Abstract
Background
Acute postoperative pain is induced by most incisional surgeries and usually resolves with wound repair. However, many patients experience moderate to severe pain despite receiving currently available postoperative pain relief. Accumulating evidence suggests that inflammatory cells, neutrophils, and macrophages infiltrating the wound site contribute to the acute inflammation, pain, and subsequent wound repair. Colchicine is commonly used to relieve pain in gout by inhibiting the infiltration of granulocytes and other motile cells. In this study, we examined the effects of colchicine on acute postoperative pain and wound repair by correlating the infiltration of neutrophils and macrophages in a mouse model of postoperative pain induced by plantar incision. Furthermore, these effects of colchicine were compared with clodronate liposomes, which selectively deplete circulating macrophages.
Results
Plantar incision induced mechanical hypersensitivity in the ipsilateral hind paw that peaked one day and lasted for three days after the surgery. Treatment with colchicine significantly attenuated the early infiltration of Gr1-positive cells (neutrophils) around the incision site and mechanical hypersensitivity, which was accompanied with inhibition of the subsequent infiltration of Iba1-positive cells (macrophages) and macrophage polarization toward the proinflammatory M1 phenotype. By contrast, an intravenous injection of clodronate liposomes significantly inhibited the infiltration of macrophages around the incision site but had little effect on the infiltration of neutrophils or mechanical hypersensitivity. Importantly, colchicine treatment significantly delayed wound closure after the incisional surgery, whereas clodronate liposome administration had no effect on wound closure.
Conclusion
These results suggest that colchicine can alleviate acute postoperative pain and also enhance the risk of delayed wound repair, which are associated with the suppression of neutrophil and subsequent proinflammatory M1 macrophage infiltration around the incision site, while the involvement of macrophages may be limited.
Keywords: Colchicine, mechanical hypersensitivity, inflammatory cells, clodronate liposomes, macrophage phenotype
Introduction
Acute postoperative pain (POP) is a common feature in patients after the surgery, and patients generally recover from acute POP with wound repair. Effective treatments for POP can improve the quality of life and clinical outcome as well as avoid complications and decrease the risk of developing chronic pain.1,2 However, despite the utilization of preventive strategies and analgesic agents, such as nonsteroidal anti-inflammatory drugs, opioids, gabapentin, and pregabalin, 50%–70% of surgical patients still experience moderate to severe POP.3
Colchicine, an alkaloid derived from colchicum autumnale, is commonly used to treat gout and familial Mediterranean fever. It binds to tubulins and interferes with microtubule polymerization, thereby impairing the function and infiltration of granulocytes and other motile cells and exerting anti-inflammatory and antifibrotic effects.4–6 Colchicine can suppress mechanical and thermal hypersensitivity in a variety of animal pain models.7–9 However, the effect of colchicine on POP and wound repair has not been clarified.
Several lines of evidence for human and animal models of POP suggest that inflammatory cells, such as neutrophils and macrophages, contribute to POP. Acute inflammatory responses are initiated by an early infiltration of neutrophils at the incision site, which peaks within 24 h after the surgery. Infiltrated neutrophils secrete antimicrobial and inflammatory substances, including proteases, reactive oxygen species, cytokines, and chemokines, and phagocytose pathogens to fight against infections, but trigger intense pain around the injury site.10 The depletion of neutrophils reduces mechanical hypersensitivity in a plantar incision-induced mouse model of POP.11 Depleting circulating neutrophils or blocking neutrophil infiltration in inflammatory, allergen-evoked, or neuropathic pain models also attenuates the early responses of hypersensitivity.12–14 Furthermore, early infiltrated neutrophils induce subsequent recruitment of monocytes that differentiate into macrophages at the incision site. Inflammatory mediators produced by activated macrophages sensitize nociceptors, resulting in the generation and prolongation of inflammatory pain.15 In a mouse model of POP, a population of proliferating CD11b+Ly6G− myeloid cells (primarily monocytes and skin-resident macrophages) contributes to mechanical hypersensitivity.16
However, neutrophils and macrophages also play essential roles in the wound repair processes.17–19 Neutrophils infiltrating the injured site early phagocytose microbes, dead cells, and wound debris to clean the injured site,4 whereas depleting neutrophils delays the wound repair20 and peripheral axon regeneration after peripheral nerve injury.21 Subsequent to neutrophil infiltration, infiltrating macrophages voraciously phagocytose the wound matrix and cell debris, including fibrin and apoptotic neutrophils, and produce a variety of cytokines and growth and angiogenic factors for fibrogenesis and angiogenesis.17–19 For wound repair, the phenotypes of macrophages, “classically activated” proinflammatory (M1) and “alternatively activated” anti-inflammatory (M2) cells, are focused.22 M1 macrophages are characterized by their expression of high levels of proinflammatory cytokines, which exacerbate tissue injury,23 whereas M2 macrophages are responsible for inflammation resolution and wound repair.24 That macrophage polarization can be affected by the depletion of neutrophils25 suggests an interaction between the early infiltrating neutrophils and the subsequently infiltrating macrophages for the wound repair.
In the present study, we therefore aimed to investigate the effects of colchicine on acute POP, inflammatory responses, and wound repair in a mouse model of POP induced by plantar incision, by comparing with clodronate liposomes, which selectively deplete circulating macrophages.
Methods
Animals
This article adheres to the applicable EQUATOR guidelines. This study was carried out in strict accordance with the ethical guidelines recommended by the Kyoto University Animal Research Committee. The protocol was approved by the Kyoto University Animal Research Committee (Permit Number: 13–38). All efforts were made to minimize the number of animals used and to limit experimentation to that necessary to produce reliable scientific information. Male C57BL/6 J mice aged six weeks (18–22 g) were purchased from Japan SLC (Shizuoka, Japan). All mice were housed four or five per plastic cage with woodchip bedding under constant ambient temperature (24℃ ± 1℃) and humidity (55% ± 10%) with free access to food and water and maintained on a 12 h light/dark cycle (light from 8:00 a.m. to 8:00 p.m.) for at least three days before the studies.
Drug administration
Mice were randomly assigned to the colchicine or clodronate group and then were further divided into control and treatment groups. Colchicine (Sigma-Aldrich, C9754) was dissolved in sterile saline. Colchicine (0.75 mg/kg) or saline was intraperitoneally injected once daily beginning two days before the plantar incision surgery. Clophosome-N (FormuMax Scientific, Inc., F70101C-N), a commercially available liposome-clodronate reagent, was intravenously administered to mice without dilution 1 h before the surgery (100 µL/mouse). The control group for the clodronate treatment comprised mice intravenously administered control liposomes (FormuMax Scientific, Inc., F70101-N).
POP model
For the mouse model of POP, the surgery was performed as previously described with some modifications.26 To minimize the number of animals used, all mice were used as POP model and assessed by comparing the incised right hind paw (ipsilateral) with the nonoperated left hind paw (cotralateral). Briefly, mice were anesthetized with isoflurane. After sterile preparation of the right hind paw, a 5-mm longitudinal incision was made through the skin and fascia of the plantar surface using a No. 11 scalpel blade. The incision started 2 mm from the proximal edge of the heel and extended toward the toes. The plantaris muscle was elevated with forceps, leaving the muscle origin and insertion intact. Although the skin was apposed with a single mattress suture in the previous study, the incision was not sutured in this study to enable the evaluation of wound repair.
von Frey filament test
The same trained experimenter handled and tested animals in the experiment and was blinded to the treatment of each animal. Mice were habituated to the testing environment of small boxes composed of metal mesh floor for at least 30 min. For the duration of the experiments, the experimenter was blind to the drug treatment. Mechanical sensitivity was assessed by measuring the frequency of paw withdrawal responses to a calibrated von Frey filament (0.04 g). The von Frey filament was applied to the plantar surface of the hind paw until the filament bent slightly for a few seconds. The filament was applied 10 times to the hind paw, and the frequency of paw withdrawal was expressed as a percentage of the total number of trials calculated as follows: (the number of trials accompanied by licking and lifting/the total number of trials) × 100. Mechanical sensitivity was assessed before the surgery as well as one, three, and seven days after the surgery.
Immunohistochemistry
For assessing neutrophil and macrophage infiltration, immunohistochemical analyses were performed one, three, and seven days after the surgery. Mice were deeply anesthetized with sodium pentobarbital (64.8 mg/kg, i.p.) and perfused through the ascending aorta with phosphate-buffered saline (PBS) followed by 4% (W/V) paraformaldehyde in phosphate buffer. The contralateral and ipsilateral hind paws including skin and underlying muscle were removed from the mice. These samples were postfixed with 4% paraformaldehyde for 4 h and cryoprotected overnight at 4℃ in 30% sucrose. The tissues were frozen and sectioned with a cryostat (Leica, Nussloch, Germany). The sections (14 µm thick) were treated with 4% normal goat serum for 1 h at room temperature. After being washed with PBS, the sections were incubated with a primary antibody directed against granulocyte receptor-1 (Gr1; rat anti-Gr1 antibody, 1:200; R&D Systems, MAB1037, #FVF0313121) and ionized calcium-binding adapter molecule-1 (Iba1; rabbit anti-Iba-1 antibody, 1:500; Wako Pure Chemical Industries, 019-19741, #SAJ2266) at 4℃ overnight. The sections were washed three times in PBS and labeled with Alexa Fluor 594-labeled secondary antibody (Alexa Fluor 594-labeled donkey anti-rat IgG, 1:200; Jackson ImmunoResearch Laboratories, Inc., 712-585-150, #116700) and Alexa Fluor 488-labeled secondary antibody (Alexa Fluor 488-labeled goat anti-rabbit IgG, 1:500; Molecular Probes, Invitrogen, Life Technologies, A11008, #1678787) as appropriate at room temperature for 1 h in the dark. After being washed three times in PBS, the sections were mounted in the anti-fading medium Vectashield (Vector Laboratories, H-1400). Confocal fluorescence images were observed using a confocal laser scanning microscope (Fluoview FV10i system, Olympus, Tokyo, Japan). Fluorescence images were captured around the incision site for each hind paw section. The number of Gr1-positive (Gr1+) cells and Iba1 immunoreactivity in three slices per each animal were measured in an area 100 × 180 µm with Image J software (National Institute of Mental Health, Bethesda, MD).
Real-time polymerase chain reaction
For assessing the messenger RNA (mRNA) expression levels, real-time polymerase chain reaction (PCR) analyses were performed one day after the surgery. Total RNAs were extracted from the contralateral and ipsilateral hind paws including skin and underlying muscle using ISOGEN Reagent (Nippon Gene, Tokyo, Japan) or NucleoSpin RNA (TaKaRa Bio Inc., Shiga, Japan), and the extracted RNAs were reverse transcribed (RT) using the ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan). Real-time quantitative RT-PCR was performed using the StepOne Real-Time PCR System (Life Technologies, Carlsbad, CA) and the Thunderbird SYBR qPCR Mix (Toyobo). Each PCR amplification consisted of heat activation for 10 min at 95℃, followed by 40 cycles at 95℃ for 15 s and 60℃ for 1 min. The oligonucleotide primers used for RT-PCR were as follows: 5′-GCA ATT ATT CCC CAT GAA CG-3′ and 5′-GGC CTC ACT AAA CCA TCC AA-3′ for the 18S ribosomal RNA gene (18S rRNA); 5′-TGT AAT GAA AGA CGG CAC ACC-3′ and 5′-TCT TCT TTG GGT ATT GCT TGG-3′ for IL1β; 5′-AAC TCT CAC TGA AGC CAG CTC T-3′ and 5′-GTG GGG CGT TAA CTG CAT-3′ for CCL2; 5′-AGA GGT CTC GGT TGG GTT GT-3′ and 5′-CAC TGT CTT TGA GGC TTG TTG C-3′ for CCR2; 5′-TTG TGT GTG TTC TGG AAA CGG AG-3′ and 5′-AAC TTA GAG GCT GTG TTG CTG GG-3′ for CD86; 5′-ACA TTG GCT TGC GAG ACG TA-3′ and 5′-ATC ACC TTG CCA ATC CCC AG-3′ for Arg1; 5′-TGA CGT CAC TGG AGT TGT ACG G-3′ and 5′-GGT TCA TGT CAT GGA TGG TGC-3′ for TGFβ; 5′-GTC ACG GAA ATA CTC CAG TTG GT-3′ and 5′-CCC GTT TTG GAT CCG AGT TT-3′ for FGF2. The mRNA expression levels of each gene were normalized to that of 18S rRNA, which was measured in parallel in each sample. Except for the mRNA expression level of IL1β, the mRNA expression level of each gene was expressed relative to each contralateral group. The mRNA expression level of IL1β was expressed relative to the saline-treated ipsilateral group because of the undetectable IL1β mRNA expression in the contralateral hind paw.
Macroscopic examination of wound closure
For assessing wound closure following a plantar incision, each incision site was digitally photographed one, two, and three days after the surgery. Changes in the wound areas were expressed as the width of the incised site.
Statistical analysis
Data are presented as means ± S.E.M. and were analyzed using GraphPad Prism version 6.0 software. Mechanical hypersensitivity data were analyzed by two-way analysis of variance for repeated measures, followed by the Bonferroni post hoc test. Statistical analyses for immunofluorescence, real-time PCR, and wound repair were performed using two-way analysis of variance, followed by the Bonferroni post hoc test, although the expression level of IL1β mRNA was analyzed with an unpaired t test. In all cases, differences of P < 0.05 were considered statistically significant.
Results
Plantar incision induces mechanical hypersensitivity
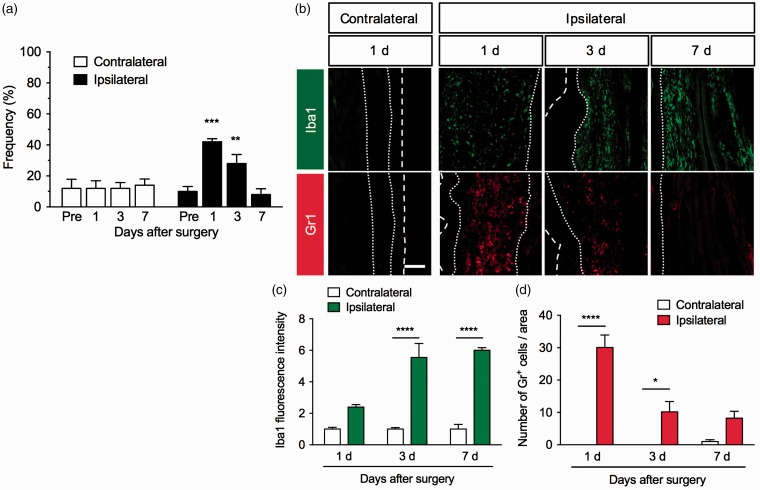
Acute POP was induced by an incision in the plantar surface of the mouse hind paw. The withdrawal response frequency of the ipsilateral hind paw to mechanical stimulation by a 0.04 g von Frey filament was significantly increased one and three days after the plantar incision (F3,24 = 16.21, P < 0.0001), while there was no change in that of the contralateral hind paw. The mechanical hypersensitivity was severest at one day and lasted for three days, before returning to the baseline seven days after the surgery (Figure 1(a)). Iba1 immunoreactivity (a marker for macrophages) and Gr1+ cells (a marker for neutrophils) were rarely observed in the contralateral hind paw but were markedly increased around the incision in the ipsilateral hind paw after the surgery. Iba1 immunofluorescence around the incision was significantly increased (F2,12 = 12.24, P < 0.01), and it was gradually increased and remained increased even seven days after the surgery. The number of Gr1+ cells around the incision site was also significantly increased (F2,12 = 12.55, P < 0.01), but it peaked one day after the surgery and then declined (Figure 1(b) to (d)).
Figure 1.
Mechanical hypersensitivity and infiltration of macrophages and neutrophils around the incision site in a mouse model of postoperative pain. Plantar incision surgery was performed on the mouse right hind paw. (a) To assess mechanical sensitivities in the contralateral and ipsilateral hind paws, the frequency of paw withdrawal responses to von Frey filament stimulation was measured before the surgery (pre) as well as one, three, and seven days after the surgery. Data are expressed as means of the percentage ± S.E.M. n = 5. **P < 0.01, ***P < 0.001, compared with pre. (b) Representative photographs for immunofluorescence staining of Iba1 (green) and Gr1 (red) in the contralateral (one day) and ipsilateral hind paws one, three, and seven days after the surgery. Scale bar = 100 µm. White dashed lines mark the outer of epidermis. White dotted lines separate the dermis from the epidermis and fascia. Graphs show Iba1 fluorescence intensity (c) and the number of Gr1+ cells (d) in the contralateral and ipsilateral hind paws, n = 3. Data are expressed as means ± S.E.M. ****P < 0.0001, *P < 0.05.
Colchicine attenuates plantar incision-induced mechanical hypersensitivity and neutrophil infiltration
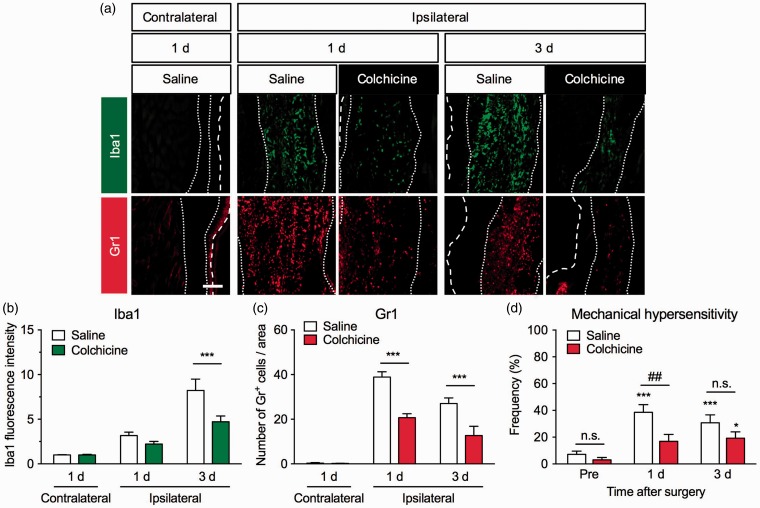
To examine the effect of colchicine on the infiltration of inflammatory cells around the incision site and acute POP, it was administered intraperitoneally once daily beginning two days before the surgery. The number of Gr1+ cells around the incision site was significantly decreased by colchicine treatment (F2,56 = 21.15, P < 0.0001). Significant decreases were observed one and three days after the surgery. Furthermore, Iba1 immunoreactivity around the incision site was also significantly attenuated in the colchicine-treated group (F2,54 = 7.718, P < 0.01), with a significant difference observed three days after the surgery (Figure 2(a) to (c)). The colchicine treatment also significantly attenuated plantar incision-induced mechanical hypersensitivity (F2,87 = 1.888, P = 0.1575). A significant difference was observed on day 1, and mechanical hypersensitivity tended to be attenuated three days after the surgery (Figure 2(d)).
Figure 2.
Effects of colchicine treatment on the infiltration of macrophages and neutrophils and mechanical hypersensitivity in a mouse model of postoperative pain. Colchicine (0.75 mg/kg) or saline was intraperitoneally injected once daily beginning two days before the plantar incision surgery. (a) Representative photographs of immunofluorescence staining for Iba1 (green) and Gr1 (red) in the contralateral and ipsilateral hind paws one and three days after the surgery. Scale bar = 100 µm. White dashed line marks the outer of epidermis. White dotted lines separate the dermis from the epidermis and deeper layer. Graphs show Iba1 fluorescence intensity (b) and the number of Gr1+ cells (c) in vehicle- and colchicine-treated groups, n = 6–16. Data are expressed as means ± S.E.M. ***P < 0.001. (d) To assess mechanical sensitivities in the ipsilateral hind paws of vehicle- (n = 14) or colchicine-treated groups (n = 13), the frequency of paw withdrawal responses to von Frey filament stimulation was measured before the surgery (pre) and one and three days after the surgery. Data are expressed as means of the percentage ± S.E.M. *P < 0.05, ***P < 0.001, compared with pre in each group. ##P < 0.01. n.s.: not significant.
Effects of colchicine on plantar incision-induced inflammatory responses and macrophage phenotype
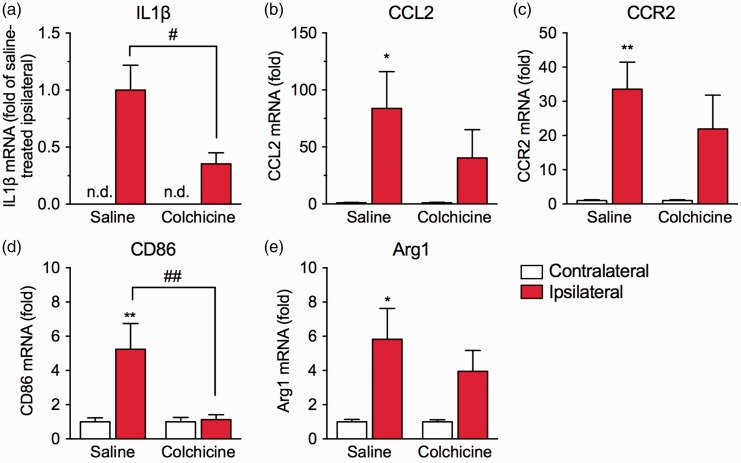
We next investigated the effects of colchicine treatment on plantar incision-induced inflammatory responses. Quantitative real-time PCR analysis revealed that the mRNA expression level of the proinflammatory cytokine interleukin-1β (IL1β) in the ipsilateral hind paw was markedly increased one day after the surgery, although the expression in the contralateral hind paw was not detected. Colchicine treatment significantly inhibited the upregulation of IL1β mRNA (Figure 3(a)). The chemokine C-C motif chemokine 2 (CCL2) is mainly produced by neutrophils and macrophages and participates in the migration of macrophages through the C-C chemokine receptor type 2 (CCR2).27 In the saline-treated group, the mRNA expression levels of CCL2 (Figure 3(b)) and CCR2 (Figure 3(c)) in the ipsilateral hind paw were significantly upregulated, compared with those in the contralateral hind paw, whereas significant upregulation was not observed in the colchicine-treated group. Colchicine treatment tended to suppress the upregulation of CCL2 and CCR2 mRNA levels, although the inhibitory effects were not significant (CCL2, F1,22 = 1.176, P = 0.2898; CCR2, F1,22 = 0.8611, P = 0.3635).
Figure 3.
Effects of colchicine on the inflammatory responses and macrophage phenotype around the incision site. Colchicine (0.75 mg/kg) or saline was intraperitoneally injected once daily beginning two days before the plantar incision surgery. Samples of contralateral and ipsilateral hind paws derived from mice in saline- or colchicine-treated groups were collected one day after the surgery. The mRNA expression levels of IL1β (a; n = 13), CCL2 (b; n = 6–7), CCR2 (c; n = 6–7), CD86 (d; n = 7–8), and Arg1 (e; n = 8) were measured by real-time quantitative PCR. Each expression level was normalized to that of 18S rRNA and presented relative to each contralateral group. The mRNA expression level of IL1β was presented relative to the saline-treated ipsilateral group, because IL1β mRNA expression was undetectable in the contralateral hind paw. Data are expressed as means ± S.E.M. *P < 0.05, **P < 0.01, compared with the contralateral hind paw; #P < 0.05; ##P < 0.01. n.d.: not detected.
Macrophage polarization can be affected by early infiltration and apoptosis of neutrophils.25 To determine whether colchicine treatment affects the phenotype of infiltrating macrophages in this mouse model of POP, we investigated the mRNA expression levels of CD86 (a marker for proinflammatory M1 macrophages) and arginase 1 (Arg1; a marker for anti-inflammatory M2 macrophages) one day after the surgery. The expression of CD86 mRNA in the ipsilateral hind paw was significantly upregulated in the saline-treated group. Colchicine treatment significantly inhibited the upregulation of CD86 mRNA (F1,26 = 7.767, P = 0.0098; Figure 3(d)). Similarly, the expression of Arg1 mRNA in the ipsilateral hind paw was significantly upregulated in the saline-treated group, whereas significant upregulation was not observed in the colchicine-treated group. Colchicine treatment tended to suppress the upregulation, although the effect was not significant (F1,28 = 0.7424, P = 0.3962; Figure 3(e)).
Macrophage depletion by clodronate liposomes has little effect on plantar incision-induced mechanical hypersensitivity
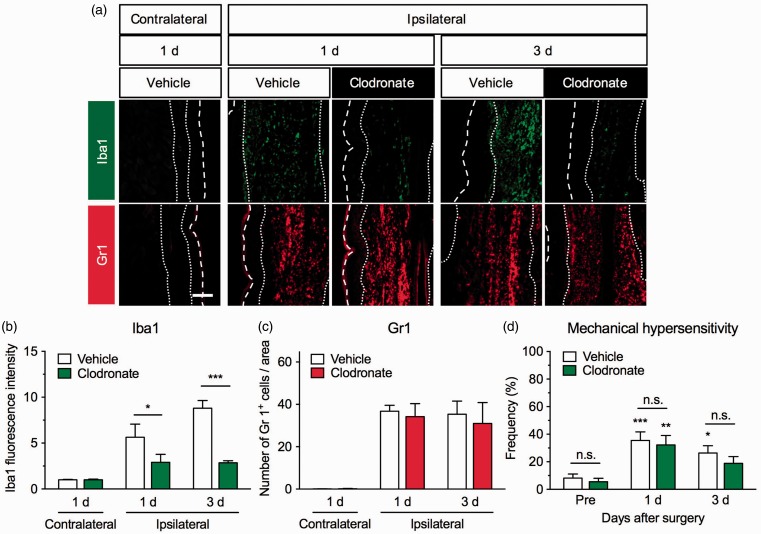
We depleted circulating macrophages by an intravenous administration of clodronate liposomes 1 h before the surgery. Intravenous liposome-encapsulated clodronate administration results in selective and temporary depletion of macrophages and circulating monocytes by phagocytosis of the mononuclear phagocyte system.28 We found that treatment with clodronate liposomes significantly inhibited the increase in Iba1 immunofluorescence around the incision site (F2,36 = 19.62, P < 0.0001). Significant differences were observed one and three days after the surgery when compared with the vehicle-treated group. By contrast, the number of Gr1+ cells around the incision site was not affected by the clodronate liposome treatment (F2,24 = 0.1894, P = 0.8287; Figure 4(a) to (c)). The clodronate liposome treatment also had little effect on plantar incision-induced mechanical hypersensitivity; there was no significant difference between the vehicle- and clodronate liposome-treated groups (F2,36 = 0.142, P = 0.8681; Figure 4(d)).
Figure 4.
Effects of clodronate liposomes on the infiltration of macrophages and neutrophils and mechanical hypersensitivity in a mouse model of postoperative pain. Clodronate (100 µL/mouse) or control liposomes were intravenously administered to mice 1 h before the plantar incision surgery. (a) Representative photographs for immunofluorescence staining of Iba1 (green) and Gr1 (red) in the contralateral and ipsilateral hind paws one and three days after the surgery. Scale bar = 100 µm. White dashed line marks the outer of epidermis. White dotted lines separate the dermis from the epidermis and deeper layer. Graphs show Iba1 fluorescence intensity (b, n = 4–11) and the number of Gr1+ cells (c, n = 3–8) in vehicle- and clodronate-treated groups. Data are expressed as means ± S.E.M. *P < 0.05, ***P < 0.001. (d) To assess mechanical sensitivities in the ipsilateral hind paws of the vehicle- (n = 9) or clodronate-treated group (n = 11), the frequency of paw withdrawal responses to von Frey filament stimulation was measured before the surgery (pre) and one and three days after the surgery. Data are expressed as means of the percentage ± S.E.M. *P < 0.05, **P < 0.01, *** P < 0.001, compared with pre in each group. n.s.: not significant.
Effect of colchicine and clodronate liposomes on wound closure
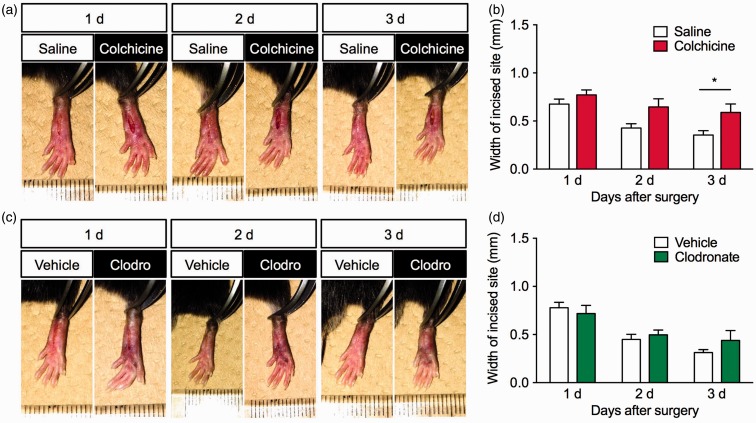
Finally, we examined the effects of colchicine or clodronate liposomes on wound closure in this mouse model of POP. The incision site gradually closed in both vehicle-treated groups. Colchicine treatment significantly delayed wound closure after the surgery (F2,28 = 1.709, P = 0.1995); a significant difference between the vehicle- and colchicine-treated groups was observed three days after the surgery (Figure 5(a) and (b)). By contrast, there was no significant difference in wound closure between the vehicle- and clodronate liposome-treated groups (F2,16 = 0.8921, P = 0.4292; Figure 5(c) and (d)).
Figure 5.
Effects of colchicine or clodronate treatment on wound closure after the surgery. (a and b) Colchicine (0.75 mg/kg) or saline was intraperitoneally injected once daily beginning two days before the plantar incision surgery, n = 8. (c and d) Clodronate (100 µL/mouse) or control liposomes were intravenously administered to mice 1 h before the plantar incision surgery, n = 3–5. (a and c) Representative photographs of the incision site on the ipsilateral hind paw one, two, and three days after the plantar incision surgery. (b and d) Graphs show the width (mm) of the incision in (b) colchicine- or (d) clodronate-treated groups. Data are expressed as means ± S.E.M. *P < 0.05.
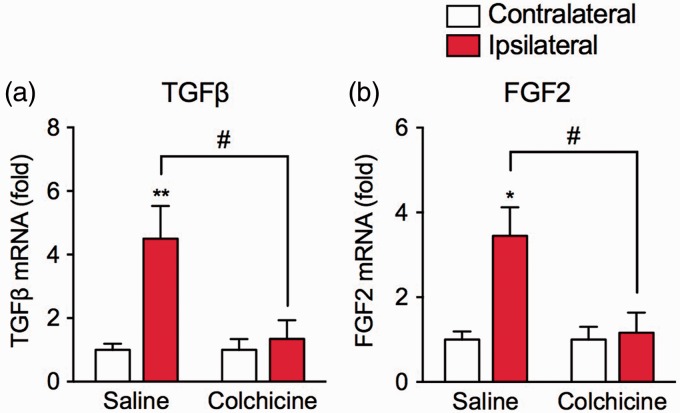
We also examined the effect of colchicine treatment on the mRNA expression levels of fibrogenesis-promoting factors, transforming growth factor-β (TGFβ), and fibroblast growth factor-2 (FGF2), one day after the surgery. In the saline-treated group, mRNA expression levels of TGFβ and FGF2 in the ipsilateral hind paw were significantly upregulated, compared with those in the contralateral hind paw. The upregulation of TGFβ (F1,12 = 6.434, P < 0.05) and FGF2 (F1,12 = 6.517, P < 0.05) mRNA levels was significantly attenuated in the colchicine-treated group (Figure 6).
Figure 6.
Effects of colchicine treatment on the expression of fibrogenesis-promoting factors. Colchicine (0.75 mg/kg) or saline was intraperitoneally injected once daily beginning two days before the plantar incision surgery. Samples of contralateral and ipsilateral hind paws of saline- or colchicine-treated groups were collected one day after the surgery. The mRNA expression levels of (a) TGFβ and (b) FGF2 were measured by real-time quantitative PCR. Each expression level was normalized to that of 18S rRNA and presented relative to each contralateral group. Data are expressed as means ± S.E.M, n = 4. *P < 0.05, **P < 0.01, compared with the contralateral group, #P < 0.05.
FGF2: fibroblast growth factor-2; TGFβ: transforming growth factor-β.
Discussion
In the present study, we provided evidence indicating that colchicine alleviates acute POP but delays the wound repair in the plantar incision-induced mouse model of POP. Furthermore, we showed that colchicine inhibited the infiltration of neutrophil and subsequent inflammatory M1 macrophages around the incision site after the surgery, while depletion of circulating macrophages by clodronate liposomes had little effect on mechanical hypersensitivity, wound closure, and neutrophil infiltration, suggesting that the involvement of macrophages may be limited.
We generated a mouse model of acute POP by longitudinally incising the skin and fascia and elevating, but leaving intact, the insertion and origin of the plantaris muscle, as described in the first report of this mouse model.26 This POP model showed shorter mechanical hypersensitivity, peaking one day and recovering within seven days after the surgery, than a POP model created by incising the plantaris muscle.29 This temporal pattern of mechanical hypersensitivity likely parallels that of neutrophil infiltration, rather than macrophage infiltration, of the incision site. Although abundant infiltration of macrophages was observed seven days after the surgery, mechanical hypersensitivity was already resolved at this time, suggesting that the delayed accumulation of macrophages has little effect on acute POP.
This hypothesis is further supported by the present results showing that mechanical hypersensitivity was not affected by clodronate liposome-induced depletion of macrophages. After being intravenously injected, liposome-encapsulated clodronate is phagocytosed by resident macrophages and circulating monocytes through the mononuclear phagocyte system, resulting in their elimination by apoptosis. Since these liposomes are not internalized by nonphagocytic cells or by granulocytes, clodronate liposome administration leads to specific depletion of macrophages and monocytes.28 Consistent with this mechanism of action, we confirmed that clodronate liposomes had no effect on plantar incision-induced neutrophil infiltration. Thus, the present results suggest that the involvement of delayed accumulation of macrophages is limited in the acute POP observed in a plantar incision-induced model. Consistent with these results, mechanical hypersensitivity in the plantar incision-induced mouse model of POP is not affected by a deficiency in CCR2,16 a key chemokine receptor for macrophages infiltrating inflamed sites in response to CCL2.27 The acute POP observed following the plantar surface incision may depend on acute inflammation but not be linked with the delayed accumulation of macrophages. However, we cannot fully exclude the possibility of the involvement of skin-resident macrophages, because cell-specific depletion of CD11b+Ly6G− myeloid cells (primarily monocytes and skin-resident macrophages) attenuates mechanical hypersensitivity in a plantar incision-induced POP model.16 This discrepancy may be due to the failure of depleting skin-resident macrophages by an intravenous injection of clodronate liposomes.
Consistent with the present findings, a previous report using vinblastine sulfate or an antineutrophil antibody shows that neutrophils around the incision site contribute to acute POP.5 Infiltrated neutrophils evoke pain through the production of a variety of inflammatory substances, including cytokines and chemokines.4 A mechanism of action for colchicine is to suppress the NACHT, LRR, and PYD domains-containing protein 3 inflammasome, which is responsible for processing and releasing IL1β, an inflammatory pronociceptive cytokine.4,5,30 In the present study using a mouse model of acute POP, the inhibition of mechanical hypersensitivity by colchicine treatment was accompanied by a decrease in the upregulation of IL1β mRNA, suggesting an involvement of the decrease in inflammatory pronociceptive substances. However, we also showed that colchicine treatment reduced the subsequent infiltration of macrophages around the incision site following decreased neutrophil infiltration, which was accompanied by a partial reduction in the mRNA expression levels of CCL2 and CCR2. Thus, we could not determine whether the IL1β upregulation was derived from the infiltrating neutrophils or macrophages. The present results for the markers for M1 and M2 macrophages CD86 and Arg1, respectively, further suggest that macrophage polarization toward the M1 phenotype is inhibited by colchicine treatment. Thus, it is possible that the inhibition of mechanical hypersensitivity by colchicine may be the synergistic result of neutrophil dysfunction and the inhibition of subsequent macrophage infiltration and polarization toward the M1 phenotype.
A previous study showed that the depletion of neutrophils by an anti-Gr1 antibody had no effect on thermal or mechanical hypersensitivity after a plantar incision in mice.11 This discrepancy with our results may be due to differences in the POP model; that is, both the skin and underlying muscle were cut with a scalpel in the aforementioned study, whereas the muscle was only elevated with forceps and received minimum injury in the present study. The former severe POP model with skin and deep tissue incision shows mechanical and thermal hyperalgesia and guarding pain with increased spontaneous activity of the dorsal horn neurons, while the latter skin incision POP model shows only mechanical and thermal hyperalgesia.31 Similarly, the activation of p38 mitogen-activated protein kinase in the dorsal root ganglia neurons is observed only in the former model, but not the latter model.32 These distinct phenotypes among POP models may be caused by the differential contribution of neutrophils and macrophages. Thus, depleting only neutrophils may be insufficient to attenuate severe POP when the wounds of both muscle and nerve terminals are severe. The most apparent difference from our results is the lack of an anti-Gr1 antibody effect on macrophage infiltration, whereas a large number of studies indicate that neutrophil depletion can reduce macrophage infiltration in inflamed sites.33,34 A body of evidence suggests direct inhibitory effects of colchicine on macrophage activation,5,30,35,36 although the effects on infiltration and phenotype polarization of macrophages have not been clarified. Further investigations will be needed to determine whether the inhibitory effect on neutrophil infiltration or the direct effects on macrophages are responsible for the reduced infiltration and polarization toward the M1 phenotype of macrophages induced by colchicine in this mouse model of acute POP.
The sequential roles of inflammatory cells, neutrophils, and macrophages in the wound repair are well documented.17–19 Nevertheless, the effect of neutrophil depletion on wound repair remains poorly defined; it may delay,20 promote,37 or fail to affect38 the wound repair. The present results showed that the colchicine-induced inhibition of neutrophil infiltration delayed wound closure following a plantar incision. As described above, colchicine treatment also reduced macrophage infiltration and polarization toward the M1 phenotype, which play crucial roles in the phagocytosis of tissue debris for wound closure.39 Furthermore, colchicine treatment tended to reduce anti-inflammatory M2 macrophages, which participate in fibrogenesis and tissue remodeling in the late phase of the wound repair.40 Thus, the delay of wound closure by colchicine may be due not only to the lack of neutrophil infiltration but also to the insufficient function of nonpolarized macrophages. In addition, the decrease in the early production of fibrogenesis-promoting factors, such as TGFβ and FGF2, which are released from stimulated neutrophils41 and M2 macrophages,42 may contribute to the delay in wound closure. However, in the present study, the macrophage depletion by clodronate liposomes had little effect on wound closure. Although previous studies demonstrated that macrophages can contribute to the diverse phases of wound repair, from inflammatory to tissue formation processes,43–45 it has also been suggested that macrophages may not be absolutely required for the basic wound repair.17 Depleting only the macrophages may have little impact on early wound closure after a small plantar incision, such as that used in the present study.
Colchicine can affect gene expression, differentiation, and proliferation of multiple cells other than neutrophils and macrophages, including endothelial cells, mast cells, dendritic cells, and fibroblasts.5 For example, colchicine shows antifibrotic effect, as well as anti-inflammatory effect.6 Thus, we cannot fully exclude the possibility that colchicine affects these cells to induce analgesic effect and delay of wound repair in the acute POP model.
In conclusion, the present results suggest that colchicine alleviates acute POP but delays the wound repair after a plantar incision, which may be due to the suppression of neutrophil and subsequent macrophage infiltration. However, the involvement of inhibition of macrophage infiltration and polarization toward M1 phenotype may be limited. Therapeutic strategies reducing acute neutrophil-driven inflammation after an incisional surgery may increase the risk of delaying wound repair. Thus, for rapid wound repair after the surgery, POP must be controlled without affecting the neutrophil function.
Author Contributions
AS, KI, TN, and SK designed the project. AS, KI, and HH conducted the experiments. AS, KI, HH, and TN analyzed the data. SI, KN, and HS helped design the study and provided technical advices. AS, KI, and TN wrote the manuscript, and SK finalized the manuscript.
Declaration of Conflicting Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI) from the Japanese Society for the Promotion of Science (Grants-in-Aid for Scientific Research (B) to TN [26293019, 17H04008], for Challenging Exploratory Research to TN [25670285], and Scientific Research on Innovative Area “Thermal Biology” to TN [16H01386]), and by grants from The Shimizu Foundation for Immunology and Neuroscience Grant for 2015 and The Nakatomi Foundation to TN.
References
- 1.Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg 2007; 104: 689–702. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012; 116: 248–273. [DOI] [PubMed] [Google Scholar]
- 3.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet 2011; 377: 2215–2225. [DOI] [PubMed] [Google Scholar]
- 4.Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep 2008; 10: 218–227. [DOI] [PubMed] [Google Scholar]
- 5.Leung YY, Yao Hui LL, Kraus VB. Colchicine – update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015; 45: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solak Y, Siriopol D, Yildiz A, et al. Colchicine in renal medicine: new virtues of an ancient friend. Blood Purif 2017; 43: 125–135. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Yaksh TL. Effects of colchicine applied to the peripheral nerve on the thermal hyperalgesia evoked with chronic nerve constriction. Pain 1993; 55: 227–233. [DOI] [PubMed] [Google Scholar]
- 8.Schuligoi R. Effect of colchicine on nerve growth factor-induced leukocyte accumulation and thermal hyperalgesia in the rat. Naunyn Schmiedebergs Arch Pharmacol 1998; 358: 264–269. [DOI] [PubMed] [Google Scholar]
- 9.Colburn RW, DeLeo JA. The effect of perineural colchicine on nerve injury-induced spinal glial activation and neuropathic pain behavior. Brain Res Bull 1999; 49: 419–427. [DOI] [PubMed] [Google Scholar]
- 10.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13: 159–175. [DOI] [PubMed] [Google Scholar]
- 11.Carreira EU, Carregaro V, Teixeira MM, et al. Neutrophils recruited by CXCR1/2 signalling mediate post-incisional pain. Eur J Pain 2013; 17: 654–663. [DOI] [PubMed] [Google Scholar]
- 12.Perkins NM, Tracey DJ. Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience 2000; 101: 745–757. [DOI] [PubMed] [Google Scholar]
- 13.Lavich TR, Siqueira Rde A, Farias-Filho FA, et al. Neutrophil infiltration is implicated in the sustained thermal hyperalgesic response evoked by allergen provocation in actively sensitized rats. Pain 2006; 125: 180–187. [DOI] [PubMed] [Google Scholar]
- 14.Cunha TM, Verri WA, Jr, Schivo IR, et al. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol 2008; 83: 824–832. [DOI] [PubMed] [Google Scholar]
- 15.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med 2010; 16: 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghasemlou N, Chiu IM, Julien JP, et al. CD11b+Ly6G− myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci USA 2015; 112: E6808–E6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 2005; 15: 599–607. [DOI] [PubMed] [Google Scholar]
- 18.Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature 2008; 453: 314–321. [DOI] [PubMed] [Google Scholar]
- 19.Leoni G, Neumann PA, Sumagin R, et al. Wound repair: role of immune-epithelial interactions. Mucosal Immunol 2015; 8: 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishio N, Okawa Y, Sakurai H, et al. Neutrophil depletion delays wound repair in aged mice. Age (Dordr) 2008; 30: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadeau S, Filali M, Zhang J, et al. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain. J Neurosci 2011; 31: 12533–12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012; 43: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 24.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol 2013; 93: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen F, Wu W, Millman A, et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol 2014; 15: 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology 2003; 99: 1023–1027. [DOI] [PubMed] [Google Scholar]
- 27.Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab 2010; 30: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 1994; 174: 83–93. [DOI] [PubMed] [Google Scholar]
- 29.Romero A, Romero-Alejo E, Vasconcelos N, et al. Glial cell activation in the spinal cord and dorsal root ganglia induced by surgery in mice. Eur J Pharmacol 2013; 702: 126–134. [DOI] [PubMed] [Google Scholar]
- 30.Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440: 237–241. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Brennan TJ. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain 2009; 144: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizukoshi K, Sasaki M, Izumi Y, et al. Activation of p38 mitogen-activated protein kinase in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision. Neuroscience 2013; 234: 77–87. [DOI] [PubMed] [Google Scholar]
- 33.Janardhan KS, Sandhu SK, Singh B. Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci 2006; 11: 1569–1576. [DOI] [PubMed] [Google Scholar]
- 34.Soehnlein O, Zernecke A, Eriksson EE, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008; 112: 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters-Golden M, McNish RW, Davis JA, et al. Colchicine inhibits arachidonate release and 5-lipoxygenase action in alveolar macrophages. Am J Physiol 1996; 271: L1004–L1013. [DOI] [PubMed] [Google Scholar]
- 36.Viktorov AV, Yurkiv VA. Albendazole and colchicine modulate LPS-induced secretion of inflammatory mediators by liver macrophages. Bull Exp Biol Med 2011; 151: 683–685. [DOI] [PubMed] [Google Scholar]
- 37.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol 2003; 73: 448–455. [DOI] [PubMed] [Google Scholar]
- 38.Martin P, D’Souza D, Martin J, et al. Wound healing in the PU.1 null mouse-tissue repair is not dependent on inflammatory cells. Curr Biol 2003; 13: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 39.Sica A, Erreni M, Allavena P, et al. Macrophage polarization in pathology. Cell Mol Life Sci 2015; 72: 4111–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A, Biswas SK, Galdiero MR, et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229: 176–185. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A, Cassatella MA, Costantini C, et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011; 11: 519–531. [DOI] [PubMed] [Google Scholar]
- 42.Laskin DL, Sunil VR, Gardner CR, et al. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol 2011; 51: 267–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goren I, Allmann N, Yogev N, et al. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol 2009; 175: 132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010; 184: 3964–3977. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Z, Ding J, Ma Z, et al. Systemic depletion of macrophages in the subacute phase of wound healing reduces hypertrophic scar formation. Wound Repair Regen 2016; 24: 644–656. [DOI] [PubMed] [Google Scholar]