Abstract
We prepared a stable cell line expressing the glucagon receptor to characterize the effect of Gs-coupled receptor stimulation on extracellular signal-regulated protein kinase 1/2 (ERK1/2) activity. Glucagon treatment of the cell line caused a dose-dependent increase in cAMP concentration, activation of cAMP-dependent protein kinase (PKA), and transient release of intracellular calcium. Glucagon treatment also caused rapid dose-dependent phosphorylation and activation of mitogen-activated protein kinase kinase/ERK kinase (MEK1/2) and ERK1/2. Inhibition of either PKA or MEK1/2 blocked ERK1/2 activation by glucagon. However, no significant activation of several upstream activators of MEK, including Ras, Rap1, and Raf, was observed in response to glucagon treatment. In addition, chelation of intracellular calcium reduced glucagon-mediated ERK1/2 activation. In transient transfection experiments, glucagon receptor mutants that bound glucagon but failed to increase intracellular cAMP and calcium concentrations showed no glucagon-stimulated ERK1/2 phosphorylation. We conclude that glucagon-induced MEK1/2 and ERK1/2 activation is mediated by PKA and that an increase in intracellular calcium concentration is required for maximal ERK activation.
Glucagon exerts its regulatory effects on hepatic glucose production by binding to the glucagon receptor (GR) (1). GR belongs to the superfamily of heptahelical transmembrane G protein-coupled receptors (GPCRs), which is divided into subfamilies based on amino acid sequence comparison. Most GPCRs fall into family A, the opsin/adrenergic receptor subfamily. GR is in family B, which shares few amino acid sequence similarities with the other GPCRs. Nevertheless, family B receptors generally couple to the same heterotrimeric G protein classes and activate the same sets of downstream effectors and signaling networks as the family A receptors.
One set of downstream effectors is the mitogen-activated protein (MAP) kinases, which include extracellular signal-regulated protein kinase 1/2 (ERK1/2), p38, and c-Jun N-terminal kinase/stress-activated protein kinase. These cytoplasmic serine/threonine protein kinases are critical points of convergence for cellular signal transduction pathways leading to cellular differentiation, proliferation, and transformation (2). ERK1/2 MAP kinases originally were shown to be activated by receptor tyrosine kinases that relied on a cascade of protein–protein interactions and phosphorylations proceeding through Ras, Raf, and MAP kinase kinase/ERK kinase (MEK1/2) (3). It has since been shown that each of the four families of heterotrimeric G proteins (Gs, Gi, Gq, and G12) activates, or sometimes inhibits, MAP kinase activity (4, 5). Although much attention has been focused on regulation of MAP kinases by family A GPCRs, much less is known about how family B receptors regulate MAP kinase activity.
We prepared a clonal cell line from human embryonic kidney (HEK) 293 cells that stably expressed a synthetic gene for the rat GR. Glucagon treatment of the cells caused dose-dependent activation of ERK1/2 MAP kinases and increases in cAMP and intracellular calcium. We used pharmacological and molecular approaches to identify the signaling pathway leading from GR activation to ERK1/2 activation. Although activation of MEK1/2 was required for glucagon-induced ERK1/2 activation, no significant activation of Ras, Rap1, or Raf was observed. Glucagon-induced MEK1/2 and ERK1/2 activation was mediated by cAMP-dependent protein kinase (PKA), and an increase in intracellular calcium concentration ([Ca2+]i) was required for PKA to activate ERK1/2 maximally.
Materials and Methods
Cell Lines Expressing GR.
HEK 293 cells (ATCC CRL 1573) were grown in DMEM supplemented with 10% FBS. The synthetic rat GR gene was subcloned into the pcDNA3 mammalian expression vector (Invitrogen) and transfected into HEK 293 cells via Lipofectamine (GIBCO/BRL). Clones were selected in the presence of 200 μg/ml G418 (GIBCO/BRL) and expanded. Clone H22 was selected for stable expression of GR based on specific glucagon binding and glucagon-induced signaling assays (1, 6, 7). Transient transfection of HEK 293 cells was performed on 80% confluent monolayers in 60-mm plates by using Lipofectamine. Empty pcDNA3 vector was added when needed to keep the amount of DNA/dish constant.
Immunoblot Analysis of ERK1/2 and MEK1/2 Activity.
Cells were serum-starved overnight and then changed to 2 ml of fresh DMEM/0.5% FBS for another 2 h before exposure to agonist [glucagon, forskolin, or epidermal growth factor (EGF)]. Cells were exposed to the desired concentration of agonist for 5 min and then lysed with Nonidet P-40 lysis buffer [25 mM Tris⋅HCl, pH 7.5/137 mM NaCl/10% (vol/vol) glycerol/1% (vol/vol) Nonidet P-40/2 mM EDTA/0.1 mM PMSF/10 μg/ml leupeptin/10 μg/ml aprotinin/5 mM sodium orthovanadate]. The cellular debris was removed by centrifugation at 7,000 × g for 10 min, and the total protein content was measured by using Bio-Rad DC Protein Assay. Aliquots (20–30 μg of protein) were separated on a 10% SDS/polyacrylamide gel and transferred semidry onto poly(vinylidene difluoride) membrane (Millipore). Phosphorylation and activation of ERK1/2 or MEK1/2 were detected by immunoblotting using rabbit polyclonal anti-phospho-ERK1/2 antibody or anti-phospho-MEK1/2 antibody (New England Biolabs), and visualized by enhanced chemiluminescence using horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody. In addition, aliquots were immunoblotted with anti-ERK1/2 antibody or anti-MEK1/2 antibody (phosphorylation-state independent) to detect total ERK1/2 or MEK1/2. For quantitation of ERK1/2 phosphorylation, films were scanned for analysis by using Scion image software.
Ras and Rap1 Activity Assays.
Glutathione S-transferase (GST)-RalGDS-Rap1 binding domain was expressed in Escherichia coli and affinity-purified on a glutathione-Sepharose column (CLONTECH) from the supernatant fraction of a lysate prepared by sonicating a bacterial suspension in PBS then adding 1% (vol/vol) Triton X-100. Rap1 activation was measured as described (8). Ras activation in H22 cells was measured with agarose-coupled GST-Raf-1-Ras binding domain according to the manufacturer's protocol (Upstate Biotechnology, Lake Placid, NY). Briefly, serum-starved H22 cells were stimulated at 37°C with different agonists for 5 min and immediately lysed. Equal amounts of supernatant fractions were incubated with agarose-coupled GST-Raf-1-Ras binding domain at 4°C for 30 min. The bound proteins were separated on a 15% SDS/polyacrylamide gel and transferred onto poly(vinylidene difluoride) membrane. The membrane was probed for activated Ras with anti-Ras antibody.
Assays of Intracellular cAMP, Inositol Phosphates, and Calcium Concentrations.
Adenylyl cyclase (AC) activity was determined by measuring cAMP levels as a function of hormone concentration essentially as reported (6). Intracellular calcium flux was measured by using Fluo-3AM (Molecular Probes) indicator dye as described (9). To measure inositol phosphates, H22 cells were incubated with [3H]myo-inositol (NEN) at 37°C for 20 h and then washed once with EBSSH buffer [26 mM Hepes, pH 7.4/125 mM NaCl/20 mM LiCl/5 mM KCl/2 mM CaCl2/1 mM MgSO4/1 mM NaH2PO4/5.6 mM glucose/0.1% (wt/vol) BSA). The cells were incubated in EBSSH at 37°C for 30 min, followed by incubation with glucagon or carbachol at 37°C for another 30 min. Inositol phosphates were extracted by aspirating buffer and adding 20 mM formic acid at 4°C for 60 min, followed by neutralization with 60 mM ammonium formate. The extracts were loaded onto AG 1-X8 200–400 mesh prepacked anion exchange columns that were pre-equilibrated with 3 M ammonium formate/100 mM formic acid followed by 10 mM formic acid/10 mM inositol. Columns were washed with 10 mM formic acid/10 mM inositol to wash off the [3H]inositol, then 5 mM borax/60 mM sodium formate to remove the glycerophosphate inositol. Columns were developed with 1 M ammonium formate/0.1 M formic acid. Radioactivity was measured after adding ReadySafe scintillation mixture (Beckman Coulter). The dpm was converted to fmol of inositol phosphates by comparing the counts of the eluates with a set of standards eluted in parallel.
Data Analysis.
The cAMP, inositol phosphate, and ERK1/2 activity assays were performed at least three times on independent samples. For an individual experiment, the data at each concentration of glucagon were fit to a logistic function. The effective concentration at 50% stimulation (EC50) was calculated from the inflection point of the best-fit curve (sigmaplot, Jandel, San Rafael, CA).
Results
To study glucagon-dependent ERK1/2 MAP kinase phosphorylation, we prepared HEK 293 cells stably expressing the GR (H22). H22 cell membranes were incubated with radiolabeled glucagon and increasing concentrations of unlabeled glucagon. The competition binding curves were well described by a logistic function characteristic of ligand-receptor equilibrium binding (data not shown) (7). The concentration of unlabeled glucagon required to displace 50% of receptor-bound 125I-glucagon (IC50 value) was 13 nM, which is consistent with previous reports for the GR in both tissue samples and heterologous expression systems (1, 6, 7).
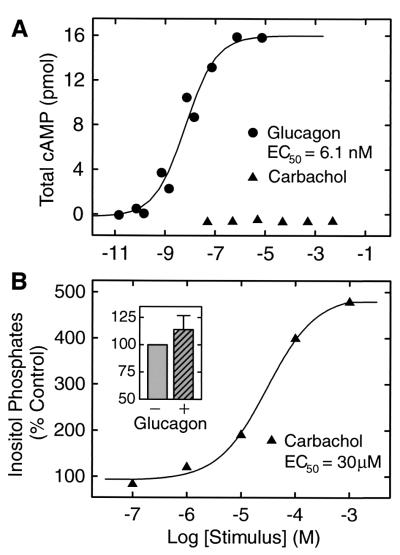
GR coupled to the stimulatory heterotrimeric G protein, Gs, in the H22 cells as demonstrated by glucagon-dependent AC stimulation (Fig. 1A). Treatment of H22 cells with glucagon led to a dose-dependent increase in cAMP level with an EC50 value of 6.1 ± 2.8 nM. As a control, carbachol stimulation of endogenous Gq-coupled muscarinic receptors caused no change in cAMP accumulation above basal levels (Fig. 1A). Glucagon has been reported to induce small increases in inositol phosphates in some cell types (10, 11). In the H22 cells, glucagon treatment did not cause a significant increase in inositol phosphates (Fig. 1B). As a positive control, carbachol induced a Gq-mediated stimulation of phospholipase C-β (PLC-β) to generate a nearly 5-fold increase in inositol phosphates (Fig. 1B). The results of the cAMP and inositol phosphate assays suggest that the expressed GR couples primarily to Gs and not to Gq to mediate signal transduction in the H22 cells.
Figure 1.
AC and PLC activities in H22 clonal cell line stably expressing the synthetic GR gene. (A) Intracellular cAMP levels were measured in H22 cells incubated at 37°C for 30 min with increasing concentrations of glucagon or carbachol. The EC50 value for glucagon is 6.1 ± 2.8 nM (mean ± SE). (B) H22 cells were loaded with [3H]myo-inositol for 20 h and incubated at 37°C for 30 min with increasing concentrations of carbachol. The EC50 value for carbachol is 30 ± 8.3 μM (mean ± SE). Each symbol represents the mean of duplicate determinations. Data shown are representative of at least three sets of determinations. (Inset) The glucagon-induced change in [3H]inositol phosphates at a glucagon concentration of 300 nM is shown. Glucagon did not cause a statistically significant increase in inositol phosphates when expressed as a percent of control (114.0 ± 12.8, mean ± SE, n = 5).
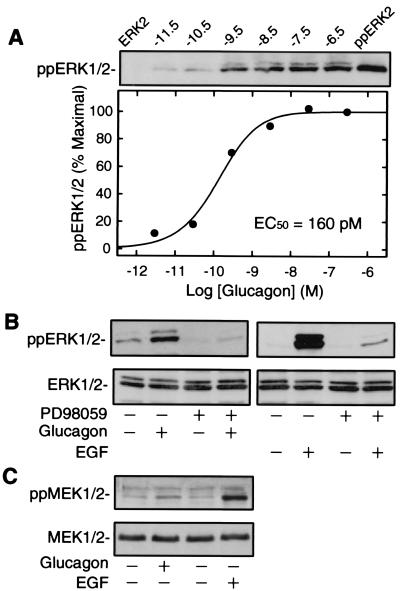
Many GPCRs have been shown either to activate or inhibit ERK1/2 MAP kinase activity, but little is known about regulation of ERK1/2 activation by GR. We set out to evaluate ERK1/2 activation in H22 cells in response to glucagon stimulation. ERK1/2 is activated by the phosphorylation of both Thr-183 and Tyr-185 residues. Therefore, ERK1/2 activation was detected by immunoblot using phospho-specific anti-ERK1/2 antibody, which only recognizes the dually phosphorylated ERK1/2 (12). Fig. 2A shows the dose response of glucagon-induced phosphorylation of ERK1/2. There was a characteristic dose-response curve with an EC50 of 160 ± 10 pM. The glucagon-induced phosphorylation of ERK1/2 peaked at 5 min and maintained a high level for at least 1 h (data not shown).
Figure 2.
GR mediates ERK1/2 activation via MEK1/2 in H22 cells. (A) H22 cells were stimulated at 37°C for 5 min with indicated concentrations of glucagon and lysed to determine ERK1/2 phosphorylation (ppERK1/2). Phosphorylation and activation of ERK1/2 was detected by immunoblotting (Upper). ERK2 and ppERK2 represent 1 ng of nonphosphorylated and phosphorylated forms of ERK2, respectively. The quantification of band intensities from the immunoblot is expressed as the percent of maximal ERK1/2 phosphorylation (Lower). The EC50 value for ERK1/2 phosphorylation is 160 ± 10 pM (mean ± SE). (B) H22 cells were preincubated at 37°C for 20 min with 10 μM PD98059 before stimulation for 5 min with 100 nM glucagon or 10 ng/ml EGF. Cells were lysed to determine ERK1/2 phosphorylation (Upper) and the total ERK1/2 (Lower). (C) H22 cells were stimulated at 37°C for 5 min with 100 nM glucagon or 10 ng/ml EGF. Cells were lysed to determine MEK1/2 phosphorylation (ppMEK1/2, Upper) and the total MEK1/2 (Lower). The data shown are representative of three independent sets of experiments with identical results.
To investigate whether or not glucagon-induced ERK1/2 phosphorylation was mediated by MEK1/2 activation, we analyzed the effect of a MEK-specific inhibitor (PD98059) on the phosphorylation of ERK1/2. As shown in Fig. 2B, ERK1/2 phosphorylation induced by glucagon or EGF was mostly suppressed by pretreatment of H22 cells with PD98059. Because MEK1/2 is activated through phosphorylation of Ser-217 and Ser-221 residues, MEK1/2 activation was examined by immunoblot using phospho-specific anti-MEK1/2 antibody, which recognizes only the Ser-217/221-phosphorylated MEK1/2. As shown in Fig. 2C, both glucagon and EGF stimulated MEK1/2 phosphorylation to the extent consistent with ERK1/2 phosphorylation. These results indicate that MEK1/2 activation mediates glucagon-induced ERK1/2 phosphorylation. However, in glucagon-stimulated H22 cells, we did not observe a significant increase in B-Raf activity or c-Raf-1 activity in immune complex kinase assays using MEK as a substrate (data not shown). These data suggest that GR may mediate its effect on MEK and ERK activation through signaling molecules other than the serine/threonine kinases of the Raf family.
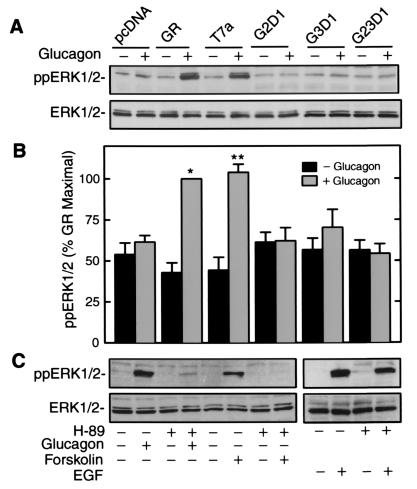
GR couples to Gs to elevate intracellular cAMP concentration. Depending on cell type, cAMP can activate PKA to exert either stimulatory or inhibitory effects on MAP kinase (13–17). We previously prepared a series of GR mutants that have essentially normal affinity for glucagon but different abilities to produce cAMP (7, 9). These mutants were expressed in HEK 293 cells to assess the regulatory effect of cAMP on ERK1/2 activity. The C-terminal-truncated GR mutant T7a, which can bind glucagon and increase cAMP comparably to wild-type GR, exerted the same effect on ERK1/2 phosphorylation as GR (Fig. 3 A and B). The chimeric receptor mutants G2D1 and G3D1, whose second or third intracellular loops, respectively were replaced with the first intracellular loop of the D4 dopamine receptor, were severely impaired in their ability to elevate intracellular cAMP (9). In response to glucagon treatment, the G2D1 and G3D1 mutants caused significantly lower ERK1/2 phosphorylation than that caused by GR. The chimeric receptor mutant G23D1, in which both intracellular loops were replaced, was completely unable to elevate intracellular cAMP in response to glucagon. G23D1 also completely failed to induce glucagon-dependent ERK1/2 phosphorylation (Fig. 3 A and B). These data suggest that glucagon-induced cAMP generation might be critical for ERK1/2 phosphorylation.
Figure 3.
GR-mediated ERK1/2 phosphorylation is cAMP- and PKA-dependent. (A) HEK 293 cells were transiently transfected with pcDNA3 vector or pcDNA3 vectors containing GR, a C-terminal truncation mutant of GR (T7a), and chimeric mutants of GR (G2D1, G3D1, and G23D1). Cells were stimulated at 37°C for 5 min with glucagon (100 nM) and lysed to determine ERK1/2 phosphorylation (Upper) and total ERK1/2 (Lower). (B) ERK1/2 phosphorylation from several independent experiments is expressed as the percent of maximal ERK1/2 phosphorylation in cells expressing GR. Each bar is the mean ± SE of pooled data from multiple experiments. * indicates P < 0.01 and ** indicates P < 0.05, by paired Student's t test. GR and GR mutants were expressed to the same levels as determined by immunoblot (data not shown). (C) HEK 293 cells were transiently transfected with GR expression vector. Serum-starved cells were preincubated at 37°C for 30 min with H-89 (20 μM) before stimulation for 5 min with glucagon (100 nM), forskolin (10 μM), or EGF (10 ng/ml). Cells were lysed to determine ERK1/2 phosphorylation (Upper) and total ERK1/2 (Lower). The data shown are representative of three independent experiments with identical results.
We next determined whether or not cAMP-dependent PKA played a role in mediating ERK1/2 phosphorylation. As shown in Fig. 3C, pretreatment with PKA-specific inhibitor (H-89) significantly abolished ERK1/2 phosphorylation by glucagon in HEK 293 cells transiently transfected with GR. The role of PKA in glucagon-mediated ERK1/2 phosphorylation was further confirmed by evaluating the effect of forskolin, which stimulates AC directly. Pretreatment with H-89 also eliminated forskolin-induced ERK1/2 phosphorylation in HEK 293 cells. As a control, H-89 pretreatment had no inhibitory effect on EGF-induced ERK1/2 phosphorylation, which is mediated via the Ras and Raf kinase cascade (Fig. 3C).
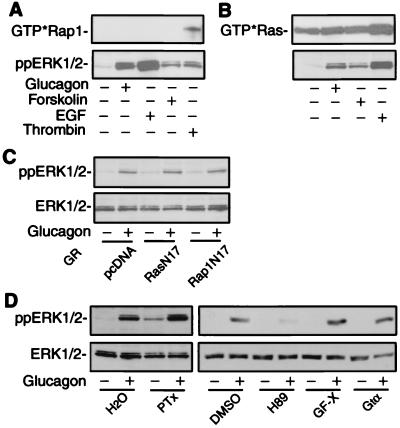
In some cell types, PKA stimulates MAP kinase activity via a Ras-related small G protein, Rap1, and the downstream kinase, B-Raf (15). Therefore, we examined whether glucagon activates endogenous Rap1 in H22 cells. Rap1 activation was measured with a GST-RalGDS-Rap1 binding domain fusion protein, which associates exclusively with the GTP-bound form, but not the GDP-bound form of Rap1 in vitro (8). Thrombin stimulated endogenous Rap1 activity and induced ERK1/2 activation in H22 cells. However, EGF, glucagon, and forskolin each failed to activate Rap1 under conditions where ERK1/2 was phosphorylated in H22 cells (Fig. 4A). Thus, in response to glucagon and forskolin stimulation, PKA-mediated ERK1/2 phosphorylation was independent of Rap1 activation.
Figure 4.
Effects of Rap1, Ras, and different inhibitors on GR-mediated ERK1/2 phosphorylation. H22 cells were stimulated at 37°C for 5 min with glucagon (100 nM), forskolin (10 μM), EGF (10 ng/ml), or thrombin (0.1 units/ml). (A) GTP-bound Rap1 in cell lysates was affinity-precipitated and immunoblotted with Rap-1/Krev-1 antibody (Upper). The corresponding ERK1/2 phosphorylation is shown (Lower). (B) GTP-bound Ras was affinity-precipitated and immunoblotted with anti-Ras antibody (Upper). The corresponding ERK1/2 phosphorylation is shown (Lower). (C) HEK 293 cells were transiently cotransfected with GR and dominant negative Ras (RasN17) or dominant negative Rap1 (Rap1N17) expression vectors. Cells were incubated at 37°C for 5 min with glucagon and lysed to determine ERK1/2 phosphorylation (Upper) and the total ERK1/2 (Lower). (D) HEK 293 cells were transiently transfected with GR expression vector. Cells were preincubated at 37°C for 24 h with pertussis toxin (PTx, 100 ng/ml) or for 30 min with H-89 (20 μM) or GF 109203X (GF-X, 2 μM) before stimulation for 5 min with glucagon. HEK 293 cells were transiently cotransfected with Gtα and GR expression vectors and stimulated at 37°C for 5 min with glucagon. Cells were lysed to determine ERK1/2 phosphorylation (Upper) and the total ERK1/2 (Lower). The data shown are representative of three independent experiments with identical results.
Because some GPCRs have been reported to activate MAP kinase via the small G protein Ras (18–20), we examined the activation of endogenous Ras in H22 cells in response to agonist stimulation. As shown in Fig. 4B, a high basal level of the GTP-bound form of Ras was detected by GST-Raf-1-Ras binding domain, which binds exclusively to activated Ras. As a positive control, EGF treatment increased the GTP-bound form of Ras. However, glucagon or forskolin treatment did not induce a significant increase in the GTP-bound form of Ras, suggesting that glucagon-stimulated ERK1/2 phosphorylation was independent of Ras activation (Fig. 4B). To further confirm that glucagon-stimulated ERK1/2 activation was independent of Ras or Rap1 activation, we cotransfected GR with the dominant-negative Ras (N17) or Rap1 (N17) and measured ERK1/2 phosphorylation induced by glucagon. As shown in Fig. 4C, the dominant negative Ras and Rap1 did not prevent ERK1/2 phosphorylation in response to glucagon stimulation. Consistent with this result, overexpression of Rap1 had no effect on GR-mediated ERK1/2 phosphorylation (data not shown).
In HEK 293 cells, the β2 adrenergic receptor switches its G protein coupling specificity from Gs to Gi upon PKA-dependent phosphorylation (21). Pertussis toxin, an irreversible Giα/Goα-specific antagonist that uncouples receptor and G protein, was used to determine whether GR couples to pertussis toxin-sensitive G proteins in MAP kinase activation. Preincubation with pertussis toxin did not inhibit ERK1/2 phosphorylation by glucagon in HEK 293 cells transiently transfected with GR (Fig. 4D). In combination with the findings in Fig. 1 on Gs coupling, this result suggests that GR mediates ERK1/2 phosphorylation by coupling to Gsα and not to Gqα or Giα/Goα.
Gq-coupled receptors stimulate PLC-β to activate protein kinase C (PKC). Activation of MAP kinases by Gq-coupled receptors has been shown to be PKC-dependent or PKC-independent (reviewed in ref. 5). Because glucagon did not activate PLC-β to generate inositol phosphates in H22 cells (Fig. 1B), we hypothesized that PKC activation may not play a role in ERK1/2 activation by glucagon. Pretreatment with GF 109203X, a broad range PKC inhibitor, had no effect on glucagon-mediated ERK1/2 phosphorylation in HEK 293 cells expressing GR (Fig. 4D), suggesting that GR-mediated ERK activation was independent of PKC activation.
Activation of Gq- or Gi-coupled receptors releases Gβγ subunits, which in turn stimulate Ras to activate MAP kinase. Gβγ mediates Ras activation via signaling pathways including tyrosine kinases, adaptor molecules, phosphoinositide 3-kinases, PKC, and novel molecular mediators (reviewed in ref. 5). However, the Gβγ subunits released by Gs-coupled receptors may not mediate ERK1/2 activation (16, 17). We coexpressed the α subunit of transducin (Gtα) with GR to sequester Gβγ subunits (22) and then determined the effect of Gβγ sequestration on ERK1/2 phosphorylation. As shown in Fig. 4D, Gtα had no inhibitory effect on ERK1/2 phosphorylation, suggesting that GR-mediated ERK activation was independent of Gβγ release.
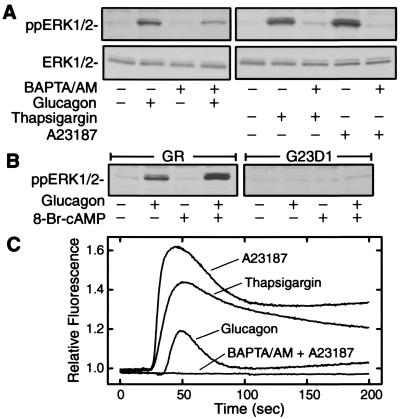
Glucagon and forskolin both induce increases in cAMP and [Ca2+]i. The origin of glucagon-dependent increase in [Ca2+]i have been discussed (9). GR chimeric mutants G2D1, G3D1, and G23D1 bind glucagon but fail to mediate increases in cAMP and [Ca2+]i levels (9). Because the effect of [Ca2+]i on ERK1/2 activation is not well characterized, we assessed whether reduction of [Ca2+]i affected ERK1/2 activation by glucagon in HEK 293 cells transiently expressing GR. As shown in Fig. 5A, glucagon-stimulated ERK1/2 phosphorylation was partially blocked when cells were pretreated with the intracellular calcium chelator BAPTA/AM [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate/acetoxymethyl ester]. The reduced ERK1/2 phosphorylation was not due to the nonspecific inhibitory effects of BAPTA/AM pretreatment. As a positive control, BAPTA/AM pretreatment abolished ERK1/2 phosphorylation that was induced by the calcium mobilizer thapsigargin and the calcium ionophore A23187 (Fig. 5A). BAPTA/AM pretreatment chelated intracellular calcium that was induced by glucagon, thapsigargin, or A23187 (Fig. 5C). These results suggest that [Ca2+]i does not appear to be essential for glucagoninduced ERK1/2 phosphorylation, but is required for maximal phosphorylation.
Figure 5.
Intracellular calcium is required for maximal ERK1/2 activation. (A) HEK 293 cells were transiently transfected with pcDNA3 vector or GR expression vector. Cells were preincubated at 37°C for 30 min with EGTA (2 mM) and BAPTA/AM (50 μM). Cells expressing GR were stimulated with glucagon (100 nM) and pcDNA3 cells were treated with thapsigargin (10 μM) or A23187 (10 μM) at 37°C for 5 min. Cells were lysed to determine ERK1/2 phosphorylation (Upper) and total ERK1/2 (Lower). (B) HEK 293 cells were transiently transfected with GR or G23D1 expression vectors. Cells were stimulated at 37°C for 5 min with glucagon (100 nM), 8-Br-cAMP (0.1 mM), or both together and lysed to determine ERK1/2 phosphorylation. (C) HEK 293 cells were transiently transfected with pcDNA3 vector or GR expression vector. Cells were collected and incubated at room temperature for 60 min with Fluo-3AM (2 μM) in the presence or absence of EGTA (2 mM) and BAPTA/AM (50 μM), and then stimulated with glucagon, thapsigargin, or A23187. Fluorescence was measured as a function of time at room temperature. The fluorescence in response to EGTA and BAPTA/AM pretreatment with A23187 stimulation is shown. The same results were observed in response to EGTA and BAPTA/AM pretreatment with glucagon or thapsigargin stimulation (data not shown). The data shown are representative of three independent experiments with identical results.
The effect of transient elevations of [Ca2+]i on PKA-mediated ERK1/2 phosphorylation was further evaluated by cotreatment with glucagon and low-dose 8-Br-cAMP. High-dose 8-Br-cAMP (1–2 mM), a partially membrane-permeable cAMP analogue, activated both PKA and ERK1/2 (data not shown). Treatment with low dose of 8-Br-cAMP (0.1 mM) neither increased [Ca2+]i (data not shown) nor stimulated ERK1/2 phosphorylation (Fig. 5B). If an increase in [Ca2+]i had no effect on PKA-mediated ERK1/2 phosphorylation, then the effect induced by cotreatment would be only slightly higher than that induced by glucagon alone, because glucagon and 8-Br-cAMP both would act on PKA to cause an additive effect on ERK1/2 phosphorylation. However, if the glucagon-induced increase in [Ca2+]i exerted some effect on PKA-mediated ERK1/2 phosphorylation, then cotreatment would stimulate significantly higher phosphorylation than either glucagon or 8-Br-cAMP alone. In fact, the cotreatment with glucagon and 8-Br-cAMP caused a synergistic phosphorylation of ERK1/2 in the cells expressing GR, but not in the cells expressing the mutant receptor G23D1 (Fig. 5B). The synergistic ERK1/2 activation suggests that a glucagon-induced elevation of [Ca2+]i is required for cAMP-dependent PKA to activate ERK1/2 maximally.
Discussion
GPCRs can regulate MAP kinase activity through various signaling pathways. For example, certain GPCRs activate receptor and nonreceptor tyrosine kinases. Tyrosine phosphorylation creates docking sites where proteins containing phosphotyrosine-binding domains can assemble in multimeric signaling complexes to stimulate a Ras-dependent Raf/MEK/MAP kinase cascade. Gq-, Gi-, and G13-coupled receptors can transactivate EGF receptor. This transactivation is mediated by GPCR-induced metalloprotease activity, which cleaves the heparin binding EGF precursor and allows the released growth factor to bind and activate EGF receptor (reviewed in ref. 23). GPCR-G protein interaction releases Gβγ dimers that mediate MAP kinase activation via additional signaling pathways: (i) activation of PLC-β to increase [Ca2+]i and diacylglycerol levels, which activate PKC and lead to MAP kinase activation; (ii) tyrosine phosphorylation of Shc to form a signaling complex for Ras activation; and (iii) recruitment of phosphoinositide 3-kinase γ to activate the Ras pathway (reviewed in ref. 5). Giα activates MAP kinase by associating with Rap1 GTPase-activating protein, Rap1GAPII, to reduce GTP-bound Rap1, which antagonizes Ras function by competing with Ras for binding to Raf-1 and A-Raf (24). However, up to now there has been no evidence to show analogous functions of Gs-coupled receptors.
The GR, which has been widely used as a model Gs-coupled GPCR, activates AC to increase intracellular cAMP and [Ca2+]i (9). In our studies, glucagon activation of cAMP-dependent PKA, in concert with an increase in [Ca2+]i, exerted a full stimulatory effect on MEK1/2 and ERK1/2 activation. GR did not activate PLC-β to generate inositol phosphates to cause ERK1/2 activation. Unlike the β2-adrenergic receptor (21), GR does not switch its G protein coupling specificity from Gs to Gi upon PKA-dependent phosphorylation. In our studies, sequestration of Gβγ dimers, inhibition of PKC, or expression of dominant negative Ras and Rap1 did not affect glucagon-stimulated ERK1/2 activation. The Gs-coupled GR seems to be unable to transactivate EGF receptor and its downstream Ras signaling pathway: (i) glucagon did not induce Ras activation and (ii) dominant-negative Ras had no inhibitory effect on glucagon-induced ERK1/2 activation. To mediate MAP kinase activation, GR does not require many of the signaling molecules that are involved in other GPCR signaling pathways, including EGF receptor, Gβγ subunits, Ras, and PKC.
Intracellular cAMP is an important second messenger that allows GR to activate downstream PKA for protein phosphorylation. Depending on cell types, PKA exerts different regulatory effects on MAP kinase activation. In the Ras-dependent Raf/MEK/MAP kinase cascade, PKA inhibits Raf-1 to suppress MAP kinase activation for cell proliferation (13, 14). On the other hand, PKA activates Rap1 to form a Rap1/B-Raf signaling complex, which in turn stimulates MEK and MAP kinase activation in PC12 cells (15). Recent reports identify other cAMP targets, including cAMP-regulated guanine nucleotide exchange factors and exchange protein directly activated by cAMP. These proteins regulate Rap1 activity in a cAMP-dependent, but PKA-independent, manner to regulate Ras signaling pathways (25, 26).
In our studies, glucagon-stimulated ERK1/2 activation was eliminated by a PKA inhibitor and was absent in GR mutants that failed to increase cAMP levels. Hence, glucagon-stimulated ERK1/2 activation was mediated by cAMP-activated PKA, but was independent of other cAMP targets such as cAMP-guanine nucleotide exchange factors and exchange protein directly activated by cAMP. Glucagon treatment could stimulate MEK1/2 and ERK1/2 activation, but neither Rap1/B-Raf activation nor Ras/Raf activation, suggesting that PKA activated the MEK/ERK kinase cascade in a Rap-, Ras-, and Raf-independent signaling pathway.
In many cells, [Ca2+]i and cAMP signal transduction pathways are tightly coupled. [Ca2+]i has been shown to stimulate MAP kinase activation through different signaling molecules, which converge to activate a Ras-dependent Raf/MEK/MAPK kinase cascade (27, 28). Thapsigargin and A23187 treatments induce a strong increase in [Ca2+]i, which may stimulate Raf to activate MAP kinase. There is evidence to show that the dominant-negative mutant of Raf inhibits thapsigargin-induced MAP kinase phosphorylation (29). BAPTA-AM treatment clamped [Ca2+]i to inactivate Raf for ERK1/2 phosphorylation by thapsigargin and A23187. However, in response to glucagon and forskolin treatment, cAMP activates PKA to inhibit Raf activation, which might prevent [Ca2+]i from directly mediating ERK1/2 phosphorylation via Raf. Under these conditions, a glucagon-mediated increase in [Ca2+]i may alternatively play a role to help PKA activate ERK1/2. In our studies, glucagon-stimulated ERK1/2 activation was partially blocked when intracellular calcium was chelated by BAPTA/AM. ERK1/2 was activated by high-dose 8-Br-cAMP, but the level of activation was not as significant as that induced by glucagon, most likely because 8-Br-cAMP caused little, if any, increase in [Ca2+]i compared with glucagon (data not shown). Cotreatment with glucagon and a low dose of 8-Br-cAMP, which alone did not significantly activate ERK1/2, caused a synergistic activation of ERK1/2. This synergistic activation did not seem to result from additive activation of PKA by 8-Br-cAMP and glucagon-induced cAMP, but rather from the coordinated action of glucagon-induced elevation of [Ca2+]i and cAMP-dependent PKA. In conclusion, although an increase in [Ca2+]i appears not to be essential, it is clearly required to observe maximal ERK1/2 activation by PKA.
Acknowledgments
We gratefully acknowledge the gifts of the Rap1 cDNA construct from Dr. D. L. Altschuler (University of Pittsburgh, Pittsburgh, PA), the dominant-negative Ras cDNA construct from Dr. X. Y. Huang (Weill Medical College, New York, NY), and the GST-RalGDS-RBD cDNA construct from Dr. J. L. Bos (Utrecht University, Utrecht, Netherlands). This research was supported in part by U.S. Public Health Service Grants DK24039 (to R.B.M.) and DK54718 (to T.P.S.), National Research Service Award Training Grant CA09673, and Medical Scientist Training Program Grant GM07739 (to A.M.C.). T.P.S. is an Associate Investigator of the Howard Hughes Medical Institute.
Abbreviations
- AC
adenylyl cyclase
- BAPTA/AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate/acetoxymethyl ester
- [Ca2+]i
intracellular calcium concentration
- EGF
epidermal growth factor
- ERK1/2
extracellular signal-regulated protein kinase 1/2
- GPCR
G protein-coupled receptor
- GR
glucagon receptor
- GST
glutathione S-transferase
- HEK
human embryonic kidney
- MAP
mitogen-activated protein
- MEK
MAP kinase kinase/ERK kinase
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PLC-β
phospholipase C-β
References
- 1.Jelinek L J, Lok S, Rosenberg G B, Smith R A, Grant F J, Biggs S, Bensch P A, Kuijper J L, Sheppard P O, Sprecher C A, et al. Science. 1993;259:1614–1616. doi: 10.1126/science.8384375. [DOI] [PubMed] [Google Scholar]
- 2.Robinson M J, Cobb M H. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 3.Marshall C J. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 4.Post G R, Brown J H. FASEB J. 1996;10:741–749. doi: 10.1096/fasebj.10.7.8635691. [DOI] [PubMed] [Google Scholar]
- 5.Gutkind J S. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers C J, Unson C G, Kim H N, Sakmar T P. J Biol Chem. 1994;269:29321–29328. [PubMed] [Google Scholar]
- 7.Unson C G, Cypess A M, Kim H N, Goldsmith P K, Carruthers C J, Merrifield R B, Sakmar T P. J Biol Chem. 1995;270:27720–27727. doi: 10.1074/jbc.270.46.27720. [DOI] [PubMed] [Google Scholar]
- 8.Franke B, Akkerman J W, Bos J L. EMBO J. 1997;16:252–259. doi: 10.1093/emboj/16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cypess A M, Unson C G, Wu C R, Sakmar T P. J Biol Chem. 1999;274:19455–19464. doi: 10.1074/jbc.274.27.19455. [DOI] [PubMed] [Google Scholar]
- 10.Wakelam M J, Murphy G J, Hruby V J, Houslay M D. Nature (London) 1986;323:68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Larocca J N, Rodriguez-Gabin A G, Charron M J. Biochim Biophys Acta. 1997;1356:229–236. doi: 10.1016/s0167-4889(96)00170-x. [DOI] [PubMed] [Google Scholar]
- 12.Payne D M, Rossomando A J, Martino P, Erickson A K, Her J H, Shabanowitz J, Hunt D F, Weber M J, Sturgill T W. EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Dent P, Jelinek T, Wolfman A, Weber M J, Sturgill T W. Science. 1993;262:1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- 14.Cook S J, McCormick F. Science. 1993;262:1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 15.Vossler M R, Yao H, York R D, Pan M G, Rim C S, Stork P J. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 16.Wan Y, Huang X Y. J Biol Chem. 1998;273:14533–14537. doi: 10.1074/jbc.273.23.14533. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt J M, Stork P J. J Biol Chem. 2000;275:25342–25350. doi: 10.1074/jbc.M003213200. [DOI] [PubMed] [Google Scholar]
- 18.Crespo P, Xu N, Simonds W F, Gutkind J S. Nature (London) 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 19.Koch W J, Hawes B E, Allen L F, Lefkowitz R J. Proc Natl Acad Sci USA. 1994;91:12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hordijk P L, Verlaan I, van Corven E J, Moolenaar W H. J Biol Chem. 1994;269:645–651. [PubMed] [Google Scholar]
- 21.Daaka Y, Luttrell L M, Lefkowitz R J. Nature (London) 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 22.Faure M, Voyno-Yasenetskaya T A, Bourne H R. J Biol Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- 23.Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Oncogene. 2001;20:1594–1600. doi: 10.1038/sj.onc.1204192. [DOI] [PubMed] [Google Scholar]
- 24.Mochizuki N, Ohba Y, Kiyokawa E, Kurata T, Murakami T, Ozaki T, Kitabatake A, Nagashima K, Matsuda M. Nature (London) 1999;400:891–894. doi: 10.1038/23738. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki H, Springett G M, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman D E, Graybiel A M. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 26.de Rooij J, Zwartkruis F J, Verheijen M H, Cool R H, Nijman S M, Wittinghofer A, Bos J L. Nature (London) 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 27.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 28.Della Rocca G J, van Biesen T, Daaka Y, Luttrell D K, Luttrell L M, Lefkowitz R J. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 29.Chao T S, Foster D A, Rapp U R, Rosner M R. J Biol Chem. 1994;269:7337–7341. [PubMed] [Google Scholar]