Abstract
Objectives:
Mortality rates at 10 years are higher in diabetic patients with chronic lower extremity peripheral arterial disease than in non-diabetic peripheral arterial disease patients. We tested the hypothesis that the predictors of mortality differ between diabetic and non-diabetic peripheral arterial disease patients.
Methods:
We studied 331 consecutive patients who were <75 years of age, symptomatic for peripheral arterial disease, and admitted to a tertiary care hospital. Our cohort included 216 patients without diabetes mellitus and 115 with diabetes mellitus. The outcome measure was all-cause mortality at 10 years post-admission.
Results:
Mortality rates at 10 years were 29% among non-diabetic peripheral arterial disease patients and 58% among diabetic peripheral arterial disease patients. We identified the following independent predictors of death in the 216 peripheral arterial disease patients without diabetes: age ≥65 years (risk ratio: 2.15; 95% confidence interval: 1.28–3.59), ankle brachial index <0.60 mmHg/mmHg (risk ratio: 1.88; 95% confidence interval: 1.14–3.08), history of peripheral arterial disease-specific intervention (risk ratio: 1.81; 95% confidence interval: 1.10–2.97), and high-sensitivity C-reactive protein ≥5.0 mg/L (risk ratio: 2.11; 95% confidence interval: 1.28–3.47). For the 115 peripheral arterial disease patients with diabetes, independent predictors of mortality were as follows: age ≥65 years (risk ratio: 1.72; 95% confidence interval: 1.05–2.83) and amino-terminal pro-B-type natriuretic peptide ≥125 ng/L (risk ratio: 2.10; 95% confidence interval: 1.22–3.60).
Conclusion:
In this study, the predictors of death at 10 years differed between peripheral arterial disease patients with and without diabetes. Among the biomarkers tested, high-sensitivity C-reactive protein was independently associated with outcomes in non-diabetic patients, whereas amino-terminal pro-B-type natriuretic peptide was an independent predictor of death in patients with diabetes. Our findings suggest that in future studies, risk assessment and treatment strategies should be differentially applied to the two peripheral arterial disease subgroups.
Keywords: Atherosclerosis, biomarkers, diabetes mellitus, mortality, peripheral artery disease
Introduction
We recently demonstrated that the 10-year mortality rates for patients with chronic lower extremity peripheral artery disease (PAD) differ between those with diabetes mellitus and those without.1 Specifically, in our previous study, of 331 individuals <75 years of age with symptomatic lower extremity PAD, 216 patients had diabetes mellitus, and 115 patients did not have diabetes mellitus.1 After a 10-year follow-up period, 63 (29%) of the 216 non-diabetic PAD patients and 67 (58%) of the 115 diabetic PAD patients had died.1 Therefore, PAD patients with diabetes had a significantly higher risk of mortality within 10 years than did non-diabetic PAD patients.1 Our finding that diabetes is a primary factor associated with increased mortality in PAD patients at 10 years might have implications for the development of a differentiated and optimized treatment strategy for non-diabetic and diabetic PAD patients. To further investigate this finding, in this study, we sought to evaluate the extent to which predictors of all-cause mortality at 10 years differ between non-diabetic and diabetic PAD patients – this specific study question was already stated in our original paper on the 10-year outcome data in PAD patients.1
It is well documented that patients with PAD have impaired survival, with a substantially increased 5- to 10-year mortality compared with healthy individuals.2 In addition, it is evident from the literature – for example – that the prevalence of diabetes mellitus and the degree of systemic inflammation are higher in patients with PAD than in patients with coronary artery disease.3,4 Interestingly, despite the increased risk of adverse outcomes in patients with lower extremity PAD, risk stratification has received relatively little attention in this population.5 This contrasts sharply with the attention paid to patients who have established coronary artery disease, as extensive investigations have been conducted to identify tools to improve risk assessment in the latter group.5 Strategies to improve risk assessment should not only consider ‘classical’ risk factors, such as smoking, diabetes mellitus, arterial hypertension, and PAD disease severity, but also involve the use of biomarkers reflecting the various pathophysiological pathways of vascular disease. The widely studied biomarkers for risk assessment in PAD patients include high-sensitivity C-reactive protein (hs-CRP) and the amino-terminal fragment of the B-type natriuretic peptide prohormone (NT-proBNP).6–11 The studies investigating biomarkers as risk prediction tools in PAD, however, have reported conflicting results.
In this study, we sought to determine predictors of death at 10 years, including ‘classical’ risk factors and biomarkers, in symptomatic PAD patients. Based on our 5-year outcome data in patients with lower extremity PAD,8 we hypothesized that the predictors of 10-year all-cause mortality would differ between diabetic and non-diabetic PAD patients. To test this hypothesis, we evaluated a cohort of PAD patients aged <75 years from the Linz Peripheral Arterial Disease (LIPAD) study as the primary analysis if this work.
Materials and methods
Study design and patient recruitment
The study design and methods were described in detail previously.1,12 Briefly, the LIPAD study was prospectively designed to evaluate possible biomarkers for PAD and to determine the predictive value of those markers for the long-term outcomes of patients with established PAD.1,12 The study protocol was approved by the local ethics committee in accordance with the Declaration of Helsinki, and all study participants provided informed consent.1,12 From April 2000 to April 2002, we consecutively enrolled all patients with symptomatic chronic atherosclerotic PAD who had been admitted to a single tertiary care hospital in Austria (Konventhospital der Barmherzigen Brüder Linz).1,12 The exclusion criteria were a history of or presence of the following: (1) any malignancy, (2) acute PAD (i.e. acute thromboembolic vascular occlusion), or (3) PAD resulting from non-atherosclerotic causes.1,12 All patients with PAD were Caucasian.
The entire LIPAD cohort included 487 consecutive patients with symptomatic lower extremity PAD as previously described.1,8,12 Patient age at the time of enrolment in the study ranged from 38 to 94 years. As described in the original article on the LIPAD cohort,12 we stratified the entire cohort into two groups according to patient age, as follows: 331 individuals <75 years of age (216 did not have diabetes mellitus and 115 did have diabetes mellitus) and 156 individuals ≥75 years of age (102 did not have diabetes mellitus and 54 did have diabetes mellitus). For this study, we assessed all-cause mortality at 10 years in our PAD cohort, and – in keeping with the nature of such a long-term study – we evaluated the 331 PAD patients <75 years of age as the primary analysis.1 However, for the information of the reader, we provide the data on the 156 PAD patients ≥75 years of age as the secondary analysis as well.
As previously described, we obtained the following data on the PAD patients in our sample:1,12 patient history, including an evaluation of comorbidities of and existing risk factors for atherosclerotic disease; Doppler segmental blood pressure of the lower limbs, including continuous-wave spectral analysis and resting ankle brachial index (ABI) measurements; and colour duplex ultrasound scanning of the carotid bifurcation and internal carotid artery. Furthermore, we performed contrast intra-arterial aortofemoral angiography in all PAD patients to confirm the presence of lower extremity PAD and to characterize the site and extent of stenoses and/or occlusions of the lower limb arteries.1,12 Symptomatic lower extremity PAD was classified as claudication or critical limb ischaemia.1,12,13
Definition of comorbidities
Coronary artery disease was defined as a documented history of percutaneous transluminal coronary angioplasty or coronary bypass surgery, previous acute coronary syndrome (i.e. myocardial infarction or stable or instable angina), or occult myocardial infarction based on electrocardiography.1,12 Cerebrovascular disease was defined as a history of stroke with permanent neurological deficit or as a history of transient or temporary stroke.1,12 Cardiovascular comorbidity was defined as having coronary artery disease, cerebrovascular disease, or both.1 Symptomatic heart failure was defined as a clinical diagnosis based on a careful history and physical examination, with typical symptoms and signs of volume overload including an enlarged heart and congestion observed on a chest X-ray, and structural or functional abnormalities observed by echocardiography when performed.11 Arterial hypertension was defined as a systolic blood pressure ≥145 mmHg, a diastolic blood pressure ≥90 mmHg, or the use of any antihypertensive medication.13 Diabetes mellitus was defined as a fasting blood glucose level ≥126 mg/dL or the use of any glucose-lowering medication.13 Current smoking was defined as any amount of tobacco use, including abstinence for less than 1 year.13
Biochemical analyses
We collected blood by venepuncture upon hospital admission after overnight fasting. Creatinine, estimated glomerular filtration rate (eGFR), lipid profiles, glucose, glycohaemoglobin A1c, hs-CRP, and NT-proBNP were analysed using the appropriate methods, which have been described in detail previously.8,11 These parameters were measured for each PAD patient enrolled in this study (no missing values). The vascular surgeons and the attending physicians were blinded to the levels of hs-CRP and NT-proBNP, but not to creatinine, eGFR, lipid profiles, glucose, and glycohaemoglobin A1c (which were routine clinical measurements) during the entire follow-up period.
Outcome assessment
Mortality data were obtained from the Austrian Mortality Registry, with the date of death and cause of death encoded either before 2002 according to the International Code of Diseases, Version 9 (ICD9), or after 2002 according to ICD10.1 The Austrian Mortality Registry includes all deaths within Austria as well as the deaths of Austrian citizens in foreign countries when reported to Austrian officials.
The outcome measure for this study was all-cause mortality, defined as death occurring during the observation period. Cardiovascular mortality was defined as ICD9/ICD10 codes 390-459/I00-I99 and cancer mortality as ICD9/ICD10 codes 140-239/C00-D48. The remaining ICD9/ICD10 codes were summarized as mortality attributable to other causes. All PAD patients completed the follow-ups, and the observation period for each patient was set at 10 years (i.e. exactly 3650 days from the date of enrolment) or until death if death occurred within 10 years after enrolment. According to Austrian laws, all patients must undergo a post-mortem autopsy if the final cause of death is not apparent from the patient history, resulting in an overall post-mortem frequency of 39% in the 487 PAD patients of the LIPAD study.
Statistical analysis
Dichotomous variables are presented as numbers (and percentages), and continuous variables are presented as medians (with interquartile ranges). Differences in variables between survivors and decedents among non-diabetic and diabetic PAD patients were calculated using the chi-square test or Mann–Whitney U-test as appropriate. We further used univariate and multivariate Cox proportional hazards regression analyses to analyse the effects of several predictor variables on survival in the PAD subgroups. Because of the limited number of events in each of the patient subgroups, multivariate risk ratios (RRs) and their confidence intervals (CIs) were calculated with Cox proportional hazards regression analysis using a stepwise forward approach. As the main approach for calculating univariate and multivariate RRs, we dichotomized the relevant continuous variables according to generally accepted cut-off values. To validate this dichotomized approach of calculating univariate and multivariate RRs, we also report a second approach with the continuous variables not dichotomized but log transformed because of the skewed distribution. We analysed our data using MedCalc 13.0.0.0 (MedCalc Software) and SPSS 13.0 (SPSS, Inc.). The p values were not adjusted for multiple comparisons and, therefore, are only descriptive.
Results
Description of the PAD cohort and mortality data at 10 years
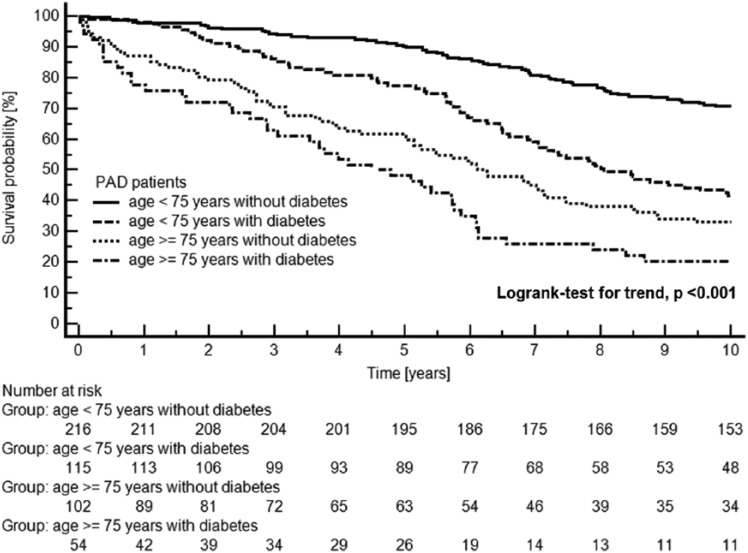
We studied 487 patients with symptomatic PAD consecutively admitted to hospital. This cohort included the following four patient subgroups: 216 PAD patients <75 years of age without diabetes mellitus, 115 PAD patients <75 years of age with diabetes mellitus, 102 PAD patients ≥75 years of age without diabetes mellitus, and 54 PAD patients ≥75 years of age with diabetes mellitus. Mortality rates at 10 years were as follows: 29% in non-diabetic PAD patients <75 years, 58% in diabetic PAD patients <75 years, 67% in non-diabetic PAD patients ≥75 years, and 80% in diabetic PAD patients ≥75 years. Figure 1 shows a Kaplan–Meier plot of 10-year all-cause mortality in the 487 PAD patients according to the four patient groups.
Figure 1.
Kaplan–Meier plot of all-cause mortality in 487 patients with symptomatic PAD separated by age groups and diabetes mellitus status.
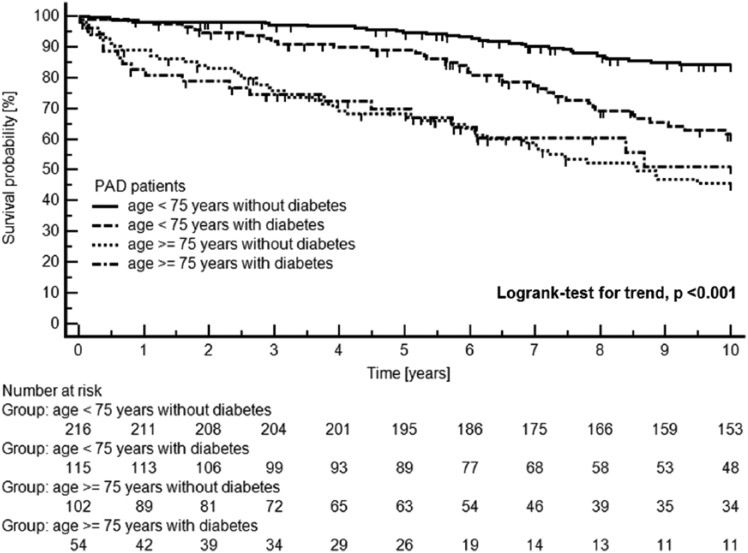
Of the 487 PAD patients, 136 died from cardiovascular disease, 34 from cancer, and 71 from other causes during the 10-year follow-up period. A Kaplan–Meier plot of cardiovascular mortality in the 487 patients with symptomatic PAD separated by age and diabetes mellitus status is shown in Figure 2.
Figure 2.
Kaplan–Meier plot of cardiovascular mortality in 487 patients with symptomatic PAD separated by age groups and diabetes mellitus status.
Censored data due to ‘cancer mortality’ and due to ‘other mortality’ (for definitions, please see the ‘Materials and methods’ section) are tagged with small vertical lines on the Kaplan–Meier curves.
Primary analysis: predictors of mortality at 10 years in non-diabetic versus diabetic PAD patients <75 years of age
Table 1 provides the baseline characteristics of the 216 non-diabetic and 115 diabetic PAD patients according to their survival status at 10 years. Continuous variables that were significantly different between survivors and decedents in either of the two patient groups were additionally dichotomized according to appropriate cut-off values.
Table 1.
Characteristics of 331 consecutive patients <75 years of age with symptomatic PAD according to survival status at 10 years.
| Variables | Non-diabetic PAD patients <75 years of age (n = 216) |
Diabetic PAD patients <75 years of age (n = 115) |
||||
|---|---|---|---|---|---|---|
| Survivors (n = 153) | Decedents (n = 63) | p valuea | Survivors (n = 48) | Decedents (n = 67) | p valuea | |
| Demographics and comorbidities | ||||||
| Patient age (years) | 62 (55–69) | 69 (60–71) | p = 0.001 | 61 (57–66) | 67 (60–72) | p = 0.002 |
| Patient age ≥ 65 years | 62 (41%) | 40 (64%) | p = 0.002 | 17 (35%) | 40 (60%) | p = 0.010 |
| Male gender | 116 (76%) | 54 (86%) | p = 0.106 | 34 (71%) | 53 (79%) | p = 0.308 |
| Body mass index (kg/m2) | 26 (24–28) | 25 (23–29) | p = 0.192 | 28 (26–30) | 27 (24–30) | p = 0.251 |
| Arterial hypertension | 64 (42%) | 32 (51%) | p = 0.228 | 28 (58%) | 55 (82%) | p = 0.005 |
| Cardiovascular comorbidityb | 42 (28%) | 28 (44%) | p = 0.015 | 21 (44%) | 40 (60%) | p = 0.091 |
| Symptomatic heart failure | 2 (1%) | 3 (5%) | p = 0.125 | 2 (4%) | 5 (7%) | p = 0.466 |
| Current smokingc | 88 (58%) | 34 (54%) | p = 0.633 | 19 (40%) | 31 (46%) | p = 0.476 |
| PAD-relevant data at the time of enrolment in the LIPAD study | ||||||
| Critical limb ischaemia | 10 (7%) | 10 (16%) | p = 0.031 | 10 (21%) | 27 (40%) | p = 0.028 |
| ABI (mmHg/mmHg) | 0.67 (0.53–0.81) | 0.56 (0.46–0.67) | p = 0.002 | 0.70 (0.53–0.89) | 0.63 (0.54–0.76) | p = 0.210 |
| ABI <0.60 mmHg/mmHg | 54 (35%) | 34 (54%) | p = 0.011 | 15 (31%) | 26 (39%) | p = 0.404 |
| Duration of symptomatic PAD (years) | 2.2 (1.1–4.8) | 3.1 (1.2–9.3) | p = 0.131 | 2.9 (1.3–8.5) | 3.6 (1.7–6.9) | p = 0.616 |
| History of PAD-specific interventiond | 50 (33%) | 32 (51%) | p = 0.013 | 21 (44%) | 33 (49%) | p = 0.560 |
| Biochemical parameters at the time of enrolment in the LIPAD study | ||||||
| eGFR (mL/min/1.73 m2) | 79 (70–91) | 78 (65–88) | p = 0.116 | 80 (65–93) | 71 (57–83) | p = 0.038 |
| eGFR <60 mL/min/1.73 m2 | 13 (9%) | 12 (19%) | p = 0.028 | 8 (17%) | 23 (34%) | p = 0.035 |
| LDL-cholesterol (mg/dL) | 156 (134–185) | 155 (125–183) | p = 0.390 | 143 (107–173) | 131 (101–168) | p = 0.452 |
| HDL-cholesterol (mg/dL) | 50 (41–60) | 49 (36–57) | p = 0.286 | 40 (33–56) | 41 (35–52) | p = 0.796 |
| Triglycerides (mg/dL) | 146 (97–206) | 132 (108–215) | p = 0.609 | 165 (118–247) | 133 (111–190) | p = 0.126 |
| Fasting glucose (mg/dL) | 96 (91–105) | 94 (85–104) | p = 0.054 | 171 (138–206) | 170 (129–206) | p = 0.746 |
| Glycohaemoglobin A1c (%) | 5.8 (5.5–6.2) | 5.8 (5.5–6.1) | p = 0.470 | 8.0 (7.1–9.4) | 7.9 (7.0–9.2) | p = 0.507 |
| hs-CRP (mg/L) | 3.7 (1.6–6.5) | 5.7 (2.7–17.2) | p = 0.001 | 4.4 (1.8–8.1) | 6.9 (1.9–24.1) | p = 0.126 |
| hs-CRP ≥ 5.0 mg/L | 55 (36%) | 36 (57%) | p = 0.004 | 20 (42%) | 36 (54%) | p = 0.202 |
| NT-proBNP (ng/L) | 97 (38–219) | 170 (65–592) | p = 0.007 | 95 (43–288) | 521 (93–1128) | p < 0.001 |
| NT-proBNP ≥ 125 ng/L | 62 (41%) | 35 (56%) | p = 0.043 | 21 (44%) | 48 (72%) | p = 0.003 |
| Mortality data | ||||||
| All-cause mortality at 10 years | 0 (0%) | 63 (100%) | 0 (0%) | 67 (100%) | ||
| Cardiovascular mortality at 10 years | 31 (49%) | 36 (54%) | ||||
| Cancer mortality at 10 years | 15 (24%) | 7 (10%) | ||||
| Other mortality at 10 years | 17 (27%) | 24 (36%) | ||||
PAD: peripheral arterial disease; LIPAD: Linz Peripheral Arterial Disease; ABI: resting ankle brachial index; eGFR: estimated glomerular filtration rate; LDL: low-density lipoprotein; HDL: high-density lipoprotein; hs-CRP: high-sensitivity C-reactive protein; NT-proBNP: amino-terminal pro-B-type natriuretic peptide.
Data are presented as numbers (and percentages) or medians (with 25th–75th percentiles).
Differences in variables in the survivors versus the decedents were calculated with the chi-square test or Mann–Whitney U-test as appropriate (p values were not corrected for multiple comparisons and are therefore only descriptive).
Cardiovascular comorbidity was defined as having coronary artery disease or cerebrovascular disease or both.
Current smoking was defined as any amount of tobacco use, including less than 1 year of abstinence.
History of a PAD-specific intervention before the index hospitalization was defined as at least one of the following: vascular surgery, percutaneous transluminal angioplasty with or without stenting, or amputation.
The proportions of patients ≥65 years of age, with critical limb ischaemia, with eGFR <60 mL/min/1.73 m2, and with NT-proBNP ≥125 ng/L differed between the survivors and decedents in both the non-diabetic and diabetic groups. In contrast, the proportions of patients with cardiovascular comorbidity, ABI <0.60 mmHg/mmHg, a history of a PAD-specific intervention, and hs-CRP ≥5.0 mg/L differed between the survivors and decedents in the non-diabetic group only. The proportion of patients with arterial hypertension differed between the survivors and decedents only in the diabetic PAD group.
We performed univariate and multivariate Cox proportional hazards regression analyses with 10-year all-cause mortality as the dependent variable and the significant dichotomous variables listed above as independent predictors (namely, patient age ≥65 years, arterial hypertension, cardiovascular comorbidity, critical limb ischaemia, ABI <0.60 mmHg/mmHg, a history of a PAD-specific intervention, eGFR <60 mL/min/1.73 m2, hs-CRP ≥5.0 mg/L, and NT-proBNP ≥125 ng/L). The same independent variables were used in the multivariate models in both the non-diabetic and diabetic patient groups using a conditional stepwise forward procedure with a stepwise entry limit of p < 0.05. The results of the univariate and multivariate analyses are shown in Table 2.
Table 2.
Cox proportional hazards regression analyses of the capability of the study variables to predict 10-year all-cause mortality in PAD patients <75 years of age according to diabetes mellitus status.
| Predictor variables | Non-diabetic PAD patients <75 years of age |
Diabetic PAD patients <75 years of age |
||
|---|---|---|---|---|
|
N = 216 (153 survivors vs 63 decedents) |
N = 115 (48 survivors vs 67 decedents) |
|||
| Univariate analyses |
Multivariate analysisa |
Univariate analyses |
Multivariate analysisa |
|
| Risk ratios (95% CI); p value | Risk ratios (95% CI); p value | Risk ratios (95% CI); p value | Risk ratios (95% CI); p value | |
| Patient age ≥65 years (vs <65 years) | 2.17 (1.30–3.63); p = 0.003 | 2.15 (1.28–3.59); p = 0.004 | 1.94 (1.19–3.17); p = 0.008 | 1.72 (1.05–2.83); p = 0.031 |
| Arterial hypertension (vs not) | 1.31 (0.80–2.15); p = 0.278 | n.e. | 2.25 (1.20–4.21); p = 0.011 | n.e. |
| Cardiovascular comorbidityb (vs not) | 1.90 (1.15–3.12); p = 0.012 | n.e. | 1.66 (1.02–2.71); p = 0.042 | n.e. |
| Critical limb ischaemia (vs claudication) | 2.29 (1.16–4.50); p = 0.016 | n.e. | 1.93 (1.18–3.15); p = 0.009 | n.e. |
| ABI <0.60 mmHg/mmHg (vs ≥0.60 mmHg/mmHg) | 1.98 (1.21–3.25); p = 0.007 | 1.88 (1.14–3.08); p = 0.013 | 1.24 (0.76–2.02); p = 0.401 | n.e. |
| History of PAD-specific interventionc (vs not) | 1.87 (1.14–3.07); p = 0.013 | 1.81 (1.10–2.97); p = 0.019 | 1.18 (0.73–1.91); p = 0.496 | n.e. |
| eGFR <60 mL/min/1.73 m2 (vs ≥60 mL/min/1.73 m2) | 1.90 (1.01–3.56); p = 0.046 | n.e. | 1.84 (1.11–3.06); p = 0.018 | n.e. |
| hs-CRP ≥ 5.0 mg/L (vs <5.0 mg/L) | 2.06 (1.25–3.39); p = 0.005 | 2.11 (1.28–3.47); p = 0.003 | 1.37 (0.85–2.21); p = 0.200 | n.e. |
| NT-proBNP ≥ 125 ng/L (vs <125 ng/L) | 1.71 (1.04–2.81); p = 0.035 | n.e. | 2.30 (1.35–3.92); p = 0.002 | 2.10 (1.22–3.60); p = 0.007 |
PAD: peripheral arterial disease; ABI: ankle brachial index; CI: confidence interval; eGFR: estimated glomerular filtration rate; hs-CRP: high-sensitivity C-reactive protein; n.e.: not entered into the model (i.e. stepwise entry limit of p < 0.05 was exceeded for each of these variables); NT-proBNP: amino-terminal pro-B-type natriuretic peptide.
Multivariate risk ratios were calculated with the Cox proportional hazards regression analysis using a conditional stepwise forward approach with all independent variables listed in Table 2. These variables were entered sequentially into the multivariate Cox proportional hazards regression analysis using a stepwise entry limit of p < 0.05.
Cardiovascular comorbidity was defined as having coronary artery disease, cerebrovascular disease, or both.
History of PAD-specific intervention before the index hospitalization was defined as at least one of the following: vascular surgery, percutaneous transluminal angioplasty with or without stenting, or amputation.
Supplementary Table 1 shows the results of the univariate and multivariate Cox proportional hazards regression analyses based on the use of the independent variables of age, ABI, eGFR, hs-CRP, and NT-proBNP as continuous variables. The results of the analysis with continuous variables were almost identical to those obtained with the dichotomized metric variables.
Secondary analysis: predictors of mortality at 10 years in non-diabetic versus diabetic PAD patients ≥75 years of age
Table 3 provides the baseline characteristics of the 102 non-diabetic and 54 diabetic PAD patients according to their survival status at 10 years. Continuous variables that were significantly different between survivors and decedents in either of the two patient groups were additionally dichotomized according to appropriate cut-off values.
Table 3.
Characteristics of 156 consecutive patients ≥75 years of age with symptomatic PAD according to survival status at 10 years.
| Variables | Non-diabetic PAD patients ≥ 75 years of age (n = 102) | Diabetic PAD patients ≥ 75 years of age (n = 54) | ||||
|---|---|---|---|---|---|---|
| Survivors (n = 34) | Decedents (n = 68) | p valuea | Survivors (n = 11) | Decedents (n = 43) | p valuea | |
| Demographics and comorbidities | ||||||
| Patient age (years) | 79 (78–81) | 80 (78–84) | p = 0.087 | 78 (75–80) | 79 (77–83) | p = 0.063 |
| Male gender | 14 (41%) | 35 (52%) | p = 0.327 | 4 (36%) | 30 (70%) | p = 0.041 |
| Body mass index (kg/m2) | 25 (23–27) | 25 (22–27) | p = 0.912 | 25 (24–28) | 25 (23–28) | p = 0.723 |
| Arterial hypertension | 20 (59%) | 52 (77%) | p = 0.065 | 9 (82%) | 36 (84%) | p = 0.880 |
| Cardiovascular comorbidityb | 13 (38%) | 36 (53%) | p = 0.161 | 6 (55%) | 26 (61%) | p = 0.721 |
| Symptomatic heart failure | 0 (0%) | 12 (18%) | p = 0.009 | 0 (0%) | 8 (19%) | p = 0.121 |
| Current smokingc | 7 (21%) | 14 (21%) | p > 0.999 | 2 (18%) | 5 (12%) | p = 0.564 |
| PAD-relevant data at the time of enrolment in the LIPAD study | ||||||
| Critical limb ischaemia | 2 (6%) | 15 (22%) | p = 0.039 | 1 (9%) | 19 (44%) | p = 0.031 |
| ABI (mmHg/mmHg) | 0.63 (0.37–0.77) | 0.57 (0.39–0.79) | p = 0.683 | 0.57 (0.38–0.65) | 0.58 (0.50–0.79) | p = 0.547 |
| Duration of symptomatic PAD (years) | 2.6 (1.0–6.5) | 2.8 (1.0–5.2) | p = 0.644 | 2.3 (1.0–3.1) | 3.7 (1.4–6.7) | p = 0.119 |
| History of PAD-specific interventiond | 15 (44%) | 25 (37%) | p = 0.473 | 4 (36%) | 20 (47%) | p = 0.546 |
| Biochemical parameters at the time of enrolment in the LIPAD study | ||||||
| eGFR (mL/min/1.73 m2) | 64 (57–77) | 64 (56–77) | p = 0.386 | 64 (57–77) | 57 (45–76) | p = 0.088 |
| LDL-cholesterol (mg/dL) | 153 (133–180) | 145 (123–168) | p = 0.201 | 149 (125–162) | 119 (98–142) | p = 0.018 |
| LDL-cholesterol ≥ 130 mg/dL | 26 (77%) | 44 (65%) | p = 0.227 | 8 (73%) | 15 (35%) | p = 0.024 |
| HDL-cholesterol (mg/dL) | 62 (50–73) | 55 (47–66) | p = 0.215 | 58 (44–59) | 44 (33–53) | p = 0.025 |
| HDL-cholesterol <40 mg/dL | 4 (12%) | 6 (9%) | p = 0.638 | 1 (9%) | 14 (33%) | p = 0.121 |
| Triglycerides (mg/dL) | 128 (93–206) | 112 (92–153) | p = 0.297 | 112 (85–193) | 113 (81–185) | p = 0.683 |
| Fasting glucose (mg/dL) | 93 (88–102) | 97 (87–101) | p = 0.895 | 124 (114–210) | 149 (118–182) | p = 0.797 |
| Glycohaemoglobin A1c (%) | 5.8 (5.5–6.2) | 5.9 (5.6–6.2) | p = 0.669 | 7.1 (6.5–8.3) | 7.6 (7.1–8.7) | p = 0.107 |
| hs-CRP (mg/L) | 2.4 (1.4–6.8) | 3.9 (2.0–12.6) | p = 0.103 | 3.7 (1.1–7.0) | 8.1 (3.1–26.9) | p = 0.021 |
| hs-CRP ≥ 5.0 mg/L | 11 (32%) | 26 (38%) | p = 0.560 | 4 (36%) | 27 (63%) | p = 0.114 |
| NT-proBNP (ng/L) | 196 (89–314) | 519 (224–1233) | p < 0.001 | 208 (70–258) | 636 (310–2538) | p < 0.001 |
| NT-proBNP ≥ 450 ng/L | 5 (15%) | 39 (57%) | p < 0.001 | 1 (9%) | 30 (70%) | p < 0.001 |
| Mortality data | ||||||
| All-cause mortality at 10 years | 0 (0%) | 68 (100%) | 0 (0%) | 43 (100%) | ||
| Cardiovascular mortality at 10 years | 49 (72%) | 20 (47%) | ||||
| Cancer mortality at 10 years | 8 (12%) | 4 (9%) | ||||
| Other mortality at 10 years | 11 (16%) | 19 (44%) | ||||
PAD: peripheral arterial disease; LIPAD: Linz Peripheral Arterial Disease; ABI: resting ankle brachial index; eGFR: estimated glomerular filtration rate; LDL: low-density lipoprotein; HDL: high-density lipoprotein; hs-CRP: high-sensitivity C-reactive protein; NT-proBNP: amino-terminal pro-B-type natriuretic peptide.
Data are presented as numbers (and percentages) or medians (with 25th–75th percentiles).
Differences in variables in the survivors versus the decedents were calculated with the chi-square test or Mann–Whitney U test as appropriate (p values were not corrected for multiple comparisons and are therefore only descriptive).
Cardiovascular comorbidity was defined as having coronary artery disease or cerebrovascular disease or both.
Current smoking was defined as any amount of tobacco use, including less than 1 year of abstinence.
History of a PAD-specific intervention before the index hospitalization was defined as at least one of the following: vascular surgery, percutaneous transluminal angioplasty with or without stenting, or amputation.
The proportions of patients with critical limb ischaemia and with NT-proBNP ≥ 450 ng/L differed between the survivors and decedents in both the non-diabetic and diabetic groups. In contrast, the proportions of patients with symptomatic heart failure differed between the survivors and decedents in the non-diabetic group only. The proportion of patients with male gender and with LDL-cholesterol ≥ 130 mg/dL differed between the survivors and decedents only in the diabetic PAD group.
We performed univariate and multivariate Cox proportional hazards regression analyses with 10-year all-cause mortality as the dependent variable and the significant dichotomous variables listed above as independent predictors (namely, male gender, symptomatic heart failure, critical limb ischaemia, LDL-cholesterol ≥130 mg/dL, and NT-proBNP ≥450 ng/L). The same independent variables were used in the multivariate models in both the non-diabetic and diabetic patient groups using a conditional stepwise forward procedure with a stepwise entry limit of p < 0.05. The results of the univariate and multivariate analyses are shown in Table 4.
Table 4.
Cox proportional hazards regression analyses of the capability of the study variables to predict 10-year all-cause mortality in PAD patients ≥75 years of age according to diabetes mellitus status.
| Predictor variables | Non-diabetic PAD patients ≥ 75 years of age |
Diabetic PAD patients ≥ 75 years of age |
||
|---|---|---|---|---|
|
N = 102 (34 survivors vs 68 decedents) |
N = 54 (11 survivors vs 43 decedents) |
|||
| Univariate analyses |
Multivariate analysisa |
Univariate analyses |
Multivariate analysisa |
|
| Risk ratios (95% CI); p value | Risk ratios (95% CI); p value | Risk ratios (95% CI); p value | Risk ratios (95% CI); p value | |
| Male gender (vs not) | 1.38 (0.86–2.22); p = 0.188 | n.e | 1.88 (0.98–3.62); p = 0.059 | 2.21 (1.13–4.33); p = 0.021 |
| Symptomatic heart failure (vs not) | 3.33 (1.76–6.31); p < 0.001 | n.e. | 4.86 (2.03–11.62); p < 0.001 | 3.11 (1.29–7.49); p = 0.011 |
| Critical limb ischaemia (vs claudication) | 3.58 (1.99–6.45); p < 0.001 | 3.39 (1.85–6.21); p < 0.001 | 2.95 (1.58–5.50); p = 0.001 | 2.54 (1.29–5.00); p = 0.007 |
| LDL-cholesterol ≥130 mg/dL (vs <130 mg/dL) | 0.60 (0.36–0.98); p = 0.042 | n.e. | 0.48 (0.25–0.90); p = 0.022 | n.e. |
| NT-proBNP ≥450 ng/L (vs <450 ng/L) | 2.98 (1.83–4.85); p < 0.001 | 2.89 (1.76–4.75); p < 0.001 | 3.34 (1.72–6.52); p < 0.001 | 2.52 (1.25–5.10); p = 0.010 |
PAD: peripheral arterial disease; CI: confidence interval; LDL: low-density lipoprotein; n.e.: not entered into the model (i.e. stepwise entry limit of p < 0.05 was exceeded for each of these variables); NT-proBNP: amino-terminal pro-B-type natriuretic peptide.
Multivariate risk ratios were calculated with the Cox proportional hazards regression analysis using a conditional stepwise forward approach with all independent variables listed in Table 4. These variables were entered sequentially into the multivariate Cox proportional hazards regression analysis using a stepwise entry limit of p < 0.05.
Supplementary Table 2 shows the results of the univariate and multivariate Cox proportional hazards regression analyses based on the use of the independent variables of LDL-cholesterol and NT-proBNP as continuous variables. The results of the analysis with continuous variables were almost identical to those obtained with the dichotomized metric variables.
Discussion
Interpretation of our results in PAD patients <75 years of age
We previously demonstrated that the probability of mortality for a given patient at 10 years strongly depends on whether the PAD patient <75 years has diabetes mellitus.1 For this reason, we evaluated the extent to which predictors of death were different in non-diabetic and diabetic PAD patients. Indeed, we found that in PAD patients <75 years of age without diabetes, in addition to increased age, the following were independent predictors of 10-year all-cause mortality: diminished ABI, a history of a PAD-specific intervention, and increased hs-CRP. In contrast, the predictors of 10-year all-cause mortality were strikingly different in PAD patients <75 years of age with diabetes. In addition to increased age, only increased NT-proBNP contributed independently to outcome prediction. Therefore, our findings could be important for clinical practice because they suggest that the development of separate risk-reduction strategies for the two PAD subgroups may be justified to improve long-term outcomes.
A diminished ABI and a history of a PAD-specific intervention should be interpreted as surrogate markers of PAD disease severity and, thus, of atherosclerotic burden. PAD disease severity is known to be associated with mortality in general.2,14 As we were the first group to divide an overall PAD cohort into non-diabetic and diabetic individuals, it was quite surprising to find that surrogate markers of PAD disease severity and, thus, of atherosclerotic burden were predictors only in PAD patients without diabetes and not in diabetic patients. Because CRP promotes vascular remodelling and plaque formation, it is considered a biomarker of vascular inflammation and atherosclerosis.15,16 Therefore, it is logical that increased hs-CRP plasma concentration, as well as a diminished ABI and a history of a PAD-specific intervention (as surrogate markers of PAD disease severity and, thus, atherosclerotic burden), predicted long-term all-cause mortality in our non-diabetic PAD patients. As a consequence, in this patient subgroup, therapeutic strategies to reduce hs-CRP plasma concentrations and thereby impede the atherosclerotic process may be favourable.
CRP has been proposed as a potential target for treatment.16 Increased CRP plasma concentrations are associated with increased mortality and more rapid functional decline among individuals with atherosclerotic PAD.17 Statin therapy reduces the relatively high rate of cardiovascular events in PAD and improves impaired walking performance in PAD, most likely because statins reduce inflammation.17 However, as the PAD population already has an indication for statin therapy,18,19 there have been no clear implications in the past for clinical management related to higher plasma concentrations of hs-CRP. Nevertheless, the specific targeting of inflammatory pathways for the treatment of cardiovascular disease has been thought to be a viable option.20–24 However, no clinical trials have been published to establish whether specific therapies that reduce circulating CRP can improve outcomes in PAD patients. Very recently, the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) has provided a deeper insight into the inflammatory hypothesis of atherosclerotic disease.25 In this study, patients with a previous myocardial infarction and an increased circulating hs-CRP concentration were assigned to receive canakinumab (a monoclonal antibody targeting interleukin-1ß) or placebo.25 As a result, canakinumab reduced hs-CRP without reduction of LDL-cholesterol and lead to a lower incidence of a composite end point of myocardial infarction, nonfatal stroke, and cerebrovascular death.25,26 Now that an agent targeting inflammation and autoimmunity has been shown to provide some clinical benefit in coronary artery disease,26 the field could be opened to further investigation in PAD patients.
In contrast to the non-diabetic PAD patients, the only independent predictors of 10-year all-cause mortality for the diabetic PAD patients were increased age and increased NT-proBNP. NT-proBNP is an established marker of functional cardiac impairment and is increased in symptomatic and asymptomatic heart disease.27 Circulating NT-proBNP increases in parallel with cardiac disease severity, and NT-proBNP is considered a therapeutic target for patients with heart failure.27 Several studies have assessed the prognostic utility of NT-proBNP in patients with PAD but have reported inconsistent results.8–11 Consequently, this study may further improve risk prediction in PAD because we stratified our patients according to their diabetes mellitus status. Indeed, it may be relevant to clinical practice that NT-proBNP independently predicted all-cause mortality at 10 years in the PAD patients with diabetes only. In contrast to the non-diabetic PAD patients, a high percentage of diabetic PAD patients may have unrecognized heart disease contributing to the high mortality rates reported in this study.28,29 This could be the reason for the conflicting results regarding the predictive value of NT-proBNP in the previously published studies, where the proportion of patients with diabetes may have been different despite a similar age distribution.10,11 Nevertheless, to reliably establish a possible benefit of natriuretic peptide measurements in PAD patients with diabetes in terms of initiating further diagnostic actions and adequate therapeutic interventions to reduce cardiovascular mortality, more prospective studies are needed. For example, NT-proBNP-guided accelerated up-titration of renin–angiotensin system antagonists and beta-blockers to maximum tolerated dosages has been an effective and safe intervention for the primary prevention of cardiac events in diabetic patients.30 We speculate that this strategy may also be beneficial for the secondary prevention of cardiac events in diabetic PAD patients. However, this remains to be tested in future studies.
As noted in the introduction, studies on biomarkers as risk prediction tools in PAD have reported inconsistent results. This inconsistency may be at least partly explained by the characteristics of the PAD cohorts studied. As seen in our study, findings most likely depend on the proportion of non-diabetic versus diabetic patients within a cohort in terms of whether hs-CRP or NT-proBNP will significantly predict outcomes in PAD patients. Furthermore, the extent to which the prediction of risk in cardiovascular disease by biomarkers (e.g. hs-CRP and NT-proBNP) depends on the length of the follow-up period remains controversial. For example, a recently published study revealed that hs-CRP was a strong predictor of short-term mortality in a cohort of PAD patients, while ‘classical’ risk factors were better at predicting long-term mortality.6 However, reports in other settings failed to reveal an unequivocal time-dependent relationship between biomarkers and outcomes.31,32 In line with the latter findings, we demonstrated that hs-CRP and NT-proBNP are risk predictors of long-term mortality in patients with symptomatic PAD although they are differentially weighted in diabetic versus non-diabetic individuals, as detailed above.
Interpretation of our results in PAD patients ≥75 years of age
The findings in PAD patients ≥75 years of age are somewhat different to those in PAD patients <75 years of age. In the older patient groups, the heart obviously matters irrespective of the diabetes mellitus status. In diabetics and in non-diabetics, increased NT-proBNP was a strong and independent predictor of 10-year all-cause mortality. We speculate that in PAD patients ≥75 years of age, functional and structural cardiac impairment is present in many patients irrespective of the diabetes mellitus status. Interestingly, hs-CRP did not predict 10-year outcome in PAD patients ≥75 years of age. However, the reason for the latter finding remains unclear with our study.
Limitations
We must first note that the LIPAD study was a single-centre study and that the number of events in the two PAD subgroups was rather low. For this reason, we applied Cox regression models with a stepwise forward approach. Although we believe that this approach is advantageous in terms of the clinical applicability of the results, it was not possible to fully adjust the multivariate analyses for all possible confounders in each of the two PAD subgroups.
Another limitation of using data from the LIPAD study is that we relied on all-cause mortality. Although we have outcome data on cardiovascular mortality (as shown in Figure 2), we relied on all-cause mortality as the main outcome parameter because this is the hardest end point and because the number of events was further reduced when assessing cardiovascular mortality, negatively affecting the study power. In addition, analyses of cardiovascular mortality bear the possibility of misdiagnoses of the cause of death (especially in those individuals who were not subjected to a post-mortem autopsy).
We further acknowledge that our cut-off values for NT-proBNP and hs-CRP were chosen arbitrarily. Nevertheless, the NT-proBNP cut-off value of 125 ng/L for individuals <75 years of age and of 450 ng/mL for individuals ≥75 years of age is described as appropriate to rule out heart failure with a high probability in the current guidelines33 and in the package insert of the assay used for this study (Roche Diagnostics), respectively. As there is no generally accepted hs-CRP cut-off value for risk prediction in manifest atherosclerotic disease, we dichotomized hs-CRP according to the upper reference limit of 5.0 mg/L that is widely used in routine clinical laboratories. However, to strengthen our findings, we used both the dichotomized approach and a continuous approach to calculate Cox regression models, revealing similar results for 10-year all-cause mortality (as shown in Supplementary Tables 1 and 2).
We did not measure cardiac troponins in our PAD cohort, which is another limitation of this study because increased cardiac troponin plasma concentrations have been reported to be predictive of higher 1-year mortality rates in PAD patients.34 However, the recruitment phase of the LIPAD study was from 2000 to 2002, and it was not common practice at the time to measure any cardiac biomarkers in the PAD population (and our frozen plasma samples are exhausted).
Another limitation of this study is related to the assessment of heart failure. The true prevalence of heart failure might be higher than that stated for our cohort; however, as outlined in the methods, the evaluation and diagnosis of heart failure was left to the discretion of the attending physicians and was based exclusively on clinical criteria without using an acknowledged heart failure classification score. Therefore, as discussed previously, the demonstration that a single NT-proBNP measurement provides reliable predictive information, especially in diabetic patients with symptomatic PAD, might have clinical implications.
Finally, as detailed in the methods section, the patients included in this study were a subset of the overall PAD population including only Caucasian patients who were admitted for inpatient diagnosis/treatment of atherosclerotic PAD. Therefore, our findings may not be generalizable to non-Caucasian individuals, patients with asymptomatic PAD, or non-hospitalized PAD patients.
Conclusion
In this study, we found high mortality rates at 10 years among patients with symptomatic PAD (especially in those with diabetes mellitus). The predictors of mortality at 10 years differed in PAD patients <75 years of age with and without diabetes. Our findings suggest that distinct risk assessment strategies should be applied to the two PAD subgroups in future studies. It is evident from the literature that all PAD patients should be treated aggressively,35,36 but – based on our results – we speculate that specific treatment strategies might be justified in non-diabetic and diabetic PAD patients. In this sense, our study might help to generate hypotheses for future trials of PAD patients to investigate whether distinct diagnostic algorithms and consecutive treatment options can improve outcomes.
Supplementary Material
Acknowledgments
Parts of this work have been presented as posters at the EAS (European Atherosclerosis Society) Congress in May 2016 in Innsbruck and at the ESC (European Society of Cardiology) Congress in September 2016 in Rome. T.M. contributed to conception and design; T.M., F.H., W.P., M.H., and B.D. contributed to acquisition, analysis and interpretation of data; T.M. and B.D. contributed to writing the article; F.H., W.P., and M.H. contributed to critical revision of the article; and T.M., F.H., W.P., M.H., and B.D. contributed to final approval of the article. Reagents for NT-proBNP measurements were provided by Roche Diagnostics (Vienna, Austria) free of charge. The company did not play a role in (1) the design of the study; (2) the data collection, analysis, and interpretation; or (3) the preparation of the manuscript. We would like to thank Barbara Leitner (Statistik Austria) for providing data from the Austrian Mortality Registry.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: T.M. and B.D. received speaking fees from Roche Diagnostics. The other authors have no conflicts of interest related to this work to declare.
Ethical approval: The study protocol was approved by the local ethics committee in accordance with the Declaration of Helsinki, and all study participants provided informed consent. Ethical approval for this study was obtained from the ETHICS COMMITTEE BARMHERZIGE BRUEDER LINZ (no approval number was assigned).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Verbal informed consent was obtained from all study participants before enrolling them into the study. Obtaining of written informed consent from study participants was waived by the ethics committee.
Trial registration: Not applicable because this is a cohort study without a study specific intervention.
References
- 1. Mueller T, Hinterreiter F, Poelz W, et al. Mortality rates at 10 years are higher in diabetic than in non-diabetic patients with chronic lower extremity peripheral arterial disease. Vasc Med 2016; 21: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eberhardt RT, Coffman JD. Cardiovascular morbidity and mortality in peripheral arterial disease. Curr Drug Targets Cardiovasc Haematol Disord 2004; 4: 209–217. [DOI] [PubMed] [Google Scholar]
- 3. Silbernagel G, Rein P, Saely CH, et al. Prevalence of type 2 diabetes is higher in peripheral artery disease than in coronary artery disease patients. Diab Vasc Dis Res 2015; 12: 146–149. [DOI] [PubMed] [Google Scholar]
- 4. Rein P, Saely CH, Silbernagel G, et al. Systemic inflammation is higher in peripheral artery disease than in stable coronary artery disease. Atherosclerosis 2015; 239: 299–303. [DOI] [PubMed] [Google Scholar]
- 5. De Lemos JA, Kumbhani DJ. Lessons from the heart: troponin elevations in patients with established peripheral artery disease. J Am Coll Cardiol 2014; 63: 1539–1541. [DOI] [PubMed] [Google Scholar]
- 6. Criqui MH, Ho LA, Denenberg JO, et al. Biomarkers in peripheral arterial disease patients and near- and longer-term mortality. J Vasc Surg 2010; 52: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stone PA, Schlarb H, Campbell JE, et al. C-reactive protein and brain natriuretic peptide as predictors of adverse events after lower extremity endovascular revascularization. J Vasc Surg 2014; 60: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mueller T, Hinterreiter F, Luft C, et al. Mortality rates and mortality predictors in patients with symptomatic peripheral artery disease stratified according to age and diabetes. J Vasc Surg 2014; 59: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 9. Skoglund PH, Arpegård J, Ostergren J, et al. Amino-terminal pro-B-type natriuretic peptide and high-sensitivity C-reactive protein but not cystatin C predict cardiovascular events in male patients with peripheral artery disease independently of ambulatory pulse pressure. Am J Hypertens 2014; 27: 363–371. [DOI] [PubMed] [Google Scholar]
- 10. Shadman R, Allison MA, Criqui MH. Glomerular filtration rate and N-terminal pro-brain natriuretic peptide as predictors of cardiovascular mortality in vascular patients. J Am Coll Cardiol 2007; 49: 2172–2181. [DOI] [PubMed] [Google Scholar]
- 11. Mueller T, Dieplinger B, Poelz W, et al. Amino-terminal pro–B-type natriuretic peptide as predictor of mortality in patients with symptomatic peripheral arterial disease: 5-year follow-up data from the Linz Peripheral Arterial Disease Study. Clin Chem 2009; 55: 68–77. [DOI] [PubMed] [Google Scholar]
- 12. Mueller T, Marschon R, Dieplinger B, et al. Factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations are not associated with chronic limb ischemia: the Linz Peripheral Arterial Disease (LIPAD) study. J Vasc Surg 2005; 41: 808–815. [DOI] [PubMed] [Google Scholar]
- 13. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26: 517–538. [DOI] [PubMed] [Google Scholar]
- 14. Dormandy J, Heeck L, Vig S. The natural history of claudication: risk to life and limb. Semin Vasc Surg 1999; 12: 123–137. [PubMed] [Google Scholar]
- 15. Yousuf O, Mohanty BD, Martin SS, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol 2013; 62: 397–408. [DOI] [PubMed] [Google Scholar]
- 16. Genest J. C-reactive protein: risk factor, biomarker and/or therapeutic target? Can J Cardiol 2010; 26(suppl. A): 41A–44A. [DOI] [PubMed] [Google Scholar]
- 17. McDermott MM, Lloyd-Jones DM. The role of biomarkers and genetics in peripheral arterial disease. J Am Coll Cardiol 2009; 54: 1228–1237. [DOI] [PubMed] [Google Scholar]
- 18. Kumbhani DJ, Steg PG, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J 2014; 35: 2864–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Westin GG, Armstrong EJ, Bang H, et al. Association between statin medications and mortality, major adverse cardiovascular event, and amputation-free survival in patients with critical limb ischemia. J Am Coll Cardiol 2014; 63: 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ridker PM. Moving beyond JUPITER: will inhibiting inflammation reduce vascular event rates? Curr Atheroscler Rep 2013; 15: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ridker PM, Thuren T, Zalewski A, et al. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 2011; 162: 597–605. [DOI] [PubMed] [Google Scholar]
- 22. Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 2013; 166: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deftereos S, Giannopoulos G, Angelidis C, et al. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation 2015; 132: 1395–1403. [DOI] [PubMed] [Google Scholar]
- 24. Khan R, Spagnoli V, Tardif JC, et al. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis 2015; 240: 497–509. [DOI] [PubMed] [Google Scholar]
- 25. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 26. Harrington RA. Targeting inflammation in coronary artery disease. N Engl J Med 2017; 377: 1197–1198. [DOI] [PubMed] [Google Scholar]
- 27. Troughton R, Michael Felker G, Januzzi JL. Natriuretic peptide-guided heart failure management. Eur Heart J 2014; 35: 16–24. [DOI] [PubMed] [Google Scholar]
- 28. Haugen S, Casserly IP, Regensteiner JG, et al. Risk assessment in the patient with established peripheral arterial disease. Vasc Med 2007; 12: 343–350. [DOI] [PubMed] [Google Scholar]
- 29. Anand RG, Ventura HO, Mehra MR. Is heart failure more prevalent in patients with peripheral arterial disease? A meta-analysis. Congest Heart Fail 2007; 13: 319–322. [DOI] [PubMed] [Google Scholar]
- 30. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62: 1365–1372. [DOI] [PubMed] [Google Scholar]
- 31. Tzoulaki I, Murray GD, Lee AJ, et al. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation 2007; 115: 2119–2127. [DOI] [PubMed] [Google Scholar]
- 32. Zethelius B, Berglund L, Sundström J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 2008; 358: 2107–2116. [DOI] [PubMed] [Google Scholar]
- 33. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 34. Linnemann B, Sutter T, Herrmann E, et al. Elevated cardiac troponin T is associated with higher mortality and amputation rates in patients with peripheral arterial disease. J Am Coll Cardiol 2014; 63: 1529–1538. [DOI] [PubMed] [Google Scholar]
- 35. Bonaca MP, Creager MA. Pharmacological treatment and current management of peripheral artery disease. Circ Res 2015; 116: 1579–1598. [DOI] [PubMed] [Google Scholar]
- 36. Olin JW, White CJ, Armstrong EJ, et al. Peripheral artery disease: evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol 2016; 67: 1338–1357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.