Abstract
Objectives: To examine physiological influences of adolescent sexual behaviour, including associated psychosocial factors. Methods: Systematic review. Results: Thirteen studies met the inclusion criteria relating to adolescents, physiology and sexual behaviour. We excluded studies relating to abnormal development. Findings highlighted hormonal and gender differences. Females appear to be more influenced by psychosocial aspects, including the effects of peers, than males. Males may be more inclined to engage in unprotected sex with a greater number of partners. Early maturing adolescents are more likely to be sexually active at an early age. Conclusions: Hormonal, psychosocial context, and sexual preference need to be acknowledged in intervention development. Stage of readiness to receive information may differ according to gender and physiological maturity.
Keywords: adolescent sexuality, sexual development, hormones, physiology, systematic review
1. Introduction
The sexual behaviour of adolescents is of importance due to the increasing number of sexually active adolescents globally (World Health Organisation [WHO], 2012). While initiation of sexual activity is a part of normal behaviour and development, it may also be associated with negative outcomes, if sexual behaviour involves engagement in sexual activity at too early an age, or without due attention to the risks involved (Maswikwa, Richter, Kaufman, & Nandi, 2015). Teenagers and young adults may face many sexual and reproductive health risks stemming from early, unprotected, or unwanted sexual activity (WHO, 2012). For example, early initiation of sexual activity increases the period of time adolescents are exposed to the risk of sexually transmitted infections or unintended pregnancy (WHO, 2011). Although there is no universal definition of early initiation of sexual activity, it is often classified as sexual intercourse during initial high school years (Johnson & Tyler, 2007) or sexual intercourse before the age of legal consent (Girma & Paton, 2015).
For many adolescents, sexual activity may start earlier than permitted by law (Klettke & Mellor, 2012, Yarrow et al., 2014); in the USA, for example, 62% of students were reported to have engaged in sexual activity before leaving school (Martinez, Copen, & Abma, 2011), and in many instances, young people may initiate sexual relations before the age of 14 years (WHO, 2011). There may be associated risks of early pregnancy, for example, if adolescents do not have a good understanding of contraception (WHO, 2012).
While rates of teenage pregnancy may be falling in some middle to high income countries (Girma & Paton, 2015), early sexual activity and pregnancy are still prevalent in other areas and cultures, and may be associated with increased morbidity and mortality (Maswikwa et al., 2015; WHO, 2012). Encouraging appropriate attitudes to sexual behaviour and activity during adolescence can help to ensure that contraception is understood, pregnancy is intended (Sher, 2016), and the cycle of deprivation associated with pregnancy in young people under the age of 18 is reduced (Scottish Government, 2016). Promoting healthy sexual behaviour can help reduce these risks, and further negative outcomes, such as sexually transmitted infections.
Sexual behaviour may be influenced by many physiological factors, in addition to the cultural and social pressures which can change rapidly from one generation to the next. Understanding the physiological influences that drive adolescent sexual activity, such as hormonal, chemical and neurological reactions and changes, can help inform interventions to support adolescents in making appropriate choices regarding their sexual behaviour. Exploring the nature of these physiological processes, and associated consequences, is essential for designing effective responses to meet the sexual and reproductive health needs of adolescents (WHO, 2011), and may help to protect their rights in relation to their choices. Improved understanding of factors that may predispose adolescents to certain behaviours can not only help to inform adolescents, but also those involved in their care.
This review aimed to examine the physiological factors that influence adolescent sexual behaviour. The review forms the first phase of a comprehensive project to examine all aspects of adolescent development and potential influences on health behaviour. This paper reports on the findings specifically relating to sexual behaviour. Other aspects of adolescent physiological development and health behaviour (e.g. substance use, sexual behaviour, physical activity) are discussed elsewhere, including our main report (McAteer et al., 2017).
2. Methods
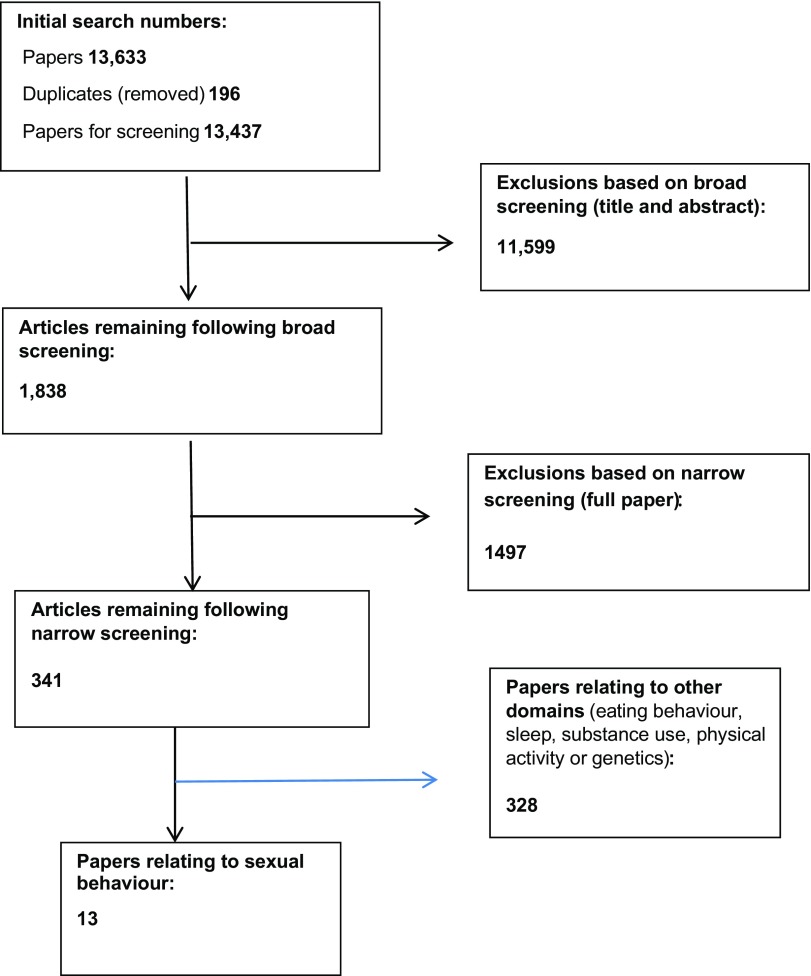
A systematic review was conducted from June 2015–January 2016, using a protocol aligned to PRISMA-R guidelines, with pre-specified inclusion/exclusion criteria. The review aimed to capture an extensive range of studies with different methodological approaches, and both published and unpublished literature, in order to produce an extensive understanding and avoid publication bias (de Souza, da Silva, & de Carvalho, 2010). Details of the overall review process are noted in the PRISMA flow chart (Figure 1).
Figure 1.
PRISMA flow diagram.
2.1. Search strategy
A detailed review protocol and search strategy was developed and agreed between the project team and advisory group members (Pringle et al., 2016). In brief, we searched MEDLINE, Pubmed, PsycInfo, Embase, ERIC, ASSIA, Discovery, Cinahl, and Cochrane databases, using key words relating to adolescence and young adults, physiological development, and (sexual) health behaviours. We also searched webpages, scanned reference lists, and included papers identified by experts in the field.
2.2. Study selection and inclusion criteria
We set no search limits on study design, and scoped a wider age range (9–24 years) than the “second decade” definition (WHO, 2015) in order to include the initial pubescent years and subsequent early adulthood period.
Inclusion dates ranged from 1980–2016. Our focus was on studies relating the impact of physiological systems on health behaviour rather than examining the impact of health behaviour on physiology, since we were not seeking to conduct a review of intervention or behavioural effect. Our focus was on either physiological impact on health behaviour, or on research that indicated a bidirectional influence. We aimed to acknowledge associated psychosocial influences as they related to behavioural outcome findings. In order to include a broad range of potential outcomes, we did not put any predetermined definitions in place regarding sexual activity or behaviour; we were therefore able to include any such outcomes that had been examined in relation to physiological processes.
Two reviewers (JP and KM) screened papers independently using the inclusion and exclusion criteria. A third reviewer from the project team examined papers where there was lack of agreement.
2.3. Data extraction, assessment of study validity, and analysis
Data relating to study aim, country, date, method, population, outcome measures, findings and conclusions were extracted and tabulated. We critically examined method, population and outcome measures to assess validity and limitations. Due to the heterogeneity of the study populations, methods, and outcome measures, meta-analysis and comparable quality appraisal were not feasible. A thematic narrative summation was therefore undertaken, to allow for a deductive approach to the development of key categories. Within identified key categories, we sought to extrapolate data relating to physiological influences (e.g. hormonal changes) and potential impact on sexual health behaviour. The resulting analysis provides a summary of three main topics of focus: hormonal influences, brain physiology, and associated psychosocial factors (summarised in Table 1).
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Studies/reports involving: | Studies/reports involving: |
|
|
No definitive age criteria were set to avoid excluding potentially relevant research.
3. Results
The PRISMA flow chart (Figure 1) summarises the review screening process, and results.
Studies relating to sexual behaviour formed one of six subdivisions within the larger review process. As mentioned above, other areas of interest (sleep, eating behaviour, genetic influences, physical activity, and substance use) are reported elsewhere.
The review identified 13 individual studies that focussed on adolescent sexual behaviour. Details of these studies are given in Table 2.
Table 2. Studies relating to adolescent sexual behaviours.
| Authors (Date) Country | Population/topic | Method | Outcomes/variables | Findings | Conclusions | Limitations/reviewer comments |
|---|---|---|---|---|---|---|
| Baams et al. (2015) | Youth 10.5–22.4 years pubertal timing/status & sexual behaviour | Systematic review with meta-analysis; 1980–2012 |
|
50 included studies. Early development associated with earlier and more (risky) sexual behaviour, especially in girls | Further research to explain variations and assist intervention development | Variations in how pubertal timing assessed, plus the range of sexual behaviours examined |
| Various countries; Dutch study team | ||||||
| Campbell et al. (2005) | Boys living in Zimbabwe, aged 12–18 years; To explore the relative timing of puberty and the relationship with sexual behaviour | Cross-sectional study. Anonymous questionnaires and blood specimens collected |
|
Data from 442 boys living in Zimbabwe; First spontaneous nocturnal emission was a stronger predictor of sexual behaviour than secondary sexual characteristics, and may be used as a marker of pubertal timing. Variation in testosterone is associated with onset of sexual behaviour, beyond its relationship with developmental timing | The mechanism of testosterone influence on the onset of sexual behaviour is unclear, and further research is warranted | Sample sizes of subsets was small and relied on self-report measures. Sexual activity was only related to sex with a girl |
| Zimbabwe | ||||||
| Dornbusch et al. (1981) | 12–17 year old youths; dating, age and sexual maturation | Survey data from US National Health Examination Survey |
|
6710 participants; Individual levels of physiological/sexual maturation add little to explain variation in dating after age has been taken into account. Social pressures determine onset of dating behaviour | Social standards can reduce the impact of physiological processes on behaviour | Data limited to a simple yes or no response as to whether participants had ever been on a date; therefore conclusions drawn from this study may not be comparable with more specific romantic or sexual data |
| USA | ||||||
| Feldstein et al. (2015) | Adols 14–18 years. To investigate brain neural activation responses and adolescents’ frequency of risky sex and substance use | Cross-sectional study |
|
95 participants; in high-risk youth, there was a negative correlation between past month substance use and response inhibition within the left inferior frontal gyrus (IFG) and right insula, but a positive correlation between past month risky sex and activation within the right IFG and left middle occipital gyrus. | Different health risk behaviours are related to different neurocognitive patterns. | All participants had started engaging in health risk behaviours by age 11–12 years. Earlier data collection might have been beneficial |
| USA | ||||||
| Graber and Sontag (2006) | Adolescent girls. To examine the psychological and social impacts of pubertal development on changes in girls’ feelings about themselves (their bodies) and their sexuality | Review and comparison of models that link puberty and sexuality e.g. models that indicate that sexual desires and behaviours are in part the result of brain development and physiological processes | Puberty/physiological development and:
|
Sexuality begins to develop more fully during puberty, develops extensively over adolescence, and is interconnected with changes in self and social context during this period. As such, sexuality is likely to have important connections to engagement in sexual behaviours and experiences, which in turn stimulate re-evaluation of beliefs and attitudes about one’s sexuality | A fuller understanding of the impact of puberty, self-evaluations, and peers among different subgroups of girls is needed e.g. girls who are more and less heterocentric in their developmental path, racial or ethnic subgroups of girls, girls who differ in maturational timing, and girls who have romantic relationships at younger versus older ages | Discussion paper, rather than research paper |
| USA | ||||||
| Halpern et al. (1998) | Adolescent males 12–14 years at start of study. To examine the relationship between testosterone and sexual activity through more frequent data collection | Longitudinal cohort study; 2–3 year follow-up. Questionnaires, interviews and hormone levels taken. Appears to be an extension of earlier research, as detailed in Halpern et al. (1993) |
|
127 adolescent males. Over 80% prepubescent at start of study (by testosterone levels). Higher levels of salivary testosterone were associated with sexual activity initiation and more frequent coital and non coital activity | Findings are consistent with a biosocial model of adolescent sexual development that pubertal changes in testosterone are a causal factor in the timing of sexual initiation and the frequency of activity during adolescence | Behavioural items were self-report, but appeared to have additional questions to capture accurate responses. Coitus limited to vaginal intercourse with a girl. Authors acknowledge heterosexual focus, and that social context could also have influenced behaviour and findings |
| USA | ||||||
| Halpern et al. (1997) | Post menarcheal adolescent females.To examine pubertal rise in testosterone and associations with subsequent increases in female sexual interest and activity, within the context of a social control variable | Longitudinal study over 2-year period, involving questionnaires and interviews |
|
200 female adolescents.Testosterone and changes in testosterone were significantly related to the timing of subsequent transition to first coitus for blacks and whites females. Frequency of attendance at religious services operated as a social control variable, and was found to moderate effects of testosterone on sexual transition | These results are consistent with a biosocial model proposing testosterone as a causal factor in female sexual activity, and suggest that biological effects are moderated by relevant social variables | Later maturing girls may have been omitted due to the sample being only post-menarcheal girls; since black girls tend to mature earlier, this may also have affected the findings.Religious attendance appeared to be the only biosocial marker used |
| USA | ||||||
| Halpern et al. (1993) | Adolescent males, 12–13 years. To examine if sexual activity is initiated and increases in relation to testosterone levels | Longitudinal cohort study. Behavioural questionnaires and blood samples (for testosterone) collected every 6 months for 3 years |
|
100 adolescent males. Pubertal development is significantly related to sexual ideation, non-coital behaviour, and transition to sexual intercourse. Hormone levels did not predict changes in ideation or non-coital sexual activity over the 3 years of the study | Results do not provide support for the idea of a direct causal link between testosterone change and change in sexual motivation or behaviour | Homosexual activity was asked about in final round of questionnaires, but authors acknowledge that due to the heterosexual orientation of the study questions, homosexual males may have opted out of participation.Over and under reporting of personal data also acknowledged |
| USA | ||||||
| Moore et al. (2014) | Adolescent girls from the National Longitudinal Study of Adolescent Health.Pubertal timing, sexual behaviour, and genetic influences | Sibling-comparison study, to establish genetic factors. Longitudinal: 4 time-points over 14 years |
|
923 sibling pairs.Shared genetic pathways influencing age at menarche and perceived pubertal timing, predicted age of first sex.Genetic factors relating only to perceived pubertal timing predicted dating, romantic and non-romantic sex | A girl’s interpretation of her pubertal timing beyond objective timing is important to consider in relation to sexual behaviour | Sex before 11 years not analysed due to non-consensual implications (n=9). Gay sexual encounters not recorded, or sex other than vaginal |
| USA | ||||||
| Smith et al. (1985) | To examine pubertal development effects on sexual behaviour, to determine which are socially motivated and effects which are attributed to biological motivation | Adolescents 14–17 years. Cross-sectional data from a longitudinal study on early adolescent sexual behaviour |
|
The biosocial model indicates that a simultaneous consideration of pubertal development and friend’s behaviour provides a different and clearer picture of the process than examination of the effects separately | The sexual behaviour of the female adolescents in this study was positively affected by androgen development, oestrogen development, and friend’s sexual behaviour. For males, greater pubertal development (regardless of age) was associated with greater sexual involvement. For males, a higher levels of pubertal development was not related to friend’s sexual behaviour | Data were only from white adolescents, potentially limiting the applicability of the findings.Study was able to differentiate between the androgen effect (i.e. stage of pubic hair development) and the oestrogen effect (i.e. breast and hip development) for girls. Such differentiation was not possible in males |
| USA | ||||||
| Udry et al. (1985) | Adolescent boys 12–14 years.To examine hormonal and social effects on adolescent male sexual behaviour | Cross sectional study |
|
102 boys; free testosterone was a strong predictor of sexual motivation and behaviour, with no additional contribution of other hormones.Including measures of pubertal development and age indicated no additional effects | Free testosterone, appears to affect sexual motivation directly and does not work through the social interpretation of accompanying pubertal development | SR data and cross sectional design were limitations |
| USA | ||||||
| Zimmer-Gembeck and Collins (2008) | Adolescents 16–26 years. To determine sexual partnering from age 16–26 years, and to test whetherbiological and social factors influenced these growth patterns | Longitudinal data gathered over 10 years |
|
176 adolescents; adolescents had accumulated a higher number of sexual partners by age 16 years when they looked older, drank alcohol more frequently, and were more involved with dating in early to middle adolescence. Male gender was associated with accumulation of sexual partners more rapidly between ages 16 and 26 years; little indication that the accumulation of different sexual partners had begun to slow by age 26 for the average participant | These findings indicate that interventions targeted at teens to reduce sexual risk behaviour may be just as necessary in the later teen years/emerging adulthood. FR to examine patterns of contraceptive use over a similar age period may to help guide the content of interventions and the groups that could benefit the most from continued access to advice and clinical services | Correlation was lower than ideal for observer assessment of maturity. Other measures via SR, therefore open to bias |
| USA | ||||||
| Zimmer-Gembeck and Helfand (2008) | Adolescents <15 years to >18 years. Review; to provide a summary of what is known about the factors that precede and covary with the onset of adolescent sexual intercourse | Review; analysis of findings from 35 longitudinal studies relating to the onset of heterosexual intercourse |
|
35 longitudinal studies. When studies were organized by age of participants, the onset of intercourse was more strongly associated with alcohol use, delinquency, school problems and (for girls) depressive symptoms following sexual intercourse by age 15 than in later years | Results highlight differences in the correlates of girls’ versus boys’ sexual intercourse and how race/ethnicity moderates associations. These gender and racial/ethnic differences were found largely in analyses of family processes, school and religion, and parent education. Further research with consideration of different sets of covariates that depend on age and other developmental features is required | This review highlights the lack of study or inclusion of same sex sexual activity, and also the lack of the influence of peers on sexual behaviour. However, it is comprehensive in the other aspects that are considered |
| USA |
Abbreviations: SR = self report; fMRI = functional Magnetic Resonance Imaging; BOLD = blood oxygen level dependent (contrast imaging).
Of the 13 studies, all but two were conducted in the USA; one study involved Zimbabwean boys (Campbell, Prossinger, & Mbzivo, 2005) and the other was conducted by a Dutch research team (Baams, Dubas, Overbeek, & van Aken, 2015). There was therefore an overall lack of diversity in the populations studied. Dates of publication were broad, ranging from 1981–2015. There were three review papers; five of the remaining ten papers involved cross-sectional study designs, which limited any causality inferences. Follow-up periods for the 5 longitudinal studies ranged from 2–10 years. None of the studies involved data from participants under 12 years of age, and the upmost age of participants was 26 years of age. All papers examined factors affecting the onset of sexual activity, rather than other aspects of sexual behaviour. Generally onset of sexual activity was related to first coitus. Subset analysis divided the topics of focus into three main, albeit overlapping, areas: hormonal influences, brain physiology, and associated psychosocial factors.
3.1. Hormonal influences
With regard to the onset of sexual activity, several papers examined the hormonal influence of testosterone. Although a longitudinal study by Halpern, Udry, Campbell, and Suchindran (1993) did not provide support for the idea of a direct causal link between testosterone levels and change in sexual motivation or behaviour, two later longitudinal studies, with more frequent data collection, were able to demonstrate such links (Halpern, Udry, & Suchindran, 1997, 1998). The first, reported that higher levels of salivary testosterone were associated with more frequent sexual activity in adolescent males, with the second reporting similar associations in adolescent females, and the timing of their first coitus. For adolescent females, frequency of attendance at religious services was also examined as a variable (Halpern et al., 1997), and found to moderate the effects of testosterone on sexual transition. Psychosocial influences are discussed further below.
In contrast, Udry, Billy, Morris, Groff, and Raj (1985) examined hormonal and social effects on adolescent male sexual behaviour, and concluded that testosterone appeared to affect sexual motivation directly in boys, and did not operate through social influences, such as peer pressure, in the same way that it may do for girls. In addition to the effects of testosterone, Campbell et al. (2005) reported that boys’ first spontaneous nocturnal emission was a stronger predictor of initiation of sexual activity than secondary sexual characteristics, such as the development of genital hair. Both of these studies were cross-sectional in design, so no causal links could be established. However, taken in conjunction with the previous longitudinal studies, they add weight to evidence of the influence of hormonal and developmental physiological processes on sexual activity (coitus) initiation.
Alongside testosterone, other hormonal changes (not withstanding peer influences) may also account for gender variations. For example, Smith, Udry, and Morris (1985) found that the sexual behaviour of female adolescents was positively affected by increasing levels of the sex hormones, testosterone and oestrogen. In contrast, for males, greater pubertal/physiological development in terms of Tanner staging (Marshall & Tanner, 1970), regardless of age, was associated with greater sexual involvement, which was unrelated to friends’ sexual behaviour. Although the study by Smith et al. (1985) was cross-sectional in method, such gender differences were also found in a review of studies by Graber and Sontag (2006) who concluded that, under the influence of gonadal hormones, sexuality begins to develop more fully during puberty, develops extensively over adolescence, and is interconnected with changes in self and social context for girls during this period. Longitudinal research by Moore, Harden, and Mendle (2014) also found that girls’ perceived pubertal timing, in addition to actual age at menarche, predicted age of first sexual intercourse. This is concurrent with the large review by Baams et al. (2015), who were in agreement that early physiological development (i.e. early onset of puberty) was associated with earlier sexual activity, especially in girls.
3.2. Brain physiology
Although there was limited data relating to brain physiology and sexual behaviour in the review, in a sample of high-risk adolescents, one study correlated how an individual’s brain activity and associated inhibiting behaviour related to his/her frequency of unprotected sexual intercourse and substance use (Feldstein, Houck, & Bryan, 2015). These high-risk adolescents were recruited from a court ordered diversion program in the USA. The study examined the relationship between brain responses during a response inhibition task and past month health-risking behaviour. They found a negative correlation between substance use and brain responses (specifically in the left inferior frontal gyrus and right insula), and a positive correlation between risky sex and brain responses in the right inferior frontal gyrus and left middle occipital gyrus during response inhibition. The authors suggest that these findings indicate that engaging in risky sexual behaviour or substance use reflects more than the individual’s cognitive control capacities, but also their ability to assess the relevant socioemotional factors of that decision. This fits with the frameworks considering how adolescents incorporate social factors when assessing the value of engaging in a health risk (Blakemore & Mills, 2014).
3.3. Associated psychosocial factors
While the main focus of the review was on physiological aspects of adolescence, there were other factors that were important to take into account from the findings. For example, in addition to physiological maturation, a review identified psychosocial factors that preceded the onset of adolescent sexual intercourse, which included alcohol use, delinquency, and school problems (Zimmer-Gembeck & Helfand, 2008). Zimmer-Gembeck and Helfand (2008) also found that the onset of adolescent sexual intercourse was correlated with depressive symptoms (especially for girls engaging in early sexual activity). With regard to other consequences, apart from early physiological development being associated with earlier and more (risky) sexual behaviour, especially in girls, early sexual activity may also result in increased incidence of infection or unplanned pregnancy (Baams et al., 2015).
Zimmer-Gembeck and Collins (2008) found that adolescents accumulated a higher number of sexual partners by age 16 years if they were more physiologically advanced (e.g. looked older), drank alcohol more frequently, and were more involved with dating in early to mid-adolescence (13–16 years of age). Male gender (and thereby male physiological hormonal profile), in particular, was associated with accumulation of sexual partners more rapidly between ages 16 and 26 years. There was little indication that the accumulation of different sexual partners had begun to slow by age 26 in this study. These findings indicate that interventions targeted at teens to reduce sexual risk behaviour may be just as necessary in the later teen years/emerging adulthood, and may need to be gender sensitive in terms of timing and content.
As discussed above, frequency of attendance at religious services was found to temper the effects of testosterone on sexual transition (Halpern et al., 1997), which is consistent with a biosocial model suggesting that physiological influences can be moderated by relevant social variables.
4. Discussion
This review reports on adolescent physiological development and its impact on sexual behaviour. The papers relating to adolescent sexual behaviour highlight the effects of differing hormonal profiles, and associated gender differences. In addition to the effects of hormonal factors, females appear to be more influenced than males by psychosocial factors, including the effect of peers. An associated paper (Skinner et al., 2015), which did not focus on physiological factors, reported that behavioural issues (e.g. anger, antisocial behaviour) from as early as 5 years of age in boys and 10 years of age in girls were a significant risk factor for earlier age at first sexual intercourse. This is something that adolescent sexual health programmes may therefore want to consider when developing interventions.
In further relation to the exact specifics of sexual activity, most of the studies defined this as engagement in vaginal coitus, either implicitly or explicitly. However, an early study by Dornbusch et al. (1981) reported on data limited to whether participants had ever been on a date. Conclusions regarding the timing of dating behaviour drawn from this study may therefore not be comparable with the timing of more specific sexual activity (e.g. coitus) data from other studies. This perhaps reflects the less open attitudes that prevailed during earlier eras, and possibly the acceptability of researching such personal topics. It does, however, provide a historical view of past research approaches and findings, to help situate current research findings.
Within the sexual behaviours that were examined in the included studies, and the method(s) of assessing pubertal status, the studies varied in terms of outcome measures, which made direct comparisons difficult. For example, pubertal status measures ranged from Tanner staging assessed by a professional, to parental or self-assessment. Although these latter measures may be considered reliable, there may also be elements of self-assessed psychological and behavioural maturity incorporated in such considerations (Dorn & Biro, 2011), which may not be acknowledged as confounding factors, thereby limiting the findings.
The review papers highlighted links between early sexual maturity and early sexual activity. With regard to the measurement of developmental changes, Halpern, Kaestle, and Hallfors (2007) used “perceived physical maturity” (i.e. the individual’s perception of their maturity) as a proxy for pubertal status, and concluded that, although advanced physical maturity was associated with increased risk (in terms of alcohol, substance use, sexual risk-taking etc.), it was also associated with greater likelihood of having a romantic partner, which further increased risk, especially for girls with an older (≥2 years) partner. However, as Halpern et al. (2007) noted themselves, although self-perceived measures are meaningful and may be valid, they are not the same as objective measures. It is possible that adolescents with older partners may see themselves as more mature by association. Past sexual experience may also impact on an individual’s maturity self-perception. In addition, girls on average enter puberty two years in advance of boys (Kail & Cavanuagh, 2015), so an age gap of this range would indicate a physical maturity match, and potentially a match with regard to readiness to engage in sexual activity; however, as the review has highlighted, this latter consideration may be mediated by culture, society or religion. Whilst these factors may be acknowledged as influencing issues, the impact on individual behaviours is difficult to ascertain, unless these are specific elements under study.
With further regard to sexual behaviours, these were often not specified in sufficient detail for comparison between studies; for example, intercourse may or may not be explicitly specified as vaginal, and there is often little acknowledgement that sexual activity may involve actions other this. Anal intercourse, for example, may be increasingly likely regardless of sexual orientation, possibly due to changing social norms (Marston & Lewis, 2014). Similarly, homosexual sexual activity, either as a behaviour or in relation to greater risk, is not acknowledged. Baams et al. (2015), for example, noted only one study (out of 50 studies in their review) as specifically relating to homosexual activity, but this was not discussed in their analysis. Similarly, Halpern et al. (1997), although noting such behaviour as increasing risk, did not discuss data from within this category.
Likewise, adolescent males may be generally less positive about condom use (Rich, Mullan, Sainsbury, & Kuczmierczyk, 2014), with subsequent increased infection risk, particularly for homosexual men. Sexual minority females (e.g. bisexual, lesbian) may be more likely to experience a teen pregnancy and less likely to receive preventative advice (Charlton, Missmer, DiVasta, Rosario, & Austin, 2016). In the past, this lack of emphasis on anything other than vaginal intercourse may have been due to a primary focus on pregnancy prevention (Albert, Brown, & Flanigan, 2003; Teenage Pregnancy Independent Advisory Group, 2010). It would therefore seem important that current research and interventions recognise a broad range of potential sexual behaviour, and acknowledge the physiological influences that impact on adolescent sexual activity during this time.
A recently published review of romance and sex during adolescence by Suleimann, Galvan, Harden, and Dahl (2016) highlights the fact that support “scaffolding” can help adolescents to negotiate the relationship terrain during their physiological and maturational development. Support services are not only welcome by adolescents, but can help to reduce health disparities (Mason-Jones et al., 2012). We hope that our findings will help to inform the knowledge base for such interventions.
5. Conclusions
This review has highlighted the role that hormones play in influencing sexual behaviour during adolescence. It has also discussed the influence of psychosocial factors, including peers, and how these may differ between males and females.
With regard to maturity outcome measures (e.g. Tanner stage), the influence of maturity perceptions needs to be taken into account. For example, perceived maturity may be a confounding factor, irrespective of objective maturity, in terms of engagement in sexual activity.
Social context and cultural or religious persuasion may also be mediating factors in the decisions adolescents make about sexual activity, in addition to physiological drivers. To more fully establish these influences, social context, cultural and religious affiliation need to be made more explicit for the populations under study.
Furthermore, sexual orientation needs to be considered in terms of sexual behaviour to avoid potential discriminatory reporting, or the under reporting of potentially risky sexual behaviours.
Further research relating to the impact of physiology on adolescent sexual behaviours would be beneficial, especially in a wider variety of populations. In particular, longitudinal research is needed to more fully clarify the causal links between hormones and their influence on sexual behaviour. In addition, there is a need for greater consistency and clarity regarding specific sexual behaviour outcomes to allow comparison between study findings. For example, coitus needs to be defined in terms of vaginal intercourse, or otherwise, for results (and risks) to be more fully evaluated.
5.1. Intervention development
The review has highlighted specific physiological gender differences that need to be taken into account when designing interventions to promote sexual health. Since many physiological factors, such as hormonal levels and brain development are non-modifiable, understanding how these interact with more modifiable factors, such as peer and social influences, can contribute to more nuanced intervention development (Wight, Wimbush, Jepson, & Doi, 2016).
Stage of readiness to receive advice and information relating to sexual behaviour may differ according to physiological maturity. However, early behavioural problems may indicate the need for earlier intervention, especially if linked to early physiological maturity. Interventions for those at risk may need to be adapted to be appropriate for both the age and maturity of the intended audience.
Intervention development needs to take account of the influence of social context, culture and religious affiliation. Some of these factors may offer protection against risk, by opposing physiological drivers. However, further research is needed to more fully establish how these protective factors may be harnessed in intervention development, or the identification of populations at greater risk.
Funding
MRC (MR/KO 023209/1), CSO and NHS Health Scotland. The views expressed in this publication are those of the authors and not necessarily those of the funders.
Author contribution
Review conceptualisation JMcA, RJ, EH, NA, SJB; protocol construction JP, JMcA, KM, RJ; searches, screening and analysis: JP, KM; manuscript draft JP, KM, JMcA; manuscript review JMcA, RJ, EH, NA, SJB.
Acknowledgements
We would like to thank our advisory group members (Prof John Coleman, Prof Divya Jindal-Snape, and Dr Anne-Lise Goddings) for supporting this project. We would also like to thank Professor John Frank for reviewing an earlier draft of the paper.
Biography
Jan Pringle has worked as a research fellow and systematic reviewer for the Scottish Collaboration for Public Health Research and Policy (SCPHRP) for over two years. SCPHRP’s vision is to develop Scotland as a leader in public health research through catalysing strong researcher/research-user collaborations. The adolescent sexual behaviour paper published here is part of a larger project to examine all aspects of adolescent development, with a view to informing interventions and policy. The project as a whole was developed in partnership with key personnel at NHS Health Scotland, and collaborators from the Scottish Government.
Other aspects of physiological influence on adolescent health behaviours (e.g. sleep, substance use, eating behaviour, physical activity) are discussed in a wider report, as detailed in the paper. Our intention is to widen this focus, and examine psychological, sociological, environmental, and cultural influences in future work.
Footnotes
Sexual activity is a normal part of growing up. However, it may also cause harm, if sex takes place when children are too young, or do not know the risks. Risks may include sexually transmitted infections or unintended pregnancy.
Our work aimed to gather information about factors within the bodies of adolescents that affect their sexual behaviour. This information came from a large number of sources.
We found that sexual behaviour is affected by many things, such as hormones and chemical changes. Family, friends, and social groups were also important in changing the way adolescents behaved. We found that young women were more influenced by their friends than young men. Those who matured early were more likely to have sex at a younger age. This could increase the risks for these adolescents. Understanding such things can help us to provide better support for adolescents and those who care for them.
References
- Albert B., Brown S., & Flanigan C. (2003). 14 and younger: The sexual behavior of young adolescents. Washington, DC: National Campaign to Prevent Teen Pregnancy. [Google Scholar]
- Baams L., Dubas J. S., Overbeek G., & van Aken M. A. G. (2015). Transitions in body and behavior: A meta-analytic study on the relationship between pubertal development and adolescent sexual behavior. Journal of Adolescent Health , , 586–598. 10.1016/j.jadohealth.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., & Mills K. L. (2014). Is adolescence a sensitive period for socio-cultural processing? Annual Review of Psychology , , 187–207. 10.1146/annurev-psych-010213-115202 [DOI] [PubMed] [Google Scholar]
- Campbell B. C., Prossinger H., & Mbzivo M. (2005). Timing of pubertal maturation and the onset of sexual behavior among Zimbabwe school boys. Archives of Sexual Behavior , , 505–516. 10.1007/s10508-005-6276-7 [DOI] [PubMed] [Google Scholar]
- Charlton B. M., Missmer S. A., DiVasta A. D., Rosario M., & Austin S. B. (2016). Female sexual orientation differences in contraceptive choices and utilization. Journal of Pediatric and Adolescent Gynecology , , 196–197. 10.1016/j.jpag.2016.01.089 [DOI] [Google Scholar]
- de Souza M. T., da Silva M. D., & de Carvalho R. (2010). Integrative review: What is it? How to do it? Einstein , , 102–106. 10.1590/s1679-45082010rw1134 [DOI] [PubMed] [Google Scholar]
- Dorn L. D., & Biro F. M. (2011). Puberty and its measurement: A decade in review. Journal of Research on Adolescence , , 180–195. 10.1111/j.1532-7795.2010.00722.x [DOI] [Google Scholar]
- Dornbusch S. M., Carlsmith J. M., Gross R. T., Martin J. A., Jennings D., Rosenberg A., & Duke P. (1981). Sexual development, age, and dating: A comparison of biological and social influences upon one set of behaviors. Child Development , , 179–185. 10.2307/1129228 [DOI] [PubMed] [Google Scholar]
- Feldstein E. S. W., Houck J. M., & Bryan A. D. (2015). Neural activation during response inhibition is associated with adolescents’ frequency of risky sex and substance use. Addictive Behaviors , , 80–87. 10.1016/j.addbeh.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girma S., & Paton D. (2015). Is education the best contraception: The case of teenage pregnancy in England? Social Science & Medicine , , 1–9. 10.1016/j.socscimed.2015.02.040 [DOI] [PubMed] [Google Scholar]
- Graber J. A., & Sontag L. M. (2006). Puberty and girls' sexuality: Why hormones are not the complete answer. New Directions in Child and Adolescent Development , , 23–38. 10.1002/(ISSN)1534-8687 [DOI] [PubMed] [Google Scholar]
- Halpern C. T., Kaestle C., & Hallfors D. (2007). Perceived physical maturity, age of romantic partner, and adolescent risk behaviour. Prevention Science , , 1–10. 10.1007/s11121-006-0046-1 [DOI] [PubMed] [Google Scholar]
- Halpern C. T., Udry J. R., Campbell B., & Suchindran C. (1993). Testosterone and pubertal development as predictors of sexual activity: A panel analysis of adolescent males. Psychosomatic Medicine , , 436–447. 10.1097/00006842-199309000-00007 [DOI] [PubMed] [Google Scholar]
- Halpern C. T., Udry J. R., & Suchindran C. (1997). Testosterone predicts initiation of coitus in adolescent females. Psychosomatic Medicine , , 161–171. 10.1097/00006842-199703000-00008 [DOI] [PubMed] [Google Scholar]
- Halpern C. T., Udry J. R., & Suchindran C. (1998). Monthly measures of salivary testosterone predict sexual activity in adolescent males. Archives of Sexual Behavior , , 445–465. 10.1023/A:1018700529128 [DOI] [PubMed] [Google Scholar]
- Johnson K. A., & Tyler K. A. (2007). Adolescent sexual onset: An intergenerational analysis. Journal of Youth and Adolescence , , 939–949. 10.1007/s10964-006-9165-z [DOI] [Google Scholar]
- Kail R. V., & Cavanuagh J. C. (2015). Human development: A life-span view (5th ed.). Boston, MA: Cengage Learning. [Google Scholar]
- Klettke B., & Mellor D. (2012). At what age can females consent to sexual activity? A survey of jury-eligible Australians. Psychiatry, Psychology and Law , , 198–208. 10.1080/13218719.2011.559901 [DOI] [Google Scholar]
- Marshall W. A., & Tanner J. M. (1970). Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood , , 13–23. 10.1136/adc.45.239.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston C., & Lewis R. (2014). Anal heterosex among young people and implications for health promotion: A qualitative study in the UK. BMJ Open , , e00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G., Copen C. E., & Abma J. C. (2011). Teenagers in the United States: Sexual activity, contraceptive use, and childbearing; 2006–2010 National Survey of Family Growth. Vital Health Statistics , , 1–10. [PubMed] [Google Scholar]
- Mason-Jones A. J., Crisp C., Momberg M., Koech J., De Koker P., & Mathews C. (2012). A systematic review of the role of school-based healthcare in adolescent sexual, reproductive, and mental health. Systematic Reviews , , 369. 10.1186/2046-4053-1-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswikwa B., Richter L., Kaufman J., & Nandi A. (2015). Minimum marriage age laws and the prevalence of child marriage and adolescent birth: Evidence from sub-saharan Africa. International Perspectives on Sexual and Reproductive Health , , 58–68. 10.1363/4105815 [DOI] [PubMed] [Google Scholar]
- McAteer J., Mills K.M., Pringle J., Jepson R., Hogg E., Anand N., & Blakemore S.-J. (2017). Adolescent physiological development and its relationship with health-related behaviour: Findings from a systematic review (Final report). Scottish Collaboration for Public Health Research and Policy and NHS Health Scotland, Edinburgh. [Google Scholar]
- Moore S. R., Harden K. P., & Mendle J. (2014). Pubertal timing and adolescent sexual behavior in girls. Developmental Psychology , , 1734–1745. 10.1037/a0036027 [DOI] [PubMed] [Google Scholar]
- Pringle J., Mills K. M., McAteer J., Jepson R., Hogg E., Anand N., & Blakemore S.-J. (2016). A systematic review of adolescent physiological development and its relationship with health-related behaviour: A protocol. BMC Systematic Reviews , , e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Mullan B. A., Sainsbury K., & Kuczmierczyk A. R. (2014). The role of gender and sexual experience in predicting adolescent condom use intentions using the theory of planned behaviour. The European Journal of Contraception & Reproductive Health Care , , 295–306. 10.3109/13625187.2014.917624 [DOI] [PubMed] [Google Scholar]
- Scottish Government (2016). Pregnancy and parenthood in young people strategy. Edinburgh: Scottish Government. [Google Scholar]
- Sher J. (2016). Missed periods: Scotland’s opportunities for better pregnancies, healthier parents and thriving babies. The first time … and every time. Glasgow: NHS Greater Glasgow and Clyde (Public Health). [Google Scholar]
- Skinner S. R., Robinson M., Smith M. A., Robbins S. C., Mattes E., Cannon J., … Doherty D. A. (2015). Childhood behavior problems and age at first sexual intercourse: A prospective birth cohort study. Pediatrics , , 255–263. 10.1542/peds.2014-1579 [DOI] [PubMed] [Google Scholar]
- Smith E. A., Udry J. R., & Morris N. M. (1985). Pubertal development and friends: A biosocial explanation of adolescent sexual behavior. Journal of Health & Social Behavior , , 183–192. 10.2307/2136751 [DOI] [PubMed] [Google Scholar]
- Suleimann A. B., Galvan A., Harden K. P., & Dahl R. E. (2016, September). Becoming a sexual being: The ‘elephant in the room’ of adolescent brain development. Developmental Cognitive Neuroscience, pp. e1–e46). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teenage Pregnancy Independent Advisory Group (2010). Teenage pregnancy: Past successes – Future challenges. London: UK Government. [Google Scholar]
- Udry J. R., Billy J. O., Morris N. M., Groff T. R., & Raj M. H. (1985). Serum androgenic hormones motivate sexual behavior in adolescent boys. Fertility and Sterility , , 90–94. 10.1016/S0015-0282(16)48324-X [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO) (2011). The sexual and reproductive health of younger adolescents. Research issues in developing countries. Geneva: WHO. [Google Scholar]
- World Health Organisation (WHO) (2012). Expanding access to contraceptive services for adolescents. Geneva: WHO. [Google Scholar]
- World Health Organisation (WHO) (2015). Adolescent development . Retrieved May 6, 2016 from https://www.who.int/maternal_child_adolescent/topics/adolescencedevelopment [Google Scholar]
- Wight D., Wimbush E., Jepson R., & Doi L. (2016). Six steps in quality intervention development (6SQuID). Journal of Epidemiology & Community Health , , 520–525. 10.1136/jech-2015-205952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarrow E., Anderson K., Apland K., & Watson K. (2014). Can a restrictive law serve a protective purpose? The impact of age-restrictive laws on young people’s access to sexual and reproductive health services. Sexuality & Culture , , 257–278. [DOI] [PubMed] [Google Scholar]
- Zimmer-Gembeck M., & Collins W. A. (2008). Gender, mature appearance, alcohol use, and dating as correlates of sexual partner accumulation from ages 16–26 years. Journal of Adolescent Health , , 564–572. 10.1016/j.jadohealth.2007.11.004 [DOI] [PubMed] [Google Scholar]
- Zimmer-Gembeck M., & Helfand M. (2008). Ten years of longitudinal research on U.S. adolescent sexual behavior: Developmental correlates of sexual intercourse, and the importance of age, gender and ethnic background. Developmental Review , , 153–224. 10.1016/j.dr.2007.06.001 [DOI] [Google Scholar]