Significance
Understanding the molecular mechanisms underlying drug resistance of antiangiogenic therapy is crucial to improvement of therapeutic efficacy in cancer patients. Our data uncover a mechanism by which the off-tumor targets compromise anti-VEGF drug sensitivity. The therapeutic implication of our findings poses a concept that blocking the off-tumor targets of antiangiogenic drugs are crucial for improvement of therapeutic efficacy. Based on our findings, modest inhibition of excessive EPO production is recommended for improvement of antiangiogenic therapy. Our work will result in a significant paradigm shift and conceptual advances as to improvement of both quality-of-life and overall survivals of antiangiogenic drug-treated cancer patients.
Keywords: tumor, angiogenesis, erythropoietin, drug resistance, hematopoiesis
Abstract
Anti-VEGF drugs are commonly used for treatment of a variety of cancers in human patients, and they often develop resistance. The mechanisms underlying anti-VEGF resistance in human cancer patients are largely unknown. Here, we show that in mouse tumor models and in human cancer patients, the anti-VEGF drug-induced kidney hypoxia augments circulating levels of erythropoietin (EPO). Gain-of-function studies show that EPO protects tumor vessels from anti-VEGF treatment and compromises its antitumor effects. Loss of function by blocking EPO function using a pharmacological approach markedly increases antitumor activity of anti-VEGF drugs through inhibition of tumor angiogenesis. Similarly, genetic loss-of-function data shows that deletion of EpoR in nonerythroid cells significantly increases antiangiogenic and antitumor effects of anti-VEGF therapy. Finally, in a relatively large cohort study, we show that treatment of human colorectal cancer patients with bevacizumab augments circulating EPO levels. These findings uncover a mechanism of desensitizing antiangiogenic and anticancer effects by kidney-produced EPO. Our work presents conceptual advances of our understanding of mechanisms underlying antiangiogenic drug resistance.
Anti-VEGF–based antiangiogenic drugs are commonly used for treatment of various cancer types in human patients. Since the approval of the first anti-VEGF drug, bevacizumab (Avastin), that is, a neutralizing antibody targeting VEGF, by the US Food and Drug Administration in 2004 for treatment of metastatic colorectal cancer (1), more than 12 y of clinical experiences have shown relatively low therapeutic efficacies of anti-VEGF drugs owing to several clinically unresolved issues (2). Both intrinsic and evasive resistances are major hindrances for beneficial clinical responses (3–7). Additionally, other issues include the following: defining reliable biomarkers for selection of patient populations who are likely to benefit from treatment; minimizing adverse effects; prolonging timeline of therapy; and understanding the fundamental mechanism of beneficial effects, especially in combination with chemotherapeutics (2, 7). At the time of this writing, these clinically related issues remain largely unresolved.
Cancer patients who receive anti-VEGF treatment often show intrinsic and acquired resistance. It is speculated that the anti-VEGF drug-altered tumor microenvironment contributes to drug resistance. For example, it has been shown that anti-VEGF treatment increases expression levels of non-VEGF angiogenic factors, which stimulates tumor angiogenesis through VEGF-independent mechanisms (8, 9). Further, anti-VEGF drug-induced tumor hypoxia has been claimed as a trigger for the compensatory mechanism of anti-VEGF resistance (10). Other studies show that anti-VEGF therapy-associated recruitments of leukocytes and stromal fibroblasts in the tumor microenvironment also contribute to antiangiogenic resistance (9, 11). However, these findings are refined in the tumor microenvironment and the global impact of off-tumor targets of these anti-VEGF drugs in cancer patients in development of drug resistance is unknown.
Anti-VEGF drugs are systemically delivered to cancer patients, and these drugs have significant impacts on healthy vasculatures distributed in various tissues and organs. For example, systemic treatment of mice with an anti-mouse VEGF neutralizing antibody results in marked regression of microvasculatures in endocrine organs (12). In thyroid, more than 70% of microvessels are regressed in response to anti-VEGF treatment (12). Similarly, vascular homeostasis in adrenal gland and pancreatic islets are also largely dependent on VEGF. Additionally, anti-VEGF drugs also significantly reduce vascular density in kidney, liver, ovary, and other organs. Reduction of vascular density in these tissues and organs may potentially result in tissue hypoxia. Independent findings with tyrosine kinase inhibitors targeting VEGFRs show the VEGF-VEGFR signaling is crucial for vascular homeostasis in various tissues and organs (13).
Erythropoietin (EPO) is essential for erythropoiesis during adulthood, and it is mainly produced by interstitial fibroblasts and tubular epithelial cells in the kidney (14, 15). EPO exerts its biological functions through activation of EPO receptor (EpoR), which is mainly expressed in erythrocyte progenitor cells (16). However, EpoR is also found in a variety of cell types including vascular endothelial cells (17, 18). EPO is considered as a multifunctional molecule through activation of the Jak-Stat3 signaling pathway (19). EPO induces angiogenesis by directly acting on endothelial cells to stimulate their proliferation and migration although it can also induce expression of other angiogenic factors (15, 20). Similar to VEGF, EPO expression is regulated by hypoxia through the hypoxia-inducible factor (HIF)-regulatory mechanism. Recently HIF-2α has been shown to be the dominant regulator for EPO expression (21, 22).
In this work, we show that systemic treatment of mice with an anti-VEGF drug significantly increases circulating levels of EPO through the mechanism of anti-VEGF drug-induced hypoxia in the kidney. High circulating EPO levels significantly contribute to anti-VEGF drug resistance. Similarly, exogenously administrated EPO also protects tumor vessels from anti-VEGF therapy. Our findings present a concept of anti-VEGF drug resistance by an off-tumor target mechanism, suggesting that EPO-based erythropoiesis-stimulating agents (ESA) and anti-VEGF drugs should not be simultaneously used in cancer patients. These data also imply that adequate inhibition of EPO function in cancer patients might improve therapeutic efficacy of anti-VEGF drugs.
Results
Systemic Anti-VEGF Treatment Augments Kidney EPO Production.
In clinical settings, anti-VEGF drugs are systemically administrated to cancer patients. We hypothesized that systemic delivery of these antiangiogenic drugs would produce global impacts of nontumor vasculatures in various tissues and organs. Our previous work showed that systemic delivery of an anti-VEGF neutralizing antibody (equivalent to bevacizumab) to mice induces vascular regression in kidney (12). To test this hypothesis, we treated nontumor-bearing healthy mice and Lewis lung cancer (LLC)-bearing mice with a previously characterized anti-mouse VEGF neutralizing antibody (VEGF blockade) (23–26). In both healthy tumor-free and LLC-bearing mice, systemic treatment with VEGF blockade caused marked vessel regression in the kidney cortex (Fig. 1A). Quantification of CD31+ vascular density showed that 40–50% of microvessels in kidney cortex were regressed after 4-wk anti-VEGF treatment (Fig. 1A). These findings demonstrate that VEGF is a crucial survival factor for vascular homeostasis in the kidney cortex. To further validate these findings, we have used an independent anti-VEGFR2 neutralizing antibody (DC101) for treating tumor-free mice. Similar to the anti-VEGF blockade, DC101 treatment produced very similar vascular regressive effects as VEGF blockade in the kidney cortex (SI Appendix, Fig. S1 A and B). Likewise, systemic treatment with DC101 also resulted in a significant increase of circulating EPO protein to the level comparable to the anti-VEGF–treated mice (SI Appendix, Fig. S1C). Thus, two independent anti-VEGF agents produced nearly identical vascular regressive effects in the kidney. For convenience, we have used the VEGF blockade for the rest of our studies.
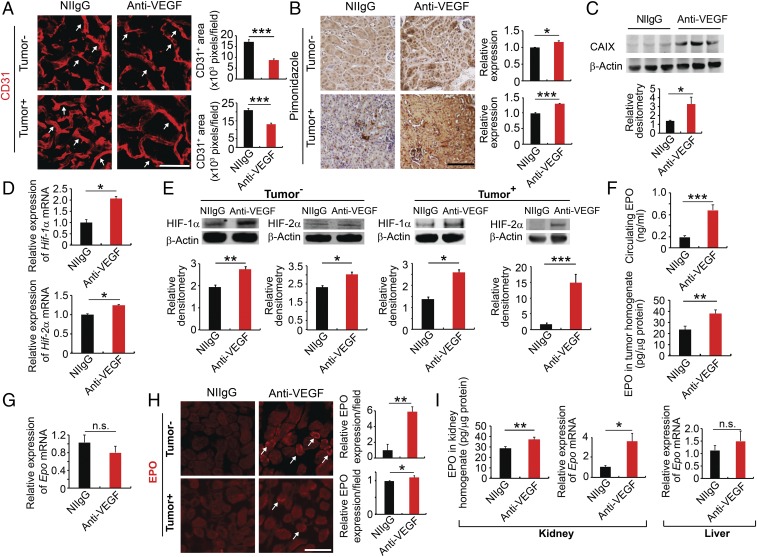
Fig. 1.
Systemic anti-VEGF treatment augments kidney EPO production. (A and B) Kidney cortex CD31+ microvessels and hypoxia pimonidazole+ signals of NIIgG- and anti-VEGF–treated tumor-free and LLC tumor-bearing mice. White arrows point to CD31+ microvessels. CD31+ microvessels and pimonidazole+ signals were randomly quantified from 12 fields (n = 6 samples per group). (C) Western blotting analysis of CAIX in the NIIgG- and anti-VEGF–treated kidney cortex (n = 5 samples per group). (D) qPCR analysis of Hif1α mRNA and Hif2α mRNA expression levels of NIIgG- and anti-VEGF–treated kidney cortex of LLC tumor-bearing animals (n = 5 samples per group). (E) Western blotting analysis of HIF-1α and HIF-2α of the kidney cortex of tumor-free and LLC tumor-bearing animals (n = 3 samples per group). (F) ELISA measurements of EPO protein levels (n = 5 samples per group). (G) Epo mRNA levels of NIIgG- and anti-VEGF–treated tumors (n = 6 samples per group). (H) EPO protein staining of NIIgG- and anti-VEGF–treated kidney cortex of tumor-free and LLC tumor-bearing animals. White arrows point to EPO+ staining. Quantification of EPO positive signals (n = 6–8 random fields per group). (I, Left and Center) ELISA analysis of EPO protein levels and qPCR analysis of Epo mRNA levels of the kidney cortex of tumor-bearing animals (n = 6 tissue samples per group). (I, Right) Epo mRNA expression levels of livers of LLC tumor-bearing animals (n = 6 tissue samples per group). Data are means ± SEM. Animal experiments were repeated twice. *P < 0.05; **P < 0.01; ***P < 0.001. n.s., not significant. (Scale bars: 50 μm.)
We further hypothesized that anti-VEGF–induced vascular regression might cause tissue hypoxia in kidney, which could subsequently alter expression levels of hypoxia-inducible genes. Indeed, systemic treatment of tumor-free and LLC tumor-bearing mice with VEGF blockade created severe hypoxia in the kidney cortex (Fig. 1B). In contrast, nonimmune IgG (NIIgG) treatment did not induce hypoxia in the kidney cortex (Fig. 1B). Consistently, expression of CAIX protein was markedly elevated in the kidney cortex of anti-VEGF–treated LLC tumor-bearing mice (Fig. 1C). In concordance with the increase of tissue hypoxia, expression levels of hypoxia-inducible factor-1α (Hif1a) mRNA were significantly elevated by systemic delivery of VEGF blockade (Fig. 1D). Similar elevated levels of Hif2a were also detected in the kidney of VEGF blockade-treated animals compared with NIIgG-treated controls (Fig. 1D). Consistent with the elevated mRNA levels, expression levels of HIF-1α and HIF-2α protein were also elevated accordingly (Fig. 1E). It appeared that anti-VEGF–induced kidney hypoxia and HIF expression were independent from tumor implantation since tumor-free and tumor-bearing mice produced very similar responses upon anti-VEGF treatment.
Since EPO-producing peritubular interstitial cells are anatomically located in the kidney cortex (16) and EPO production is markedly regulated by hypoxia, we next measured EPO levels in the kidney and plasma. Notably, systemic anti-VEGF treatment of LLC tumor-bearing mice induced a more than threefold increase of circulating EPO level relative to NIIgG-treated tumor-bearing animals (Fig. 1F). Similarly, systemic delivery of VEGF blockade in tumor-free mice also increased circulating EPO levels (SI Appendix, Fig. S1D). High EPO protein levels were also found in tumor tissues of anti-VEGF–treated animals relative to those of control NIIgG-treated mice (Fig. 1F).
To distinguish kidney-derived EPO from tumor-synthesized EPO, Epo mRNA levels were measured in tumor tissues. Despite high EPO protein levels in tumor tissues, Epo mRNA level was not significantly altered in anti-VEGF–treated tumors compared with those in control NIIgG-treated tumor tissues (Fig. 1G). These findings show that kidney, but not tumor tissues, is the primary site for anti-VEGF–elevated production of the circulating EPO, and high levels of tumor EPO protein are derived from kidney. To further validate the kidney source of EPO production, the anti-VEGF–treated kidney cortex was stained with a specific anti-EPO antibody as previously described (18). Expectedly, anti-VEGF treatment increased EPO protein signals in the kidney cortex (Fig. 1H). Consistent with high levels of kidney EPO protein, an approximately fourfold increase of Epo mRNA was also detected in the kidney cortex of VEGF blockade-treated tumor-bearing mice (Fig. 1I), validating the kidney source of EPO production. Since liver could be a potential site for EPO production, we measured Epo mRNA levels in NIIgG- and anti-VEGF–treated livers and found no difference, excluding the possibility of liver source of excessive EPO production (Fig. 1I). Similar findings were also found in tumor-free animals (SI Appendix, Fig. S1 E and F).
Most cancer types including colorectal cancer (CRC) occur during adulthood and anti-VEGF drugs are given adult human patients. To recapitulate clinical relevance and to exclude the possibility of a specific response to anti-VEGF therapy in younger mice, we also treated 1-y-old mice that are equivalent to ∼50+-y-old adult humans. Similar to younger mice, VEGF blockade markedly regressed microvessels in the kidney cortex (SI Appendix, Fig. S1 G and H). The robust regression in older mice was equivalently comparable to that in younger mice. Consistently, circulating EPO level was also significantly increased along with kidney vessel regression (SI Appendix, Fig. S1I). These findings show that the anti-VEGF–induced vessel regression occurs in both younger and older mice through a nonantiangiogenic but rather a disparaging vascular hemostasis mechanism.
EpoR-Mediated Endothelial Cell Signaling and Functions.
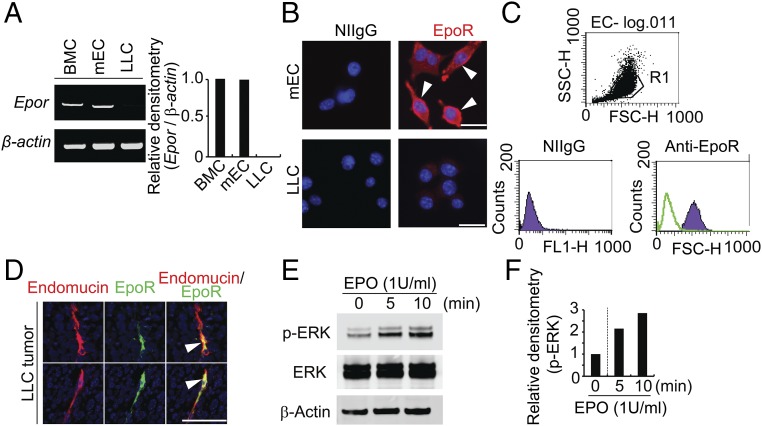
Given high EPO protein levels in anti-VEGF–treated tumor tissues, we defined EPO-targeted cell types in the tumor microenvironment. In the LLC tumor model, tumor cells completely lacked Epor mRNA expression, suggesting that these cells lacked responses to EPO stimulation (Fig. 2 A and B). In contrast to tumor cells, isolated murine blood vessel endothelial cells expressed Epor mRNA to the level equivalent to that of primary bone marrow cells (BMCs) (Fig. 2A). Indeed, stimulation of LLC tumor cells with EPO did not alter tumor cell proliferation (SI Appendix, Fig. S2). EPO receptor (EpoR) was localized on the surface of primary endothelial cells isolated from the tumor tissues (Fig. 2B). Again, LLC tumor cells lacked any detectable expression of EpoR. These findings from immunostaining were further validated by FACS analysis, showing abundant EpoR signals on vascular endothelial cells (Fig. 2C). We next performed immunohistochemical staining on tumor tissues with the anti-EpoR antibody. Interestingly, EpoR was almost exclusively expressed in endomucin+ microvessels in the tumor microenvironment although other stromal cells have been claimed to express EpoR (Fig. 2D). These findings show that vascular endothelial cells are the primary target of EPO protein in the tumor microenvironment.
Fig. 2.
EPO-EpoR–triggered endothelial cell signaling and functions. (A) RT-PCR analysis of Epor mRNA levels in different cell types. Bone marrow cell (BMC) served as a positive control. (B) Immunocytochemical staining of EpoR-positive signals in mEC cells (Upper) and LLC cells (Lower). (C) FACS analysis of EpoR expression on mEC cells. (D) Immunocytochemical staining of EpoR positive signals in LLC tumor tissue. (E and F) Western blotting analysis of phospho-ERK, total-ERK, and β-actin levels of each treated and nontreated samples. Mouse endothelial cells were stimulated with 1 U/mL human recombinant EPO for indicated time points. Densitometry of p-ERK–positive signals. Experiments were repeated twice. (Scale bars: 50 μm.)
Consistent with high EpoR, stimulation of tumor-derived endothelial cells with recombinant EPO protein significantly induced ERK phosphorylation, an intracellular component mediating EPO-triggered signaling (Fig. 2 E and F). Stimulation of vascular endothelial cells with EPO significantly increased cell motility and tube formation (SI Appendix, Fig. S3). Taken together, these data show that EPO targets endothelial cells but does not lack an autonomous effect on tumor cells in this lung cancer model.
Endogenous EPO Compromises Anti-VEGF Effects by Augmenting Tumor Angiogenesis.
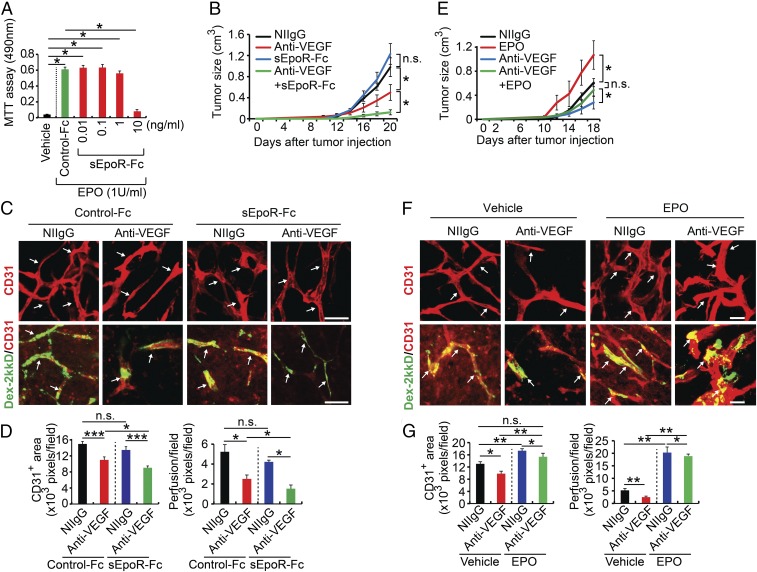
We next investigated the involvement of EPO protein in anti-VEGF response in in vivo tumor models. To approach the functional impact of EPO in tumor growth and angiogenesis, we generated a soluble EpoR consisting of the extracellular domain of human EpoR fused with the Fc portion of IgG (sEpoR-Fc). In an EPO-dependent UT-7/EPO cell proliferation assay (27), a concentration of 10 ng·mL−1 sEpoR-Fc completely blocked EPO-induced proliferation of UT-7/EPO cells (Fig. 3A), indicating that sEpoR-Fc displays a potent effect on suppression of EPO-triggered cell functions. With this available EPO blockade, we treated LLC tumor-bearing mice with sEpoR-Fc alone or in combination with VEGF blockade by systemic delivery. Intriguingly, treatment of LLC tumors with sEpoR-Fc alone did not produce any effect on tumor growth compared with NIIgG-treated control tumors (Fig. 3B). In contrast, treatment with VEGF blockade alone significantly inhibited tumor growth (51% inhibition) (Fig. 3B). Importantly, combination of VEGF blockade with EPO blockade markedly enhanced the antitumor activity (88% inhibition) (Fig. 3B).
Fig. 3.
Kidney-derived EPO confers anti-VEGF resistance. (A) Proliferation assay of sEpoR-Fc and control Fc-treated UT-7/EPO in the presence or absence of EPO protein (n = 6 samples per group). (B) Tumor growth rates (n = 6 animals per group). (C and D) Immunohistochemical analyses of CD31+ microvessels and vascular perfusion of 2,000-kDa dextran. Arrows in C, Upper point to CD31+ blood vessels and in Lower indicate perfused dextran+ signals. Quantifications of CD31+ vessel density and dextran blood perfusion (n = 10 samples per group). (E) Growth rates of various monotherapy- or combination therapy-treated LLC tumors (n = 6 animals per group). (F and G) Immunohistochemical analyses of CD31+ microvessels and vascular perfusion of 2,000-kDa dextran. Arrows in F, Upper point to CD31+ blood vessels and in Lower indicate perfused dextran+ signals (n = 10 samples for each group). Quantifications of CD31+ vessel density and dextran blood perfusion (n = 10 samples per group). *P < 0.05; **P < 0.01; ***P < 0.001. n.s., not significant. Data are means ± SEM. (Scale bars: 50 μm.)
To further substantiate our findings in LLC tumor models, we performed a similar experiment using a known anti-VEGF–resistant human glioma model (28, 29). Anti-VEGF alone produced a significant antitumor effect compared with those tumors receiving NIIgG vehicle treatment (SI Appendix, Fig. S4A). We should emphasize that our anti-VEGF antibody neutralizes both human and mouse VEGF. Most previously published studies employ anti-human VEGF antibody for treatment of U87 glioma in immunodeficient mice (28, 29), thus ignoring the role of stromal cell-derived VEGF in supporting tumor growth. It has been shown that stroma cell-derived VEGF plays a crucial role in supporting tumor angiogenesis (24, 30). Clinically approved anti-VEGF drugs block both tumor cell- and stromal cell-derived VEGF. Thus, our anti-VEGF treatment recapitulates the clinical situation. Human U87 glioma also demonstrated a significantly enhanced antitumor activity after receiving anti-VEGF plus soluble EpoR combination treatment compared with the concurrent anti-VEGF alone (SI Appendix, Fig. S4). Consistently, anti-VEGF plus soluble EpoR also showed a superior antiangiogenic effect (SI Appendix, Fig. S4). These data demonstrate that anti-EPO and anti-VEGF produce additive antitumor activity.
We have used our standard method to measure hematopoietic parameters in these mice (18, 31). It should be emphasized that treatment of tumor-bearing mice with sEpoR-Fc alone or sEpoR-Fc plus VEGF blockade did not alter red blood cell counts, hemoglobin, and hematocrits during the experimental period (SI Appendix, Table S1). Perhaps, the treatment duration was too short to see hematocrit changes because the lifespan of mouse red blood cells is relatively long (about 40 d) (32). Prolonged treatment would most likely alter hematocrit. Another notion is that tumor-bearing mice had generally lower hematocrit than tumor-free healthy mice (SI Appendix, Table S1). This is perhaps not surprising because of the tumor-associated anemia is often seen in cancer animals and patients.
Accordant with the enhanced antitumor effect, addition of sEpoR-Fc to VEGF blockade significantly enhanced the antiangiogenic activity in tumor tissues (Fig. 3 C and D). Additionally, tumor blood vessel perfusion was decreased to a minimal level in animals receiving anti-VEGF and anti-EPO combination therapy (Fig. 3 C and D). Thus, endogenous EPO significantly compromises the antiangiogenic effect of anti-VEGF drugs.
Exogenous EPO Compromises Antiangiogenic and Antitumor Effects of Anti-VEGF Drugs.
In some regions of the globe, ESAs are still commonly prescribed as therapeutic drugs for treatment of cancer-associated anemia and chemotherapy-related anemia (33), although it is generally banned in the Western society. However, the impact of administration of ESAs together with anti-VEGF drugs such as bevacizumab on tumor growth and tumor angiogenesis has not been investigated. To address this important issue, we treated tumor-bearing mice with a combination of a recombinant EPO protein and VEGF blockade. Treatment of LLC tumor-bearing mice with EPO alone resulted in an accelerated tumor growth rate relative to vehicle-treated tumors (Fig. 3E). The EPO-augmented tumor growth was unlikely due to its direct effect on LLC tumor cells as these tumor cells lacked EpoR expression (Fig. 2A). Again, treatment with VEGF blockade produced 53% inhibition of tumor growth in this lung tumor model (Fig. 3E). However, treatment of EPO protein together with VEGF blockade completely ablated the antitumor effect by VEGF blockade (Fig. 3E). In another tumor model of fibrosarcoma, anti-VEGF treatment also markedly augmented high levels of EPO production (SI Appendix, Fig. S5A). These data were supported by independent experimental evidence using T241 fibrosarcoma tumor model (SI Appendix, Fig. S5B).
Reconciling with restoration of vascular density, EPO treatment completely rescued anti-VEGF–decreased vascular perfusion (Fig. 3 F and G). Thus, exogenous administration of EPO protein compromises anti-VEGF effects by augmenting tumor angiogenesis. Similar anti-VEGF–resistant effects of EPO protein were also found in T241 tumors (SI Appendix, Fig. S5 C–E). Although EPO-induced tumor angiogenesis significantly contributed to anti-VEGF resistance in suppression of tumor growth and angiogenesis, other mechanisms could not be completely excluded. It was plausible that EPO-stimulated hematopoiesis also contributed to anti-VEGF resistance. Indeed, EPO treatment increased hematocrits in tumor-bearing mice (SI Appendix, Fig. S6). Interestingly, EPO plus anti-VEGF further increased red blood cell count, hemoglobin, and hematocrit relative to anti-VEGF alone (SI Appendix, Fig. S6). These findings suggest that EPO and anti-VEGF stimulate hematopoiesis through different mechanisms. However, this possibility was unlikely to significantly contribute to anti-VEGF resistance because genetic deletion of EpoR in nonhematopoietic cells in tumor-bearing mice recovered anti-VEGF sensitivity.
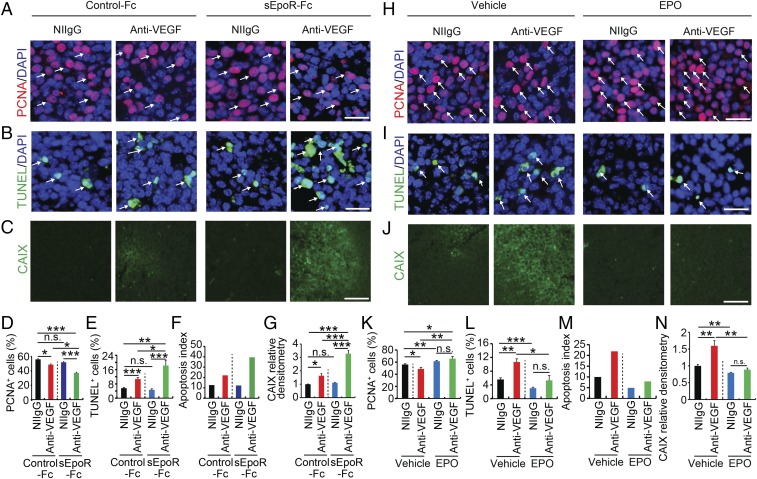
EPO Blockade Enhances Anti-VEGF–Triggered Antiproliferation and Apoptosis of Tumor Cells.
To gain further mechanistic insights of EPO inhibition-potentiated anti-VEGF sensitivity, VEGF blockade-, EPO blockade-, and VEGF blockade plus EPO blockade-treated tumor tissues were stained with proliferative and apoptotic markers. Anti-VEGF treatment alone significantly inhibited LLC tumor cell proliferation, whereas sEpoR-Fc treatment did not significantly alter tumor cell proliferation relative to Fc-treated control tumors (Fig. 4 A and D). Notably, treatment of LLC tumor-bearing mice with combination of anti-VEGF antibody and sEpoR-Fc produced a markedly inhibitory effect on tumor cell proliferation (Fig. 4 A and D). These findings show that VEGF blockade and EPO blockade synergistically inhibit tumor cell proliferation in an in vivo tumor model. Conversely, anti-VEGF and anti-EPO combination treatment markedly induced tumor cell apoptosis (Fig. 4 B and E). After 3-wk treatment with the combination regimen, nearly 20% of tumor cells underwent apoptosis, resulting in an exceptionally high apoptosis index (Fig. 4 B, E, and F). In the anti-VEGF alone-treated LLC tumors, about 10% of apoptotic tumor cells were detected. Consistent with the synergistic antiangiogenic effects (Fig. 3), anti-VEGF and anti-EPO combination markedly induced tumor tissue hypoxia as measured by CAIX expression (Fig. 4 C and G). Taken together, treatment of in vivo tumors with a combination of anti-VEGF and anti-EPO drugs synergistically inhibits tumor cell proliferation, augments tumor cell apoptosis, and induces severe hypoxia.
Fig. 4.
EPO inhibition increases antitumor activity by anti-VEGF drugs. (A–C) Immunohistochemical analyses of PCNA+ proliferating tumor cells, TUNEL+ apoptotic tumor cells, and tumor hypoxia. Arrows in A indicate PCNA+ proliferating tumor cells and arrows in B point to TUNEL+ apoptotic tumor cells. CAIX positive hypoxic signals are indicated with green signals in C. DAPI in blue was used for counterstaining of cell nuclei. (D–G) Quantification of PCNA+ proliferating tumor cells, TUNEL+ apoptotic tumor cells, apoptotic index, and CAIX positive hypoxic signals (n = 10 random fields per group; six animals per group). (H–J) Immunohistochemical analyses of PCNA+ proliferating tumor cells, TUNEL+ apoptotic tumor cells, and tumor hypoxia. Arrows in H indicate PCNA+ proliferating tumor cells and arrows in I point to TUNEL+ apoptotic tumor cells. CAIX-positive hypoxic signals are indicated with green signals in J. DAPI in blue was used for counterstaining of cell nuclei. (K–N) Quantification of PCNA+ proliferating tumor cells, TUNEL+ apoptotic tumor cells, apoptosis index, and CAIX-positive hypoxic signals (n = 10 random fields per group; six animals per group). *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. Data are means ± SEM. (Scale bars: A, B, H, and I, 50 μm; C and J, 100 μm.)
EPO Protein Induces Tumor Hyperproliferation, Protection of Apoptosis, and Improvement of Hypoxia.
In contrast to sEpoR-Fc, exogenous administration of EPO protein to LLC tumor-bearing animals further stimulated tumor cell proliferation compared with vehicle-treated control tumors (Fig. 4 H and K). It should be emphasized that LLC tumor cells lacked EpoR expression and did not show any proliferative response to EPO in vitro (Fig. 2A and SI Appendix, Fig. S2) and, thus, EPO promoted tumor cell proliferation in vivo through an independent mechanism, most likely through its potent angiogenic effect in vivo. Interestingly, treatment of tumor-bearing mice with EPO protein alone significantly protected tumor cells from apoptosis (Fig. 4 I and L). Stimulation of hyperproliferation and antiapoptosis indicates that EPO is a potent protumorigenic factor that primarily modulates the tumor microenvironment. Additionally, EPO treatment completely ablated the anti-VEGF–induced antiproliferative (Fig. 4 H and K) and proapoptotic effects (Fig. 4 I and L). Consequently, EPO treatment reduced the anti-VEGF–augmented apoptotic index to the control level (Fig. 4M). Ultimately, treatment with EPO protein markedly improved tumor hypoxia (Fig. 4 J and N). Thus, delivery of EPO protein to LLC tumor-bearing mice neutralizes the anti-VEGF–triggered antiproliferative and proapoptotic effects on tumor cells. These conclusions were further supported by experimental evidence using an independent T241 fibrosarcoma model (SI Appendix, Fig. S7).
Genetic Deletion of Epor in Nonhematopoietic Cells Increases Anti-VEGF Sensitivity.
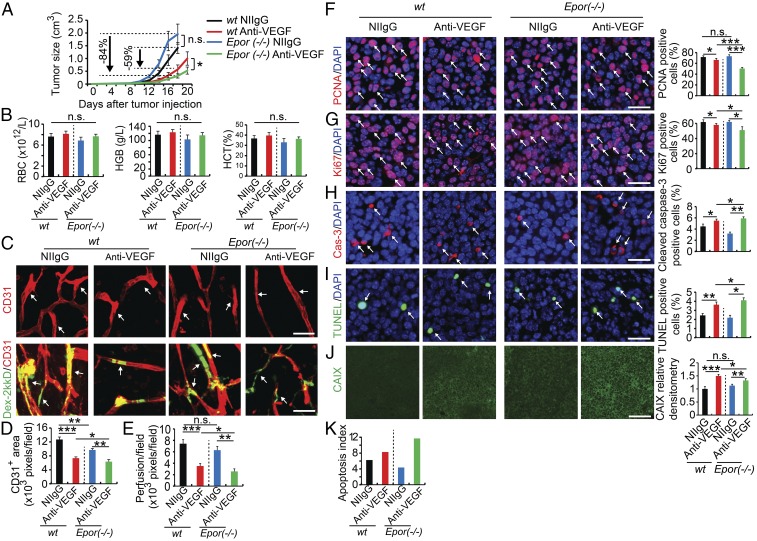
To further strengthen our conclusions, we took a genetic approach to selectively delete Epor in nonhematopoietic cells in mice, that is, EpoR (−/−)::HG1-EpoR strain as previously described (34). Implantation of LLC tumors in these EpoR (−/−)::HG1-EpoR syngeneic mice did not significantly alter tumor growth rates compared with wild-type (WT) mice (Fig. 5A). Similarly, red blood cell counts, hemoglobin, and hematocrits in LLC tumor-bearing EpoR (−/−)::HG1-EpoR mice were nearly identical to those of tumor-bearing WT mice (Fig. 5B), supporting the fact of nonhematopoietic deletion of Epor gene in these mice. Interestingly, anti-VEGF treatment of LLC tumor-bearing EpoR (−/−)::HG1-EpoR mice resulted in 84% tumor suppression relative to NIIgG-treated control tumors in the same mice (Fig. 5A). In the WT mice, VEGF blockade only inhibited 59% of tumor growth. These findings indicate that the nonhematopoietic EpoR signaling compromises the antiangiogenic and antitumor effects of anti-VEGF drugs. In consistence with pharmacological approaches, genetic deletion of Epor in nonhematopoietic cells also significantly enhanced the antiangiogenic effect of VEGF blockade in the LLC tumor model (Fig. 5 C and D). Accordingly, blood perfusion in tumor vessels were markedly decreased in VEGF blockade-treated LLC-bearing EpoR (−/−)::HG1-EpoR mice (Fig. 5 C and E). Thus, enhancement of antiangiogenic activity of VEGF blockade by genetic deletion of Epor reconcile with improvement of antitumor activity by this drug. Both genetic and pharmacological approaches support the conclusion that increased levels of EPO production are responsible for desensitizing anti-VEGF drugs for cancer therapy.
Fig. 5.
Nonhematopoietic ablation of EpoR increases anti-VEGF sensitivity. (A) Tumor growth rates (n = 6 animals per group). (B) Analyses of red blood cells, hemoglobin levels, and hematocrits (n = 6 animals per group). (C–E) Immunohistochemical analyses of CD31+ microvessels and vascular perfusion of 2,000-kDa dextran (n = 6 animals per group). Arrows in C, Upper point to CD31+ blood vessels and in Lower indicate perfused dextran+ signals (n = 10 samples per group). Quantifications of CD31+ vessel density and dextran blood perfusion (n = 10 samples per group). (F–K) Immunohistochemical analyses of PCNA+ proliferating tumor cells, Ki67+ proliferating tumor cells, cleaved caspase-3+ apoptotic tumor cells, TUNEL+ apoptotic tumor cells, and tumor hypoxia (n = 10 random fields per group; six animals per group). Epor (−/−) indicates EpoR(−/−)::HG1-EpoR mice. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. Data are means ± SEM. (Scale bars: 50 μm.)
Similar to sEpoR-Fc treatment, deletion of Epor in EpoR (−/−)::HG1-EpoR tumor-bearing mice markedly improved antiproliferative and proapoptotic effects of tumor cells by VEGF blockade (Fig. 5 F–I and K). Owing to the decreases of vascular density and blood perfusion, anti-VEGF–treated LLC tumors in EpoR (−/−)::HG1-EpoR mice also encountered a higher degree of hypoxia relative to anti-VEGF–treated tumors in WT mice (Fig. 5J). Thus, this genetic loss-of-function model reproduced the findings from the pharmacological loss-of-function experiments.
Chemotherapy in Combination of Anti-VEGF Therapy Elevated EPO Expression.
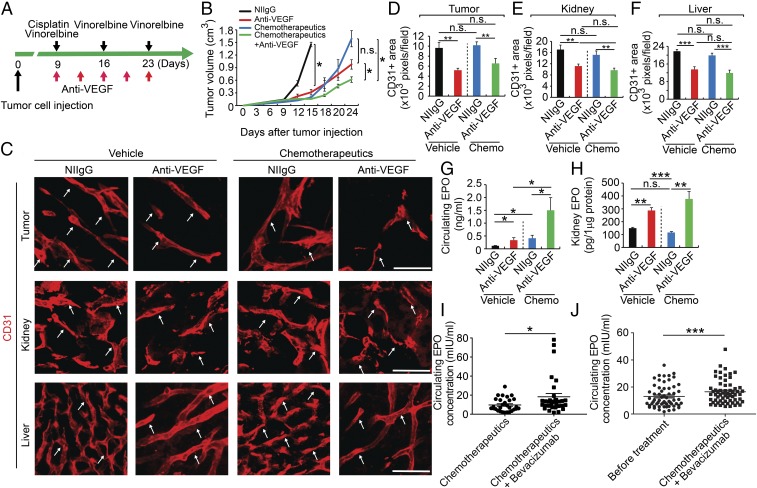
In clinical settings, anti-VEGF drugs are often combined with chemotherapeutics for treatment of cancer patients. To recapitulate the clinical situation of combination therapy, we designed a combination therapy regimen for lung cancer. Cisplatin and vinorelbine are two commonly used chemotherapeutics for treatment of lung cancers, and these chemotherapeutics were combined with anti-VEGF treatment (Fig. 6A). Expectedly, a clinically relevant dose of chemotherapy alone significantly inhibited tumor growth without affecting tumor angiogenesis (Fig. 6 B–D), endorsing the direct antitumor effect. Indeed, chemotherapy alone for treatment of lung cancer significantly inhibited tumor cell proliferation, but markedly induced cellular apoptosis (SI Appendix, Fig. S8). Additionally, chemotherapy alone had no impact on vasculatures in the EPO-producing organs such as kidney and liver (Fig. 6 C, E, and F). Addition of chemotherapeutic to anti-VEGF drugs did not change the anti-VEGF–induced antiangiogenic effects in tumors, kidney, and liver (Fig. 6 C–F).
Fig. 6.
Chemotherapy in combination of anti-VEGF therapy elevated EPO expression. (A) Treatment schedule with anti-VEGF drug in combination of cisplatin and vinorelbine. (B) Tumor growth rates (n = 5 animals per group). (C) Immunohistochemical analyses of CD31+ microvessels Arrows point to CD31+ blood vessels. (Scale bars: 50 μm.) (D–F) Quantifications of CD31+ vessel density in tumor, kidney, and liver (n = 8 random fields per group). (G) ELISA analysis of plasma EPO protein levels (n = 5 samples per group). (H) ELISA analysis of kidney EPO protein levels (n = 4–5 samples per group). (I) ELISA analysis of plasma EPO protein levels of bevacizumab plus chemotherapeutics (9.31 ± 1.17 mIU) and chemotherapeutics alone-treated (18.32 ± 3.44 mIU) colorectal cancer patients (n = 34 patients in chemotherapy alone group; n = 32 patients in bevacizumab plus chemotherapy group). (J) ELISA analysis of plasma EPO protein levels of control (12.5 ± 0.107 mIU) and bevacizumab plus chemotherapeutic docetaxel-epirubicin–treated (16.8 ± 0.124 mIU) breast cancer patients (n = 61 patients per group). *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. Data are means ± SEM.
It appeared that chemotherapy plus anti-VEGF treatment augmented an even higher level of circulating EPO than anti-VEGF alone (Fig. 6G). Similarly, the combination-treated group showed a trend of higher EPO production in the kidney (Fig. 6H). These findings show that EPO expression levels were also elevated in the chemotherapy plus anti-VEGF–treated tumor-bearing animals, recapitulating the clinical relevance of our findings.
Bevacizumab Treatment Increases Circulating EPO Levels in Human Colorectal Cancer Patients.
Bevacuizumab is a currently used anti-VEGF drug as the first-line and second-line treatment of metastatic colorectal cancer (mCRC) and is systemically delivered to cancer patients. The impact of systemic bevacizumab on EPO production in CRC patients is unknown. To recapitulate our findings to clinical relevance, we measured circulating EPO levels in human CRC patients who received bevacizumab treatment for three cycles. In this study, two arms of patient populations were compared. In the first group (n = 34), mCRC patients received conventional chemotherapy alone. In another group (n = 32), mCRC patients received bevacizumab plus chemotherapy combination therapy. Plasma samples were collected and circulation EPO protein levels were measured by an ELISA. The average circulating EPO level was significantly higher in the bevacizumab plus chemotherapy group compared with chemotherapy alone (Fig. 6I and SI Appendix, Table S2). These pilot clinical findings validate our data from preclinical cancer models.
Having known high-circulating EPO levels in CRC cancer patients, we performed a relatively large cohort clinical study on breast cancer patients, who are often resistant to anti-VEGF treatment. We recruited 61 patients with localized HER2 negative breast cancers. These patients received a combination therapy consisting of docetaxel-epirubicin plus bevacizumab. Again, statistically higher circulating EPO levels were found in the combination-treated patients, validating the fact that systemic treatment of breast cancer patients with an anti-VEGF drug augments EPO production (Fig. 6J and SI Appendix, Table S3). These findings provide clinical evidence that anti-VEGF treatment increases circulating EPO production in human cancer patients.
Discussion
Antiangiogenic drugs are originally designed to specifically target the tumor vasculature as physiological angiogenesis rarely occurs in healthy adult tissues (35). However, systemic delivery of anti-VEGF–based antiangiogenic drugs inevitably exposes drugs to the entire vasculature of all tissues and organs in the cancer patients. Would the off-tumor targets of antiangiogenic drugs have any functional impact on cancer patients? The short answer is definitive. While VEGF is a potent angiogenic factor under physiological and pathological conditions, it is also a key factor for maintaining vascular hemostasis in various tissues and organs. We recently showed that systemic treatment of tumor-free healthy mice with an anti-VEGF neutralizing antibody produces broad impacts on vascular regression in various tissues and organs. For example, substantial vascular regression is observed in anti-VEGF–treated kidney, thyroid, pancreas, liver, adrenal gland, intestinal villi, and ovary (12). An independent study using axitinib, a tyrosine kinase inhibitor (TKI) targeting VEGFRs, produces very similar vascular regression in these tissues and organs (13). However, TKIs are often nonspecific for VEGFRs and block phosphorylation of many other kinases. For example, axitinib inhibits all three types of VEGFRs, PDGFRs, c-Kit, and other kinases (36).
We originally hypothesized that anti-VEGF treatment-induced vascular regression in the kidney cortex would induce tissue hypoxia, which alters gene expression profiles. Our experimental data support this hypothesis, and HIF-1α and HIF-2α are significantly up-regulated in the anti-VEGF–treated kidneys. Among hypoxia-regulated genes, Vegf, Epo, and Glucose transporter 1 (Glut-1) are a few direct targets of HIFs (37). Unlike many other factors, production of EPO hormone is specifically restricted to kidney and a limited number of other organs. Another study shows that delivery of a soluble VEGFR by an adenovirus increases EPO production in the liver but not in the kidney (38). It is known that systemic delivery of an adenovirus preferentially infects liver cells because of the abundant expression of adenoviral receptors in hepatocytes (38). Thus, this is a local delivery approach rather than a systemic expression. With this approach, it is perhaps unsurprising that kidney EPO production is not altered. EPO displays broad biological functions on various cell types other than hematopoietic lineage-committed erythroblasts. EPO is a potent angiogenic factor that stimulates tumor growth and invasion (18). Interestingly, EpoR is predominately expressed in vascular endothelial cells, supporting the robust angiogenic function of hormone. Several phase 3 clinical trials with EPO for treatment of cancer-associated anemia showed significant shortening of patient survival (39, 40), concluding prohibition of ESA treatment in cancer patients. Despite this fact, ESA is still used in some countries for treatment of cancer-associated anemia in human patients (33). Taking globalization of anti-VEGF drugs for cancer therapy into consideration, the impact of simultaneous treatment of anti-VEGF drugs and ESA on patient survival is completely overlooked. Therefore, our work provides evidence that EPO desensitizes antiangiogenic and anticancer effects of anti-VEGF drugs. In addition to exogenously administrated EPO protein, we show that kidney-derived endogenous EPO significantly compromises anti-VEGF drug sensitivity in two tumor models (Fig. 7). The mechanism does not involve its hematopoietic function because deleting Epor gene in nonhematopoietic cell types completely abrogates the EPO-mediated effect.
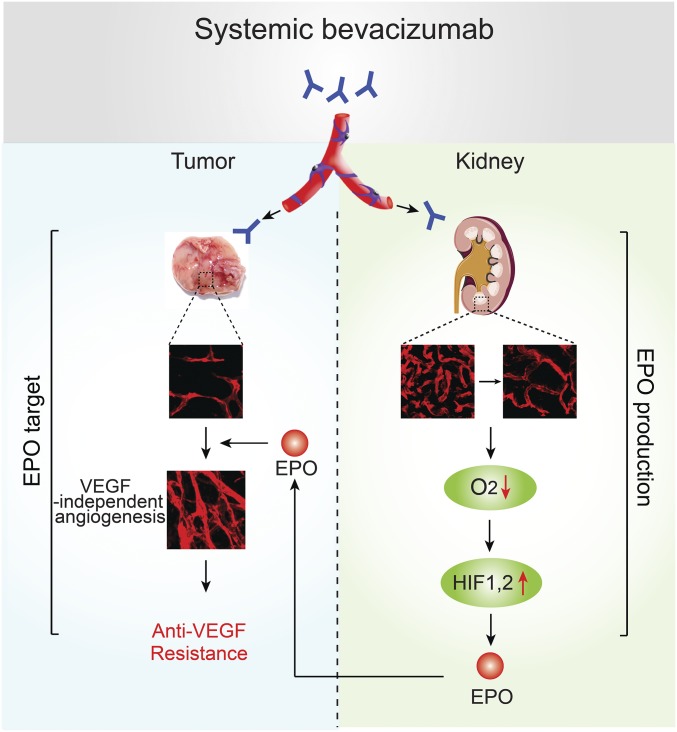
Fig. 7.
Schematic diagram of systemic anti-VEGF treatment-induced drug resistance. Systemic administration of anti-VEGF drugs such as bevacizumab enters into the circulation to target both tumor and healthy vasculatures such as kidney blood vessels. Anti-VEGF–induced vessel regression in the kidney cortex leads to tissue hypoxia, which induces HIF-1α and HIF-2α expressions. HIF-1α and HIF-2α at transcription level target the Epo promoter for high production of EPO in peritubular interstitial cells in kidney cortex. Kidney-derived EPO enters the circulation to stimulate angiogenesis and eventually contributes to antiangiogenic drug resistance. These findings provide mechanistic insights of off-tumor targets of antiangiogenic drugs in the cancer patients for development of drug resistance.
If these findings from genetic mouse models can be extended to clinical therapy, targeting nonhematopoietic EpoR would offer an attractive approach to improve drug sensitivity and clinical benefits of anti-VEGF therapy. Based on our findings, we reasonably propose that modest inhibition of EPO function or decrease of circulating EPO levels would be potentially beneficial for cancer patients who receive anti-VEGF treatment. Perhaps, low doses of EPO neutralizing agents and anti-VEGF drugs should be simultaneously given to cancer patients to maximize anticancer effects. If so, bone marrow hematopoiesis would not be significantly affected by the low-dose anti-EPO agents. Conversely, measurement of circulating EPO levels would potentially predict therapeutic outcome, and EPO serves as a surrogate marker for anti-VEGF treatment. This possibility warrants future clinical validation. Our preclinical findings are relevant to clinical situations. In two independent patient populations with CRC and breast cancer, anti-VEGF treatment significantly elevates similar circulating EPO levels as seen in our mouse models. Based on preclinical findings, various possible mechanisms that underlie anti-VEGF drug resistance have been proposed. These include tumor cell-organized vascular tunnels-vascular mimicry, tumor vessel remodeling, VEGF-independent angiogenesis, and vessel cooption (8, 9, 41–46). In the tumor local microenvironment, anti-VEGF treatment would create a local hypoxia that serves as an important trigger for up-regulation of non-VEGF angiogenic factors such as FGFs, PDGFs, and angiopoietins. These non-VEGF factors may significantly contribute to development of anti-VEGF drug resistance. In the kidney, exposure of anti-VEGF drugs elevates EPO expression, which might together with other factors synergistically or additively augment tumor angiogenesis and eventually development of drug resistance. Thus, blocking EPO together with other non-VEGF factors should be considered as an effective combination therapy.
Taken together, our findings provide an example of off-tumor targets of antiangiogenic drugs significantly contribute to development of drug resistance. Systemic delivery of antiangiogenic drugs produces profound impacts of nonmalignant tissues that significantly alters drug responses in the cancer microenvironment and probably overall survival of cancer patients. Our findings have also paved potential avenues for defining biomarkers predicting antiangiogenic responses by detecting gene expression profiles in noncancer tissues.
Materials and Methods
Tumor Experiment and Treatment.
Cultured tumor cells were suspended to make a final concentration of 1 × 106 or 6 × 106 cells in 100 μL of PBS and were s.c. injected into the middle region of dorsal back of each mouse. Tumor sizes were measured with a caliper every other day and were calculated according to a standard formula (length × width2 × 0.52). A rabbit anti-mouse VEGF-A–specific neutralizing antibody (kindly provided by the Simcere Pharmaceutical Company) and a rabbit nonimmune IgG (catalog no. 10500C; Invitrogen) were intraperitoneally (i.p.) administered twice per week at a dose of 5.0 mg·kg−1 starting at day 4 after tumor injection. Recombinant EPO was i.p. administered three times per week at a dose of 2,000 U·kg−1. sEpoR-Fc protein or control-Fc protein (7 μg per mouse) were i.p. administered twice a week. In the chemotherapy plus anti-VEGF combination therapy, 8.5 mg·kg−1 vinorelbine once per week, 30 mg/kg cisplatin once per month, and 5.0 mg·kg−1 anti-VEGF twice per week, were administered at day 9 after tumor implantation. At the end of all experiments, mice were killed with a lethal dose of CO2, followed by cervical dislocation. Tumor tissues were immediately removed and fixed overnight with 4% paraformaldehyde (PFA) at 4 °C or were freshly frozen in liquid nitrogen, and kept in −80 °C until further use.
Immunoblotting.
Proteins from total lysates, along with a protein ladder (catalog no. SM1811; Fermentas), were subjected to SDS/PAGE (catalog no. NP0301; Invitrogen), followed by wet transferring onto nitrocellulose membranes (catalog no. 88018; Thermo Scientific). Membranes were blocked at room temperature with 5% BSA (catalog no. A8806; Sigma) for 1 h before incubation overnight with a rabbit anti-ERK antibody (catalog no. 4695, 1:1,000; Cell Signaling), a rabbit anti-phospho-ERK (catalog no. 9101, 1:1,000; Cell Signaling) antibody, a rabbit anti-CAIX antibody (catalog no. NB100-417, 1:1,000; Novus Biologicals), or a monoclonal antibody to mouse β-Actin (catalog no. 3700, 1:2,000; Cell Signaling). Membranes were incubated at room temperature for 1 h with a donkey anti-rabbit IgG antibody (catalog no. 926–68073, IRDye 680RD, 1:15,000; LI-COR) and a donkey anti-mouse IgG antibody (catalog no. 926–32212, IRDye 800CW, 1:15,000; LI-COR). Protein bands were visualized and quantified using the ODYSSEY CLx (LI-COR) detection system at 700- and 800-nm wavelengths.
Human Patient Samples.
Blood samples were collected from colorectal cancer patients and healthy donors with written informed permission. All human studies were approved by the Ethical Review Committee in Jinling Hospital, Nanjing, China. Blood samples were collected from patients participating in the PROMIX clinical trial who had localized primary breast cancer including inflammatory breast cancer. Blood sampling were performed before the start of treatment and after two cycles of chemotherapy and two cycles of chemotherapy plus bevacizumab. Details of PROMIX clinical trial are available at https://clinicaltrials.gov/; identifier NCT00957125.
Animals.
All mouse studies were approved by the Animal Ethics Committee of Northern Stockholm.
Statistics.
Student’s t tests (Microsoft Excel) were used to measure statistical differences. P < 0.05 was considered statistically significant; P < 0.01 was considered very significant; P < 0.001 was considered extremely significant.
Supplementary Material
Acknowledgments
The Y.C. laboratory is supported through European Research Council Advanced Grant ANGIOFAT (project no. 250021) and research grants from the Swedish Research Council, the Swedish Cancer Foundation, the Karolinska Institute Foundation, the Torsten Soderbergs Foundation, the Maud and Birger Gustavsson Foundation, the NOVO Nordisk Foundation, and the Knut and Alice Wallenbergs foundation, and a Karolinska Institute distinguished professor award. M.N. is supported by a Swedish Cancer Foundation Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703431114/-/DCSupplemental.
References
- 1.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, et al. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3:114rv3. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y, Langer R. Optimizing the delivery of cancer drugs that block angiogenesis. Sci Transl Med. 2010;2:15ps3. doi: 10.1126/scitranslmed.3000399. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Zhong W, Sun Y. Improvement of antiangiogenic cancer therapy by understanding the mechanisms of angiogenic factor interplay and drug resistance. Semin Cancer Biol. 2009;19:338–343. doi: 10.1016/j.semcancer.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Chung AS, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 7.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Crawford Y, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, et al. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc Natl Acad Sci USA. 2013;110:12018–12023. doi: 10.1073/pnas.1301331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamba T, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 14.Eckardt KU, Ratcliffe PJ, Tan CC, Bauer C, Kurtz A. Age-dependent expression of the erythropoietin gene in rat liver and kidneys. J Clin Invest. 1992;89:753–760. doi: 10.1172/JCI115652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Y. Erythropoietin in cancer: A dilemma in risk therapy. Trends Endocrinol Metab. 2013;24:190–199. doi: 10.1016/j.tem.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Koury MJ, Bondurant MC. The mechanism of erythropoietin action. Am J Kidney Dis. 1991;18(Suppl 1):20–23. [PubMed] [Google Scholar]
- 17.Anagnostou A, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci USA. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue Y, et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med. 2011;18:100–110. doi: 10.1038/nm.2575. [DOI] [PubMed] [Google Scholar]
- 19.Remy I, Wilson IA, Michnick SW. Erythropoietin receptor activation by a ligand-induced conformation change. Science. 1999;283:990–993. doi: 10.1126/science.283.5404.990. [DOI] [PubMed] [Google Scholar]
- 20.Ribatti D, et al. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- 21.Grimm C, et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 22.Rankin EB, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, et al. VEGF-B promotes cancer metastasis through a VEGF-A-independent mechanism and serves as a marker of poor prognosis for cancer patients. Proc Natl Acad Sci USA. 2015;112:E2900–E2909. doi: 10.1073/pnas.1503500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, et al. Vascular endothelial growth factor-dependent spatiotemporal dual roles of placental growth factor in modulation of angiogenesis and tumor growth. Proc Natl Acad Sci USA. 2013;110:13932–13937. doi: 10.1073/pnas.1309629110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim S, et al. VEGFR2-mediated vascular dilation as a mechanism of VEGF-induced anemia and bone marrow cell mobilization. Cell Rep. 2014;9:569–580. doi: 10.1016/j.celrep.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Yu Y, et al. A humanized anti-VEGF rabbit monoclonal antibody inhibits angiogenesis and blocks tumor growth in xenograft models. PLoS One. 2010;5:e9072. doi: 10.1371/journal.pone.0009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu N, et al. Establishment and characterization of an erythropoietin-dependent subline, UT-7/Epo, derived from human leukemia cell line, UT-7. Blood. 1993;82:456–464. [PubMed] [Google Scholar]
- 28.Piao Y, et al. Acquired resistance to anti-VEGF therapy in glioblastoma is associated with a mesenchymal transition. Clin Cancer Res. 2013;19:4392–4403. doi: 10.1158/1078-0432.CCR-12-1557. [DOI] [PubMed] [Google Scholar]
- 29.Castro BA, et al. Macrophage migration inhibitory factor downregulation: A novel mechanism of resistance to anti-angiogenic therapy. Oncogene. 2017;36:3749–3759. doi: 10.1038/onc.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong J, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23:2800–2810. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue Y, et al. Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome. Proc Natl Acad Sci USA. 2008;105:18513–18518. doi: 10.1073/pnas.0807967105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Dale GL, Song P, Viollet B, Zou MH. AMPKalpha1 deletion shortens erythrocyte life span in mice: Role of oxidative stress. J Biol Chem. 2010;285:19976–19985. doi: 10.1074/jbc.M110.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, et al. Erythropoiesis-stimulating agents in the management of cancer patients with anemia: A meta-analysis. Chin J Cancer Res. 2014;26:268–276. doi: 10.3978/j.issn.1000-9604.2014.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki N, et al. Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood. 2002;100:2279–2288. doi: 10.1182/blood-2002-01-0124. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 36.Cohen EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: Results from a phase II study. J Clin Oncol. 2008;26:4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam BY, et al. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12:793–800, and erratum (2009) 15:462. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 39.Bohlius J, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 40.Henke M, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 41.Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: Vasculogenic mimicry in tumor cells: Diagnostic and therapeutic implications. Clin Cancer Res. 2012;18:2726–2732. doi: 10.1158/1078-0432.CCR-11-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinto MP, Sotomayor P, Carrasco-Avino G, Corvalan AH, Owen GI. Escaping antiangiogenic therapy: Strategies employed by cancer cells. Int J Mol Sci. 2016;17:E1489. doi: 10.3390/ijms17091489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helfrich I, et al. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med. 2010;207:491–503. doi: 10.1084/jem.20091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.di Tomaso E, et al. Glioblastoma recurrence after cediranib therapy in patients: Lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerbel RS. A decade of experience in developing preclinical models of advanced- or early-stage spontaneous metastasis to study antiangiogenic drugs, metronomic chemotherapy, and the tumor microenvironment. Cancer J. 2015;21:274–283. doi: 10.1097/PPO.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 46.Bridgeman VL, et al. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J Pathol. 2017;241:362–374. doi: 10.1002/path.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.