Abstract
A number of published case–control studies reported that the apolipoprotein E (ApoE) gene polymorphism was associated with the mild cognitive impairment (MCI). However, previous reports still remain conflicting. To estimate the association between ApoE polymorphism and MCI susceptibility, we searched the electronic databases including PubMed, Wanfang, CNKI (China National Knowledge Infrastructure), VIP, and EMBASE to retrieve all available studies. A total of 18 studies with 2,004 cases and 3,705 controls were included in this meta-analysis. The pooled analysis based on selected studies showed that statistically significant risk association was found between ApoE gene polymorphism and MCI in overall population (ε4 vs ε3: odds ratio [OR] =2.38, 95% confidence interval [CI]: 2.11–2.68; ε4/ε4 vs ε3/ε3: OR =4.45, 95% CI: 3.06–6.48; ε2/ε4 vs ε3/ε3: OR =2.57, 95% CI: 1.77–3.73; ε3/ε4 vs ε3/ε3: OR =2.31, 95% CI: 1.99–2.69). However, no significant association was detected in two genetic models: ε2 versus ε3 (OR =0.90, 95% CI: 0.77–1.05) and ε2/ε2 versus ε3/ε3 (OR =0.91, 95% CI: 0.50–1.65). Furthermore, ApoE ε2/ε3 genotype provided a slight protection for MCI in overall population (ε2/ε3 vs ε3/ε3: OR =0.80, 95% CI: 0.66–0.97). In the stratified analysis based on ethnicity, similar results were also observed in Chinese population (significant risk: ε4 vs ε3: OR =2.52, 95% CI: 2.19–2.90; ε4/ε4 vs ε3/ε3: OR =5.45, 95% CI: 3.41–8.70; ε2/ε4 vs ε3/ε3: OR =2.59, 95% CI: 1.74–3.86; ε3/ε4 vs ε3/ε3: OR =2.34, 95% CI: 1.97–2.79; slight protection: ε2/ε3 vs ε3/ε3: OR =0.79, 95% CI: 0.64–0.98; no association: ε2 vs ε3: OR =0.92, 95% CI: 0.78–1.09; and ε2/ε2 vs ε3/ε3: OR =1.04, 95% CI: 0.55–1.99). In summary, this meta-analysis of 5,709 subjects suggested that ApoE ε4 allele was associated with an increased risk of MCI. In addition, ApoE ε2/ε3 genotype provided a slight protection for MCI.
Keywords: mild cognitive impairment, apolipoprotein E, polymorphism, meta-analysis
Introduction
Mild cognitive impairment (MCI) is a transitional state between normal aging and Alzheimer’s disease (AD).1,2 Approximately 18.5% of Chinese people over the age of 55 years were estimated to have MCI.3 In fact, patients with MCI represented a conversion rate of 10%–15% per year for developing AD.4,5 Therefore, discussing the associations between the risk factors and MCI susceptibility is of great significance.
The apolipoprotein E (ApoE) gene, located on the chromosome 19q13, is closely related to MCI and AD.6,7 ApoE protein plays a vital role in the transport of lipid and cholesterol in the central nervous system (CNS).8 ApoE gene polymorphism has three common alleles: ε2, ε3, and ε4, which determine three homozygous (ε2/ε2, ε3/ε3, and ε4/ε4) and heterozygous (ε2/ε4, ε3/ε4, and ε2/ε3) genotypes.9 Of those, ApoE ε3 allele is the most prevalent, followed by ε4 and ε2 alleles.10 The ApoE ε4 allele has been highly associated with MCI;11,12 its presence is associated with the elevated serum β-amyloid and age-related cognitive decline.13,14 In addition, it is well known that ε4 allele was associated with an increased risk of AD.15
To date, numerous studies have been conducted to estimate the association between ApoE polymorphism and MCI susceptibility. However, the reports still conflict. The sample sizes of the published studies have been relatively small, and individual study may lack powerful power to obtain a more reliable conclusion. In addition, no meta-analysis was performed to explore those associations. Therefore, we conducted a comprehensive meta-analysis to clarify those varying associations.
Materials and methods
Search strategy
All published studies assessing the association of ApoE polymorphism with MCI susceptibility were identified by comprehensive literature searches of the PubMed, EMBASE, Wanfang, VIP, and CNKI (China National Knowledge Infrastructure) databases from May 2002 to October 2016. The key terms used for searching are (“MCI” OR “mild cognitive impairment”) AND (“ApoE” OR “apolipoprotein E”) AND (“polymorphism” OR “variant”). Moreover, the references in all selected studies were searched for other potential studies.
Inclusion and exclusion criteria
Studies included in our meta-analysis must meet the following criteria: 1) case–control or cohort study; 2) estimate the association between ApoE polymorphism and MCI susceptibility; 3) allelic and genotype frequencies are available for calculating odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs); 4) genotype distribution of control must be in Hardy–Weinberg equilibrium (HWE); 5) not overlapping samples; and 6) studies with full-text. The exclusion criteria for the studies were as follows: case reports, reviews, in vitro studies, clinical trials, incomplete genotype data, and meta-analysis.
Data extraction
Relevant data from each selected studies, including the first author, publication year, country of region, genotyping methods, sample size, genotype distributions and allele frequencies of cases and controls, and the diagnosis criteria of MCI, were extracted independently by two investigators (TH and WSD).
Statistical analysis
The ORs and corresponding 95% CIs were used to evaluate the relationship between ApoE polymorphism and MCI susceptibility. The risk of variant genotypes ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε4, and ε4/ε4 was evaluated compared with the ε3/ε3 genotype. In addition, ε2 versus ε3 and ε4 versus ε3 were also analyzed. The test of heterogeneity for selected studies was assessed by I2-statistics.16,17 When a significant heterogeneity (no heterogeneity: I2<25%; moderate heterogeneity: I2=25%–50%; significant heterogeneity: I2≥50%) appeared across the selected studies, the random effects model was used.18,19 Otherwise, the fixed effects model was adopted. To estimate whether our results were stable, a sensitivity analysis was performed by sequentially omitting each individual study and recalculating the remaining studies. The potential publication bias was examined by Begg’s tests and funnel plot.20 Statistical tests were carried out by Stata software v12.0 (Stata Corp, College Station, TX, USA).
Results
Characteristics of eligible studies
The initial search identified 721 references. Of those, 18 publications21–38 with 2,004 cases and 3,705 controls were included in our meta-analysis. The study selection process was shown in Figure 1. Of all eligible studies focusing on the association between ApoE polymorphism and MCI susceptibility, 14 studies were performed in China,21,23–35 two in Caucasians,22,37 one in Brazil,36 and one in India.38 The genotype distributions of all control samples are consistent with the HWE. The detailed characteristics of selected studies are summarized in Table 1.
Figure 1.
Flow diagram of the article selection process.
Table 1.
Characteristics of the selected studies
| Study | Year | Geographical location | Sample size (case/control) | Case
|
Control
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ε4ε4 | ε4ε3 | ε4ε2 | ε3ε3 | ε3ε2 | ε2ε2 | ε4 | ε3 | ε2 | ε4ε4 | ε4ε3 | ε4ε2 | ε3ε3 | ε3ε2 | ε2ε2 | ε4 | ε3 | ε2 | ||||
| Wang et al21 | 2002 | China (Heifei) | 28/30 | 2 | 9 | 7 | 6 | 3 | 1 | 20 | 24 | 12 | 1 | 4 | 2 | 18 | 4 | 1 | 8 | 44 | 8 |
| Gamarra et al22 | 2015 | Spain | 124/125 | 20 | 66 | 2 | 119 | 9 | 0 | 108 | 313 | 11 | 0 | 49 | 2 | 170 | 22 | 2 | 51 | 411 | 28 |
| Wang et al23 | 2014 | China (Wuzhong) | 216/743 | 1 | 24 | 1 | 135 | 17 | 3 | 27 | 311 | 24 | 6 | 34 | 2 | 580 | 105 | 16 | 48 | 1,299 | 139 |
| Borenstein et al24 | 2010 | China (Shanghai) | 30/32 | 2 | 5 | 2 | 20 | 1 | 0 | 11 | 46 | 3 | 0 | 2 | 1 | 23 | 6 | 0 | 3 | 54 | 7 |
| Wang et al25 | 2014 | China (Beijing) | 56/75 | 0 | 23 | 1 | 26 | 3 | 3 | 24 | 78 | 10 | 0 | 8 | 3 | 51 | 12 | 1 | 11 | 122 | 17 |
| Chen et al26 | 2016 | China (Shanghai) | 583/1,149 | 27 | 129 | 16 | 353 | 56 | 2 | 199 | 891 | 76 | 8 | 156 | 21 | 802 | 154 | 8 | 193 | 1,914 | 191 |
| Chen et al27 | 2016 | China (Ningbo) | 64/54 | 5 | 20 | 0 | 35 | 4 | 0 | 30 | 94 | 4 | 0 | 7 | 0 | 37 | 10 | 0 | 7 | 91 | 10 |
| Hu et al28 | 2005 | China (Guangxi) | 16/96 | 1 | 3 | 1 | 10 | 1 | 0 | 6 | 24 | 2 | 0 | 4 | 1 | 79 | 7 | 5 | 5 | 169 | 18 |
| Xu et al29 | 2009 | China (Guangzhou) | 120/120 | 2 | 36 | 1 | 65 | 16 | 0 | 41 | 182 | 17 | 1 | 16 | 1 | 81 | 21 | 0 | 19 | 199 | 22 |
| Lv and Zhong30 | 2012 | China (Shanghai) | 84/106 | 3 | 20 | 2 | 52 | 6 | 1 | 28 | 130 | 10 | 0 | 15 | 1 | 76 | 12 | 2 | 16 | 179 | 15 |
| He et al31 | 2015 | China (Nanchang) | 120/120 | 4 | 23 | 1 | 79 | 12 | 1 | 32 | 193 | 15 | 0 | 17 | 1 | 83 | 17 | 2 | 18 | 200 | 22 |
| Ye and Zhang32 | 2008 | China (Wuhan) | 56/89 | 2 | 15 | 5 | 31 | 3 | 0 | 24 | 80 | 8 | 1 | 11 | 1 | 64 | 12 | 0 | 14 | 151 | 13 |
| He et al33 | 2015 | China (Shenyang) | 63/60 | 6 | 18 | 1 | 32 | 6 | 0 | 31 | 88 | 7 | 2 | 8 | 1 | 39 | 10 | 0 | 13 | 96 | 11 |
| Yue et al34 | 2013 | China (Nanjing) | 111/90 | 4 | 25 | 3 | 66 | 13 | 0 | 36 | 170 | 16 | 0 | 8 | 0 | 69 | 13 | 0 | 8 | 159 | 13 |
| Chen et al35 | 2011 | China (Guiyang) | 76/152 | 9 | 18 | 28 | 13 | 6 | 2 | 64 | 50 | 38 | 10 | 29 | 38 | 57 | 14 | 4 | 87 | 157 | 60 |
| Kerr et al36 | 2016 | Brazil | 43/144 | 1 | 14 | 1 | 25 | 1 | 1 | 17 | 65 | 4 | 1 | 31 | 2 | 91 | 18 | 1 | 35 | 231 | 22 |
| Lanni et al37 | 2012 | Italy | 70/248 | 2 | 18 | 2 | 41 | 7 | 0 | 24 | 107 | 9 | 0 | 27 | 0 | 201 | 20 | 0 | 27 | 449 | 20 |
| Jairani et al38 | 2016 | India | 87/152 | 4 | 40 | 2 | 35 | 6 | 0 | 50 | 116 | 8 | 20 | 34 | 3 | 82 | 8 | 5 | 81 | 206 | 21 |
Quantitative synthesis
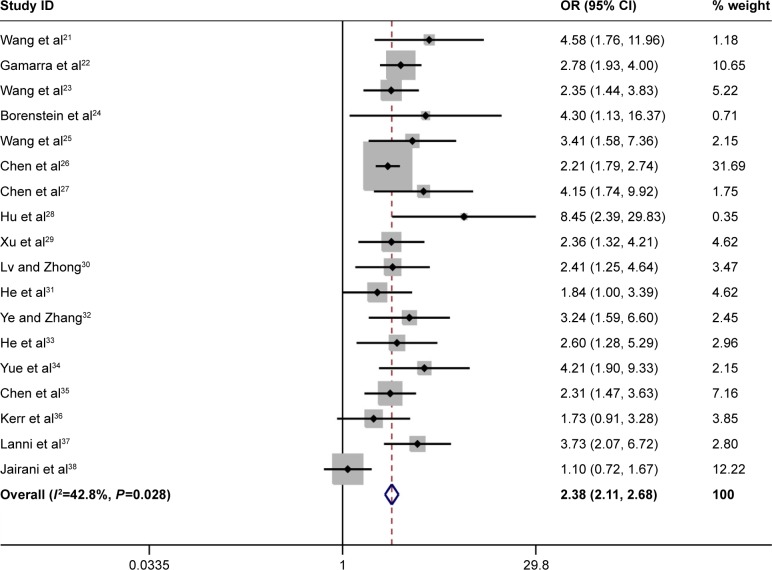
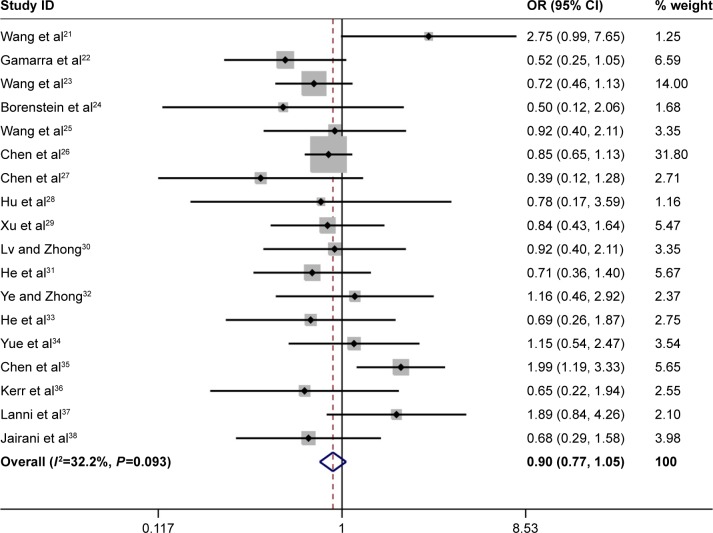
The overall results showed that ApoE variants were associated with an increased risk of MCI in the following genetic models: ε4 versus ε3: OR =2.38, 95% CI: 2.11–2.68; ε4/ε4 versus ε3/ε3: OR =4.45, 95% CI: 3.06–6.48; ε2/ε4 versus ε3/ε3: OR =2.57, 95% CI: 1.77–3.73; ε3/ε4 versus ε3/ε3: OR =2.31, 95% CI: 1.99–2.69 (Figure 2 and Table 2). The results also showed that a slight protection was observed in ε2/ε3 versus ε3/ε3 analysis (OR =0.80, 95% CI: 0.66–0.97, Table 2). However, no association was detected in ε2 versus ε3 (OR =0.90, 95% CI: 0.77–1.05, Figure 3 and Table 2) and ε2/ε2 versus ε3/ε3 models (OR =0.91, 95% CI: 0.50–1.65, Table 2). In the stratified analysis based on ethnicity, we only analyzed the Chinese population due to rare publications on other ethnicities. Stratified analysis indicated that ApoE variants contributed to increase the risk of MCI in Chinese population (ε4 versus ε3: OR =2.52, 95% CI: 2.19–2.90; ε4/ε4 versus ε3/ε3: OR =5.45, 95% CI: 3.41–8.70; ε2/ε4 versus ε3/ε3: OR =2.59, 95% CI: 1.74–3.86; ε3/ε4 versus ε3/ε3: OR =2.34, 95% CI: 1.97–2.79, Table 2). No significant association was observed in two genetic models in Chinese population (ε2 versus ε3: OR =0.92, 95% CI: 0.78–1.09 and ε2/ε2 versus ε3/ε3: OR =1.04, 95% CI: 0.55–1.99, Table 2). It is noted that only slight protection was found under the comparison of ε2/ε3 versus ε3/ε3 genotype (OR =0.79, 95% CI: 0.64–0.98, Table 2) in Chinese population. In addition, no significant heterogeneity was detected in all genetic models (Table 2).
Figure 2.
Forest plot for the association of ApoE polymorphism with MCI susceptibility in the overall populations (ε4 vs ε3).
Abbreviations: ApoE, apolipoprotein E; MCI, mild cognitive impairment; OR, odds ratio; CI, confidence interval.
Table 2.
Meta-analysis of apolipoprotein E gene polymorphism and MCI risk
| Genetic models | Variables | Number of studies | Test of association
|
Test of heterogeneity
|
|||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | I2 (%) | Model | |||
| ε2/ε2 vs ε3/ε3 | Overall | 18 | 0.91 | 0.50–1.65 | 0.758 | 0 | F |
| Chinese | 14 | 1.04 | 0.55–1.99 | 0.902 | 0 | F | |
| ε2/ε4 vs ε3/ε3 | Overall | 18 | 2.57 | 1.77–3.73 | <0.001 | 0 | F |
| Chinese | 14 | 2.59 | 1.74–3.86 | <0.001 | 0 | F | |
| ε2/ε3 vs ε3/ε3 | Overall | 18 | 0.8 | 0.66–0.97 | 0.026 | 0 | F |
| Chinese | 14 | 0.79 | 0.64–0.98 | 0.03 | 0 | F | |
| ε3/ε4 vs ε3/ε3 | Overall | 18 | 2.31 | 1.99–2.69 | <0.001 | 0 | F |
| Chinese | 14 | 2.34 | 1.97–2.79 | <0.001 | 5.8 | F | |
| ε4/ε4 vs ε3/ε3 | Overall | 18 | 4.45 | 3.06–6.48 | <0.001 | 39.6 | F |
| Chinese | 14 | 5.45 | 3.41–8.70 | <0.001 | 0 | F | |
| ε4 allele vs ε3 allele | Overall | 18 | 2.38 | 2.11–2.68 | <0.001 | 42.8 | F |
| Chinese | 14 | 2.52 | 2.19–2.90 | <0.001 | 0 | F | |
| ε2 allele vs ε3 allele | Overall | 18 | 0.9 | 0.77–1.05 | 0.179 | 32.2 | F |
| Chinese | 14 | 0.92 | 0.78–1.09 | 0.346 | 30.2 | F | |
Note: P-value corresponding to the Z-test for the summary effect estimate (P<0.05 considered statistically significant).
Abbreviations: F, fixed effects model; OR, odds ratio; CI, confidence interval; I2, heterogeneity index; MCI, mild cognitive impairment.
Figure 3.
Forest plot for the association of ApoE polymorphism with MCI susceptibility in the overall populations (ε2 vs ε3).
Abbreviations: ApoE, apolipoprotein E; MCI, mild cognitive impairment; OR, odds ratio; CI, confidence interval.
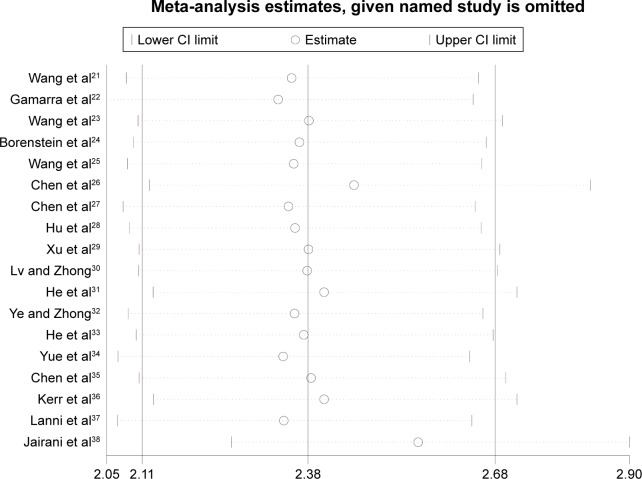
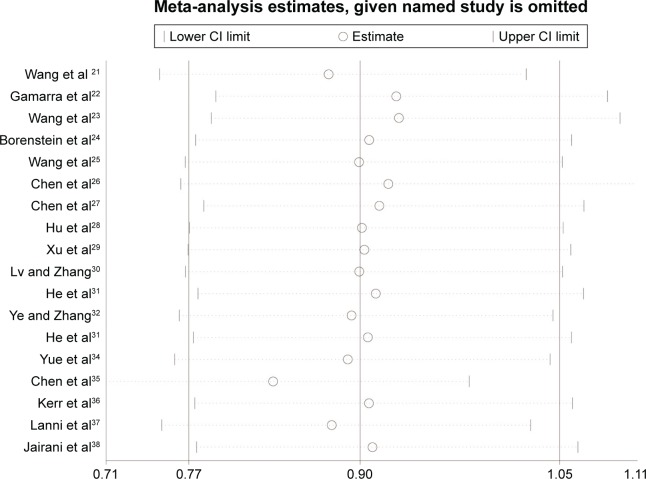
Sensitivity analysis and publication bias
The stability of results is assessed by sequential omission of one study in turn. The pooled ORs are not materially altered (Figures 4 and 5), indicating that no single study could influence the stability of the results of this meta-analysis.
Figure 4.
Sensitivity analysis of the summary of OR coefficients in the overall populations (ε4 vs ε3).
Abbreviations: OR, odds ratio; CI, confidence interval.
Figure 5.
Sensitivity analysis of the summary of OR coefficients in the overall populations (ε2 vs ε3).
Abbreviations: OR, odds ratio; CI, confidence interval.
To assess the potential publications bias of studies, Begg’s test was performed. For ε2 versus ε3, the funnel plot seemed nearly symmetry (Figure 6), and the P-value for Begg’s test (P=0.820) suggests no obvious publication bias. With regard to ε4 versus ε3 model, the funnel plot seemed asymmetry (Figure 7), and the P-value (P<0.05) revealed that a significant publication existed. By using the trim and fill method, six studies are filled for ε4 versus ε3 model, in order to balance the funnel plot. The adjusted risk estimate for ε4 versus ε3 was 2.255 (95% CI: 2.141–2.370, P<0.001), remaining statistically significant, suggesting that the results of our meta-analysis was stable.
Figure 6.
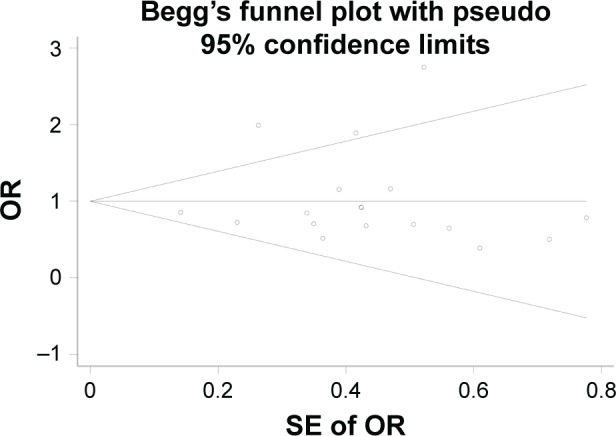
Begg’s funnel plot of ApoE polymorphism with MCI susceptibility in overall populations (ε2 vs ε3).
Abbreviations: ApoE, apolipoprotein E; MCI, mild cognitive impairment; OR, odds ratio; SE, standard error.
Figure 7.
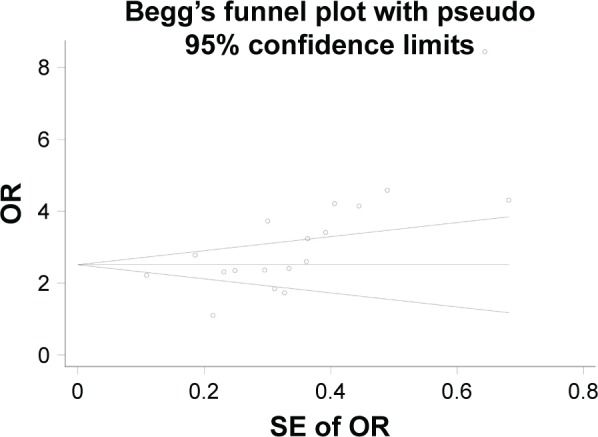
Begg’s funnel plot of ApoE polymorphism with MCI susceptibility in overall populations (ε4 vs ε3).
Abbreviations: ApoE, apolipoprotein E; MCI, mild cognitive impairment; OR, odds ratio; SE, standard error.
Discussion
The ApoE gene is one of the most studied genes for associations with MCI susceptibility. The ApoE polymorphism has been associated with an increased risk of several CNS disorders. Although the exact mechanisms by which ApoE variants lead to MCI are still unclear, ApoE may have many important functions for developing MCI. Studies showed that carrying ε4 allele could increase the aggregation and deposition of amyloid β-protein (Aβ) in brain compared to other polymorphisms.39,40 In addition, higher tau levels, lower CSF Aβ 42 levels, and greater brain atrophy were found in the ε4 allele carriers than noncarriers.41 ApoE gene polymorphisms also play an important role in the neuronal repair,42 cerebral glucose metabolism,43 lipid metabolism,44 maintaining synaptic plasticity,45,46 neuroinflammation,47–49 and neurogenesis.50–52 Those functions of ApoE may also be involved in the pathology of MCI.
In 1998, Smith et al53 first demonstrated that ApoE gene ε4 allele was highly associated with an increased risk of MCI. Subsequently, a number of studies were performed to estimate the association of ApoE gene polymorphism with MCI. However, the results were still controversial. To further explore and evaluate the association between ApoE gene polymorphism and MCI susceptibility, we performed a meta-analysis of 2,004 cases and 3,705 controls. Overall, we detected that ApoE polymorphism contributed to increase the risk of MCI under the ε4 versus ε3, ε4/ε4 versus ε3/ε3, ε2/ε4 versus ε3/ε3, and ε3/ε4 versus ε3/ε3 genetic models. However, no association was found under the ε2 versus ε3 and ε2/ε2 versus ε3/ε3 genetic models. Furthermore, a slight protection was discovered under the ε2/ε3 versus ε3/ε3 genetic model. In the stratified analysis, we analyzed only the Chinese population, and the results were similar to overall population. Interestingly, we found that ApoE ε4 allele increased MCI risk in a dose-dependent manner (ε4 versus ε3: OR =2.52, 95% CI: 2.19–2.90; ε4/ε4 versus ε3/ε3: OR =5.45, 95% CI: 3.41–8.70), which was in accordance with several previous studies.23,24,54,55 No significant heterogeneity was identified in any genetic models.
In this meta-analysis, we detected a potential publication bias in the ε4 versus ε3 genetic model, which may generate false-positive results. By using the trim and fill method, the results suggested that six studies were needed to balance the asymmetric funnel plot and the adjusted results for ε4 versus ε3 remained significant (OR =2.255, 95% CI: 2.141–2.370, P<0.001), indicating that the results were stable. It was emphasized that the potential publications may partly influence the results, but not deeply.
There are several limitations in the present meta-analysis. First, our meta-analysis was based predominantly on Chinese population. Only one study focused on the African, two studies on Caucasians, and one study on Indian, which might generate a partial result. Second, due to rare publications on other ethnicities, we analyzed only the Chinese population and other ethnicities were not evaluated in our meta-analysis. Finally, MCI is a complex disease. Gene–gene or gene–environment factors play an important role in MCI susceptibility. However, most selected studies did not analyze those interacted factors.
Conclusion
Our meta-analysis first showed that ApoE ε4 allele, ε4ε4, ε4ε3, and ε2ε4 genotypes were the risk factors of MCI, while ε2ε3 genotype was a protective factor, especially in Chinese population. We boldly supposed that ApoE polymorphism may be used as a useful potential therapeutic target to prevent, delay, or revert the healthy elderly to MCI conversion. Considering several limitations mentioned above, the results should be interpreted with caution. Further well-designed studies with larger sample size are required to validate the association between ApoE polymorphism and MCI risk.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No 81671305), key Development Projects of Shandong Province (Grant No 2015GSF118177), and the Major Science and Technology Project of Independent Innovation of Qingdao (Grant No 14-6-1-6-zdzx).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275(3):214–228. doi: 10.1111/joim.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 3.Su X, Shang L, Xu Q, et al. Prevalence and predictors of mild cognitive impairment in Xi’an: a community-based study among the elders. PLoS One. 2014;9(1):e83217. doi: 10.1371/journal.pone.0083217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67(12):2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 6.Kitagawa K, Matsumoto M, Kuwabara K, et al. Protective effect of apolipoprotein E against ischemic neuronal injury is mediated through antioxidant action. J Neurosci Res. 2002;68(2):226–232. doi: 10.1002/jnr.10209. [DOI] [PubMed] [Google Scholar]
- 7.Tangirala RK, Praticó D, FitzGerald GA, et al. Reduction of isoprostanes and regression of advanced atherosclerosis by apolipoprotein E. J Biol Chem. 2001;276(1):261–266. doi: 10.1074/jbc.M003324200. [DOI] [PubMed] [Google Scholar]
- 8.Mahley RW, Rall SC. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 9.Lahiri DK, Sambamurti K, Bennett DA. Apolipoprotein gene and its interaction with the environmentally driven risk factors: molecular, genetic and epidemiological studies of Alzheimer’s disease. Neurobiol Aging. 2004;25(5):651–660. doi: 10.1016/j.neurobiolaging.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 11.Brainerd CJ, Reyna VF, Petersen RC, et al. The apolipoprotein E genotype predicts longitudinal transitions to mild cognitive impairment but not to Alzheimer’s dementia: findings from a nationally representative study. Neuropsychology. 2013;27(1):86–94. doi: 10.1037/a0030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiung GY, Sadovnick AD, Feldman H. Apolipoprotein E epsilon4 genotype as a risk factor for cognitive decline and dementia: data from the Canadian Study of Health and Aging. CMAJ. 2004;171(8):863–867. doi: 10.1503/cmaj.1031789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghebremedhin E, Schultz C, Thal DR, et al. Gender and age modify the association between APOE and AD-related neuropathology. Neurology. 2001;56(12):1696–1701. doi: 10.1212/wnl.56.12.1696. [DOI] [PubMed] [Google Scholar]
- 14.Millar K, Nicoll JA, Thornhill S, Murray GD, Teasdale GM. Long term neuropsychological outcome after head injury: relation to APOE genotype. J Neurol Neurosurg Psychiatry. 2003;74(8):1047–1052. doi: 10.1136/jnnp.74.8.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Bian C, Zhang J, Wen F. Apolipoprotein E gene polymorphism and Alzheimer’s disease in Chinese population: a meta-analysis. Sci Rep. 2014;4:4383. doi: 10.1038/srep04383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–137. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 18.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 19.Martin WJ. Statistical aspects of poliomyelitis in England and Wales in recent years. Mon Bull Minist Health Public Health Lab Serv. 1959;18:54–64. [PubMed] [Google Scholar]
- 20.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang QS, Tian L, Huang YL, Qin S, He LQ, Zhou JN. Olfactory identification and apolipoprotein E epsilon 4 allele in mild cognitive impairment. Brain Res. 2002;951(1):77–81. doi: 10.1016/s0006-8993(02)03137-2. [DOI] [PubMed] [Google Scholar]
- 22.Gamarra D, Elcoroaristizabal X, Fernández-Martínez M, de Pancorbo MM. Association of the C47T polymorphism in SOD2 with amnestic mild cognitive impairment and Alzheimer’s disease in carriers of the APOEε4 allele. Dis Markers. 2015;2015:746329. doi: 10.1155/2015/746329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Ma W, Rong Y, Liu L. The association between apolipoprotein E gene polymorphism and mild cognitive impairment among different ethnic minority groups in China. Int J Alzheimers Dis. 2014;2014:150628. doi: 10.1155/2014/150628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borenstein AR, Mortimer JA, Ding D, et al. Effects of apolipoprotein E-epsilon4 and -epsilon2 in amnestic mild cognitive impairment and dementia in Shanghai: SCOBHI-P. Am J Alzheimers Dis Other Demen. 2010;25(3):233–238. doi: 10.1177/1533317509357736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Wang H, Li H, Li T, Yu X. Frequency of the apolipoprotein E ε4 allele in a memory clinic cohort in Beijing: a naturalistic descriptive study. PLoS One. 2014;9(6):e99130. doi: 10.1371/journal.pone.0099130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen KL, Sun YM, Zhou Y, Zhao QH, Ding D, Guo QH. Associations between APOE polymorphisms and seven diseases with cognitive impairment including Alzheimer’s disease, frontotemporal dementia, and dementia with Lewy bodies in southeast China. Psychiatr Genet. 2016;26(3):124–131. doi: 10.1097/YPG.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Yao Q, Zhuo RJ, Wang YQ, Pang YY, Yu JB. The relationship of plasma homocysteine level and apolipoprotein E gene polymorphism with Alzheimer’s disease. Chinese Journal of Geriatrics. 2016;35(5):467–470. [Google Scholar]
- 28.Hu CY, Yang Z, Zheng CG, et al. Cognitive ability and apolipoprotein E genotypes in long lived elderly in Bama area of Guangxi. Chinese Mental Health Journal. 2005;19(6):383–386. [Google Scholar]
- 29.Xu MM, Yi YH, Zhang M. Role of apolipoprotein E gene polymorphism and plasma lipids in mild cognitive impairment. J Shandong Univ. 2009;47(11):103–107. [Google Scholar]
- 30.Lv XR, Zhong Y. Association between Alzheimer’s disease and mild cognitive impairment and the apolipoprotein E gene. Chinese J Gerontol. 2012;32(5):917–919. [Google Scholar]
- 31.He L, Yuan YF, Ren CF, et al. The study on the relation between the distribution of apolipoprotein E (ApoE) genotypes and mild cognitive impairment. Acta Universitatis Medicinalis Anhui. 2015;50(10):1468–1470. [Google Scholar]
- 32.Ye N, Zhang LH. The study on the association between apolipoprotein E polymorphism and mild cognitive impairment. Chin J Neuroimmunol Neurol. 2008;15(1):74–75. [Google Scholar]
- 33.He ZY, Liu BW, Bai Q, Tao L, Xian JF, Zheng DM. Association between genetic polymorphisms of apolipoprotein E and amnestic mild cognitive impairment. Pract Geriatr. 2015;29(2):109–111. [Google Scholar]
- 34.Yue CX, Zhang ZJ, Yu H, et al. Association of polymorphisms of apolipoprotein E and genes associated with beta-amyloid with amnestic mild cognitive impairment. Chinese J Psychiatry. 2013;46(5):295–300. [Google Scholar]
- 35.Chen J, Huang WY, Yang JY, et al. Relationship between Apo E gene polymorphism and risk of different subtypes of mild cognitive impairment. China Public Health. 2011;27(7):836–838. [Google Scholar]
- 36.Kerr DS, Stella F, Radanovic M, Aprahamian I, Bertollucci PH, Forlenza OV. Apolipoprotein E genotype is not associated with cognitive impairment in older adults with bipolar disorder. Bipolar Disord. 2016;18(1):71–77. doi: 10.1111/bdi.12367. [DOI] [PubMed] [Google Scholar]
- 37.Lanni C, Garbin G, Lisa A, et al. Influence of COMT Val158Met polymorphism on Alzheimer’s disease and mild cognitive impairment in Italian patients. J Alzheimers Dis. 2012;32(4):919–926. doi: 10.3233/JAD-2012-120358. [DOI] [PubMed] [Google Scholar]
- 38.Jairani PS, Aswathy PM, Gopala S, Verghese J, Mathuranath PS. Interaction with the MAPT H1H1 genotype increases dementia risk in APOE ε4 carriers in a population of Southern India. Dement Geriatr Cogn Disord. 2016;42(5–6):255–264. doi: 10.1159/000447446. [DOI] [PubMed] [Google Scholar]
- 39.Kaushal R, Woo D, Pal P, et al. Subarachnoid hemorrhage: tests of association with apolipoprotein E and elastin genes. BMC Med Genet. 2007;8:49. doi: 10.1186/1471-2350-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu WQ, Zhu H, Dean M, et al. Amyloid-associated depression and ApoE4 allele: longitudinal follow-up for the development of Alzheimer’s disease. Int J Geriatr Psychiatry. 2016;31(3):316–322. doi: 10.1002/gps.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vemuri P, Wiste HJ, Weigand SD, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67(3):308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buttini M, Orth M, Bellosta S, et al. Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: isoform-specific effects on neurodegeneration. J Neurosci. 1999;19(12):4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buckner RL. Human functional connectivity: new tools, unresolved questions. Proc Natl Acad Sci U S A. 2010;107(24):10769–10770. doi: 10.1073/pnas.1005987107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buttini M, Yu GQ, Shockley K, et al. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J Neurosci. 2002;22(24):10539–10548. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaDu MJ, Shah JA, Reardon CA, et al. Apolipoprotein E and apolipoprotein E receptors modulate A beta-induced glial neuroinflammatory responses. Neurochem Int. 2001;39(5–6):427–434. doi: 10.1016/s0197-0186(01)00050-x. [DOI] [PubMed] [Google Scholar]
- 48.Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114(1–2):107–113. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- 49.Keene CD, Cudaback E, Li X, Montine KS, Montine TJ. Apolipoprotein E isoforms and regulation of the innate immune response in brain of patients with Alzheimer’s disease. Curr Opin Neurobiol. 2011;21(6):920–928. doi: 10.1016/j.conb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang CP, Gilley JA, Zhang G, Kernie SG. ApoE is required for maintenance of the dentate gyrus neural progenitor pool. Development. 2011;138(20):4351–4362. doi: 10.1242/dev.065540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrews-Zwilling Y, Bien-Ly N, Xu Q, et al. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30(41):13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li G, Bien-Ly N, Andrews-Zwilling Y, et al. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5(6):634–645. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith GE, Bohac DL, Waring SC, et al. Apolipoprotein E genotype influences cognitive ‘phenotype’ in patients with Alzheimer’s disease but not in healthy control subjects. Neurology. 1998;50(2):355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- 54.Boyle PA, Buchman AS, Wilson RS, Kelly JF, Bennett DA. The APOE epsilon4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology. 2010;34(1):43–49. doi: 10.1159/000256662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albert M, Soldan A, Gottesman R, et al. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Curr Alzheimer Res. 2014;11(8):773–784. doi: 10.2174/156720501108140910121920. [DOI] [PMC free article] [PubMed] [Google Scholar]