Abstract
A tailored therapy to patient requirements by combining endovascular and surgical steps can be necessary to prolong the life of a vascular access. Stent grafts play a growing role for the therapy of dialytic access complications. Randomized multi-center trials, however, support the on-label use of stent grafts in the treatment of graft venous outflow and in-stent restenosis. The main contraindication to their use is an ongoing infection. We report two cases of new off-label application of Viabahn (Gore, flagstaff, USA) stent graft. In the first case, the failure of a radiocephalic early cannulation graft was treated by stent graft placement on the arterial inflow anastomosis, when emergent angiographic examination revealed the previously unknown high takeoff of the radial artery from the axillary artery. At 13-month follow-up, the target lesion remained untreated. In the second case, elbow stent graft occlusion with extended thrombosis occurred in a right radiocephalic fistula after 3 years of unassisted patency. Being the last option for vascular access, successful endovascular recanalization was carried out. After 3 months, however, the clinical setting relapsed. A two-stage hybrid strategy with vascular surgeon was arranged due to ongoing signs of local infection. Flow was restored by emergent thromboaspiration associated with a new stent graft placement as a endovascular bridge to subsequent surgical treatment. After 2 days, the overlapped stent grafts were excised as planned. Surgical rerouting was completed by polytetrafluoroethylene prosthetic bridge implantation across the elbow. At 4 months, the follow-up remained uneventful. In selected instances, the off-label use of stent grafts may expand the therapeutic options of the vascular access team.
Keywords: Arteriovenous access, early cannulation, hemodialysis, infection, nephrology, radiology, stent graft, surgery, trans-anastomotic
Introduction
The multi-disciplinary team dealing with hemodialysis (HD) access should evaluate systemic and local patient features before choosing a therapeutic strategy. Comorbidities and clinical settings, residual vein capital, anatomic challenges and future perspectives regarding HD access and life expectancy, as well as local availability of technical resources should be included in the judgment.1 We present two HD patients, one dialyzing through an arteriovenous fistula (AVF) and the other one through an arteriovenous graft (AVG). Both patients were treated with off-label endovascular stent grafting combined with surgical treatment for malfunctioning vascular access (VA). In both cases, the reported treatment ensured the continued efficient functioning of the native or prosthetic VA for HD, and prevented the need for a bridge central venous catheter (CVC).
Case 1
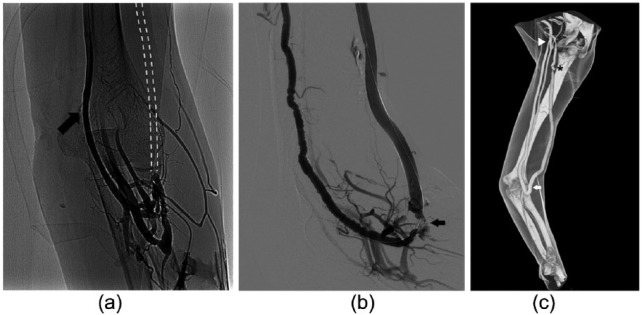
A 68-year-old female on HD for 1 year as a result of glomerulonephritis was seen for a malfunctioning proximal radiocephalic AVF, created by an interventional nephrologist of the VA team. Diagnostic angiography showed a patent arteriovenous (AV) anastomosis and perianastomotic vein and chronic occlusion of a long segment of the cephalic vein (Figure 1(a)). An “early cannulation graft” (Acuseal, Gore, Flagstaff USA) was implanted as venous bridge on the cephalic vein, maintaining her native AV anastomosis as inflow. After 2 months, she was newly admitted for VA dysfunction. US-guided phlebography showed low flow along the graft (Figure 1(b)). No definite opacification of the AV anastomosis was obtained. A 0.014″ guide wire was negotiated up the arterial side via a retrograde approach. A 4F angled diagnostic catheter was exchanged on the wire for diagnostic angiography. The previously unknown high takeoff of the radial artery from the axillary artery, an anatomical variation referred to as brachioradial artery, was discovered. A critical stenosis of >90% on the arterial anastomosis was clearly depicted. Initially, repeated long inflation angioplasty (percutaneous transluminal angioplasty (PTA)) with high-pressure low-profile catheter balloons of 4 and 5 mm was attempted, but immediate elastic recoil occurred with persisting stenosis. A 6 mm × 100 mm Viabahn stent graft (Gore, Flagstaff, USA) was thus deployed through a 6F sheath from the radial artery across the anastomosis and the swing vein, to land in the healthy zone of the cephalic vein. Trans-anastomotic intrastent post dilation to 6 mm was carried out along the entire length of the stent graft. The side-to-end AVG was transformed into an end-to-end anastomosis with the exclusion of the distal radial artery. Thrill was perceived immediately, and the postprocedure period was uneventful. The patient underwent efficient HD the following day without problem to the hand that presents a good ulnar artery flow. A computed tomography angiography (CTA) performed 4 months later to study central veins confirmed the neo-anastomotic target lesion patency. It revealed a stenosis on the cephalic arch, which was treated by PTA with drug eluting balloon 1 month later (Figure 1(c)). Circuit access and target lesion were patent at the 13-month follow-up.
Figure 1.
(a) Venography of the AVF by direct puncture of the juxta-anastomotic vein during manual compression of the brachial artery: regular patency of the arteriovenous anastomosis and the occlusion of the cephalic vein above the anastomosis. Main drainage through the basilic vein (arrow). Dashed line: schematic representation of the occluded cephalic vein. (b) Fistulography showing vasospasm of the high takeoff radial artery, occlusion of the graft’s arterial inflow (arrow) and the patency of the “early cannulation” graft. (c) Volume rendering CTA 4 months after trans-anastomotic Viabahn stent graft (Gore, Flagstaff, USA) (arrow) release showing circuit patency. High takeoff of the radial artery from the axillary artery is clearly depicted (arrowhead). Patency of the ulnar and interosseus artery to the forearm from the brachial artery. Residual stenosis at the graft-vein anastomosis, treated 1 month later (star).
Case 2
A 75-year-old man on HD for 7 years was referred to us as tertiary center for a malfunctioning right radiocephalic fistula. Comorbidities included coronary artery disease (CAD) with previous aortic coronary bypass and peripheral arterial disease (PAD). Access history included implanted cardiac defibrillator–related chronic occlusion of the left central venous axis, right brachiocephalic vein angioplasty and the implantation 3 years earlier of a 8 mm × 50 mm Viabahn stent graft at the elbow for a relapsing obstructive lesion of the basilic vein without necessity for further intervention. On admittance, the patient presented an abrupt occlusion of the stent graft with fresh thrombus spreading extensively above the elbow detected at ultrasound and confirmed by fistulography. A 4-mm skin ulcer overlying the stent was present. Skin swab culture was negative and other signs of infection were absent (white blood cell count (WBC) of 6160 µL, HB of 10.5 g\dL, erythrocyte sedimentation rate (ESR) of 37 mm\h, C-reactive protein (CRP) of 3.1 mg\dL). The thrombus was aspirated percutaneously and a new stent graft (8 mm × 100 mm) was placed overlapping the previous one (Figure 2(a)) gaining AVF’s thrill. Patient refused surgical revision and returned to the referring dialysis center. After 3 months, the patient was readmitted with fever (WBC of 13,170 µL, ESR of 62 mm\h, CRP of 8.3 mg\dL). A mild edema from the hand to the elbow was appreciated on the AVF side. Another skin ulcer, found to be infected with Gram positive bacteria, was present at a HD puncture site. Broad spectrum antibiotic therapy was started. The AVF anastomosis was pulsing and still patent on ultrasound examination but thrill was absent. Phlebography revealed complete occlusion of the stent grafts with an extended thrombosis of 14 cm along the basilic vein above the elbow (Figure 2(b)). After VA team evaluation, a surgical prosthetic bridge was deemed unfeasible at the time due to the extended basilic thrombosis. A two-stage hybrid repair was opted for. First, a new percutaneous recanalization as endovascular bridge was performed. Manual thrombus aspiration and multiple high-pressure PTAs were done through a 8Fr sheath. Due to unsuccessful hemodynamic result, a third 8 mm × 50 mm Viabahn stent graft was finally released to restore AVF flow. Thrill reappeared and patency of the circuit access including the basilic vein was confirmed at completion angiography. The patient underwent efficient HD through the AVF the same day. After 3 days, he underwent the planned surgical removal of the three overlapping infected stent grafts. Because an “early cannulation” graft was not available, surgical rerouting was done with a polytetrafluoroethylene (PTFE) graft (Propaten, Gore, Flagstaff, USA) to bridge the forearm AVF efferent vein and the basilic vein. During the graft maturation time, HD puncture was allowed on the proximal and distal vein segments (Figure 2(c)). The association of endovascular and surgical treatment gave a double benefit: the salvage of the last VA and the excision of the infected stent grafts avoiding the placement of a temporary venous access. The patient remained asymptomatic at 4 months control.
Figure 2.
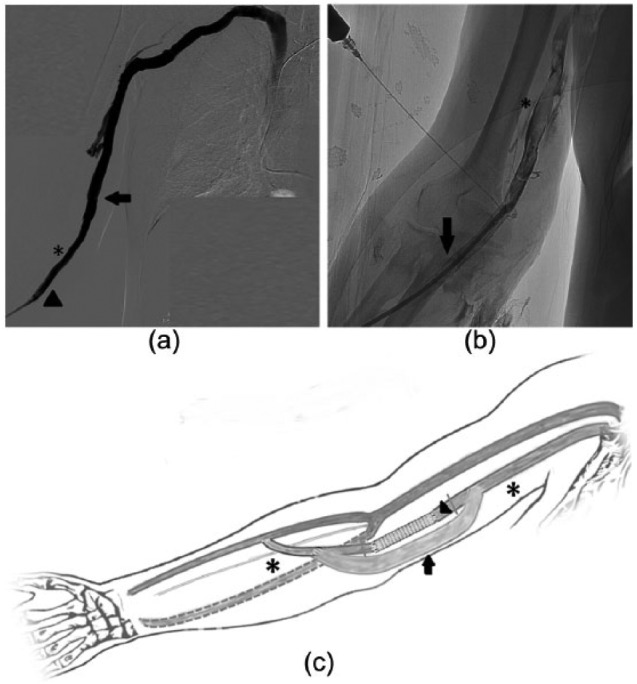
(a) Completion venography after first endovascular reintervention. Restored flow along the venous axis through a new Viabahn stent graft (arrowheads) overlapping the old stent graft (stars), no residual thrombus in the efferent basilic vein (arrow). (b) After 3 months: angiography through the occlusion of the second Viabahn stent graft in correspondence of the proximal edge of the first one (arrow). Some encroaching of the Nitinol mesh seems appreciable. Floating thrombus extended above the elbow in the basilic vein (star). (c) Schematic drawing illustrates final situation: removal of the stent grafts (arrowhead) and surgical implantation of a PTFE bridge (arrow) on the venous line preserving the native arteriovenous fistula (early cannulation graft was not available). Stars indicate the available native areas for HD venipuncture during graft maturation.
Discussion
While angioplasty remains the gold standard for treating venous stenoses causing dysfunctions in HD access, frequent reinterventions are required to maintain the patency of target lesions. Bare metal stents, useful in preventing elastic recoil, are subject to restenosis and give no long-term improvement in patency compared to PTA.1,2 PTFE stent grafts combine the scaffolding property of Nitinol with the capability of PTFE to block the transmesh overgrowth of neointimal hyperplasia, considered the main cause of restenosis and thrombosis. They are currently recommended as rescue devices for venous rupture, exclusion of venous aneurysms and pseudoaneurysms in HD access.2 The on-label indication for stent grafts, which are based on the data from multi-center randomized studies,3–6 is the treatment of graft venous outflow and in-stent restenosis in AVGs and AVFs.4–6 Unlike the FLAIR study, the REVISE study—on Viabahn stent grafts—included thrombosed AVG and location across the elbow.4 The deployment of a trans-anastomotic stent graft from the arterial anastomosis to the perianastomotic vein has not been reported in the literature. Swinnen et al.7 reported the use of trans-anastomotic bare metal stents in wrist radiocephalic AVFs stenosis, where distal ischemia risk is negligible even in the presence of end-to-end anastomosis. In the first case, a patient who had already suffered the failure of two AVFs and one AVG, stenosis of the arterial inflow anastomosis caused severe access malfunction, entailing the risk of completely losing the left upper side for future Vascular access (VAs). The off-label deployment of a stent graft on the arterial inflow of the surgical graft created a neo-anastomosis. The blood flow in the HD circuit was restored and the AVG placed on the cephalic vein was preserved. The stenting of the AVG arterial inflow was enabled by the discovery of the high takeoff of the radial artery: in a patient with normal vessel anatomy, the deployment of a trans-anastomotic stent graft extended to the brachial artery would have excluded the distal forearm arterial branches, causing hand ischemia. According to Rodríguez-Niedenführ et al.,8 this anatomical pattern, the most frequently encountered arterial variation in the arm and forearm, should be referred to as brachioradial artery. The presence at the antecubital fossa of an anastomosis between the brachioradial variant and the brachial artery distinguishes this anatomical variant from superficial brachioradial artery8 and may explain the missed diagnosis at the first phlebography, in which the forearm arterial branches but not the anatomic radial artery variant, were visible. In final analysis of this case, we can assume that the positioning of the stent graft in emergency clinical setting allowed to correct a previously misdiagnosed anatomic picture which had led to early failure of AVF and AVG. The second patient presented two times with acute occlusion of the AVF circuit at the elbow, with extended thrombosis from the stent graft (SG) in the basilic vein. The wrist radiocephalic AVF was functioning, however. Surgical rerouting was proposed, but the extended thrombosis above the infected stent grafts impeded to adopt this solution as first line therapy. Given the chronic occlusion of the left central axis, a right jugular CVC through an already treated anonymous vein was kept as an obliged last therapeutic option. Stent graft revascularization alone has been reported as successful9 therapy for infected AVG at risk for life-threatening hemorrhage. The early relapse of infected thrombosis prompted us for a resolutive strategy combining an endovascular recanalization as bridge to a surgical rerouting. Subtotal and partial graft excision associated with graft rerouting through adjacent sterile tissue has been proposed by Ryan et al.10 to treat local AVG infection without the need of temporary venous access in the latter case. Re-infection after less than total excision of infected AVG has been reported as low as 15%.11 This strategy has been applied to general surgery. Combinations of endovascular and surgical steps to treat aorto-enteric and iliac-enteric fistulas have been described.12,13 In such situations, an emergent endovascular stent graft is deployed to stop or prevent a life-threatening hemorrhage caused by local infection in order to buy time for a less risky and demolitive elective open surgical intervention. The second step may include the excision of the previously implanted stent graft to allow the durable correction of the infected area.12 This strategy proved to be correct to save the VA. A temporary stent graft was thus deployed as an endovascular bridge to allow the successive placement of an elective surgical prosthetic bridge with rerouting of the VA. Although questionable, the maneuver preserved the VA and expanded the room for HD venipuncture. Among the concerns related to stent grafts, for VA, salvage is the risk of infections. Indeed, they are prone to metastatisation by hematogenous infective agents prior to full endothelization.14 Venipuncture and thrombosis of the stent graft increase the risk of infection, particularly in the presence of skin breaks.14–20 However, although not authorized in the product instructions, the venipuncture of SGs for dialysis has been reported as being carried out routinely without complications.17,18 Only one stent graft in 106 patients treated for venous stenosis with PTFE stent graft required excision following infection as reported by Dolmatch et al.16
Conclusion
Stent grafts play a relevant role in the treatment of dysfunctional AV dialytic accesses: their peculiar properties may extend therapeutic options, enabling tailored off-label solutions in selected instances. The AV access should be always pursued as it is the preferred lifeline for dialysis in prevalent patients, and TCVC time dependence should be minimized for the related infectious complications rate. Furthermore, the stent graft can enable to fully restore flow in thrombosed access when angioplasty alone is not capable to overcome stenosis causing the clot formation. In our experience, stent graft allowed both the salvage of the arterial early cannulation graft inflow anastomosis in the first case and the hybrid strategy combining endovascular bridge with a staged surgical rerouting graft in the second.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The authors confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies. The principles outlined in the Declaration of Helsinki have been followed.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: The authors confirm that written patient consent and permission to publish have been obtained.
References
- 1. National Kidney Foundation. Clinical practice guidelines for vascular access. Am J Kidney Dis 2006; 48: S176–S275. [DOI] [PubMed] [Google Scholar]
- 2. Gray RJ, Sacks D, Martin LG, et al. Reporting standards for percutaneous interventions in dialysis access. J Vasc Interv Radiol 2003; 14: S433–S442. [DOI] [PubMed] [Google Scholar]
- 3. Haskal ZJ, Trerotola S, Dolmatch B, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med 2010; 362(6): 494–503. [DOI] [PubMed] [Google Scholar]
- 4. Vesely T, DaVanzo W, Behrend T, et al. Balloon angioplasty versus Viabahn stent graft for treatment of failing or thrombosed prosthetic hemodialysis grafts. J Vasc Surg 2016; 64(5): 1400–1410. [DOI] [PubMed] [Google Scholar]
- 5. Haskal ZJ, Saad TF, Hoggard JG, et al. Prospective, randomized, concurrently-controlled study of a stent graft versus balloon angioplasty for treatment of arteriovenous access graft stenosis: 2-year results of the RENOVA study. J Vasc Interv Radiol 2016; 27(8): 1105–1114. [DOI] [PubMed] [Google Scholar]
- 6. Falk A, Maya ID, Yevzlin AS, et al. A prospective, randomized study of an expanded polytetrafluoroethylene stent graft versus balloon angioplasty for in-stent restenosis in arteriovenous grafts and fistulae: two-year results of the RESCUE study. J Vasc Interv Radiol 2016; 27: 1465–1476. [DOI] [PubMed] [Google Scholar]
- 7. Swinnen J, Lean Tan K, Allen R, et al. Juxta-anastomotic stenting with aggressive angioplasty will salvage the native radiocephalic fistula for dialysis. J Vasc Surg 2015; 61(2): 436–442. [DOI] [PubMed] [Google Scholar]
- 8. Rodríguez-Niedenführ M, Vázquez T, Nearn L, et al. Variations of the arterial pattern in the upper limb revisited: a morphological and statistical study, with a review of the literature. J Anat 2001; 199(Pt 5): 547–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith G, Dunne J, Singh N, et al. Emergency hybrid repair of an infected pseudo-aneurysm of an axillary dialysis necklace graft. J Vasc Endovasc Surg 2016; 1: 4. [Google Scholar]
- 10. Ryan SV, Calligaro KD, Dougherty MJ. Management of hemodialysis access infections. J Vasc Surg 2004; 39(1): 73–78. [DOI] [PubMed] [Google Scholar]
- 11. Sgroi MD, Kirkpatrick VE, Resnick KA, et al. Less than total excision of infected prosthetic PTFE graft does not increase the risk of reinfection. Vasc Endovascular Surg 2015; 49(1–2): 12–15. [DOI] [PubMed] [Google Scholar]
- 12. Franchin M, Tozzi M, Piffaretti G, et al. Emergency endovascular “bridge” treatment for iliac-enteric fistula. Cardiovasc Intervent Radiol 2011; 34(5): 1106–1108. [DOI] [PubMed] [Google Scholar]
- 13. Marone EM, Mascia D, Kahlberg A, et al. Emergent endovascular treatment of a bleeding recurrent aortoenteric fistula as a “bridge” to definitive surgical repair. J Vasc Surg 2012; 55(4): 1160–1163. [DOI] [PubMed] [Google Scholar]
- 14. Asif A, Gadalean F, Eid N, et al. Stent graft infection and protrusion through the skin: clinical considerations and potential medico-legal ramifications. Semin Dial 2010; 23(5): 540–542. [DOI] [PubMed] [Google Scholar]
- 15. Beathard GA. Bacterial colonization of thrombosed dialysis arteriovenous grafts. Semin Dial 2015; 28(4): 446–449. [DOI] [PubMed] [Google Scholar]
- 16. Dolmatch BL, Duch JM, Winder R, et al. Salvage of angioplasty failures and complications in hemodialysis arteriovenous access using the FLUENCY plus stent graft: technical and 180-day patency results. J Vasc Interv Radiol 2012; 23(4): 479–487. [DOI] [PubMed] [Google Scholar]
- 17. Bent CL, Rajan DK, Tan K, et al. Effectiveness of stent-graft placement for salvage of dysfunctional arteriovenous hemodialysis fistulas. J Vasc Interv Radiol 2010; 21(4): 496–502. [DOI] [PubMed] [Google Scholar]
- 18. Rhodes ES, Silas AM. Dialysis needle puncture of Wallgrafts placed in polytetrafluoroethylene hemodialysis grafts. J Vasc Interv Radiol 2005; 16(8): 1129–1123. [DOI] [PubMed] [Google Scholar]
- 19. Kim CY, Guevara CJ, Engstrom BI, et al. Analysis of infection risk following covered stent exclusion of pseudoaneurysms in prosthetic arteriovenous hemodialysis access grafts. J Vasc Interv Radiol 2012; 23(1): 69–74. [DOI] [PubMed] [Google Scholar]
- 20. Benrashid E, Youngwirth LM, Mureebe L, et al. Operative and perioperative management of infected arteriovenous grafts. J Vasc Access 2017; 18(1): 13–21. [DOI] [PubMed] [Google Scholar]