]. [cited May 16, 2006]. Available from: http://www.nature.com/gimo/contents/pt1/full/gimo28.html.
- 11.Aviv JE, Kim T, Sacco RL, et al. FEESST: a new bedside endoscopic test of the motor and sensory components of swallowing. Ann Otol Rhinol Laryngol. 1998;107(5 Pt 1):378–387. doi: 10.1177/000348949810700503. [DOI] [PubMed] [Google Scholar]
- 12.Kondo E, Jinnouchi O, Ohnishi H, Kawata I, Takeda N. Aural stimulation with capsaicin ointment improved the swallowing function in patients with dysphagia: evaluation by the SMRC scale. Nippon Jibiinkoka Gakkai Kaiho. 2015;118(11):1319–1326. doi: 10.3950/jibiinkoka.118.1319. Japanese. [DOI] [PubMed] [Google Scholar]
- 13.Gupta D, Verma S, Vishwakarma SK. Anatomic basis of Arnold’s ear-cough reflex. Surg Radiol Anat. 1986;8(4):217–220. doi: 10.1007/BF02425070. [DOI] [PubMed] [Google Scholar]
- 14.Bloustine S, Langston L, Miller T. Ear-cough (Arnold’s) reflex. Ann Otol Rhinol Laryngol. 1976;85(3 Pt 1):406–407. doi: 10.1177/000348947608500315. [DOI] [PubMed] [Google Scholar]
- 15.Canning BJ. Afferent nerves regulating the cough reflex: mechanisms and mediators of cough in disease. Otolaryngol Clin North Am. 2010;43(1):15–25. vii. doi: 10.1016/j.otc.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekizawa K, Ujiie Y, Itabashi S, Sasaki H, Takishima T. Lack of cough reflex in aspiration pneumonia. Lancet. 1990;335(8699):1228–1229. doi: 10.1016/0140-6736(90)92758-a. [DOI] [PubMed] [Google Scholar]
- 17.Sekizawa K, Matsui T, Nakagawa T, Nakayama K, Sasaki H. ACE inhibitors and pneumonia. Lancet. 1998;352(9133):1069. doi: 10.1016/S0140-6736(05)60114-6. [DOI] [PubMed] [Google Scholar]
- 18.Ebihara S, Ebihara T, Kohzuki M. Effect of aging on cough and swallowing reflexes: implications for preventing aspiration pneumonia. Lung. 2012;190(1):29–33. doi: 10.1007/s00408-011-9334-z. [DOI] [PubMed] [Google Scholar]
- 19.Shinohara Y, Origasa H. Post-stroke pneumonia prevention by angiotensin-converting enzyme inhibitors: results of a meta-analysis of five studies in Asians. Adv Ther. 2012;29(10):900–912. doi: 10.1007/s12325-012-0049-1. [DOI] [PubMed] [Google Scholar]
- 20.Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107(4):490–502. doi: 10.1093/bja/aer260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neubauer PD, Hersey DP, Leder SB. Pharyngeal residue severity rating scales based on fiberoptic endoscopic evaluation of swallowing: a systematic review. Dysphagia. 2016;31(3):352–359. doi: 10.1007/s00455-015-9682-6. [DOI] [PubMed] [Google Scholar]
- 22.Baijens L, Barikroo A, Pilz W. Intrarater and interrater reliability for measurements in videofluoroscopy of swallowing. Eur J Radiol. 2013;82(10):1683–1695. doi: 10.1016/j.ejrad.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29(6):1256–1276. doi: 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]