Abstract
Objectives:
We used mediation models to examine the mechanisms underlying the relationships among physical fitness, sleep-disordered breathing (SDB), symptoms of depression, and cognitive functioning.
Methods:
We conducted a cross-sectional secondary analysis of the cohorts involved in the 2003-2006 project PLAY (a trial of the effects of aerobic exercise on health and cognition) and the 2008-2011 SMART study (a trial of the effects of exercise on cognition). A total of 397 inactive overweight children aged 7-11 received a fitness test, standardized cognitive test (Cognitive Assessment System, yielding Planning, Attention, Simultaneous, Successive, and Full Scale scores), and depression questionnaire. Parents completed a Pediatric Sleep Questionnaire. We used bootstrapped mediation analyses to test whether SDB mediated the relationship between fitness and depression and whether SDB and depression mediated the relationship between fitness and cognition.
Results:
Fitness was negatively associated with depression (B = –0.041; 95% CI, –0.06 to –0.02) and SDB (B = –0.005; 95% CI, –0.01 to –0.001). SDB was positively associated with depression (B = 0.99; 95% CI, 0.32 to 1.67) after controlling for fitness. The relationship between fitness and depression was mediated by SDB (indirect effect = –0.005; 95% CI, –0.01 to –0.0004). The relationship between fitness and the attention component of cognition was independently mediated by SDB (indirect effect = 0.058; 95% CI, 0.004 to 0.13) and depression (indirect effect = –0.071; 95% CI, –0.01 to –0.17).
Conclusions:
SDB mediates the relationship between fitness and depression, and SDB and depression separately mediate the relationship between fitness and the attention component of cognition.
Keywords: pediatric obesity, sleep, physical fitness, depression, cognitive functioning
Childhood obesity is a public health concern, and black children are disproportionately affected. In 2014, 19.5% of non-Hispanic black children were obese, whereas only 14.7% of non-Hispanic white children were obese.1 In addition to the negative impact that obesity has on physical health (eg, risks of type 2 diabetes, high blood pressure, high cholesterol),2,3 it adversely affects sleep quality,4 mood,5 and cognitive functioning.6,7 For overweight children, regular aerobic exercise leads to decreased adiposity (percentage body fat) and improved fitness.8
Excess body weight is also associated with higher rates of sleep-disordered breathing (SDB) among children and adolescents.9,10 SDB is characterized by abnormal breathing patterns that result in disturbances in the sleep cycle, and it encompasses obstructive sleep apnea (ie, partial or complete closure of airways during sleep), upper airway resistance syndrome, and chronic snoring. Physical activity leads to improved SDB. In a study of inactive overweight children, of whom 25% screened positive for SDB, exercise improved snoring and reduced SDB symptoms overall.11 However, the number of studies on the potential relationship between childhood physical fitness and SDB is limited. Apart from preliminary results suggesting that decreased fitness is associated with shorter sleep duration for adolescents,12 little is known about the relationship between fitness and SDB in children.
More is known about the relationship among activity, fitness, and mood, or affective states (particularly depression), in children. Physical activity and symptoms of depression appear to be inversely related among children and adolescents.13 One study of overweight children found a dose-response relationship such that more time spent in vigorous daily exercise led to a greater reduction in symptoms of depression.14 In addition, emerging research suggests that although physical activity may be prospectively associated with fewer symptoms of depression in young people, the underlying mechanism may actually involve physical fitness. For example, a recent study found that middle schoolers with low fitness levels were likely to report more symptoms of depression than those with higher fitness levels.15 In a large population-based study, lower cardiovascular fitness among 18-year-old men was prospectively linked to a higher risk of developing depression.16
Similarly, sleep may also affect depression, although the exact nature of the relationship is less clear. Disrupted sleep is 1 symptom of depression, with up to 73% of adolescents diagnosed with depression reporting comorbid sleep difficulties.17 Conversely, up to 58% of adults diagnosed with breathing-related sleep disorders meet the criteria for depression.18 Furthermore, sleep difficulties in childhood have been implicated as a risk factor for the development of depression,19 and a recent meta-analysis suggested that sleep disturbance is actually a precursor to symptoms of depression among adolescents20 rather than merely a symptom or result of depression.21
Aside from being linked with mood and possibly sleep, physical activity and physical fitness may also affect the cognition of young people. In a study of sedentary overweight 7- to 11-year-old children, aerobic training increased not only their physical fitness but also their cognitive performance.22 In addition, physical fitness has been shown to be directly associated with better cognition among children.23–25
We aimed to clarify the mechanisms underlying the relationships between physical fitness and SDB and between fitness and depression with a sample of overweight, racially diverse children at risk for SDB. We also aimed to examine the potential links among physical fitness, SDB, symptoms of depression, and cognitive functioning. We hypothesized that physical fitness would be negatively related to SDB and symptoms of depression and positively related to cognition. Furthermore, we hypothesized that the relationship between physical fitness and symptoms of depression would be mediated by SDB. Finally, we hypothesized a serial relationship between physical fitness and cognition, in which physical fitness would be related to SDB, which in turn would be related to symptoms of depression, which in turn would be related to cognitive functioning.
Methods
Participants
Our study was a secondary analysis of combined baseline data from project PLAY8,11,22 and the SMART study.26 In project PLAY, we recruited 6 waves of 30 to 40 children each (N = 222) during a 4-year period (2003-2006) to participate in a trial of the effects of aerobic exercise on health and cognition.8,22 We recruited children through presentations and flyers distributed at 15 elementary schools in the Augusta, Georgia, metropolitan area. We used the following inclusion criteria: white or black race, age 7-11, overweight or obese (≥85th percentile body mass index),27 inactive (ie, no regular physical activity program >1 hour per week), no medical condition or medications that would affect study results or limit physical activity, and an ability to provide a fasting blood sample at the study’s baseline.
In the SMART study, which examined the effects of exercise on the brain, we recruited 4 waves of 25 to 50 children each (N = 175) during a 4-year period (2008-2011)26 through presentations and flyers distributed at 14 elementary schools in Augusta. We used the following inclusion criteria: age 8-11, any race or ethnicity, and the same requirements as project PLAY for overweight or obese status, physical activity, and medical history. Unlike in project PLAY, however, children did not have to provide a fasting blood sample to be enrolled in the SMART study. For both studies, we obtained informed consent and assent orally and in writing from a parent or guardian and from the child. The Augusta University Medical College of Georgia Institutional Review Board approved both studies.
Measures
Fitness
We measured fitness using the Modified Balke Protocol for Poorly Fit Children on a treadmill to determine VO2 peak.28 A higher VO2 peak indicates a higher level of aerobic fitness.
Sleep-Disordered Breathing
In project PLAY and the SMART study, the parents of participating children reported sleep behaviors with the Pediatric Sleep Questionnaire, which has been validated against polysomnography for children.29 The questionnaire includes 22 dichotomous questions about children’s sleeping patterns, and the answers are scored as no = 0 and yes = 1. For each participant, we added the scores for all questions and then calculated a total average score. We considered a total average score ≥0.33 to be a positive screen for SDB.
Symptoms of Depression
Participants in project PLAY completed the Reynolds Child Depression Scale (RCDS), a 30-item self-report questionnaire of symptoms of depression among children. We reported responses using a 4-point Likert scale, where 1 = almost never and 4 = all the time. Total scores range from 30 to 120, and higher scores indicate more symptoms of depression. We considered a score ≥74 indicative of a clinically relevant level of depression.30 Fourteen (6.3%) RCDS results were invalid; these scores were not included in the analyses.
SMART study participants completed the Children’s Depression Inventory (CDI), a 27-item multiple-choice self-report questionnaire of symptoms of depression among children and adolescents.29 Each item consists of statements with a rating ranging from 0 (absence of symptoms) to 2 (definite symptoms). CDI scores range from 0 to 54; we considered a score ≥19 to be a positive screen for depression (in this pediatric nonclinical population). In a study of children aged 8-12, the RCDS and CDI were each internally consistent and highly positively correlated.31 We converted RCDS and CDI scores into z scores for our analysis.
Cognition
To assess children’s cognitive functioning, we administered the Cognitive Assessment System (CAS), a standardized individual assessment that yields 4 scale scores (Planning, Attention, Simultaneous, and Successive) and a composite Full Scale score.32 The Planning Scale requires the child to consider how to solve items, develop a plan of action, apply the plan, modify the plan as needed, and control the impulse to act without careful consideration. The Attention Scale requires the child to focus cognitive activity, detect particular stimuli, and inhibit responses to competing stimuli. The Simultaneous Scale requires the child to synthesize separate stimuli into an interrelated group involving spatial and logical content. The Successive Scale requires the child to integrate material into a specific serial order in which each element is related to those that precede and follow it, and it involves the repetition or comprehension of the serial organization of elements. The Full Scale score is the equally weighted composite of the Planning, Attention, Simultaneous, and Successive Scale scores. A child’s performance on each scale, as well as the Full Scale, yields a standard score. The standard scores, which are set at a mean of 100 and a standard deviation of 15, show performance relative to a historical population of children of the same age; higher scores reflect higher functioning in each cognitive domain.
Demographic Characteristics
The children’s primary caregiver (eg, parent) self-reported his or her education level as less than high school graduate, high school graduate, some college, college degree, or postgraduate degree. Socioeconomic status was coded based on primary caregiver’s education level. Parents also reported the race (black or white) and age of participating children.
Adiposity
We used dual-energy x-ray absorptiometry (QDR-4500 W or Discovery W; Hologic Inc, Bedford, MA) of the whole body to measure percentage fat (fat mass/body mass × 100).
Data Analysis
We conducted all analyses using IBM SPSS version 21.0.33 For all statistical tests, we considered an α level of .05 to be significant. We examined relationships among variables of interest using partial correlations, controlling for socioeconomic status and adiposity. We reported standardized coefficients, r, to indicate the relative strengths of relationships among the variables in our study.
We conducted mediation analyses using PROCESS macro version 2.13.34 Mediation analyses examine hypotheses about whether a proposed mediator acts as an intermediate variable between a proposed cause and a proposed outcome. For these analyses, we reported unstandardized coefficients, B, to indicate the relative strengths of mediation relationships. We used a 95% bootstrap CI with 5000 resamples to determine whether these mediation effects, also called indirect effects, were significant.35,36
We estimated a mediation model in which the proposed cause was fitness (measured as VO2 peak), the outcome variable was symptoms of depression (measured with RCDS or CDI), and the proposed mediator was SDB (measured with the Pediatric Sleep Questionnaire). In these analyses, we controlled for socioeconomic status and adiposity, and we used Model 4 in PROCESS (simple mediation). If the causal order of the variables is correctly specified, mediation models can provide evidence about whether the proposed cause might influence the outcome through the proposed mediator.
We then conducted moderated mediation analyses to determine whether other variables (study cohort, race, sex) affected our mediation results. We used model 59 in PROCESS to determine whether the study cohort (PLAY vs SMART) played a moderating role. Moderation by study cohort would suggest that the mediation findings were not consistent between the 2 study cohorts, whereas the absence of moderation would show consistent relationships among the variables in both study cohorts. We used model 58 in PROCESS to test whether race (black or white) and sex were moderators of the relationships among fitness (the proposed cause), SDB (the proposed mediator), and symptoms of depression (the proposed outcome), again controlling for socioeconomic status and adiposity. Here, moderation would indicate that the fitness effect on SDB and/or the SDB effect on symptoms of depression was dependent on race or sex.
In addition, we used a serial multiple mediator model (model 6 in PROCESS) to determine whether the relationship between fitness and another outcome variable, cognition, was mediated by SDB and/or symptoms of depression. In this model, we hypothesized 3 indirect effects: (1) fitness would be associated with cognition through SDB; (2) fitness would be associated with cognition through symptoms of depression; and (3) fitness would be associated with cognition through a serial relationship in which fitness would be associated with SDB, which in turn would be associated with symptoms of depression, which in turn would be associated with cognition.
Recent methodologic discussions about mediation analysis suggest that it is not necessary that total effects (relationship between cause and outcome) be significant when testing indirect effects (mediator acting as intermediate variable in relationship between cause and outcome),34 and tests of indirect effects may actually have higher power than tests of total effects.37 Also, although these models do not provide evidence for causal claims, they do provide estimates of the indirect effects through which causal mechanisms may operate.
Results
Of 397 study participants, 282 (71%) children were black (1 Pacific Islander child was included in the white category), and 235 (59%) children were female (Table 1). Of the 397 parents (or primary caregivers), 21 (5%) reported their highest education level as less than high school, 99 (25%) as high school, 178 (45%) as some college, 75 (19%) as a college degree, and 24 (6%) as a postgraduate degree.
Table 1.
Characteristics of overweight and obese inactive children aged 7-11 in public schools, by sex and race, based on study cohorts from project PLAY8,11,22 (2003-2006) and the SMART study26 (2008-2011), Augusta, Georgiaa
| Girls (n = 235) | Boys (n = 162) | ||||
|---|---|---|---|---|---|
| Characteristics | Total (N = 397) | Black (n = 175) | White (n = 60) | Black (n = 107) | White (n = 55) |
| Age, y | 9.5 ± 1.0 | 9.5 ± 1.0 | 9.4 ± 1.0 | 9.5 ± 1.0 | 9.6 ± 1.2 |
| Percentage fat of body mass | 39.19 ± 6.81 | 40.40 ± 6.07 | 42.17 ± 4.72 | 35.97 ± 7.80 | 38.34 ± 6.50 |
| VO2 peak, mL/kg/minb | 28.43 ± 5.49 | 27.19 ± 5.03 | 28.59 ± 4.85 | 30.14 ± 6.32 | 28.94 ± 4.99 |
| Positive screen for SDB | 70 (18) | 27 (15) | 13 (22) | 20 (19) | 10 (18) |
| Pediatric Sleep Questionnaire scorec | 0.22 ± 0.20 | 0.20 ± 0.20 | 0.23 ± 0.20 | 0.22 ± 0.20 | 0.22 ± 0.20 |
| Positive screen for depression | 24 (6) | 13 (7) | 3 (5) | 7 (7) | 1 (2) |
| RCDS scored | 51 ± 10 | 52 ± 12 | 53 ± 11 | 50 ± 8 | 49 ± 10 |
| CDI scoree | 7.9 ± 7.1 | 7.5 ± 6.6 | 8.9 ± 8.2 | 7.9 ± 7.5 | 11.0 ± 8.2 |
| CAS scoresf | |||||
| Planning Scale | 96 ± 13 | 97 ± 12 | 97 ± 16 | 92 ± 12 | 99 ± 12 |
| Attention Scale | 97 ± 13 | 100 ± 12 | 97 ± 14 | 92 ± 12 | 95 ± 11 |
| Simultaneous Scale | 101 ± 11 | 100 ± 11 | 106 ± 12 | 99 ± 10 | 105 ± 12 |
| Successive Scale | 98 ± 12 | 99 ± 12 | 99 ± 14 | 97 ± 11 | 99 ± 11 |
| Full Scale | 97 ± 12 | 98 ± 11 | 100 ± 14 | 93 ± 11 | 99 ± 11 |
Abbreviations: CAS, Cognitive Assessment System; CDI, Children’s Depression Inventory; RCDS, Reynolds Child Depression Scale; SDB, sleep-disordered breathing.
aValues are presented as mean ± SD or No. (%).
bVO2 peak is a measure of aerobic physical fitness.
cPediatric Sleep Questionnaire includes 22 dichotomous questions for primary caregivers (eg, parents) about children’s sleeping patterns (no = 0, yes = 1). Scores for all questions are added, and then a total average score is calculated; a total average score ≥0.33 was considered to be a positive screen for SDB.29
dCompleted by participants in project PLAY, the RCDS30 is a 30-item self-report questionnaire of symptoms of depression among children. Responses are reported with a 4-point Likert scale, where 1 = almost never and 4 = all the time. RCDS scores range from 30 to 120, with higher scores indicating more symptoms of depression. A score ≥74 indicated a clinically relevant level of depression. Fourteen (6.3%) RCDS results were invalid; these scores were not included in the analyses.
eCompleted by participants in the SMART study, the CDI is a 27-item multiple-choice self-report questionnaire of symptoms of depression among children and adolescents.29 Each item consists of statements with a rating ranging from 0 (absence of symptoms) to 2 (definite symptoms). CDI scores range from 0 to 54, with higher scores indicating higher depression levels. A score ≥19 indicated a positive screen for depression.
fCAS is a standardized individual assessment of children’s cognitive processes that yields 4 scale scores (Planning, Attention, Simultaneous, and Successive) and a composite Full Scale score.32 Standard scores, which show how a child’s performance compares with peers of the same age, are provided for each scale (normative mean = 100, SD = 15). The Planning Scale requires the child to consider how to solve each item and control the impulse to act without thinking. The Attention Scale requires the child to focus cognitive activity and inhibit responses to competing stimuli. The Simultaneous Scale involves spatial and logical content. The Successive Scale requires the child to integrate material into a specific serial order. The Full Scale score is the equally weighted composite of scores from the Planning, Attention, Simultaneous, and Successive Scales.
Fitness was negatively correlated with SDB (r = –0.14) and symptoms of depression (r = –0.18). SDB was positively correlated with symptoms of depression (r = 0.13; Table 2). Fitness was marginally positively correlated with scores of the CAS Attention Scale (r = 0.10) and Simultaneous Scale (r = 0.11). Symptoms of depression were negatively correlated with scores from the CAS Planning, Attention, Successive, and Full Scales (r = –0.19 to –0.22) but not the CAS Simultaneous Scale.
Table 2.
Partial correlations among physical fitness, sleep-disordered breathing, symptoms of depression, and cognition, adjusted for socioeconomic status and adiposity, among overweight and obese inactive children aged 7-11 (n = 314)a in public schools, based on study cohorts from project PLAY8,11,22 (2003-2006) and the SMART study26 (2008-2011), Augusta, Georgia
| Measure | Physical Fitness | P Valueb | Sleep-Disordered Breathing | P Valueb | Symptoms of Depression | P Valueb |
|---|---|---|---|---|---|---|
| Sleep-disordered breathingc | –0.14 | .009 | ||||
| Symptoms of depressiond | –0.18 | .001 | 0.13 | .034 | ||
| Cognitione | ||||||
| Planning Scale | 0.06 | .262 | –0.08 | .198 | –0.19 | .001 |
| Attention Scale | 0.10 | .033 | –0.19 | <.001 | –0.19 | .001 |
| Simultaneous Scale | 0.11 | .025 | 0.01 | .861 | –0.04 | .741 |
| Successive Scale | –0.02 | .900 | 0.01 | .541 | –0.21 | .001 |
| Full Scale | 0.08 | .063 | –0.09 | .135 | –0.22 | <.001 |
aOf the full study sample (N = 397), 83 participants had missing data for at least 1 variable of interest or 1 covariate and were not included in the partial correlation analyses, resulting in a final sample for the partial correlations of 314 participants.
bSignificant at P < .05.
cPediatric Sleep Questionnaire includes 22 dichotomous questions for primary caregivers (eg, parents) about children’s sleeping patterns (no = 0, yes = 1). Scores for all questions are added, and then a total average score is calculated; a total average score ≥0.33 was considered to be a positive screen for sleep-disordered breathing.29
d Z scores were calculated from Reynolds Child Depression Scale (RCDS)30 scores (project PLAY) and Childhood Depression Inventory (CDI)29 scores (SMART study). The RCDS30 is a 30-item self-report questionnaire of symptoms of depression among children. Responses are reported with a 4-point Likert scale, where 1 = almost never and 4 = all the time. RCDS scores range from 30 to 120, with higher scores indicating more symptoms of depression (a score ≥74 is considered to be indicative of a clinically relevant level of depression). Fourteen (6.3%) RCDS results were invalid; these scores were not included in the analyses. The CDI is a 27-item multiple-choice self-report questionnaire of symptoms of depression among children and adolescents.29 Each item consists of statements with a rating ranging from 0 (absence of symptoms) to 2 (definite symptoms). CDI scores range from 0 to 54, with higher scores indicating higher depression levels (a score ≥19 is considered to be indicative of a positive screen for depression in this pediatric nonclinical population).
eThe Cognitive Assessment System is a standardized individual assessment of children’s cognitive processes that yields 4 scale scores (Planning, Attention, Simultaneous, and Successive) and a composite Full Scale score.32 Standard scores, which show how a child’s performance compares with peers of the same age, are provided for each scale (normative mean = 100, SD = 15). The Planning Scale requires the child to consider how to solve each item and control the impulse to act without thinking. The Attention Scale requires the child to focus cognitive activity and inhibit responses to competing stimuli. The Simultaneous Scale involves spatial and logical content. The Successive Scale requires the child to integrate material into a specific serial order. The Full Scale score is the equally weighted composite of the scores from the Planning, Attention, Simultaneous, and Successive Scales.
Mediation Models
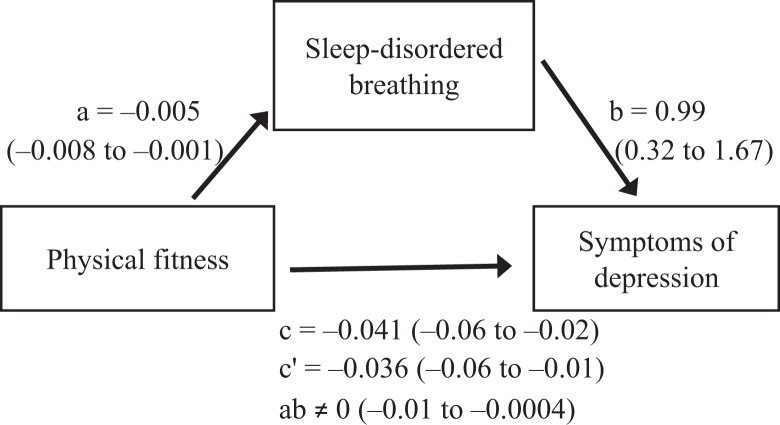
In the model with fitness as the independent variable, SDB as the mediator, and symptoms of depression as the dependent variable, fitness was significantly negatively associated with SDB (B = –0.005; 95% CI, –0.008 to –0.001; P = .008), and SDB was significantly positively associated with symptoms of depression (B = 0.99; 95% CI, 0.32, 1.67; P = .004), after controlling for fitness (Figure 1). These results supported the mediational hypothesis. In addition, fitness was significantly negatively associated with symptoms of depression (B = –0.041; 95% CI, –0.06 to –0.02; P = .002), even when controlling for the mediator (B = –0.036; 95% CI, –0.06 to –0.01), consistent with only partial mediation. The indirect effect of fitness on symptoms of depression mediated by SDB was different from zero (B ≠ 0; 95% bootstrap CI, –0.01 to –0.0004), indicating that those children who were 1 unit higher on VO2 peak were expected to be .005 SD lower on the depression scales, mediated by the influence of VO2 peak on SDB and, subsequently, SDB on symptoms of depression.
Figure 1.
Mediation model of the association between physical fitness and symptoms of depression mediated by sleep-disordered breathing (SDB) among overweight and obese inactive children aged 7-11 in public schools, based on study cohorts from project PLAY8,11,22 (2003-2006) and the SMART study26 (2008-2011), Augusta, Georgia. Unstandardized regression coefficients, B, and 95% CIs in parentheses are reported for each path. Abbreviations: a, direct effect of physical fitness on SDB (P = .008); b, direct effect of SDB on symptoms of depression after controlling for fitness (P = .004); c, total effect of physical fitness on symptoms of depression (P = .002); c′, direct effect of physical fitness on symptoms of depression after controlling for SDB; ab, indirect effect of physical fitness on symptoms of depression mediated by SDB.
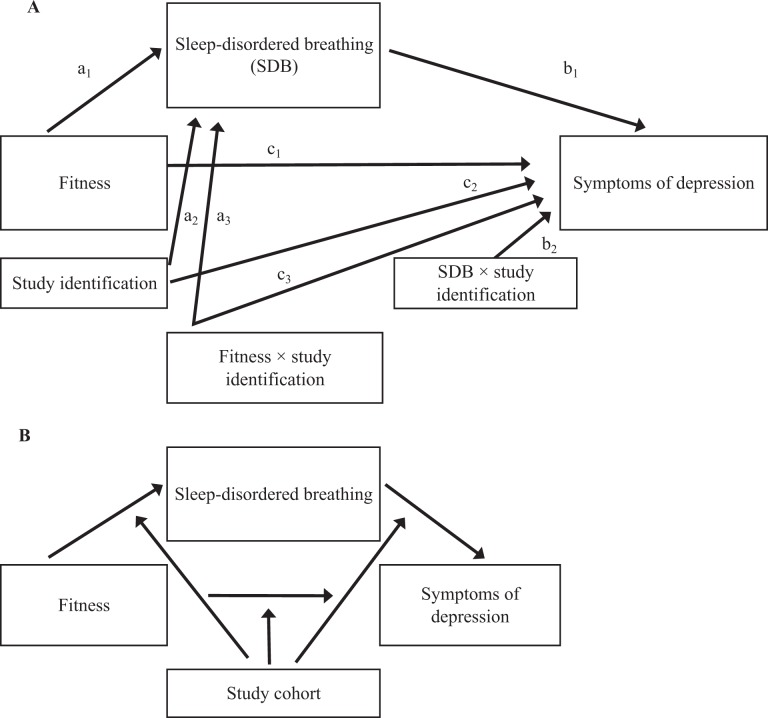
Using the moderated mediation model to examine the relationship between fitness and symptoms of depression mediated by SDB, we found no moderation by study cohort (Figure 2). We also found that the indirect effect of fitness on symptoms of depression mediated by SDB was not conditional on either race (model 58 index of moderated mediation = 0.01; 95% bootstrap CI, –0.01 to 0.02) or sex (model 58 index of moderated mediation = –0.002; 95% bootstrap CI, –0.01 to 0.01).
Figure 2.
Statistical (A) and conceptual (B) moderated mediation models, examining whether the association between fitness and symptoms of depression mediated by sleep-disordered breathing (SDB) was moderated by study cohort, W, among overweight and obese inactive children aged 7-11 in public schools, based on study cohorts from project PLAY8,11,22 (2003-2006) and the SMART study26 (2008-2011), Augusta, Georgia. Formula for the conditional indirect effect of fitness on symptoms of depression mediated by SDB: ω = (a1 + a3 W)(b1 + b2 W), where W is the moderator (study cohort). We used moderated mediation model 59 in PROCESS,34 and we found no moderation by study cohort.
Abbreviations: a1, path from physical fitness to SDB; a2, path from study cohort to SDB; a3, path from fitness × study cohort to SDB; b1, path from SDB to symptoms of depression; b2, path from SDB × study cohort to symptoms of depression; c1, path from physical fitness to symptoms of depression; c2, path from study cohort to symptoms of depression; c3, path from fitness × study cohort to symptoms of depression.
In the model with fitness as the independent variable, SDB and symptoms of depression as mediators, and the CAS Simultaneous Scale as the dependent variable, fitness was significantly negatively associated with SDB (B = –0.01; 95% CI, –0.01 to –0.001; P = .016), and SDB was significantly positively associated with symptoms of depression (B = 0.99; 95% CI, 0.32 to 1.67; P = .004). However, the total effect of fitness on the Simultaneous Scale was not significant (P = .069), and the direct effect, controlling for the mediators, was also not significant (P = .069). In addition, the separate indirect effects of fitness on the Simultaneous Scale mediated by only SDB (95% bootstrap CI, –0.05 to 0.03) and by only symptoms of depression (95% bootstrap CI, –0.04 to 0.06) were not significant. Similarly, the serial indirect effects of fitness on the Simultaneous Scale mediated by SDB and, in turn, by symptoms of depression (95% bootstrap CI, –0.004 to 0.006) were also not significant. The model including fitness, all mediators, and all covariates did not account for a significant amount of variance in the CAS Simultaneous Scale (R 2 = 0.026, P = .15). These results did not support the independent hypotheses (SDB, symptoms of depression) or serial mediation hypothesis.
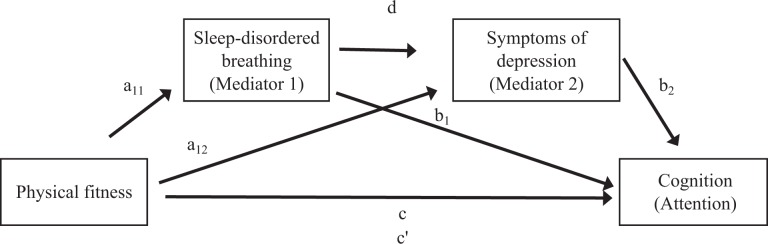
In the model with fitness as the independent variable, SDB and symptoms of depression as the mediators, and the CAS Attention Scale as the dependent variable (Figure 3), fitness was significantly negatively associated with SDB (B = –0.005; 95% CI, –0.01 to –0.001; P = .016) and symptoms of depression (B = –0.03; 95% CI, –0.06 to –0.01; P = .004). The total effect of fitness on the Attention Scale was not significant (B = 0.27; 95% CI, –0.03 to 0.57; P = .076), and the direct effect of fitness on the Attention Scale, controlling for the mediators, was also not significant (B = 0.14; 95% CI, –0.16 to 0.43; P = .371). However, the separate indirect effects of fitness on the Attention Scale mediated by only SDB (indirect effect = 0.06; 95% bootstrap CI, 0.004 to 0.13) and only symptoms of depression (indirect effect = 0.07; 95% bootstrap CI, 0.01 to 0.17) were both significant. Finally, the serial indirect effect of fitness mediated by SDB and, in turn, by symptoms of depression was not significant (indirect effect = 0.006; 95% bootstrap CI, –0.001 to 0.02). These results supported the independent hypotheses (1: fitness, SDB, attention; 2: fitness, symptoms of depression, attention) but not the serial mediation hypothesis. The model including fitness, all mediators, and all covariates accounted for 8% of the variance in the CAS Attention Scale (R 2 = 0.079, P < .001).
Figure 3.
Serial multiple mediation model examining the association between fitness and the attention component of cognition mediated by sleep-disordered breathing (SDB) and symptoms of depression among overweight and obese inactive children aged 7-11 in public schools, based on study cohorts from project PLAY8,11,22 (2003-2006) and the SMART study26 (2008-2011), Augusta, Georgia. Cognition was measured with the Cognitive Assessment System32 Attention Scale, which requires the child to focus cognitive activity and inhibit responses to competing stimuli. Abbreviations: a11, effect of physical fitness on SDB; a12, effect of physical fitness on symptoms of depression; b1, effect of SDB on attention; b2, effect of symptoms of depression on attention; c, total effect of physical fitness on attention; c′, direct effect of physical fitness on attention, controlling for SDB and symptoms of depression; d, effect of SDB on symptoms of depression. Results: Physical fitness was significantly negatively associated with SDB (B = –0.005; 95% CI, –0.01 to –0.001; P = .016) and symptoms of depression (B = –0.03; 95% CI, –0.06 to –0.01; P = .004). The total effect of fitness on attention was not significant (B = 0.27; 95% CI, –0.03 to 0.57; P = .076), and the direct effect of physical fitness on attention, controlling for the mediators, was also not significant (B = 0.14; 95% CI, –0.16 to 0.43; P = .371). The serial indirect effect of fitness mediated by SDB and, in turn, by symptoms of depression was not significant (indirect effect = 0.006; 95% bootstrap CI, –0.001 to 0.02). However, the indirect effects of fitness on attention mediated only by SDB (indirect effect = 0.06; 95% bootstrap CI, 0.004 to 0.13) and only by symptoms of depression (indirect effect = 0.07; 95% bootstrap CI, 0.01 to 0.17) were both significant. The model including fitness, all mediators, and all covariates accounted for 8% of the variance in attention (R2 = 0.079, P < .001).
Discussion
We hypothesized and tested a causal pathway between physical fitness and mood (symptoms of depression), mediated by sleep quality (SDB). Using a diverse sample of inactive 7- to 11-year-old overweight or obese children, we found that better physical fitness was linked to less SDB, which in turn was linked to fewer symptoms of depression. Previous research has suggested that higher weight is associated with lower cardiorespiratory fitness,38 increases in physical activity are associated with increases in fitness,39 obesity is associated with SDB,9,10 and increased physical activity is associated with improved sleep quality.11 Our finding that better fitness levels among overweight and obese children are associated with lower levels of SDB is consistent with these previous findings.
In addition, our finding that fitness levels are associated with mood is consistent with previous findings of children and adolescents showing beneficial effects of increased physical activity on mood.13–15 However, little is known about the mechanisms that drive the relationship between physical fitness levels and mood. Our finding that this relationship is mediated by SDB adds to the emerging literature about the involvement of sleep disturbances in the causal sequence of depression in children and adolescents. Indeed, although sleep difficulties are generally considered a symptom of depression,40 for children and adolescents, there may now be more support in the literature for the causal role of prodromal sleep difficulties in the development of depression than there is for symptoms of depression causing poor sleep quality.20 Furthermore, our findings about the fitness-sleep-mood pathway also point to additional potential prevention and treatment options, particularly focused on physical activity interventions, for childhood and adolescent depression.
We also hypothesized that changes in the fitness-sleep-mood pathway would be related to changes in cognitive functioning. We found that physical fitness was in fact marginally positively correlated with the cognitive ability to focus and inhibit responses (CAS Attention Scale). This is consistent with findings from others linking fitness to attention control as measured by laboratory reaction time and accuracy tasks.24,41 We also showed that the relationship between physical fitness and the attention component of cognition was mediated separately, but not sequentially, by SDB and symptoms of depression. The finding that SDB and attention are related is consistent with previous research showing that SDB is associated with self- and parent-reported attention problems,42,43 difficulty with cognitive processes that require high attention resources (eg, declarative memory),44 and symptoms of attention-deficit/hyperactivity disorder in young people.45 However, our finding of a link between depression and attention in children and adolescents is not well established in the published literature.46 The reason may be that previous studies examining clinical samples largely measured attention using laboratory tasks rather than standardized psychometric instruments, such as those used in our study. Also, as opposed to previous studies, our study used a community sample of children who reported mostly subthreshold symptoms of depression. Taken together, our mediation results on the attention component of cognition, integrated with findings in the literature, appear to point to fitness-related disruptions in sleep and mood as important mechanisms affecting cognitive functioning in children.
Limitations
This study had several limitations. First, our study was cross-sectional; as such, we could not ascertain temporal or causal relationships among physical fitness, SDB, symptoms of depression, and cognition. Second, serial mediation models require large sample sizes to detect indirect effects.47 Because of our modest sample size, our test of serial mediation may have failed to show significance because it was underpowered. Finally, the indirect effects described in this study accounted for a relatively small amount of variance in symptoms of depression (ie, 8%). However, our results still contribute valuable information to the growing understanding of the multiple factors involved in the development of depression.
Strengths
This study also had several strengths. First, components of cognition are commonly measured in research studies by using laboratory reaction time and accuracy computer tasks. Our use of an age-normed psychometric measure of cognitive functioning (ie, CAS), used most often in clinical settings, increased the clinical applicability and reliability of our results. Furthermore, laboratory measures of attention have demonstrated questionable test-retest reliability,48 whereas the reliability of the CAS Attention Scale has been excellent.32 Second, our use of VO2 peak to measure cardiorespiratory fitness provided us with a more objective index of aerobic capacity than if we had relied on the more subjective self-reporting of physical activity. Third, because we found no evidence that race or sex affected the relationships among fitness, SDB, and symptoms of depression, our results are generalizable to most US children, regardless of their race or sex. Finally, although causation cannot be inferred from the data in our study, the results are consistent with the posited fitness-sleep-mood-cognition causal sequence, and they suggest future directions for experimental research examining other potential mechanisms through which physical fitness might influence mood and cognition.
Conclusions
The results of our study indicate that SDB mediates the relationship between physical fitness and symptoms of depression and that SDB and symptoms of depression separately mediate the relationship between physical fitness and the attention component of cognition. They also add to the previous literature highlighting the mechanistic relationships among physical fitness, sleep, mood, and cognition. The mediation models tested in this study represent an important step in understanding the mechanisms underlying the relationships between sleep disturbance and mood and cognitive outcomes, with a broader goal of improving children’s well-being.
Future experimental studies should examine the potential causal link between SDB and symptoms of depression. Neuroimaging studies might shed light on the neural circuitry that affects SDB, mood, and cognition. Mediation models can be used to improve the understanding of the mechanisms linking sleep disturbance, mood, and cognition.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the following grants: National Institutes of Health R01 DK060692 and R01 DK070922 (NCT00108901), National Institutes of Health R01 HL087923 (NCT02227095), Health Resources and Services Administration D40HP26860, National Science Foundation DGE-1 343 012, and the Medical College of Georgia Children’s Summer Scholar Program.
References
- 1. Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150(1):12–17.e2. [DOI] [PubMed] [Google Scholar]
- 3. Whitlock EP, Williams SB, Gold R, Smith PR, Shipman SA. Screening and interventions for childhood overweight: a summary of evidence for the US Preventive Services Task Force. Pediatr. 2005;116(1):e125–e144. [DOI] [PubMed] [Google Scholar]
- 4. Nixon GM, Brouillette RT. Sleep 8: paediatric obstructive sleep apnoea. Thorax. 2005;60(6):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bell LM, Byrne S, Thompson A, et al. Increasing body mass index z-score is continuously associated with complications of overweight in children, even in the healthy weight range. J Clin Endocrinol Metab. 2007;92(2):517–522. [DOI] [PubMed] [Google Scholar]
- 6. Li Y, Dai Q, Jackson JC, Zhang J. Overweight is associated with decreased cognitive functioning among school-age children and adolescents. Obesity (Silver Spring). 2008;16(8):1809–1815. [DOI] [PubMed] [Google Scholar]
- 7. Taras H, Potts-Datema W. Obesity and student performance at school. J Sch Health. 2005;75(8):291–295. [DOI] [PubMed] [Google Scholar]
- 8. Davis CL, Pollock NK, Waller JL, et al. Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. JAMA. 2012;308(11):1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mallory GB, Fiser DH, Jackson R. Sleep-associated breathing disorders in morbidly obese children and adolescents. J Pediatr. 1989;115(6):892–897. [DOI] [PubMed] [Google Scholar]
- 10. Gupta NK, Mueller WH, Chan W, Meininger JC. Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol. 2002;14(6):762–768. [DOI] [PubMed] [Google Scholar]
- 11. Davis CL, Tkacz J, Gregoski M, Boyle CA, Lovrekovic G. Aerobic exercise and snoring in overweight children: a randomized controlled trial. Obesity (Silver Spring). 2006;14(11):1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Countryman AJ, Saab PG, Llabre MM, Penedo FJ, McCalla JR, Schneiderman N. Cardiometabolic risk in adolescents: associations with physical activity, fitness, and sleep. Ann Behav Med. 2013;45(1):121–131. [DOI] [PubMed] [Google Scholar]
- 13. Biddle SJ, Asare M. Physical activity and mental health in children and adolescents: a review of reviews. Br J Sports Med. 2011;45(11):886–895. [DOI] [PubMed] [Google Scholar]
- 14. Petty KH, Davis CL, Tkacz J, Young-Hyman D, Waller JL. Exercise effects on depressive symptoms and self-worth in overweight children: a randomized controlled trial. J Pediatr Psychol. 2009;34(9):929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rieck T, Jackson A, Martin SB, Petrie T, Greenleaf C. Health-related fitness, body mass index, and risk of depression among adolescents. Med Sci Sports Exerc. 2013;45(6):1083–1088. [DOI] [PubMed] [Google Scholar]
- 16. Åberg MA, Waern M, Nyberg J, et al. Cardiovascular fitness in males at age 18 and risk of serious depression in adulthood: Swedish prospective population-based study. Br J Psychiatry. 2012;201(5):352–359. [DOI] [PubMed] [Google Scholar]
- 17. Liu X, Buysse DJ, Gentzler AL, et al. Insomnia and hypersomnia associated with depressive phenomenology and comorbidity in childhood depression. Sleep. 2007;30(1):83–90. [DOI] [PubMed] [Google Scholar]
- 18. Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64(10):1195–1200. [DOI] [PubMed] [Google Scholar]
- 19. Sadeh A, Tikotzky L, Kahn M. Sleep in infancy and childhood: implications for emotional and behavioral difficulties in adolescence and beyond. Curr Opin Psychiatry. 2014;27(6):453–459. [DOI] [PubMed] [Google Scholar]
- 20. Lovato N, Gradisar M. A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep Med Rev. 2014;18(6):521–529. [DOI] [PubMed] [Google Scholar]
- 21. Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10(4):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davis CL, Tomporowski PD, McDowell JE, et al. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychol. 2011;30(1):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pontifex MB, Raine LB, Johnson CR, et al. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J Cogn Neurosci. 2011;23(6):1332–1345. [DOI] [PubMed] [Google Scholar]
- 24. Khan NA, Hillman CH. The relation of childhood physical activity and aerobic fitness to brain function and cognition: a review. Pediatr Exerc Sci. 2014;26(2):138–146. [DOI] [PubMed] [Google Scholar]
- 25. Davis CL, Cooper S. Fitness, fatness, cognition, behavior, and academic achievement among overweight children: do cross-sectional associations correspond to exercise trial outcomes? Prev Med. 2011;52(suppl 1):S65–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krafft CE, Schwarz NF, Chi L, et al. An 8-month randomized controlled exercise trial alters brain activation during cognitive tasks in overweight children. Obesity (Silver Spring). 2014;22(1):232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for disease control and prevention 2000 growth charts for the United States: improvements to the 1977 national center for health statistics version. Pediatrics. 2002;109(1):45–60. [DOI] [PubMed] [Google Scholar]
- 28. Rowland T. Oxygen uptake and endurance fitness in children, revisited. Pediatr Exerc Sci. 2013;25(4):508–514. [DOI] [PubMed] [Google Scholar]
- 29. Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. [DOI] [PubMed] [Google Scholar]
- 30. Reynolds WM. Reynolds Child Depression Scale. 2nd ed Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- 31. Crowley SL, Emerson EN. Discriminant validity of self-reported anxiety and depression in children: negative affectivity or independent constructs? J Clin Child Psychol. 1996;25(2):139–146. [Google Scholar]
- 32. Naglieri JA, Das JP. Cognitive Assessment System. Itasca, IL: Riverside Publishing; 1997. [Google Scholar]
- 33. IBM Corp. SPSS Version 21.0. Armonk, NY: IBM Corp; 2012. [Google Scholar]
- 34. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: The Guilford Press; 2013. [Google Scholar]
- 35. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. [DOI] [PubMed] [Google Scholar]
- 36. Hayes AF, Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychol Sci. 2013;24(10):1918–1927. [DOI] [PubMed] [Google Scholar]
- 37. Kenny DA, Judd CM. Power anomalies in testing mediation. Psychol Sci. 2014;25(2):334–339. [DOI] [PubMed] [Google Scholar]
- 38. Lohman TG, Ring K, Pfeiffer K, et al. Relationships among fitness, body composition, and physical activity. Med Sci Sport Exerc. 2008;40(6):1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407. [DOI] [PubMed] [Google Scholar]
- 40. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 41. Pontifex MB, Kamijo K, Scudder MR, et al. V: the differential association of adiposity and fitness with cognitive control in preadolescent children. Monogr Soc Res Child Dev. 2014;79(4):72–92. [DOI] [PubMed] [Google Scholar]
- 42. Lehto JE, Uusitalo-Malmivaara L. Sleep-related factors: associations with poor attention and depressive symptoms. Child Care Health Dev. 2014;40(3):419–425. [DOI] [PubMed] [Google Scholar]
- 43. Simola P, Liukkonen K, Pitkäranta A, Pirinen T, Aronen ET. Psychosocial and somatic outcomes of sleep problems in children: a 4-year follow-up study. Child Care Health Dev. 2014;40(1):60–67. [DOI] [PubMed] [Google Scholar]
- 44. Csábi E, Benedek P, Janacsek K, Katona G, Nemeth D. Sleep disorder in childhood impairs declarative but not nondeclarative forms of learning. J Clin Exp Neuropsychol. 2013;35(7):677–685. [DOI] [PubMed] [Google Scholar]
- 45. Sedky K, Bennett DS, Carvalho KS. Attention deficit hyperactivity disorder and sleep disordered breathing in pediatric populations: a meta-analysis. Sleep Med Rev. 2014;18(4):349–356. [DOI] [PubMed] [Google Scholar]
- 46. Vilgis V, Silk TJ, Vance A. Executive function and attention in children and adolescents with depressive disorders: a systematic review. Eur Child Adolesc Psychiatry. 2015;24(4):365–384. [DOI] [PubMed] [Google Scholar]
- 47. Taylor AB, MacKinnon DP, Tein JY. Tests of the three-path mediated effect. Organ Res Methods. 2008;11(2):241–269. [Google Scholar]
- 48. van Leeuwen M, van den Berg SM, Hoekstra RA, Boomsma DI. Endophenotypes for intelligence in children and adolescents. Intelligence. 2007;35(4):369–380. [Google Scholar]