Abstract
Objectives:
Measures of pregnancy associated deaths provide important guidance for public health initiatives. Record linkage studies have significantly improved identification of deaths associated with childbirth but relatively few have also examined deaths associated with pregnancy loss even though higher rates of maternal death have been associated with the latter. Following PRISMA guidelines we undertook a systematic review of record linkage studies examining the relative mortality risks associated with pregnancy loss to develop a narrative synthesis, a meta-analysis, and to identify research opportunities.
Methods:
MEDLINE and SCOPUS were searched in July 2015 using combinations of: mortality, maternal death, record linkage, linked records, pregnancy associated mortality, and pregnancy associated death to identify papers using linkage of death certificates to independent records identifying pregnancy outcomes. Additional studies were identified by examining all citations for relevant studies.
Results:
Of 989 studies, 11 studies from three countries reported mortality rates associated with termination of pregnancy, miscarriage or failed pregnancy. Within a year of their pregnancy outcomes, women experiencing a pregnancy loss are over twice as likely to die compared to women giving birth. The heightened risk is apparent within 180 days and remains elevated for many years. There is a dose effect, with exposure to each pregnancy loss associated with increasing risk of death. Higher rates of death from suicide, accidents, homicide and some natural causes, such as circulatory diseases, may be from elevated stress and risk taking behaviors.
Conclusions:
Both miscarriage and termination of pregnancy are markers for reduced life expectancy. This association should inform research and new public health initiatives including screening and interventions for patients exhibiting known risk factors.
Keywords: Maternal mortality, pregnancy associated death, longevity, pregnancy loss, termination of pregnancy, abortion, miscarriage, risk factors, pregnancy screening, health policy
Introduction
Maternal deaths associated with pregnancy are a major public health concern. Death rate calculations based on death certificates alone, however, consistently miss cases due to the fact that registrars often lack information about the deceased’s woman’s complete pregnancy history. This problem can be alleviated in part by linking death certificates to birth certificates, fetal death records, termination of pregnancy (TOP) registries, and medical treatment records.
Without such record linkage only 26% of deaths during pregnancy or after live birth or stillbirth would have been identified from the death registry or death certificates alone, according to a Finnish study.1 Using death certificates alone, only 12% of deaths following miscarriage or ectopic pregnancy and just 1% of deaths following termination of pregnancy (TOP) could be identified without record linkage.1 The importance of systematically using record linkage to identify deaths associated with pregnancy losses (TOP, miscarriage, and ectopic pregnancies) is further demonstrated by the same study’s findings, which demonstrate that the mortality rate in the year following a pregnancy loss was two to four times higher than that of delivering women.
Record linkage studies are therefore clearly necessary to properly identify the effects of pregnancy on the health and longevity of women. This methodology is especially important to understanding mortality rates associated with TOP and natural pregnancy losses precisely because such deaths are (a) much more common than deaths during pregnancy or after delivery, and (b) less likely to be identified on death certificates alone.1
Compared to women who deliver, those who miscarry or have TOP face significantly elevated rates of psychiatric disorders,2–10 substance use,5,6,10–13 suicidal behaviors,5,6,13–16 sleep disorders,17 post-traumatic stress disorders,7,18,19 a decline in general health,20 and elevated rates of recourse to medical treatments in general,21,22 most of which have been observed within the first through ten years following the pregnancy loss. Any and all of the aforementioned conditions may shorten longevity. It is therefore especially important from a public health and economic viewpoint to improve investigations regarding the mortality rates associated with pregnancy losses.
While the importance of research on maternal mortality is widely recognized, it has appeared increasingly evident to the authors that insufficient attention has been devoted to examining the subset of women’s deaths following pregnancy losses. Greater insight into this subset of deaths may help to guide and prioritize the development of proactive health initiatives that can save women’s lives and improve health.
Therefore, the authors identified the need for a systematic review which would provide (a) a description and synthesis of all the available qualifying literature, including proposals for research priorities and actionable interventions based on the best available evidence, and (b) a quantitative meta-analysis of the available evidence. To meet these goals, we determined that we should first seek to identify all record linkage studies examining mortality rates associated with pregnancy outcome regardless, without any limitation on time frame. This initial assessment would help us to identify any missed opportunities for examining pregnancy loss associated mortality. Second, we seek to identify all record linkage studies that have specifically examined death rates associated with pregnancy losses, including voluntary and therapeutic terminations. Using this subset of studies, we would then (a) develop a narrative synthesis of the common and specific findings of the relevant studies and (b) undertake a meta-analysis of any comparative mortality rates associated with different pregnancy outcomes which are appropriate to the methods of meta-analyses.
The importance of this investigation is underscored by numerous studies which have found that that parity and the exposure to various pregnancy outcomes has significant effects on life expectancy.23–25 Record linkage studies examining pregnancy associated life expectancy are needed to help to identify how the number of pregnancies, number of deliveries, and types of pregnancy outcomes may affect the health and longevity of women. These findings, in turn, may then contribute to better screening to identify the subsets of women who may most benefit from interventions to ameliorate any harmful effects and/or to enhance any beneficial effects associated with pregnancy and pregnancy management.
Definitions
Pregnancy loss, as used herein, includes all pregnancy outcomes that do not end in a live birth.2
Natural loss is a subset that includes all pregnancy losses except TOP. While the vast majority of natural losses are miscarriages, it should be noted that some researchers have chosen to report only on miscarriages while others have included ectopic pregnancies, still births and other natural losses together. Still other investigators have grouped women who had stillbirths with women who had live births since these pregnancies continued to term or near term.1
Pregnancy associated death, has been defined by the American College of Obstetricians and Gynecologists (ACOG) and the United States’ Centers for Disease Control (CDC) to include all deaths during pregnancy or within one year of a pregnancy outcome regardless of presumed cause of death.26 The identification of pregnancy associated deaths has been recognized is an important precursor to efforts to identify maternal deaths, which are defined to include only those deaths for which there is a medical opinion that some aspect of the pregnancy or pregnancy management was a contributing cause of death.26
Pregnancy associated long-term mortality is defined to include all deaths following one or more pregnancy outcomes without an imposed time limit. While the time limits used in each study reporting pregnancy associated long-term mortality should always be noted, this definition avoids establishing any arbitrary time limits and prepares the way toward calculating pregnancy associated mortality and life expectancy rates relative to variables such as gravidity, parity, live births, and exposure to pregnancy losses.
Abortion related deaths are defined by the CDC as any “death from a direct complication of an [induced] abortion (legal or illegal), an indirect complication caused by a chain of events initiated by an abortion, or an aggravation of a preexisting condition by the physiologic or psychologic effects of abortion.”27 The deliberate choice to place no time limit on the definition of TOP related deaths reflects the fact that there is no clear temporal limit on physiological and psychological effects that may contribute to subsequent death.
TOP associated deaths (or abortion associated deaths) are herein defined as the subset of pregnancy associated deaths which are within one year of a TOP. The one year limit corresponds to that for “pregnancy associated deaths.”
TOP associated long-term mortality is an extension of the CDC’s “abortion related deaths” and include all deaths among women with a history of TOP without regard to time. Just as the systematic identification of early and late maternal deaths must be preceded by a systematic identification of pregnancy history, so the identification of abortion related deaths should be preceded by the systematic identification of TOP history without a predefined time limit.
Materials and methods
PRISMA guidelines were consulted and employed where appropriate in the development and writing of this review.
Eligibility criteria
The first level of predefined eligibility criteria were: (1) the study was available in English; (2) the study examined mortality rates of women relative to one or more pregnancy outcomes; and (3) the study included systematic linking of death certificates to independent records used to identify if the deceased had one or more pregnancy outcomes within a year of her death. The independent records might be one of the following: birth certificates, fetal death certificates, TOP registries, paid insurance claims, or comprehensive hospital or medical records documenting treatments related to pregnancy.
The second level of eligibility criteria was to identify all publications meeting the first level of inclusion criteria which reported on death rates associated with any form of pregnancy loss (miscarriage, legal TOP, ectopic pregnancy, still birth, or any other failed pregnancy) as identified through records independent of the death certificates. This step eliminated studies that examined only mortality rates associated with childbirth, or which failed to distinguish between deaths associated with childbirth and pregnancy loss. This step helped to both identify missed research opportunities and to identify the eligible studies which do have information regarding mortality rates associated with pregnancy loss but failed to report this data.
The third step was to identify studies eligible for inclusion in a meta-analysis. This subset was drawn from the list of studies meeting the second level of eligibility. This third level of eligible studies included only those that (a) report mortality rates within one year for all three pregnancy outcomes of interest (childbirth, natural losses, and TOP) and (b) provided the most recently relevant data, thereby excluding duplication of results when the same population of women were examined in more than one study.
Information sources and search terms
In July of 2015, a SCOPUS search was conducted using the search ( ( ( TITLE-ABS-KEY ( maternal mortality ) OR TITLE-ABS-KEY ( maternal death ) ) ) AND ( ( TITLE-ABS-KEY ( record linkage ) OR TITLE-ABS-KEY ( linked records ) ) ) ) OR ( ( ( TITLE-ABS-KEY ( pregnancy associated mortality ) OR TITLE-ABS-KEY ( pregnancy associated death ) ) ) AND ( ( TITLE-ABS-KEY ( record linkage ) OR TITLE-ABS-KEY ( linked records ) ) ) ). A total of 458 records of potential interest was returned.
A MEDLINE search was conducted using the search ((“pregnancy associated mortality” OR “pregnancy associated death”) AND (“record linkage” OR “linked records”)) OR ((“record linkage” OR “linked records”) AND (“maternal mortality” OR “maternal death”)). This search returned 20 references.
Additional candidates were identified using the “snowball method,” the review of all references cited by eligible papers plus citations from other maternal mortality reviews.
Study selection
After elimination of duplicates, all titles and abstracts were examined to identify publications with a prospect for meeting the predefined inclusion criteria. Those deemed candidates for inclusion were retrieved for full text review and studied to determine which articles met the pre-determined inclusion criteria. Assessments of those studies qualifying for both levels of inclusion criteria were conducted by two reviewers, with disagreements resolved by discussion.
Risk of bias
Studies qualifying for both levels of inclusion were scored for bias using the Newcastle-Ottawa Quality Assessment Scale (NOQAS) for cohort studies.
Data collection for descriptive summary of literature
Each study meeting the second level of eligibility was entered into a table identifying the source, population size, time period examined, types of pregnancy outcomes examined, means of identifying deaths and pregnancy outcomes, any confounding variables that were examined in the study, NOQAS score, and a summary of major findings. The table was completed by two reviewers, with disagreements resolved by discussion.
Data collection for meta analysis
To calculate the age adjusted number of deaths in the first year for each subgroup’s population for our meta-analysis we extracted data relative to the reported age adjusted risk of death during the first year following the pregnancy outcome from each country. To avoid duplication of cases, only the most recent study for each country was used in the meta-analysis. Using the age adjusted mortality rate of delivering women as the control in each case, odds ratios and confidence limits for each subgroup (TOP vs birth, and natural losses vs birth) and for each study were calculated using EpiInfo 7’s StatCalc. These results were then entered into the Comprehensive Meta Analysis software package to produce results using the fixed effects model.
Results
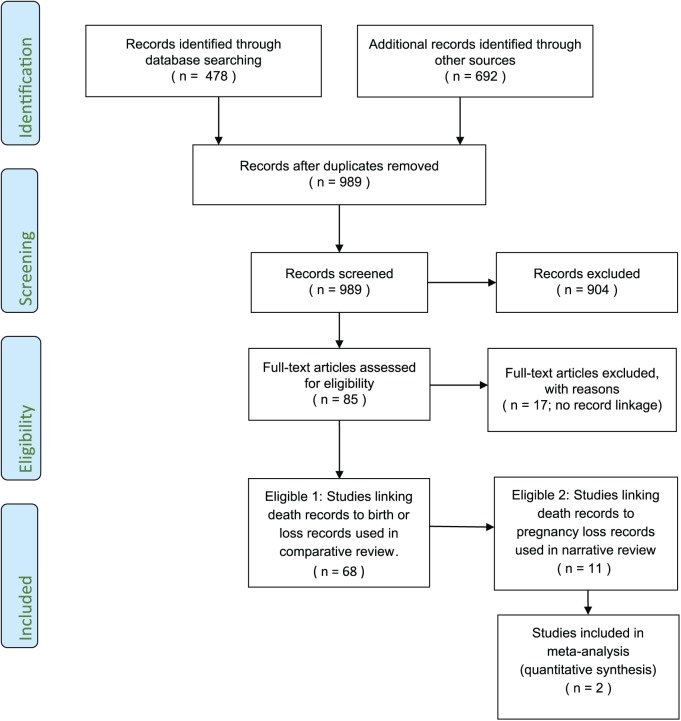
After removal of duplicates, a total of 989 titles were identified by the combination of search terms and review of additional references (Figure 1). Review of abstracts eliminated 904 references. At the second level of review, 14 more were eliminated after full text review because they did not identify pregnancy history using record linkage. Three non-English studies were also identified, but their abstracts indicated that none included data on pregnancy loss associated mortality so English translations were not sought. Thus, a total of 17 studies were eliminated at this stage.
Figure 1.
Flow chart of search results, reasons for exclusion, and three levels of inclusion.
A total of 68 studies examining populations in 11 countries met the criteria for the first level of eligibility. All of the studies identified significantly more maternal deaths than would have been identified by reliance on death certificates alone.
Of the 68 studies identified, 57 included record linkage of only birth and death records. In other words, they lacked any data on deaths associated with pregnancy losses. The distribution by country of these studies was as follows: one in Bangladesh,28 one in Brazil,29 two in Canada,30,31 one in Denmark,32 one in Italy,33 three in Netherlands,34–36 four in Sweden,37–39 one in Taiwan,40 six in the United Kingdom,41–46 thirty-four in the United States including Puerto Rico,47–79 and three reporting data from multiple countries for which at least one country’s data used record linkage which met our criteria for inclusion.80–82
The remaining 11 studies met the criteria for the second level of eligibility: reporting results of linkage of death certificates to independent records of pregnancy loss. These included seven studies from Finland,1,83–88 two from Denmark,89,90 and two from the United States.91,92 Two of these investigated only deaths in the year following TOP.88,91 The remainder investigated pregnancy associated deaths and/or pregnancy associated long-term mortality relative to both birth and pregnancy loss.
Details of the eleven studies are summarized in Table 1. The column labelled “Confounding Variables Examined” identifies factors which were either (a) controlled for statistically, such as was commonly done in regard to age of the woman, or (b) controlled for by study design, such as restriction of the population to only the lowest economic class, or exclusion of women with prior psychiatric history, or (c) controlled for by showing segregated results for discrete groups, such as married and unmarried. The NOQAS assessment revealed that quality of these studies was very high, with low risk of bias. With a possible range from 0-9, (high corresponding to the highest quality) only the one very earliest study scored below 8.
Table 1.
Record linkage studies examining deaths associated with one or more types of pregnancy loss with notes regarding key findings.
| Study (year) Country |
Population & Time Period (Births / TOP / Natural Losses / Deaths) |
Records Examined and Linked | Confounding Variables Examined | Quality Score*
Range 0–9 |
Summary of Major Findings |
|---|---|---|---|---|---|
| Shelton and Schoenbucher91
(1978) United States |
All fertile-aged Georgia women in 1975–Feb 1976 (NA / 19,877 / NA / 1,610) |
death certificates TOP certificates |
none | 6 | In this exploratory study Georgia death certificates were used to identify ten deaths preceded by an abortion, With an average observation period of 8 months, the one year abortion associated mortality rate was 75.5 per 100,000 cases. Deaths included 2 suicides (one four days after the TOP), 3 homicides (all within 4 months), 3 attributed to accidents, one sudden death from “coronary occlusion,” and one death from ovarian cancer (the woman was receiving chemotherapy at time of TOP). Record linkage was incomplete due to limited information on the TOP certificates. |
| Gissler et al.83
(1996) Finland |
All fertile-aged women, 1987–1994. (513,472 / 93,807 / 71,701 / 9,192) |
death certificates birth certificates TOP registry hospital discharge |
age social class marital status |
9 | National suicide study. 1,347 suicides identified. No suicides while pregnant were found. Compared to women not pregnant in the year prior to suicide, women who aborted were three times more likely to commit suicide (3.08, 95%CI 1.57 to 6.03), pregnant and delivering women were half as likely (0.52; 95%CI 0.19 to 1.41), and women who miscarried were not significantly different. Suicide risk was highest in first two months following the pregnancy outcome. |
| Gissler et al.84
(1997) Finland |
All fertile-aged women, 1987–1994. (513,472 / 93,807 / 71,701 / 9,192) |
death certificates birth certificates TOP registry hospital discharge |
age | 8 | All death certificates were linked to medical and TOP registry to identify pregnancy within a year prior to death. Only 22% of pregnancies were identified on death certificates. Record linkage to TOP and hospital discharge records doubled number of deaths identified compared to linkage to birth certificates alone. Compared to women not pregnant, the age adjusted mortality ratio was half for delivering women (0.50, 95%CI 0.32 to 0.78) and significantly higher following TOP (1.76, 95%CI 1.27 to 2.42). |
| Gissler and Hemminki85
(1999) Finland |
All fertile-aged women, 1987–1994. (513,472 / 93,807 / 71,701 / 9,192) |
death certificates birth certificates TOP registry hospital discharge |
age | 8 | Compared to women who were not pregnant in the year before death, women who had TOPs had an 81% higher rate of death (1.81, 95%CI 1.31 to 2.50), women who gave birth had a 53% lower risk of death (0.47, 95%CI 0.30 to 0.74), and those who miscarried were not significantly different (0.85, 95%CI 0.58 to 1.24). 34% of deaths were from external causes. Women who had TOPs had significantly elevated risk of death from suicide, accidents, and homicides. Risk of death from natural causes was significantly lower for women giving birth (0.47, 95%CI 0.25 to 0.86) and for women who miscarried (0.39, 95%CI 0.20 to 0.75). |
| Reardon et al.92
(2002) United States |
Medicaid eligible and fertile aged women in California with pregnancy outcome in 1989 (116,936 / 56,343/ NA / 1,294) |
death certificates all paid medical claims |
age economic class 12–18 months prior psychiatric history |
9 | Medical records for women with a Medicaid treated pregnancy in 1989 were linked to death certificates. After controlling for psychiatric history and age, women who had a TOP were at significantly higher risk of death. The relative risk was 2.03 (95%CI 1.33 to 3.10) in the first two years following pregnancy outcome, 1.98 (95%CI 1.25 to 3.15) in years three and four, and declined to an insignificant 1.35 (95%CI 0.89 to 2.05) in the fifth and sixth years, and 1.29 (95%CI 0.84 to 1.96) in the seventh and eighth years. Multiple pregnancy outcomes significantly affected mortality rates. During the eight years following pregnancy, women who aborted had a significantly higher age-adjusted relative risk of death compared to delivering women from all causes (1.61, 95%CI 1.30 to 1.99), suicide (3.12, 95%CI 1.25 to 7.78), and homicide (1.93, 95%CI 1.11 to 3.33), as well as from natural causes (1.44, 95%CI 1.08 to 1.91), circulatory diseases (2.00, 95%CI 1.00 to 3.99), and cerebrovascular disease (4.42, 95%CI 1.06 to 18.48). |
| Gissler et al.1
(2004) Finland |
All fertile-aged women, 1987–2000. (865,988 / 156,789 / 118,490 / 15,823) |
death certificates birth certificates TOP registry hospital discharge |
age | 8 | All death certificates were examined. A total of 419 deaths were among women pregnant in the year prior to death. Without record linkage, 73% of pregnancy associated deaths would have been missed. Following live or still birth, 27% of deaths within 42 days and 78% of deaths from 43–364 days would have been missed without record linkage. Following TOP 71% of deaths within 42 days and 97% of deaths between 43–364 days would have been missed without record linkage. Following miscarriage or ectopic pregnancy, 54% of deaths within 42 days of pregnancy outcome and 94% of deaths between 43–364 days would have been missed. |
| Gissler86
(2004) Finland |
All fertile-aged women, 1987–2000. (865,988 / 156,789 / 118,490 / 15,823) |
death certificates birth certificates TOP registry hospital discharge |
age | 8 | One-year age adjusted mortality rates were calculated for women not pregnant in the year prior to death and compared to age adjusted mortality rates of three groups of women who were pregnant at death or during the year prior to death. The death per 100,000 was 57.0 for not recently pregnant women, 28.2 for delivering or pregnant women (RR 0.49, 95% CI 0.43–0.56), 51.9 for women who miscarried (RR 0.91, 95% CI 0.71 to 1.17), and 83.1 for women who had TOPs (RR 1.45, 95% CI 1.22 to 1.73). Women aged 25–34 who had TOPs were significantly more likely to die of circulatory system disease compared to not recently pregnant women, delivering women, and those who miscarried (rates per 100,000, respectively: 8.7; 4.4; 3.3; 1.5). |
| Gissler et al.87
(2005) Finland |
All fertile-aged women, 1987–2000. (865,988 / 156,789 / 118,490 / 15,823) |
death certificates birth certificates TOP registry hospital discharge |
age | 8 | This study examined only deaths from external causes. The death rate from external causes per 100,000 was 24.2 for women who had not been pregnant, 10.2 for those giving birth, 35.2 for those with natural losses, and 60.3 for those who had TOPs. The tables present segregated results show death rates from suicide, homicide, and those classified as accidental varied significantly by age and pregnancy outcome. The authors endorse recommendations for routine post-TOP checkup screening for depression and psychosis in the weeks following a TOP. |
| Reardon and Coleman89
(2012) Denmark |
All fertile-aged whose first pregnancy was in 1980–2004. (318,646 / 119,179 / 25,648 / 2,238) |
death certificates birth certificates TOP registry hospital discharge |
first pregnancy age at time of pregnancy; year of woman’s birth |
9 | Age and maternal birth year adjusted mortality rates following first pregnancy outcomes were calculated over numerous time periods. Deaths rates for the first and second year are shown in Figure 1. Cumulative TOP associated mortality was significantly higher for every time period examined from 180 days to 10 years for both early and later TOP. The cumulative odds ratio for early TOP declined from a high at 180 days (2.03, 95% CI 1.11 to 3.71) to a low at ten years (1.39, 95% CI 1.22 to 1.60). Mortality rates associated with miscarriages were lower than for TOP and were significantly higher than for birth for periods over four years. |
| Coleman et al.90
(2013) Denmark |
All fertile-aged women, 1980–2004. (438,134 / 171,582 / 111,205 / 5,137) |
death certificates birth certificates TOP registry hospital discharge |
year of woman’s birth age at last pregnancy number of births number of TOPs number natural losses |
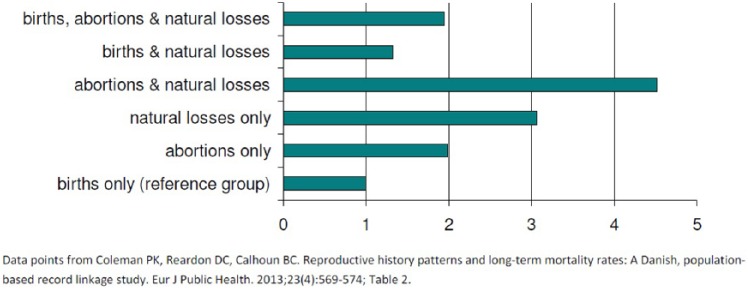
9 | This study examined all causes of death using 25 years of data using numerous control variables, including exposure rate to various pregnancy outcomes. A dose effect was observed as shown in Figure 6. Exposure to various combinations of pregnancy outcomes was significant. The rate per 100,000 was 352 experiencing only births, 365 for those with both birth and natural losses, 541 for those with both births and TOP, 549 for those with no pregnancies, 550 for those with births, TOP and natural losses, 805 for those with only natural losses, and 1281 for those with only TOP. These findings suggest that TOP combined with natural loss compounds the risk of reduced longevity while a successful birth may reduce the risks associated with pregnancy loss. |
| Gissler et al.88
(2014) Finland |
All fertile-aged women, 1987–2012. (NA / 284,751 / NA / 3,798) |
death certificates TOP registry |
age | 8 | Based on prior research associating TOP with higher suicide rates, unofficial guidelines in Sweden recommended 2–3 week post-TOP assessments of psychological adjustment. These guidelines were made official in 2001. This study sought to examine if the guidelines adopted in 1996 may have reduced TOP associated suicide rates. The elevated risk of suicide after TOP declined from 2.84 (95% CI, 2.05 to 2.93) before 1997 to 2.44 (1.80 to 3.32) for 1997 thru 2012, but the drop was not statistically significant. |
Details of the Quality Score assessment can be viewed at: https://docs.google.com/spreadsheets/d/1T0GySPufF4MXnuTNwmiDgcqHf1yh66Ulso1AotTP8IQ/edit?usp=sharing
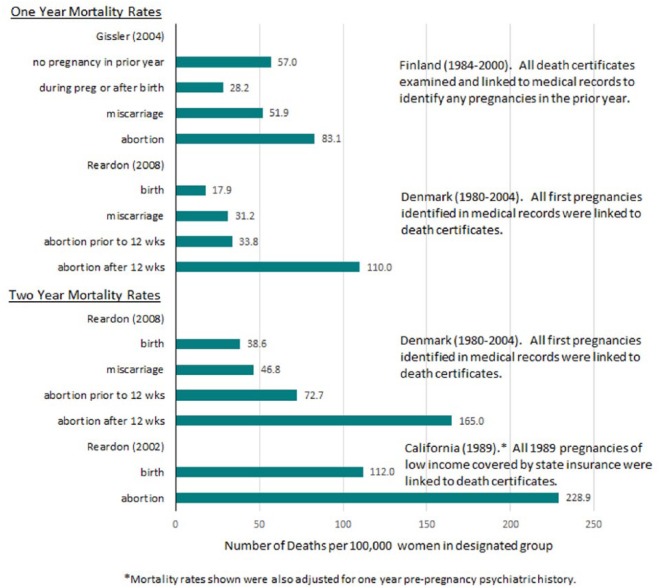
Figure 2 shows the mortality rate per 100,000 person years for each outcome reported by the latest studies from each of Finland, Denmark, and the United States, showing cumulative mortality rates for both one year and two years. The graph illustrates that mortality rates remain elevated after pregnancy loss beyond one year. Notably, the mortality rate over two years, comparing results from Denmark and California, suggest that low income women are at higher risk but that socioeconomic effects do not fully explain the results. Alternatively, the difference may be due to only first pregnancies being examined in the Denmark study.
Figure 2.
Cumulative Age Adjusted, All Cause Mortality Rates per 100,000 Women for One and Two Year Periods Following Pregnancy Outcome.
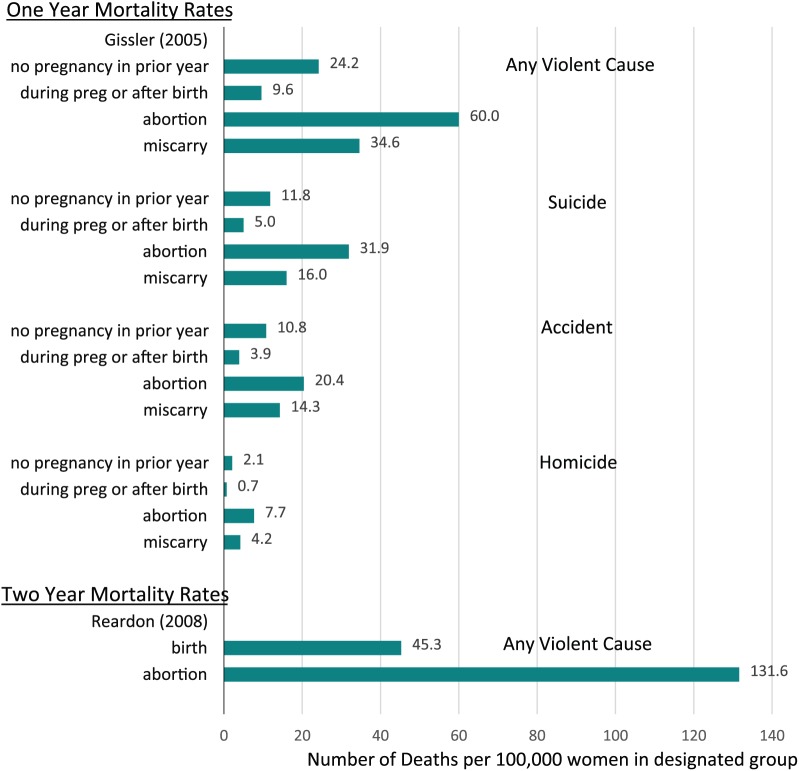
Figure 3 shows that the risk of death after pregnancy loss is most elevated in regard to deaths from external causes: suicide, homicide, and accidents compared to both delivering women and women who have not recently been pregnant.87,92 The implication that psychological effects associated with pregnancy loss may contribute to deaths resulting from self-destructive or risk taking behavior is further supported by a finding of higher rates of death attributed to mental illness (RR = 3.21, 94% CI 1.11–9.27) following TOP, even after controlling for prior psychiatric history.92
Figure 3.
Cumulative Age Adjusted, Violent Cause Mortality Rates per 100,000 Women for One and Two Year Periods Following Pregnancy Outcome.
*Mortality rates shown were also adjusted for one year pre-pregnancy psychiatric history.
As several the eleven studies undertook examined associations from a different perspective, a summary of their most important findings, including figures illustrating many of these findings, is provided below:
Pregnancy loss associated mortality may be over twice that of birth associated mortality.1 TOP associated mortality is higher than miscarriage associated mortality, which is higher than pregnancy and delivery associated mortality. (Figure 2)
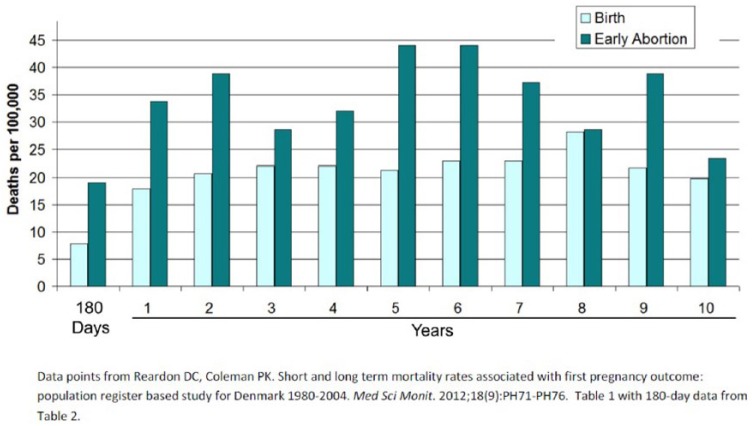
TOP associated mortality rates are higher than birth associated mortality during the first 180 days89 and remains higher for six or more years.89,90,92 (Figure 4)
Differences in pregnancy associated life expectancy vary according to the type and number of exposures to various outcomes. Successful deliveries may mitigate some of the effects of pregnancy loss.90,92 (Figure 5)
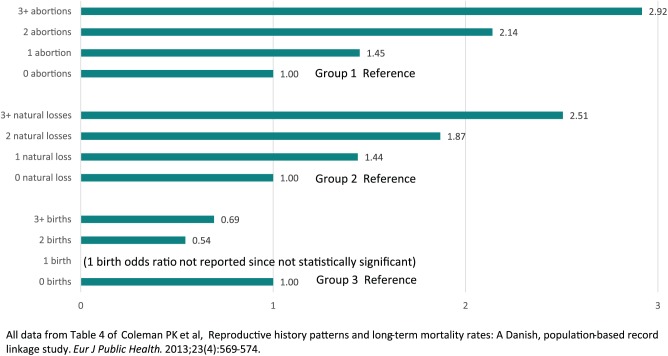
There is a dose effect, whereby exposure to multiple pregnancy losses increases the negative effect on life expectancy whereas multiple births increases life expectancy.90 (Figure 6)
The risk of death associated with pregnancy loss remains elevated even after controlling for psychological differences and economic class.92 (Figure 2)
While the risk of death after pregnancy loss is most elevated in regard to deaths from violent causes,87,92 there is also evidence that when risk of death after pregnancy loss is tracked beyond one year a significant higher risk is also associated with specific causes of natural death, such as circulatory disease (RR = 2.87, 95% CI 1.68–4.89)92
Figure 4.
Death rates following first pregnancy outcome through 180 days and during each of the first through tenth years after pregnancy outcome.
Figure 5.
Adjusted odds ratios for pregnancy associated long-term mortality by exposure to types of pregnancy outcomes. Adjusting for age at last pregnancy and number of pregnancies.
Figure 6.
Adjusted Odds Ratios for Pregnancy Associated Long Term Mortality Rates by Frequency of Exposure to Each Pregnancy Outcome—Denmark 1980–2004.
Group 1. The odds ratios for exposure to abortion are adjusted for age at last pregnancy, number of births and number of natural losses.
Group 2. The odds ratios for exposure to natural loss are adjusted for age at last pregnancy, number of births and number of abortions.
Group 3. The odds ratios for exposure to birth are adjusted for age at last pregnancy, number of natural losses and number of abortions.
All data from Table 4 of Coleman PK et al.90
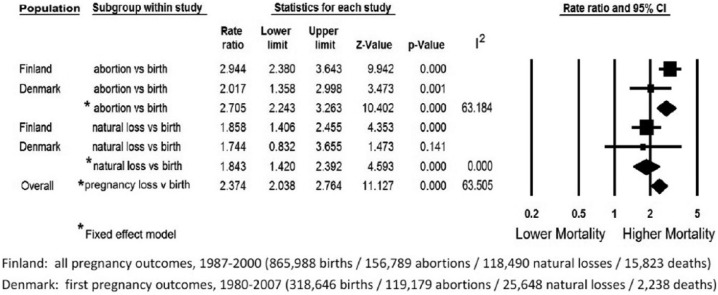
The meta-analysis used age adjusted mortality rates for each pregnancy outcome reported in most recent studies of the population of Finland86 and Denmark.89 While the eleven studies included data on women in three countries, neither American study reported age adjusted mortality rates for the first year after pregnancy outcome.
Figure 7 shows results of the meta-analysis using the fixed effects model. It illustrates the comparative risk of death in the first year after TOP compared to delivery and for the first year after natural losses compared to delivery. The risk of death during pregnancy and one year after a delivery the age adjusted pregnancy associated risk of death was 170 percent higher following a TOP (RR = 2.705; 2.243 < 95% CI < 3.263), and 84 percent higher following natural losses (RR = 1.843; 1.420 < 95% CI < 2.392). For all pregnancy losses compared to delivery, the risk was 137% higher (RR = 2.374; 2.038 < 95% < 2.764; Q-value = 8.220, P = .042). The I2 statistic indicates that about 63% of the variation in the overall results is due to heterogeneity rather than chance.
Figure 7.
Meta-Analysis of Age Adjusted One Year Mortality Rates Associated with Comparative Pregnancy Outcomes.
Discussion
Our systematic review found 68 studies employing record linkage of death certificates to independent records of pregnancy and pregnancy outcomes. In nearly every case, the authors reported that record linkage significantly improved the identification of maternal deaths and pregnancy associated deaths compared to reliance on death certificates alone. We concur with the opinion that the direct and indirect effects of pregnancy on women’s mortality rates cannot be accurately accessed without record linkage between death certificates and other medical records.1
This systematic review also revealed that every record linkage study examining mortality rates relative to different pregnancy outcomes has revealed that pregnancy loss is associated with a higher risk of death than childbirth. These studies also show that this elevated mortality risk persists over many years, is multiplied by repeat exposure to pregnancy loss, and may be reduced by successful deliveries. The quality of these eleven studies is very high, with all but the one earliest attempt scoring 8 or above on the NCQAS (with a range 0–9).
Overall, the meta-analysis revealed that pregnancy loss associated mortality is more than double that of delivery associated mortality. Notably, the Danish data used in the meta-analysis included only first pregnancy outcomes while the Finnish data included all pregnancy outcomes. This may explain the higher pregnancy loss mortality rate observed in the Finnish data since a significant portion of the Finnish subjects would have been exposed to multiple pregnancy losses for which a dose effect of increased mortality risk has been observed.90
A disproportionate share of pregnancy loss associated deaths are due to suicides, accidents, or homicide.83,86,87,92 In case study reports from mental health professionals and surveys of women struggling with pregnancy loss issues heightened risk taking and self-destructive behaviors are reported which may contribute to rates of accidents and homicide, in addition to suicide.93 Risk of death from accidents and homicide may also be impacted by the elevated risk of substance abuse associated with TOP.10–12 This hypothesis is supported by one U.K. study of pregnancy associated deaths that reported that1 a major portion of accidental deaths were due to drug overdose, and2 of eight women who died after being struck by cars as pedestrians, seven were drug users.43 These findings underscore the importance of record linkage as a precursor to efforts to evaluate “abortion related deaths,” as defined by the CDC.27
Strengths and weaknesses
A strength of the narrative portion of this review is that while only 11 of 68 record linkage studies of mortality rates associated with pregnancy included examination of deaths associated with pregnancy losses, these eleven examined a variety of different time frames and confounding variables, including economic class, marital status, age, number and types of prior pregnancy outcomes, and prior psychiatric history. At the same time, however, it is also a weakness that all of these confounding variable were not addressed in every study. The fact that all of these studies, despite variations, showed a consistent trend in findings indicates that the trend is a real one and is likely to replicated if applied to other populations.
Clearly, a priority of future research should examine a broader number of confounding variables across more populations to better understand the direct and indirect pathways and co-occurring risk factors that may guide future interventions. Future studies should seek to control for potential confounders including: income inequality, psychiatric history, access to medical care including birth control, intimate partner violence, intentionality of pregnancy, and level of maternal attachment to the pregnancy.
A major weakness of our meta-analysis is that data on mortality rates in the first year following pregnancy losses were only available from two countries, which highlights the failure of most researchers to address this issue. In addition, a minor weakness is that the Danish study included stillbirths in the natural loss grouping while in the Finnish study stillbirths were included in delivery category. Since the number of stillbirths were not reported, we could not adjust for this difference. But given the expected low number of stillbirths, this difference in categorization is very unlikely to have a major impact on the results. Another inconsistency is that all the studies from Finland included deaths during pregnancy in with deaths following a delivery (live or stillbirth), potentially adding nine months mortality risk to the one-year post-delivery mortality rate. This would tend to inflate deaths associated with delivery. Reporting deaths during pregnancy as a separate item would be preferable. These points highlight why more consistent classification standards would be helpful in future research.
In our opinion, any pregnancy that fails to produce a live birth should be treated as a pregnancy loss since there may be grief issues impacting future health. Rare cases of multiple gestations including both live birth and fetal loss are confounding and should be excluded from more general analyses or treated as a separate group.
Future research and missed opportunities
Unfortunately, many opportunities to investigate pregnancy associated mortality and long-term mortality have been missed, to date. Our literature review found that only 11 of 68 record linkage studies (and only 2 of 37 studies in the United States) explored mortality rates associated with pregnancy loss.
This oversight can and should be corrected. Even in countries without central TOP registries, such as exist in Finland and Denmark, exposure to TOP and miscarriage can be identified through medical records and insurance claims, as shown by researchers in the United Kingdom,15 Canada,22 and in the United States.91,92 Unfortunately, except for these rare exceptions, most of the leading investigations into pregnancy associated deaths in Canada, the United Kingdom and the USA have failed to use these same techniques to investigate deaths associated with TOP or miscarriage.
Another missed opportunity appears to have occurred in a study of Italian women33 in which researchers report that they did, in fact, link death certificates to records of terminations and miscarriages, but unfortunately their published analyses failed to provide any breakdown of death rates relative to each pregnancy outcome. Our request for a breakdown of deaths associated with each type of pregnancy outcome was rejected.
The failure of so many studies to report on pregnancy loss associated deaths indicates that there may be a risk of reporting bias. For example, social, political, or academic sensitivities relative to efforts to promote legalization of safe abortion in developing countries may produce a bias against investigating and/or publishing findings that may show TOP is associated with an increase in mortality rates.94,95 On the other hand, even though such findings have been reported since at least 1997,83,84 there may also be lack of sufficient awareness among researchers regarding the elevated mortality rates associated with pregnancy loss. In either case, it is clear that in most countries where record linkage studies have been performed there are no structural obstacles to expanding record linkage studies to include pregnancy loss associated mortality. What is required is simply the academic and/or political will to undertake such investigations.
What is already sufficiently clear is that mortality rates and longevity are significantly affected by exposure to pregnancy losses, whether natural or induced. Therefore, in the interests of patients, future investigations into pregnancy associated mortality should all include efforts to identify and report on the comparative effects associated with prior exposure to TOP, miscarriage, and other natural losses. Such research is necessary to guide the development of better screening and treatment strategies for those subsets of women who may most benefit from targeted interventions.
Incidental or causal relationships?
As discussed above, termination of pregnancy remains a sensitive and politically charged issue, for both those who defend it as a fundamental woman’s right and those who oppose it for moral reasons. In our experience, these passions often inspire a hypercritical level of suspicion regarding any epidemiological findings which run counter to preconceived expectations.
For readers to access their own biases regarding this subject matter, simply imagine if our results were all reversed and the risk of death in the year following a TOP was half that associated with childbirth. Would the reader consider such reversed results more comfortable or more disturbing? Would such results provoke more confidence in the value of record linkage studies or more suspicion?
In either event, it is important to interpret these findings in as balanced a perspective as possible. Correlation does not prove causation. There may be common risk factors for pregnancy loss which explain the elevated risks.96 Indeed, given the fact that a disproportionate number of deaths associated with prior pregnancy loss are due to suicide and accidents, it would appear that causal contribution would most likely be indirect and chiefly mediated by psychological effects which are known to occur among women who experience a pregnancy loss.2–10,17–19 Moreover, the finding that there pregnancy loss has a dose effect on increased risk of death90 (Figure 6) strongly parallels the finding of pregnancy loss having a dose effect on increased risk of mental illness.2,5,13
But even if the elevated risks can be entirely explained by common risk factors, it is critically important to acknowledge that these findings are still clinically relevant and very useful. Why?
Because a history of pregnancy loss is at least a useful marker for identifying women who may need additional screening, counselling and care. Therefore, alert clinicians can and should screen for a history of pregnancy loss in order to use this actionable information as detailed in our clinical recommendations below. How this marker may be used to provide better screening and referrals will be discussed more fully in the next section.
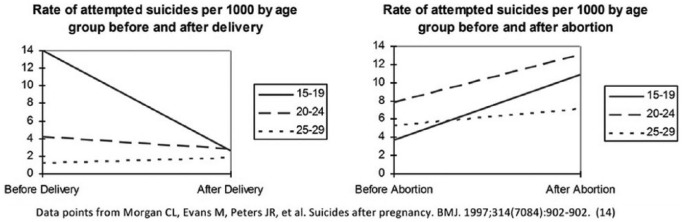
Additional support for a causal interpretation is found in studies which have identified the first onset of psychological problems, such as sleep disorders17 or substance abuse,97 soon after a pregnancy loss among women who did not previously have these problems.13 Another important study examined hospital admission rates for attempted suicide rates prior to pregnancy and after a TOP15 and revealed a significant and dramatic shift from a “normal” rate of suicide attempts to an elevated rate after TOP, as seen in Figure 8. These findings led the researchers to conclude that “the increased risk of suicide after an induced abortion may therefore be a consequence of the procedure itself.”
Figure 8.
Rate of treatments for attempted suicide before and after delivery or TOP.
Another factor to consider regarding the question of causality is that negative effects may be substantially limited to small subgroups of women who are at greater risk. For example, experts on “both sides” of the legal abortion controversy are actually in agreement regarding the evidence that women who feel coerced or pressured into unwanted TOP are at greater risk of serious complications, including elevated self-destructive tendencies.98 If we were to hypothesize, then, that all of the elevated risk of death associated with TOP reported in the studies we examined are limited to cases of coerced TOP, it would then follow that the findings reported herein may be an indirect measure of the frequency of coerced TOP. Such a conclusion would only further underscore the importance of the clinical recommendations offered in the next section.
Perhaps the most powerful evidence that pregnancy loss contributes directly to mental health problems is the frequency with which self-aware, introspective women specifically attribute the onset or worsening of substance use, depression, flashbacks, sexual dysfunction, self-destructive tendencies and other issues to their pregnancy loss experiences.93,99,100 These self-assessments are further validated by therapists treating women for pregnancy loss related issues.101,102 Additionally, evidence that post-abortion counselling programs reduce symptoms of psychological illness103 also support the hypothesis that TOP can trigger or exacerbate psychological illness; after all, an effective treatment is evidence for an accurate diagnosis.
We are not asserting that pregnancy loss is the sole cause of the elevated risk of death identified in these studies, but rather that there is ample evidence to believe pregnancy loss can be a contributing cause. The discussion above is therefore intended to emphasize the importance of research designed to better understand the causal pathways and co-occurring risk factors which can then be used to better identify women who may benefit from appropriate interventions.
Clinical recommendations
Clinician’s should be alert to the fact that a history of any pregnancy loss may impact many aspects of women’s lives. Prior pregnancy losses, voluntary or involuntary, are also sensitive issues for many women which they may hesitate to dicuss. Therefore, it is highly recommended that as a standard intake question, or in periodic updating with patients, clinicians should make a gentle, non-judgmental query: “Have you had any pregnancy losses, like a miscarriage, abortion, or still birth?” This query, which non-judgmentally names each type of pregnancy loss, gives women permission to discuss any sensitive feelings regarding past pregnancy losses and also opens up opportunities to discuss any lingering or intermittent concerns.
When women do report a prior pregnancy loss, or for women considering a termination of pregnancy, we recommend that clinicians should then investigate if additional risk factors are present. Especially useful in this regard, at least 15 risk factors for more severe reactions following TOP which have been identified by American Psychological Association Task Force on Mental Health and Abortion.104 With slight modification, these risk factors can also be applied to miscarriage and other natural losses. They are:
terminating a pregnancy that is wanted or meaningful
perceived pressure from others to terminate a pregnancy
perceived opposition to the abortion from partners, family, and/or friends
lack of perceived social support from others
various personality traits (e.g., low self-esteem, a pessimistic outlook, low-perceived control over life)
a history of mental health problems prior to the pregnancy
feelings of stigma; perceived need for secrecy
exposure to antiabortion picketing
use of avoidance and denial coping strategies
feelings of commitment to the pregnancy
ambivalence about the abortion decision
low perceived ability to cope with the abortion
history of prior abortion
late term abortion.
These risk factors can and should be used to identify women who may need more counselling and other services. Given the dose effects observed, screening for a history of pregnancy loss is especially important in preparing treatment plans for women in all subsequent pregnancies. Therefore, we recommend the APA identified screening criteria should be used on at least four occasions: (a) when women seeking mental health care report any history of pregnancy loss, (b) when women are seeking care in anticipation of becoming pregnant, (c) upon diagnosis of a pregnancy, and (d) before termination of a pregnancy.
Summary
Deaths associated with pregnancy, both within the first year and beyond, are significantly different relative to pregnancy outcome. Births have a positive effect on longevity while pregnancy losses have a negative effect, with negative effect of TOP being greater than that of natural losses. Multiple pregnancy losses are especially problematic. Pregnancy loss is at least a marker for adverse maternal outcomes, but is most likely a contributing risk factor driven by psychological stresses related to pregnancy loss.2–22
Many opportunities to investigate pregnancy loss associated long-term mortality rates have been missed. Future investigations into maternal mortality and pregnancy associated mortality should include systematic record linkage to medical and insurance records to identify pregnancy losses so that these patterns and risk factors can be better understood.
Screening for a history of pregnancy loss (induced or natural) is highly recommended as a means of identifying women who may benefit from additional counselling and interventions. Screening for risk factors associated with more psychological maladjustments following TOP, as identified by the APA,104 is also highly recommended.
Supplementary Material
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: No grants or outside funding were used. David Reardon’s efforts were funded as part of his regular duties as Director of Research with the Elliot Institute. John Thorp’s efforts were not funded.
Ethical approval: Ethical approval was not sought for the present study because it is a literature review and does involve any original research using human or animal subjects.
Informed consent: Informed consent was not sought for the present study because it is a literature review and does not involve any original research using human subjects.
- Prisma Checklist.
- Spreadsheet of Newcastle - Ottawa Quality Assessment Scale: Cohort Studies.
Trial registration: This was not a randomized clinical trial therefore it was not registered as such.
References
- 1. Gissler M, Berg C, Bouvier-Colle M-H, et al. Methods for identifying pregnancy-associated deaths: population-based data from Finland 1987–2000. Paediatr Perinat Epidemiol 2004; 18(6): 448–455. [DOI] [PubMed] [Google Scholar]
- 2. Giannandrea SAM, Cerulli C, Anson E, et al. Increased risk for postpartum psychiatric disorders among women with past pregnancy loss. J Womens Health 2013; 22(9): 760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broen AN, Moum T, Bødtker AS, et al. The course of mental health after miscarriage and induced abortion: a longitudinal, five-year follow-up study. BMC Med 2005; 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munk-Olsen T, Bech BH, Vestergaard M, et al. Psychiatric disorders following fetal death: a population-based cohort study. BMJ Open 2014; 4: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fergusson DM, Horwood LJ, Boden JM. Does abortion reduce the mental health risks of unwanted or unintended pregnancy? A re-appraisal of the evidence. Aust N Z J Psychiatry 2013; 47(9): 819–827. [DOI] [PubMed] [Google Scholar]
- 6. Coleman PK. Abortion and mental health: quantitative synthesis and analysis of research published 1995–2009. Br J Psychiatry 2011; 199: 180–186. [DOI] [PubMed] [Google Scholar]
- 7. Van den Akker OB. The psychological and social consequences of miscarriage. Expert Rev Obstet Gynecol 2011; 6(3): 295–304. [Google Scholar]
- 8. Reardon DC, Cougle JR, Rue VM, et al. Psychiatric admissions of low-income women following abortion and childbirth. CMAJ 2003; 168(10): 1253–1256. [PMC free article] [PubMed] [Google Scholar]
- 9. Coleman PK, Reardon DC, Rue VM, et al. State-funded abortions versus deliveries: a comparison of outpatient mental health claims over 4 years. Am J Orthopsychiatry 2002; 72(1): 141–152. [DOI] [PubMed] [Google Scholar]
- 10. Fergusson DM, Horwood LJ, Boden JM. Abortion and mental health disorders: evidence from a 30-year longitudinal study. Br J Psychiatry 2008; 193(6): 444–451. [DOI] [PubMed] [Google Scholar]
- 11. Coleman PK. Induced abortion and increased risk of substance abuse: a review of the evidence. Curr Womens Health Rev 2005; 1(1): 21–34. [Google Scholar]
- 12. Steinberg JR, McCulloch CE, Adler NE. Abortion and mental health: findings from the national comorbidity survey-replication. Obstet Gynecol 2014; 123(2 Pt 1): 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sullins DP. Abortion, substance abuse and mental health in early adulthood: thirteen-year longitudinal evidence from the United States. SAGE Open Med 2016; 4: 2050312116665997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shadigian E, Bauer ST. Pregnancy-associated death: a qualitative systematic review of homicide and suicide. Obstet Gynecol Surv 2005; 60(3): 183–190. [DOI] [PubMed] [Google Scholar]
- 15. Morgan CL, Evans M, Peters JR. Suicides after pregnancy. Mental health may deteriorate as a direct effect of induced abortion. BMJ 1997; 314(7084): 902. [PMC free article] [PubMed] [Google Scholar]
- 16. Tishler CL. Adolescent suicide attempts following elective abortion: a special case of anniversary reaction. Pediatrics 1981; 68(5): 670–671. [PubMed] [Google Scholar]
- 17. Reardon DC, Coleman PK. Relative treatment rates for sleep disorders and sleep disturbances following abortion and childbirth: a prospective record-based study. Sleep 2006; 29(1): 105–106. [DOI] [PubMed] [Google Scholar]
- 18. Daugirdaitė V, van den Akker O, Purewal S. Posttraumatic stress and posttraumatic stress disorder after termination of pregnancy and reproductive loss: a systematic review. J Pregnancy 2015; 2015: 646345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zulčić-Nakić V, Pajević I, Hasanović M, et al. Psychological problems sequalae in adolescents after artificial abortion. J Pediatr Adolesc Gynecol 2012; 25(4): 241–247. [DOI] [PubMed] [Google Scholar]
- 20. Ney PG, Fung T, Wickett AR, et al. The effects of pregnancy loss on women’s health. Soc Sci Med 1994; 38(9): 1193–1200. [DOI] [PubMed] [Google Scholar]
- 21. Berkeley D, Humphreys PC, Davidson D. Demands made on general practice by women before and after an abortion. J R Coll Gen Pract 1984; 34(263): 310–315. [PMC free article] [PubMed] [Google Scholar]
- 22. Østbye T, Wenghofer EF, Woodward CA, et al. Health services utilization after induced abortions in Ontario: a comparison between community clinics and hospitals. Am J Med Qual 2001; 16(3): 99–106. [DOI] [PubMed] [Google Scholar]
- 23. Dior UP, Hochner H, Friedlander Y, et al. Association between number of children and mortality of mothers: results of a 37-year follow-up study. Ann Epidemiol 2013; 23(1): 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun F, Sebastiani P, Schupf N, et al. Extended maternal age at birth of last child and women’s longevity in the long life family study. Menopause 2015; 22(1): 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McArdle PF, Pollin TI, O’Connell JR, et al. Does having children extend life span? A genealogical study of parity and longevity in the Amish. J Gerontol A Biol Sci Med Sci 2006; 61(2): 190–195. [DOI] [PubMed] [Google Scholar]
- 26. Atrash HK, Rowley D, Hogue CJ. Maternal and perinatal mortality. Curr Opin Obstet Gynecol 1992; 4(1): 61–71. [PubMed] [Google Scholar]
- 27. Pazol K, Creanga AA, Burley KD, et al. Abortion surveillance – United States, 2010. MMWR Surveill Summ 2013; 62(8): 1–44. [PubMed] [Google Scholar]
- 28. Khlat M, Ronsmans C. Deaths attributable to childbearing in Matlab, Bangladesh: indirect causes of maternal mortality questioned. Am J Epidemiol 2000; 151(3): 300–306. [DOI] [PubMed] [Google Scholar]
- 29. Sousa MH, Cecatti JG, Hardy EE, et al. Severe maternal morbidity (near miss) as a sentinel event of maternal death. An attempt to use routine data for surveillance. Reprod Health 2008; 5(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turner LA, Cyr M, Kinch RAH, et al. Under-reporting of maternal mortality in Canada: a question of definition. Chronic Dis Can 2002; 23(1): 22–30. [PubMed] [Google Scholar]
- 31. Turner LA, Kramer MS, Liu S, et al. Cause-specific mortality during and after pregnancy and the definition of maternal death. Chronic Dis Can 2002; 23(1): 31–36. [PubMed] [Google Scholar]
- 32. Andersen BR, Westergaard HB, Bødker B, et al. Maternal mortality in Denmark, 1985–1994. Eur J Obstet Gynecol Reprod Biol 2009; 142(2): 124–128. [DOI] [PubMed] [Google Scholar]
- 33. Donati S, Senatore S, Ronconi A. Maternal mortality in Italy: a record-linkage study. BJOG 2011; 118(7): 872–879. [DOI] [PubMed] [Google Scholar]
- 34. Schutte JM, Hink E, Heres MHB, et al. Maternal mortality due to psychiatric disorders in the Netherlands. J Psychosom Obstet Gynecol 2008; 29(3): 150–153. [DOI] [PubMed] [Google Scholar]
- 35. Schutte JM, de Jonge L, Schuitemaker NWE, et al. Indirect maternal mortality increases in the Netherlands. Acta Obstet Gynecol Scand 2010; 89(6): 762–768. [DOI] [PubMed] [Google Scholar]
- 36. Schutte JM, Steegers E, a P, et al. Rise in maternal mortality in the Netherlands. BJOG 2010; 117(4): 399–406. [DOI] [PubMed] [Google Scholar]
- 37. Samuelsson E, Hellgren M, Högberg U. Pregnancy-related deaths due to pulmonary embolism in Sweden. Acta Obstet Gynecol Scand 2007; 86(4): 435–443. [DOI] [PubMed] [Google Scholar]
- 38. Kvarnstrand L, Milsom I, Lekander T, et al. Maternal fatalities, fetal and neonatal deaths related to motor vehicle crashes during pregnancy: a national population-based study. Acta Obstet Gynecol Scand 2008; 87(9): 946–952. [DOI] [PubMed] [Google Scholar]
- 39. Esscher A, Högberg U, Haglund B, et al. Maternal mortality in Sweden 1988-2007: more deaths than officially reported. Acta Obstet Gynecol Scand 2013; 92(1): 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang C-C, Chiu H-F, Yang C-Y. Parity, age at first birth, and risk of death from pancreatic cancer: evidence from a cohort in Taiwan. Pancreas 2010; 39(5): 567–571. [DOI] [PubMed] [Google Scholar]
- 41. Lewis G, Drife J. (eds). Why mothers die 1997–1999: the confidential enquiries into maternal deaths in the United Kingdom. London: RCOG press, 2001. [Google Scholar]
- 42. Oates M. Perinatal psychiatric disorders: a leading cause of maternal morbidity and mortality. Br Med Bull 2003; 67: 219–229. [DOI] [PubMed] [Google Scholar]
- 43. Lewis G, Drife J. (eds). Why mothers die 2000–2002: confidential enquiry into maternal and child health. London: RCOG press, 2004. [Google Scholar]
- 44. Lewis G. (ed.). Saving mothers’ lives: reviewing maternal deaths to make motherhood safer 2003–2005. London: CEMACH, 2007. [Google Scholar]
- 45. Cantwell R, Clutton-Brock T, Cooper G, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011; 118(Suppl. 1): 1–203. [DOI] [PubMed] [Google Scholar]
- 46. Hastie CE, Smith GCS, Mackay DF, et al. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: retrospective cohort study of 750 350 singleton pregnancies. Int J Epidemiol 2011; 40(4): 914–919. [DOI] [PubMed] [Google Scholar]
- 47. Rubin G, McCarthy B, Shelton J, et al. The risk of childbearing re-evaluated. Am J Public Health 1981; 71(7): 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benedetti TJ, Starzyk P, Frost F. Maternal deaths in Washington state. Obstet Gynecol 1985; 66(1): 99–101. [PubMed] [Google Scholar]
- 49. Starzyk P, Frost F, Kobayashi J. Misclassification of maternal deaths – Washington state. MMWR Morb Mortal Wkly Rep 1986; 35(39): 621–623. [PubMed] [Google Scholar]
- 50. Comas A, Navarro A, Conde J, et al. Misreporting of maternal mortality in Puerto Rico. Bol Asoc Med P R 1990; 82(8): 343–346. [PubMed] [Google Scholar]
- 51. Allen MH, Chavkin W, Marinoff J. Ascertainment of maternal deaths in New York city. Am J Public Health 1991; 81(3): 380–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. May W, Buescher P, Murray A. Enhanced maternal mortality surveillance – North Carolina, 1988 and 1989. MMWR Morb Mortal Wkly Rep 1991; 40(28): 469–471. [PubMed] [Google Scholar]
- 53. Dye TD, Gordon H, Held B, et al. Retrospective maternal mortality case ascertainment in West Virginia, 1985 to1989. Am J Obstet Gynecol 1992; 167(1): 72–76. [DOI] [PubMed] [Google Scholar]
- 54. Floyd V, Hadley C, Lavoie M, et al. Pregnancy-related mortality – Georgia, 1990–1992. MMWR Morb Mortal Wkly Rep 1995; 44(5): 93–96. [PubMed] [Google Scholar]
- 55. Jocums S, Mitchel EF, Entman SS, et al. Monitoring maternal mortality using vital records linkage. Am J Prev Med 1995; 11(2): 75–78. [PubMed] [Google Scholar]
- 56. Harper M, Parsons L. Maternal deaths due to homicide and other injuries in North Carolina: 1992–1994. Obstet Gynecol 1997; 90(6): 920–923. [DOI] [PubMed] [Google Scholar]
- 57. Dietz PM, Rochat RW, Thompson BL, et al. Differences in the risk of homicide and other fatal injuries between postpartum women and other women of childbearing age: implications for prevention. Am J Public Health 1998; 88(4): 641–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jocums SB, Berg CJ, Entman SS, et al. Postdelivery mortality in Tennessee, 1989–1991. Obstet Gynecol 1998; 91(5 Pt 1): 766–770. [DOI] [PubMed] [Google Scholar]
- 59. Fang J, Madhavan S, Alderman MH. Maternal mortality in New York city: excess mortality of black women. J Urban Heal 2000; 77(4): 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Horon IL, Cheng D. Enhanced surveillance for pregnancy-associated mortality - Maryland, 1993–1998. J Am Med Assoc 2001; 285(11): 1455–1459. [DOI] [PubMed] [Google Scholar]
- 61. Lydon-Rochelle M, Holt VL, Easterling TR, et al. Cesarean delivery and postpartum mortality among primiparas in Washington State, 1987–1996. Obstet Gynecol 2001; 97(2): 169–174. [DOI] [PubMed] [Google Scholar]
- 62. Buescher PA, Harper M, Meyer RE. Enhanced surveillance of maternal mortality in North Carolina. N C Med J 2002; 63(2): 76–79. [PubMed] [Google Scholar]
- 63. Nannini A, Weiss J, Goldstein R, et al. Pregnancy-associated mortality at the end of the twentieth century: Massachusetts, 1990–1999. J Am Med Womens Assoc 2002; 57(3): 140–143. [PubMed] [Google Scholar]
- 64. Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance – United States, 1991–1999. MMWR Morb Mortal Wkly Rep 2003; 52(2): 1–8. [PubMed] [Google Scholar]
- 65. Baker N, Fogarty C, Stroud D, et al. Enhanced pregnancy-associated mortality surveillance: Minnesota, 1990–1999. Minn Med 2004; 87(1): 45–47. [PubMed] [Google Scholar]
- 66. Harper MA, Espeland MA, Dugan E, et al. Racial disparity in pregnancy-related mortality following a live birth outcome. Ann Epidemiol 2004; 14(4): 274–279. [DOI] [PubMed] [Google Scholar]
- 67. Horon IL, Cheng D, Chang J, et al. Underreporting of maternal deaths on death certificates and the magnitude of the problem of maternal mortality. Am J Public Health 2005; 95(3): 478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wolfe EL, Davis T, Guydish J, et al. Mortality risk associated with perinatal drug and alcohol use in California. J Perinatol 2005; 25(2): 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rosenberg D, Geller SE, Studee L, et al. Disparities in mortality among high risk pregnant women in Illinois: a population based study. Ann Epidemiol 2006; 16(1): 26–32. [DOI] [PubMed] [Google Scholar]
- 70. Watson A, Thompson D, Burch D, et al. Pregnancy-related mortality report, Florida 1999–2005. Tallahassee, FL: Florida Department of Health, 2008. [Google Scholar]
- 71. Kavanaugh VM, Fierro MF, Suttle DE, et al. Psychosocial risk factors as contributors to pregnancy-associated death in Virginia, 1999–2001. J Womens Health 2009; 18(7): 1041–1048. [DOI] [PubMed] [Google Scholar]
- 72. Horon IL, Cheng D. Effectiveness of pregnancy check boxes on death certificates in identifying pregnancy-associated mortality. Public Health Rep 2011; 126(2): 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tran T, Roberson E, Borstell J, et al. Evaluation of pregnancy mortality in louisiana using enhanced linkage and different indicators defined by WHO and CDC/ACOG: challenging and practical issues. Matern Child Health J 2011; 15(7): 955–963. [DOI] [PubMed] [Google Scholar]
- 74. Burch D, Noell D, Hill WC, et al. Pregnancy-associated mortality review: the Florida experience. Semin Perinatol 2012; 36(1): 31–36. [DOI] [PubMed] [Google Scholar]
- 75. Burlingame J, Horiuchi B, Ohana P, et al. Sauvage LMM. The contribution of heart disease to pregnancy-related mortality according to the pregnancy mortality surveillance system. J Perinatol 2012; 32(3): 163–169. [DOI] [PubMed] [Google Scholar]
- 76. Mitchell C, Lawton E, Morton C, et al. California pregnancy-associated mortality review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J 2014; 18(3): 518–526. [DOI] [PubMed] [Google Scholar]
- 77. Main EK, McCain CL, Morton CH, et al. Pregnancy-related mortality in California: causes, characteristics, and improvement opportunities. Obstet Gynecol 2015; 125(4): 938–947. [DOI] [PubMed] [Google Scholar]
- 78. Hardt N, Wong TD, Burt MJ, et al. Prevalence of prescription and illicit drugs in pregnancy-associated non-natural deaths of florida mothers, 1999–2005. J Forensic Sci 2013; 58(6): 1536–1541. [DOI] [PubMed] [Google Scholar]
- 79. Hardt NS, Eliazar J, Burt M, et al. Use of a prenatal risk screen to predict maternal traumatic pregnancy-associated death: program and policy implications. Womens Health Issues; 23(3): e187–e193. [DOI] [PubMed] [Google Scholar]
- 80. Salanave B, Bouvier-Colle M-H, Varnoux N, Alexander S, et al. Classification differences and maternal mortality: a European study. Int J Epidemiol 1999; 28(1): 64–69. [DOI] [PubMed] [Google Scholar]
- 81. Deneux-Tharaux C, Berg C, Bouvier-Colle MH, et al. Underreporting of pregnancy-related mortality in the United States and Europe. Obstet Gynecol 2005; 106(4): 684–692. [DOI] [PubMed] [Google Scholar]
- 82. Gissler M, Deneux-Tharaux C, Alexander S, et al. Pregnancy-related deaths in four regions of Europe and the United States in 1999-2000: characterisation of unreported deaths. Eur J Obstet Gynecol Reprod Biol 2007; 133(2): 179–185. [DOI] [PubMed] [Google Scholar]
- 83. Gissler M, Hemminki E, Lönnqvist J, et al. Suicides after pregnancy in Finland, 1987-94: register linkage study. BMJ 1996; 313(7070): 1431–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gissler M, Kauppila R, Meriläinen J, et al. Pregnancy-associated deaths in Finland 1987–1994 – definition problems and benefits of record linkage. Acta Obstet Gynecol Scand 1997; 76(7): 651–657. [DOI] [PubMed] [Google Scholar]
- 85. Gissler M, Hemminki E. Pregnancy-related violent deaths. Scand J Public Health 1999; 27: 54–55. [PubMed] [Google Scholar]
- 86. Gissler M, Berg C, Bouvier-Colle M-H, et al. Pregnancy-associated mortality after birth, spontaneous abortion, or induced abortion in Finland, 1987–2000. Am J Obstet Gynecol 2004; 190(2): 422–427. [DOI] [PubMed] [Google Scholar]
- 87. Gissler M, Berg C, Bouvier-Colle M-H, et al. Injury deaths, suicides and homicides associated with pregnancy, Finland 1987–2000. Eur J Public Health 2005; 15(5): 459–463. [DOI] [PubMed] [Google Scholar]
- 88. Gissler M, Karalis E, Ulander V-M. Decreased suicide rate after induced abortion, after the Current Care Guidelines in Finland 1987–2012. Scand J Public Health 2014; 43(1): 99–101. [DOI] [PubMed] [Google Scholar]
- 89. Reardon DC, Coleman PK. Short and long term mortality rates associated with first pregnancy outcome: population register based study for Denmark 1980–2004. Med Sci Monit 2012; 18(9): PH71–PH76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Coleman PK, Reardon DC, Calhoun BC. Reproductive history patterns and long-term mortality rates: a Danish, population-based record linkage study. Eur J Public Health 2013; 23(4): 569–574. [DOI] [PubMed] [Google Scholar]
- 91. Shelton JD, Schoenbucher AK. Deaths after legally induced abortion linkage. Public Health Rep 1978; 93(4): 375–378. [PMC free article] [PubMed] [Google Scholar]
- 92. Reardon DC, Ney PG, Scheuren F, et al. Deaths associated with pregnancy outcome: a record linkage study of low income women. South Med J 2002; 95(8): 834–841. [PubMed] [Google Scholar]
- 93. Burke T, Reardon DC. Forbidden grief: the unspoken pain of abortion. Springfield IL: Acorn Books, 2007, p. 334. [Google Scholar]
- 94. Raymond EG, Grimes DA. The comparative safety of legal induced abortion and childbirth in the United States. Obstet Gynecol 2012; 119(2 Pt 1)215–219. [DOI] [PubMed] [Google Scholar]
- 95. Grimes DA, Benson J, Singh S, et al. Unsafe abortion: the preventable pandemic. Lancet 2006; 368: 1908–1919. [DOI] [PubMed] [Google Scholar]
- 96. Major B. Psychological implications of abortion – highly charged and rife with misleading research. CMAJ 2003; 168(10): 1257–1258. [PMC free article] [PubMed] [Google Scholar]
- 97. Reardon DC, Ney PG. Abortion and subsequent substance abuse. Am J Drug Alcohol Abuse 2000; 26(1): 61–75. [DOI] [PubMed] [Google Scholar]
- 98. Coyle CT. Coercion and/or pressure. In: Macnair RM. (ed.) Peace psychology perspectives on abortion. Kansas City, MO: Feminism & Nonviolence Studies Association, 2016. [Google Scholar]
- 99. Stotland NL. Abortion: social context, psychodynamic implications. Am J Psychiatry 1998; 155(7): 964–967. [DOI] [PubMed] [Google Scholar]
- 100. Rue VM, Coleman PK, Rue JJ, et al. Induced abortion and traumatic stress: a preliminary comparison of American and Russian women. Med Sci Monit 2004; 10(10): SR5–SR16. [PubMed] [Google Scholar]
- 101. De Puy C, Dovitch D. The healing choice: your guide to emotional recovery after an abortion. New York: Simon & Schuster, 1997. [Google Scholar]
- 102. Torre-Bueno A. Peace after abortion. San Diego, CA: Pimpernel Press, 1997. [Google Scholar]
- 103. Layer SD. Postabortion grief: evaluating the possible efficacy of a spiritual group intervention. Res Soc Work Pract 2004; 14(5): 344–350. [Google Scholar]
- 104. Major B, Appelbaum M, Beckman L, et al. Report of the APA task force on mental health and abortion. Washington, DC: American Psychological Association, 2008, p. 105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.