Significance
Chloroplasts harbor a complex redox network formed by two systems, the FTR- thioredoxins (Trxs), which relies on photoreduced ferredoxin (Fd), and the NADPH-dependent Trx reductase C NTRC. Thus, an important issue in chloroplast biology is to establish the relationship between these redox pathways. Here we propose that the Fd-FTR-Trxs and NTRC redox systems are integrated via the redox balance of 2-Cys peroxiredoxins (Prxs), which therefore has a key role in chloroplast function. NTRC controls the redox balance of 2-Cys Prxs, which maintains the reducing capacity of the pool of chloroplast Trxs and, consequently, proper regulation of photosynthetic carbon assimilation enzymes. Therefore, redox regulation of chloroplast enzymes and hydrogen peroxide reduction are linked by the action of the NTRC-2-Cys Prxs system.
Keywords: chloroplast, peroxiredoxin, NTRC, redox signaling, thioredoxin
Abstract
Thiol-dependent redox regulation allows the rapid adaptation of chloroplast function to unpredictable changes in light intensity. Traditionally, it has been considered that chloroplast redox regulation relies on photosynthetically reduced ferredoxin (Fd), thioredoxins (Trxs), and an Fd-dependent Trx reductase (FTR), the Fd-FTR-Trxs system, which links redox regulation to light. More recently, a plastid-localized NADPH-dependent Trx reductase (NTR) with a joint Trx domain, termed NTRC, was identified. NTRC efficiently reduces 2-Cys peroxiredoxins (Prxs), thus having antioxidant function, but also participates in redox regulation of metabolic pathways previously established to be regulated by Trxs. Thus, the NTRC, 2-Cys Prxs, and Fd-FTR-Trxs redox systems may act concertedly, but the nature of the relationship between them is unknown. Here we show that decreased levels of 2-Cys Prxs suppress the phenotype of the Arabidopsis thaliana ntrc KO mutant. The excess of oxidized 2-Cys Prxs in NTRC-deficient plants drains reducing power from chloroplast Trxs, which results in low efficiency of light energy utilization and impaired redox regulation of Calvin–Benson cycle enzymes. Moreover, the dramatic phenotype of the ntrc-trxf1f2 triple mutant, lacking NTRC and f-type Trxs, was also suppressed by decreased 2-Cys Prxs contents, as the ntrc-trxf1f2-Δ2cp mutant partially recovered the efficiency of light energy utilization and exhibited WT rate of CO2 fixation and growth phenotype. The suppressor phenotype was not caused by compensatory effects of additional chloroplast antioxidant systems. It is proposed that the Fd-FTR-Trx and NTRC redox systems are linked by the redox balance of 2-Cys Prxs, which is crucial for chloroplast function.
Photosynthesis is a source of metabolic intermediates that inevitably generates reactive oxygen species (ROS), which may cause oxidative damage but have also an important signaling function (1). The dual role of chloroplasts as source of metabolic precursors and second messengers is highly dependent on light. Thiol-based redox regulation plays an important role in the rapid adaptation of chloroplast metabolism to dark/light transitions. A classic example of a chloroplast redox regulated process is the CO2 fixation by the Calvin–Benson cycle in which key regulatory enzymes are reduced and fully active during the day and oxidized, and hence inactive, during the night (2–4).
While in heterotrophic organisms redox regulation relies on NADPH via a low number of thioredoxins (Trxs) and an NADPH-dependent Trx reductase (NTR), chloroplasts harbor a specific system composed by photosynthetically reduced ferredoxin (Fd), an Fd-dependent Trx reductase (FTR), and a complex set of Trxs (5). In addition, chloroplasts contain an NTR with a joint Trx domain, termed NTRC (6, 7), which participates in the redox regulation of metabolic pathways previously reported to be regulated by Trxs, such as the biosynthesis of starch (8, 9) and tetrapyrroles (10, 11). These results suggest that both redox systems act concertedly, a notion further supported by the severe growth-retarded phenotype of an Arabidopsis double mutant combining the deficiencies of NTRC and f- or x-type Trxs (12, 13). NTRC is an efficient reductant of 2-Cys peroxiredoxins (Prxs) and was therefore proposed to have antioxidant function (14). However, 2-Cys Prxs act as peroxide scavengers but also as peroxide sensors (15).
Therefore, NTRC is simultaneously involved in redox regulation and antioxidant defense, suggesting that both processes are interconnected; however, the mechanism that allows this central function of NTRC remains poorly understood. Here we have addressed this issue by analyzing the genetic interaction of NTRC and 2-Cys Prxs. Strikingly, the ntrc-Δ2cp triple mutant, which lacks NTRC and contains severely reduced levels of 2-Cys Prxs, nearly recovered the WT phenotype, indicating that the deficiency of 2-Cys Prxs suppresses the ntrc phenotype. This suppressor effect was characterized, and, based on these results, we propose that the Fd-FTR-Trxs and NTRC redox systems are integrated by the redox balance of the 2-Cys Prxs, which is thus essential for chloroplast redox regulation.
Results
Decreased Levels of 2-Cys Prxs Suppress the Phenotype Caused by the Lack of NTRC in Arabidopsis.
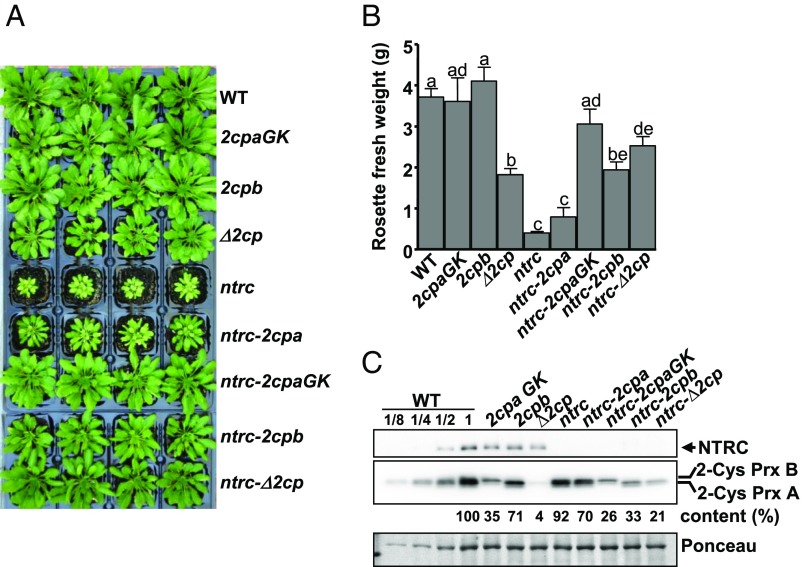
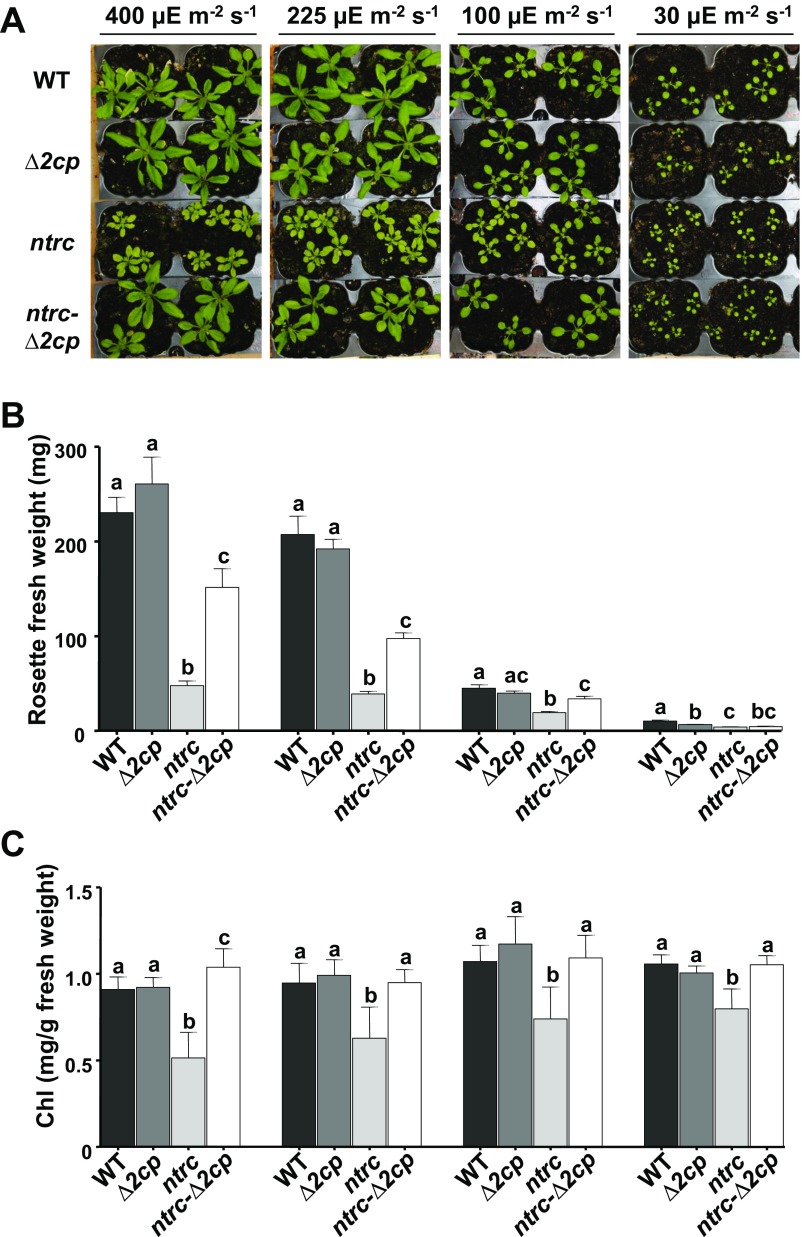
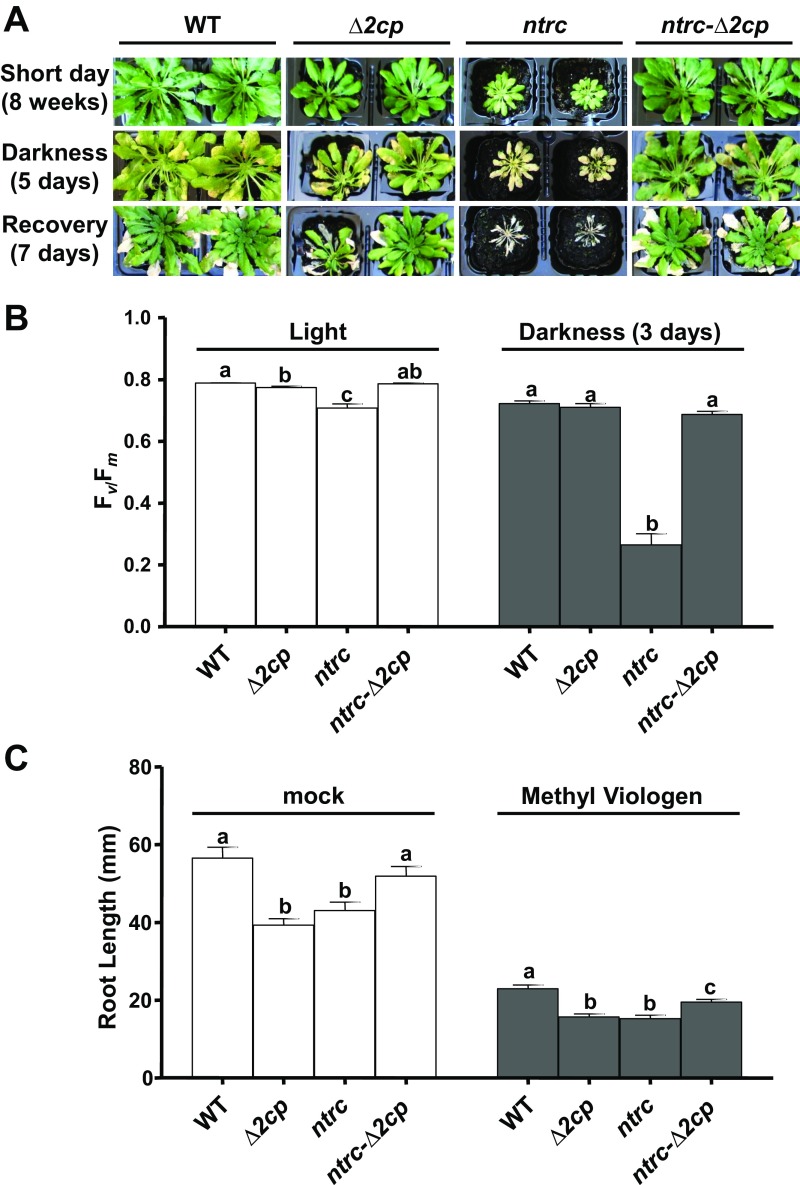
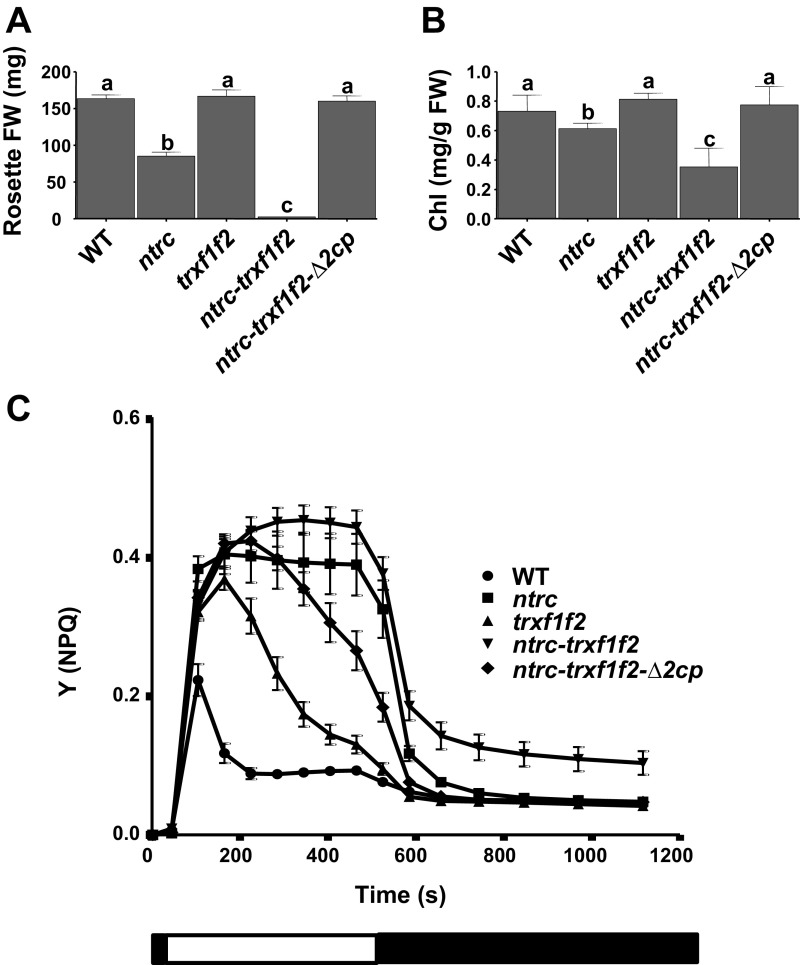
With the aim of establishing the genetic interaction of NTRC and 2-Cys Prxs, Arabidopsis mutants combining the deficiencies of both enzymes were generated. Because Arabidopsis contains two almost identical 2-Cys Prxs, termed A and B, the double mutant Δ2cp, which lacks 2-Cys Prx B but still contains a small amount of 2-Cys Prx A (16), was manually crossed with the ntrc mutant (6). Strikingly, the triple mutant, here termed ntrc-Δ2cp, despite being affected in redox regulation and antioxidant defense, partially recovered WT phenotype (Fig. 1A), as determined by rosette fresh weight (Fig. 1B), indicating that the growth phenotype of the ntrc mutant was suppressed by decreased contents of 2-Cys Prxs. It should be noted that the content of 2-Cys Prxs, compared with the WT, is higher in the ntrc-Δ2cp (21%) than in the Δ2cp mutant (4%; Fig. 1C), suggesting a compensatory effect triggered by the deficiency of NTRC. To further analyze this suppressor effect, different growth and environmental conditions were tested. The suppressor effect was also observed when plants were grown under long-day photoperiod at different light intensities (Fig. S1A), as shown by rosette fresh weight (Fig. S1B) and chlorophyll content (Fig. S1C). It is known that the ntrc mutant is extremely sensitive to treatments of prolonged darkness (14), which exert a deep effect on PSII photochemical efficiency. Interestingly, the response to prolonged darkness of the ntrc-Δ2cp mutant was similar to those of the WT and the Δ2cp mutant (Fig. S2A), as shown by the ratio of variable fluorescence (Fv) to maximal fluorescence (Fm; Fig. S2B). Finally, the ntrc-Δ2cp mutant also showed a better response to oxidative stress than the ntrc mutant, as determined by the rate of root growth in the presence of methyl viologen (Fig. S2C).
Fig. 1.
The phenotype of the Arabidopsis ntrc mutant is suppressed by decreased levels of 2-Cys Prxs. (A) WT and mutant lines were grown under short-day conditions for 9 wk. The weights of rosette leaves from at least seven plants are presented as average values ± SE. Letters indicate significant differences by Student’s t test at a 95% CI (B). (C) Western blot analysis of the content of NTRC and 2-Cys Prxs. Protein extracts were obtained from leaves of plants grown as stated in A, and aliquots of 15 μg (1×) from all lines, as well as 1:2, 1:4, and 1:8 dilutions from WT, as indicated, were subjected to SDS/PAGE under reducing conditions, transferred to nitrocellulose filters, and probed with anti-NTRC or anti-2-Cys Prxs antibodies. Even loading was monitored by Ponceau staining. Band intensities of 2-Cys Prxs and Ponceau were quantified (ScionImage), and the ratio between them, referred to the WT 1× sample (arbitrarily assigned a value of 100), are indicated.
Fig. S1.
The ntrc mutant phenotype is suppressed by decreased levels of 2-Cys Prxs under different growth conditions. WT and mutant lines, as indicated, were grown under long-day photoperiod for 3 wk under the stated light intensity (A). For each growth condition, the weight of the rosette leaves (B) and chlorophyll content (C) were determined from at least 10 plants and are presented as average values ± SE. Letters indicate significant differences by Student’s t test at a 95% CI.
Fig. S2.
The ntrc mutant phenotype is suppressed by decreased levels of 2-Cys Prxs under abiotic stress treatments. (A) WT and mutant lines, as indicated, were grown on soil under short-day photoperiod for 8 wk, incubated for 5 d under continuous darkness, and recovered under short-day conditions for 7 d. (B) The maximum PSII quantum yield, determined as Fv/Fm ratio, was measured in dark-adapted leaves of plants grown under short-day photoperiod for 8 wk or incubated for 3 d in continuous darkness. The Fv/Fm values are represented as average values ± SE of at least 10 measurements from different plants. (C) WT and mutant lines, as indicated, were grown for 11 d on Murashige and Skoog synthetic medium containing 0.5% (wt/vol) sucrose in the absence (mock) or presence of 0.025 μM methyl viologen under long-day photoperiod. Root length, determined from at least 30 seedlings, is shown as average values ± SE. Letters indicate significant differences by Student’s t test at 99% (B) or 95% (C) CI.
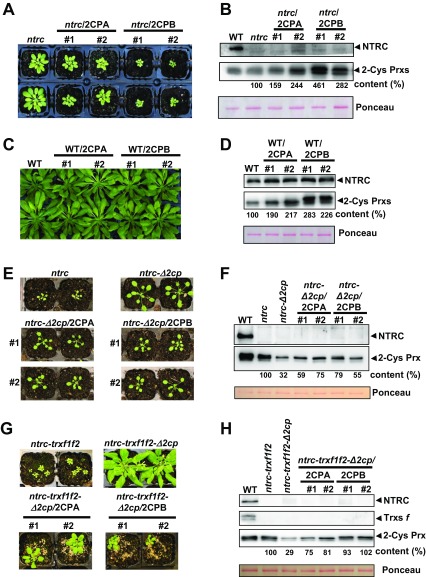
We then tested the effect of the two 2-Cys Prxs present in Arabidopsis chloroplasts. To that end, the single mutants 2cpa, still containing residual amounts of 2-Cys Prx A; 2cpb, devoid of 2-Cys Prx B (17); and a mutant KO for 2-Cys Prx A, here termed 2cpaGK, were manually crossed with ntrc, and double mutants ntrc-2cpa, ntrc-2cpaGK, and ntrc-2cpb were generated (Fig. 1A). As expected, the 2cpaGK and 2cpb single mutants showed phenotypes indistinguishable from the WT (Fig. 1 A and B). Interestingly, whereas mutants ntrc-2cpaGK and ntrc-2cpb, containing lower levels of 2-Cys Prxs (26% and 33%, respectively; Fig. 1C), showed growth phenotypes comparable to the ntrc-Δ2cp mutant (Fig. 1 A and B), the ntrc-2cpa mutant, with higher content of 2-Cys Prxs (70%; Fig. 1C), showed a subtle reversion of the ntrc phenotype (Fig. 1 A and B). Thus, the phenotype caused by the lack of NTRC depends on the dose of 2-Cys Prxs, with isoforms A and B having indistinguishable effect. A different approach was then undertaken to further test the effect of the dose of 2-Cys Prxs. Overexpression of 2-Cys Prx A or B in the ntrc mutant background showed aggravated growth retardation phenotype (Fig. S3 A and B), but had no visible effect in the WT background (Fig. S3 C and D). In contrast, an increased expression of 2-Cys Prxs A or B in the ntrc-Δ2cp background led to phenotypes intermediate between those of ntrc and ntrc-Δ2cp (Fig. S3 E and F). Overall, these results confirm that the phenotype of plants devoid of NTRC is highly dependent on the level of 2-Cys Prxs.
Fig. S3.
The effect of the overexpression of 2-Cys Prx A and 2-Cys Prx B in Arabidopsis WT, ntrc mutant, and suppressed lines. Two independent transgenic lines, nos. 1 and 2, expressing 2-Cys Prx A or 2-Cys Prx B under the CaMV 35S constitutive promoter in the ntrc (A), WT (C), ntrc-Δ2cp (E), and ntrc-trxf1f2-Δ2cp (T1 generation; G) genetic backgrounds were grown under short-day (A, C, and E) or long-day (G) photoperiod for 8 wk (A and C) or 4 wk (E and G). (B, D, F, and H) Western blot analyses of the contents of NTRC, 2-Cys Prxs, and Trxs f (H) in WT, mutants, and transgenic lines. Protein extracts [2 µg (B), 4 µg (D), 3 µg (F and H)] from leaves of plants grown as stated earlier were subjected to SDS/PAGE under reducing conditions, transferred to nitrocellulose filters, and probed with anti-NTRC, anti-2-Cys Prxs, and anti-Trxs f antibodies. Even loading was monitored by Ponceau staining. Band intensities of 2-Cys Prxs and Ponceau were quantified (ScionImage), and the ratios between them, referred to the ntrc (B and F), WT (D), or ntrc-trxf1f2 (H) samples, arbitrarily assigned a value of 100, are indicated.
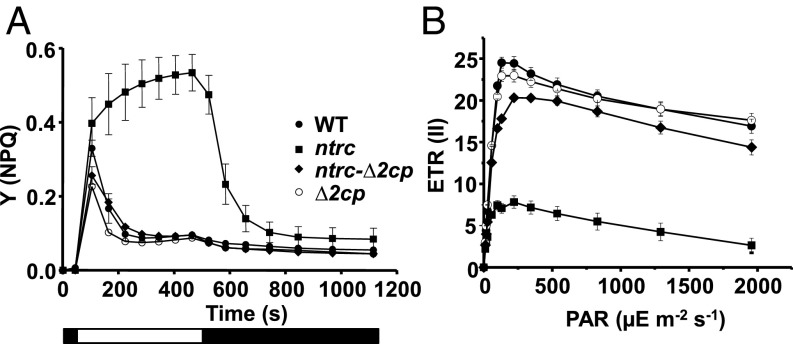
The ntrc mutant shows low efficiency of light energy utilization as revealed by the extensive nonphotochemical quenching (NPQ) at low light intensity and the decreased photosynthetic electron transport rate (ETR) (18). Thus, to further characterize the suppression of the ntrc phenotype by decreased levels of 2-Cys Prxs, these photosynthetic parameters were also analyzed. The ntrc-Δ2cp line recovered almost WT levels of NPQ (Fig. 2A) and photosynthetic ETR (Fig. 2B), which is in line with the recovery of the rate of CO2 fixation (Fig. S4).
Fig. 2.
NPQ and linear photosynthetic ETR in Arabidopsis WT and mutant lines. (A) Quantum yield of NPQ [Y(NPQ)] was performed using attached leaves from plants grown under short-day conditions for 8 wk. White and black blocks indicate light (75 μE m−2⋅s−1) and darkness periods, respectively. Each data point is the average of at least four determinations, and SEs are represented by error bars. (B) Relative ETRs of PSII, ETR(II), were determined during stepwise increasing photosynthetically active radiation (PAR) in plants grown as in A. Each data point is the mean of the ETR(II) from at least five leaves from different plants, and SEs are presented.
Fig. S4.
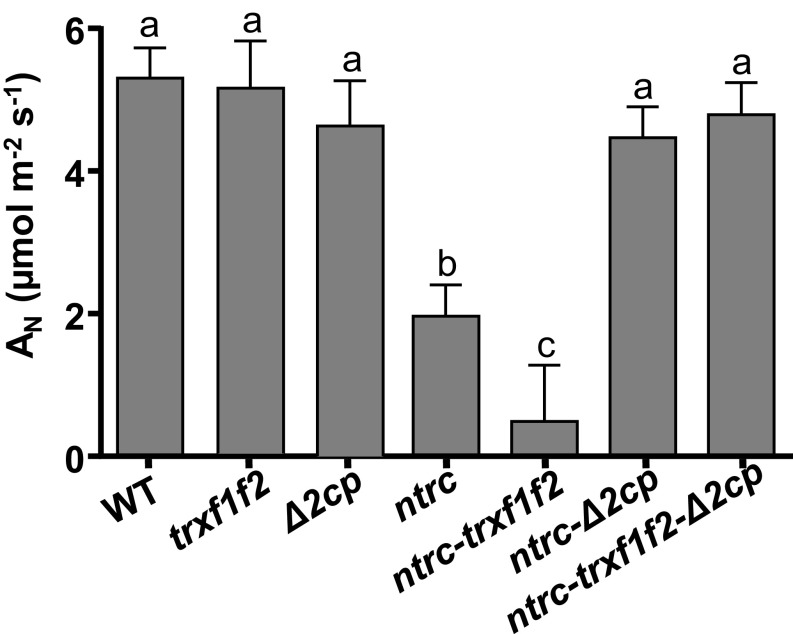
Rate of CO2 assimilation in WT and mutant lines. Net CO2 assimilation rate (AN) was determined in leaves of plants grown under long-day photoperiod (4 wk), which were dark-adapted and then illuminated with a PAR of 125 μE m−2⋅s−1. Six leaves were measured per line, and mean values ± SE are presented. Letters indicate significant differences by Tukey test at a 99.9% CI.
2-Cys Prxs Cause Imbalance of Chloroplast Redox Regulation in NTRC Deficient Plants.
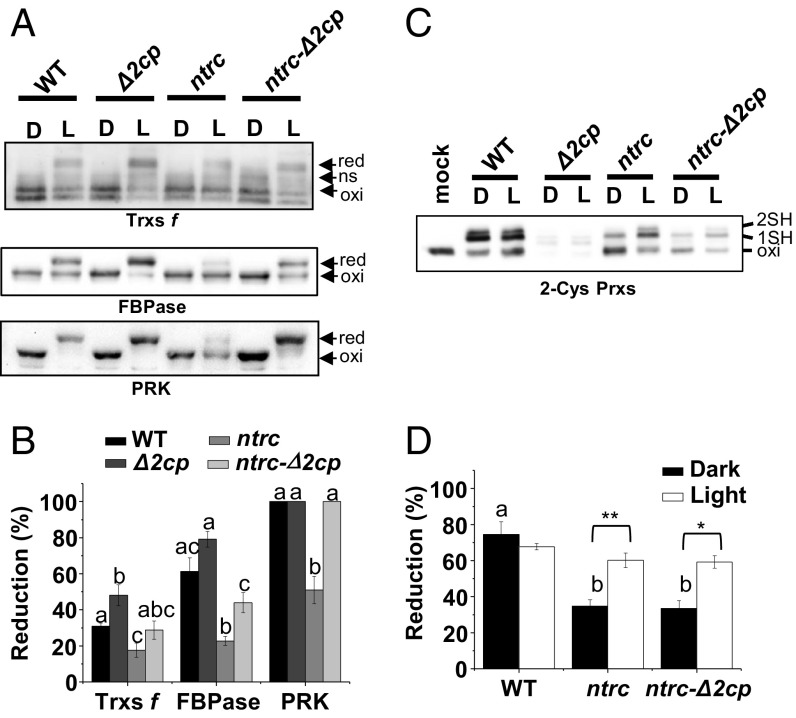
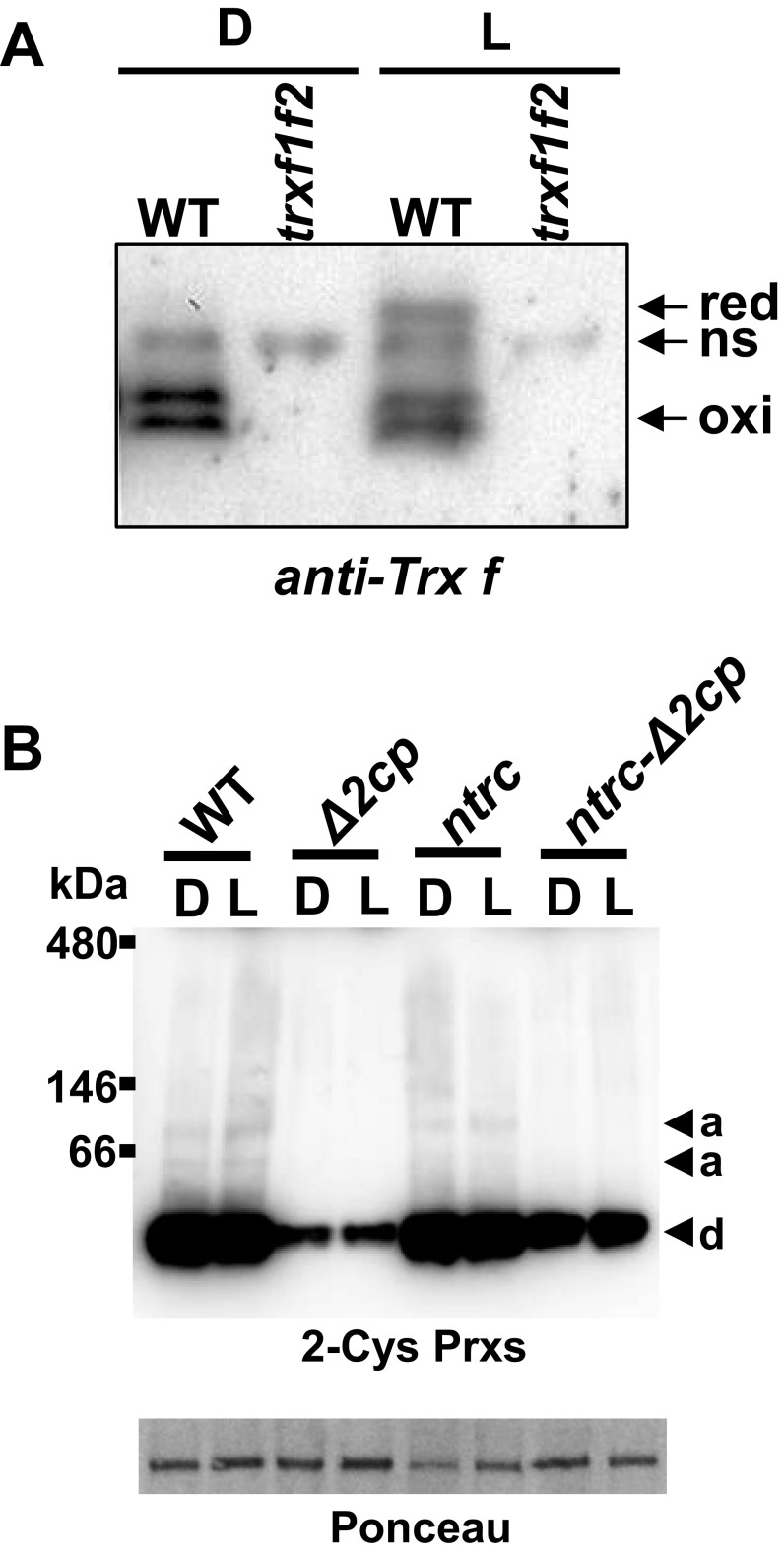
The severe growth inhibition phenotype caused by higher levels of 2-Cys Prxs in the ntrc background suggests that the harmful effect is caused by the accumulation of oxidized 2-Cys Prxs. Thus, we established the hypothesis that the increased level of oxidized 2-Cys Prxs in NTRC-deficient plants might act as a sink for electrons from reduced chloroplast electron carriers. Clear candidates for transferring electrons to the 2-Cys Prxs are Trxs because, albeit with low efficiency, different Trxs are able to reduce 2-Cys Prxs (19–24). If this were the case, such redirection of photosynthetic reducing power from the pool of Trxs would affect the light-dependent reduction of Trx-regulated enzymes. To test this possibility, the light-dependent changes of the redox state of Trxs f, chosen as representatives of the pool of Trxs, and fructose-bisphosphate phosphatase (FBPase) and phosphoribulokinase (PRK), well-known redox-regulated enzymes of the Calvin–Benson cycle, were analyzed by thiol labeling with methylmaleimide polyethylene glycol24 [MM(PEG)24]. Dark-adapted WT plants showed the previously reported double band (25), corresponding to fully oxidized Trxs f, and an additional nonspecific band (Fig. 3A) also detected in extracts from the trxf1f2 double KO mutant (Fig. S5A). In response to light, Trxs f were partially reduced, the level of reduction being similar to the WT in the ntrc-Δ2cp mutant, higher in the Δ2cp mutant, and lower in the ntrc mutant (Fig. 3 A and B). The changes in the redox state of FBPase and PRK resembled those of Trxs f (Fig. 3 A and B), except that PRK became fully reduced upon illumination in all lines except ntrc. Therefore, decreased levels of 2-Cys Prxs suppressed the impairment of redox regulation of Calvin–Benson cycle enzymes in NTRC-deficient plants.
Fig. 3.
In vivo redox changes of representative chloroplast enzymes during dark/light transitions in Arabidopsis WT and mutant plants. (A) In vivo redox state of Trxs f, FBPase, and PRK determined from leaves of 8-wk-old short-day grown plants at the end of the dark period (marked as “D”) and after 30 min of illumination at 125 μE m−2⋅s−1 (“L”). (B) Band intensities were quantified (ScionImage), and the percentage of reduction is the ratio of the reduced form and the sum of reduced and oxidized forms. Each value is the mean of three independent experiments ± SE. Letters indicate significant differences between mutants by Student’s t test at a 95% CI. (C) In vivo redox state of 2-Cys Prxs. (D) Reduction level is the ratio between the sums of the half reduced (1SH) and fully reduced (2SH) forms and the sum of reduced and oxidized forms. Each value is the mean of at least three independent experiments ± SE. Letters indicate significant differences between mutants in darkness by Tukey test at a 99% CI (there are no significant differences in light). Statistical significance (*P < 0.05 and **P < 0.01) determined with the Student’s t test comparing dark and light values for each line. ns, nonspecific; Oxi, oxidized; red, reduced. 1SH and 2SH indicate reduction of one or the two cysteines, respectively, of 2-Cys Prxs.
Fig. S5.
Bands detected by antibodies against Trxs f in MM(PEG)24-based alkylation experiments and aggregation state of 2-Cys Prxs in WT and mutant lines. (A) WT and the trxf1f2 mutant of A. thaliana were grown under long-day conditions for 4 wk. Alkylation assays were performed as described in Materials and Methods using leaves harvested at the end of the night (marked as “D”) and after 30 min of illumination (“L”) with growth light intensity. Proteins were resolved in SDS/PAGE under nonreducing conditions, transferred to nitrocellulose filters, and probed with the anti-Trx f antibodies. ns, nonspecific; oxi, oxidized; red, reduced. (B) Western blot analysis of the aggregation state of 2-Cys Prxs. Protein extracts (15 μg) from leaves were subjected to native gel electrophoresis, transferred to nitrocellulose filters, and probed with anti–2-Cys Prxs antibodies. Even loading was monitored by Ponceau staining, and molecular weight markers (in kilodaltons) are indicated. a, aggregated; d, dimer.
As our hypothesis implies the redirection of reducing power to the 2-Cys Prxs, it would be expected that there would be light-dependent reduction of these enzymes in plants devoid of NTRC. In alkylation assays, the oxidized form of 2-Cys Prxs (oxi) was detected as a band with a mobility similar to nonalkylated samples (mock), whereas the two shifted bands corresponded to the half-reduced (1SH) and fully reduced (2SH) forms, respectively (Fig. 3C). Interestingly, 2-Cys Prxs were predominantly reduced (1SH plus 2SH) in WT plants, reaching similar levels in the dark and upon illumination (Fig. 3 C and D). Although the ntrc and ntrc-Δ2cp mutants showed a significant decrease of the reduced forms of 2-Cys Prxs in darkness, a significant light-dependent increase of the reduced forms was observed (Fig. 3 C and D). It should be noted that the amount of 2-Cys Prxs in the Δ2cp mutant is too low to be reliably quantified; however, in the ntrc-Δ2cp mutant, the content of 2-Cys Prxs is made higher (Fig. 1C) by the compensatory effect of the deficiency of NTRC, thus making quantification possible. Oxidant conditions favor the aggregation of 2-Cys Prxs, which shows chaperone activity (26). Thus, an additional possibility is that the aggregation state of 2-Cys Prxs is affected in the ntrc-Δ2cp suppressed line. However, native gel electrophoresis showed that most of the 2-Cys Prxs are in dimeric form, and no significant differences in the aggregation state were observed in this preliminary test in any of the lines under analysis in dark/light transitions (Fig. S5B).
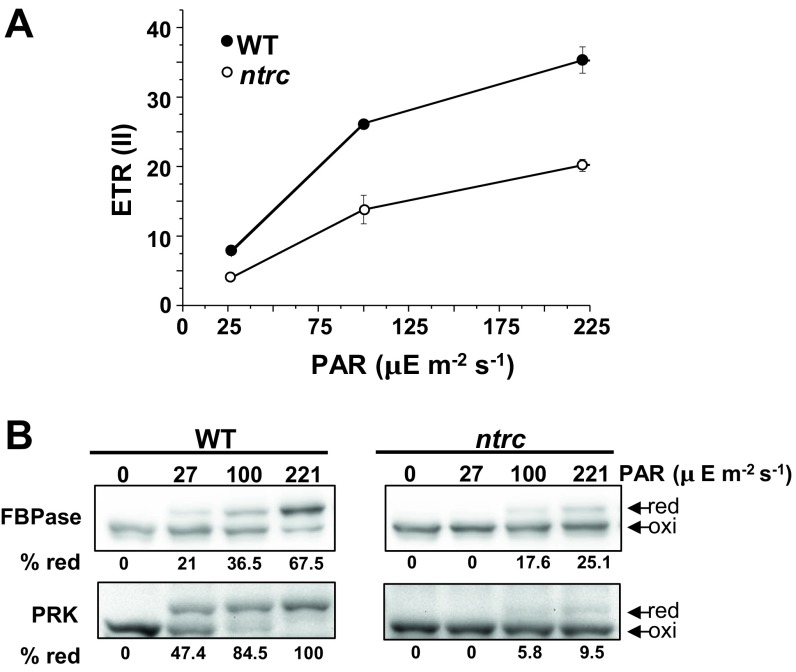
As the ntrc mutant shows lower efficiency of light energy utilization (Fig. 2), the poor reduction of Trx-regulated enzymes might be the result of the lower photosynthetic ETR rather than the redirection of reducing power from the pool of Trxs to the 2-Cys Prxs. To distinguish between these possibilities, WT and ntrc mutant plants were exposed to different light intensities, and the effects on the photosynthetic ETR and the redox state of FBPase and PRK were analyzed. As expected, the photosynthetic ETR (Fig. S6A) and, accordingly, the level of reduction of FBPase and PRK (Fig. S6B), were impaired in the ntrc mutant at all light intensities tested. However, at 100 μE m−2⋅s−1, which produced a photosynthetic ETR in the ntrc mutant slightly higher than that produced at 27 μE m−2⋅s−1 in the WT (Fig. S6A), the ntrc mutant showed lower level of reduction of FBPase and PRK than the WT (Fig. S6B), indicating that the level of reduction of these enzymes does not solely respond to photosynthetic ETR.
Fig. S6.
In vivo redox changes of representative chloroplast enzymes in response to light intensity in Arabidopsis WT and ntrc mutant plants. (A) Relative ETRs of PSII, ETR(II), were measured after 10 min of illumination with different actinic light intensities (27, 100, and 221 μE m−2⋅s−1) in 4 wk-old long-day grown plants adapted to darkness. Each data point is the mean of the ETR(II) from at least three leaves from different plants. SDs are represented by error bars. (B) Effect of PAR on the in vivo redox state of FBPase and PRK in WT and ntrc plants. Dark-adapted plants of the indicated lines, grown as in A, were kept in darkness or exposed to the stated light intensity for 10 min. After the treatment, at least three leaves of each genotype and condition were harvested and alkylated with MM(PEG)24. Protein extracts from MM(PEG)24-treated samples were separated in SDS/PAGE under nonreducing conditions, transferred to nitrocellulose filters, and probed with the stated antibodies. oxi, oxidized; red, reduced. Band intensities were quantified (ScionImage), and the percentage of reduced enzymes is indicated below each line.
As 2-Cys Prxs have peroxidase activity, an additional possibility is that the suppression of the ntrc phenotype in the ntrc-Δ2cp mutant is the result of compensatory effects of additional antioxidants. Minor differences were observed in the total content of ascorbic acid (AsA) plus dehydroascorbic acid (DHA) and reduced glutathione (GSH) plus oxidized glutathione (GSSG) in leaves of the lines under analysis (Table S1). Although both antioxidants were slightly more oxidized in the ntrc mutant, the ntrc-Δ2cp suppressed line partially recovered levels of the reduced form of both antioxidants (Table S1). Therefore, compensatory effects of nonenzymatic antioxidants could be excluded.
Table S1.
Determination of nonenzymatic antioxidants in Arabidopsis WT and mutant plants
| Nonenzymatic antioxidant | WT | ∆2cp | ntrc | ntrc-∆2cp |
| AsA+DHA (μmol⋅g−1 FW) | 3.806 ± 0.207 | 3.675 ± 0.260 | 3.334 ± 0.225 | 3.571 ± 0.276 |
| AsA/(AsA+DHA) | 0.914 ± 0.019* | 0.922 ± 0.026* | 0.829 ± 0.017† | 0.883 ± 0.016*† |
| GSH+GSSG (μmol⋅g−1 FW) | 0.924 ± 0.041 | 0.944 ± 0.064 | 0.890 ± 0.056 | 0.918 ± 0.032 |
| GSH/GSH+GSSG | 0.879 ± 0.027* | 0.856 ± 0.023* | 0.796 ± 0.025† | 0.851 ± 0.035*† |
Whole rosettes (n = 5) of 6-wk-old plants grown under short-day photoperiod were harvested to determine AsA, DHA, GSH, and GSSG (Materials and Methods). For the indicated lines, total ascorbic acid (AsA+DHA), reduced ascorbic acid /total ascorbic acid (AsA/AsA+DHA) ratios, total glutathione (GSH+GSSG), and reduced glutathione/total glutathione (GSH/GSH+GSSG) ratios are presented as mean values ± SE from two independent experiments combined.
,†Significant differences between genotypes marked with different symbols (Tukey test at a 95% CI).
Decreased Levels of 2-Cys Prxs Suppress the Phenotype Caused by the Combined Deficiencies of NTRC and f-Type Trxs.
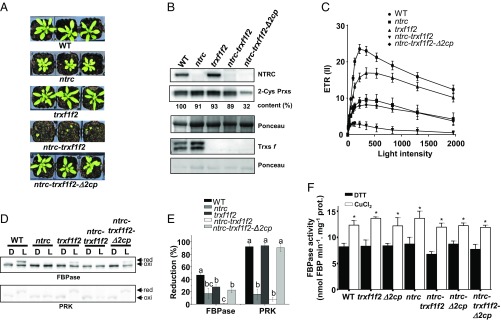
Recently, it was reported that the Arabidopsis triple mutant ntrc-trxf1f2, defective in NTRC and f-type Trxs, has a very severe growth inhibition phenotype (13). We then tested whether the suppressor effect of decreased levels of 2-Cys Prxs is also exerted in these plants. These experiments were performed with plants grown under long-day conditions because the ntrc-trxf1f2 mutant showed reduced viability under the short-day conditions used for the previous experiments. In line with its severe growth retardation phenotype (Fig. 4A and Fig. S7 A and B), the use of light energy by the ntrc-trxf1f2 mutant was very inefficient, showing the highest NPQ (Fig. S7C) and the lowest ETR (Fig. 4C) of the lines analyzed. Interestingly, the ntrc-trxf1f2-Δ2cp quintuple mutant, which is KO for NTRC and Trxs f and contains decreased levels of 2-Cys Prxs (32%; Fig. 4B), showed partial recovery of NPQ (Fig. S7C) and the photosynthetic ETR (Fig. 4C), whereas the rate of CO2 fixation (Fig. S4) and growth phenotype were similar to those of the WT (Fig. 4A and Fig. S7 A and B). In agreement with previous results (12, 25), the light-dependent redox regulation of FBPase was more affected by the deficiency of NTRC than by the deficiency of f-type Trxs, as was the redox regulation of PRK (Fig. 4 D and E). Hardly any light-dependent reduction of FBPase or PRK was observed in the ntrc-trxf1f2 mutant, whereas the ntrc-trxf1f2-Δ2cp quintuple mutant recovered levels of light-dependent reduction of both enzymes, similar to those of the trxf1f2 mutant (Fig. 4 D and E). Finally, the determination of FBPase activity from leaf extracts showed higher activity under reducing (i.e., DTT) than under oxidant (i.e., CuCl2) conditions in all lines analyzed (Fig. 4F), reflecting the activating effect of reducing conditions on the chloroplastic redox-sensitive isoform of the enzyme. The finding that decreased levels of 2-Cys Prxs suppress the dramatic growth phenotype of plants simultaneously devoid of NTRC and Trxs f uncovers the key role of 2-Cys Prxs in chloroplast redox regulation. This notion was further confirmed by the overexpression of 2-Cys Prx A or B in the ntrc-trxf1f2-Δ2cp quintuple mutant, which resulted in phenotypes intermediate between ntrc-trxf1f2 and ntrc-trxf1f2-Δ2cp (Fig. S3 G and H).
Fig. 4.
Decreased levels of 2-Cys Prxs suppress the growth inhibition phenotype of the ntrc-trxf1f2 mutant. (A) WT and mutant lines were grown under long-day conditions for 4 wk. (B) Western blot analysis of the content of NTRC, 2-Cys Prxs, and Trxs f in WT and mutant lines grown as stated in A. Band intensities of 2-Cys Prxs and Ponceau were quantified (ScionImage), and the ratios between them in reference to the WT sample (arbitrarily assigned a value of 100) are indicated. (C) Relative ETRs of PSII, ETR(II), were determined during stepwise increasing PAR in plants grown as in A. Each data point is the mean of the ETR(II) from at least five leaves from different plants, and SEs are presented. (D) In vivo redox state of FBPase and PRK was monitored at the end of the dark period (marked as “D”) or after 30 min of illumination at 125 μE m−2⋅s−1 (“L”) using plants grown as in A. (E) Band intensities were quantified (ScionImage), and the percentage of reduction for each enzyme is the ratio between the reduced form and the sum of reduced and oxidized forms. Each value is the mean of five independent experiments ± SE. Letters indicate significant differences between mutants by Student’s t test at a 95% CI. oxi, oxidized; red, reduced. (F) FBPase activity was assayed in leaf extracts, pretreated with 100 µM CuCl2 (black bars) or 10 mM DTT (white bars), from plants grown as described in A and harvested at 12 h of the day period. Results are the mean ± SE from three biological replicates. For each genotype, asterisks indicate significant differences (Student’s t test at a 95% CI) between oxidant and reducing conditions.
Fig. S7.
Decreased levels of 2-Cys Prxs suppress the phenotype of the ntrc-trxf1f2 mutant. WT and mutant lines, as indicated, were grown under long-day and growth-light conditions for 4 wk. (A) The weights of the rosette leaves was determined from at least seven plants and are presented as average values ± SE. (B) Chlorophyll levels were measured from leaf discs (n ≥ 8), and mean values ± SE are shown, with letters indicating significant differences by Student’s t test at a 95% CI. (C) After incubation of plants during 30 min in darkness, an induction/recovery curve was performed as described in Materials and Methods to determine Y(NPQ). Values are the mean ± SE of at least seven plants. White and black blocks indicate light (75 μE m−2⋅s−1) and darkness periods, respectively.
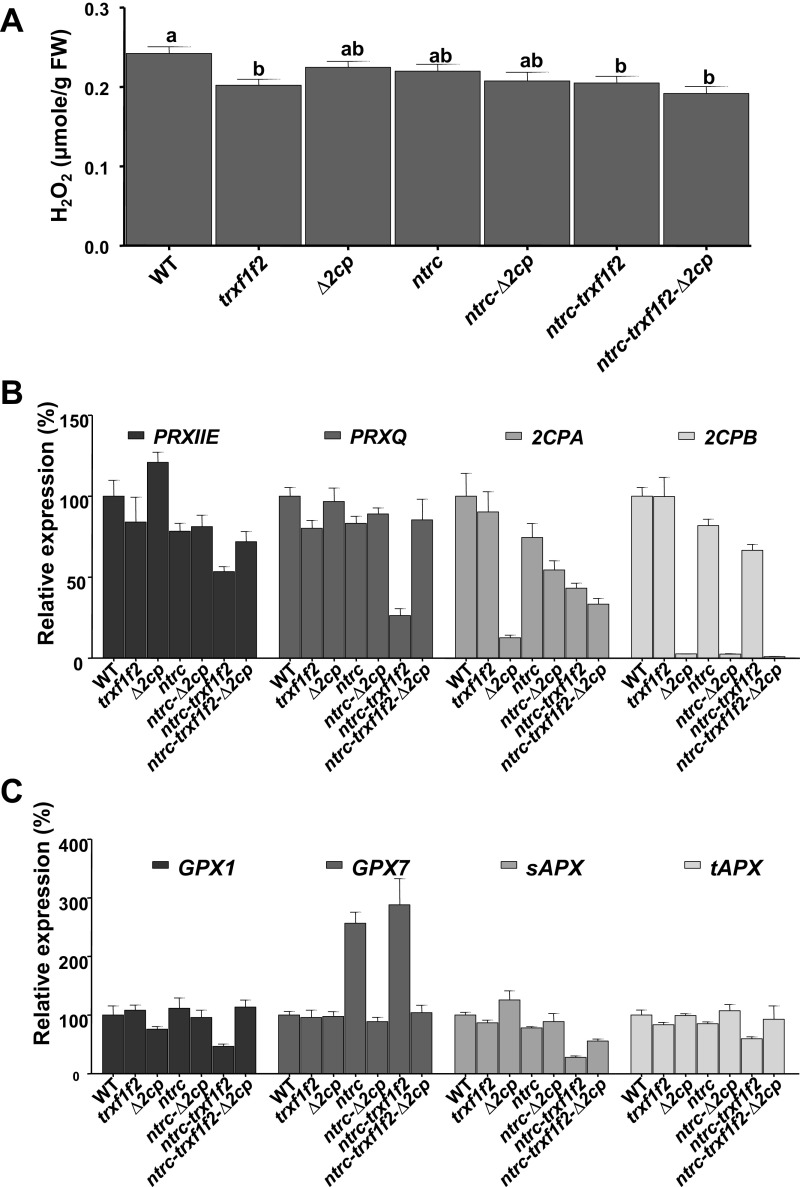
NTRC-deficient plants lack the major pathway of 2-Cys Prxs reduction, which might compromise H2O2 detoxification and provoke its accumulation. However, as previously reported (14), leaves of the ntrc mutant showed similar levels of H2O2 as WT (Fig. S8A). Even the ntrc-trxf1f2 mutant, which shows the most severe phenotype, did not show any increase of H2O2 (Fig. S8A). Such a finding might be caused by a compensatory effect exerted by additional antioxidants within chloroplasts. As the content of nonenzymatic antioxidants was very similar in the ntrc mutant and ntrc-Δ2cp suppressed line (Table S1), we analyzed the expression of genes encoding plastid-localized enzymatic peroxidases. Although the Δ2cp mutant contained the expected lower levels of 2CPA and almost undetectable levels of 2CPB transcripts, the content of 2CPA transcripts was increased in the ntrc-Δ2cp and ntrc-trxf1f2-Δ2cp mutants (Fig. S8B), indicating that the higher level of 2-Cys Prx A in these mutants (Fig. 1C) is the result of the up-regulation of the 2CPA gene triggered by the deficiency of NTRC. Minor differences were observed in the content of transcripts of genes encoding the other plastid-localized Prxs, PRXQ and PRXIIE, and peroxidases GPX1, sAPX, and tAPX, except in the ntrc-trxf1f2 triple mutant, which showed reduced amounts (Fig. S8 B and C). Interestingly, partial (PRXIIE and sAPX) or complete (PRXQ, GPX1, and tAPX) recovery of the WT levels of these transcripts were observed the ntrc-trxf1f2-Δ2cp quintuple mutant (Fig. S8 B and C). The GPX7 gene was induced in ntrc and ntrc-trxf1f2 mutants, with induction also suppressed in ntrc-Δ2cp and ntrc-trxf1f2-Δ2cp mutants (Fig. S8C). Altogether, these results suggest that the phenotypic effects caused by the deficiencies of the redox systems studied here are the result of imbalance of the chloroplast redox network rather than oxidative stress or compensatory effects of additional antioxidants.
Fig. S8.
Hydrogen peroxide content and expression of genes encoding chloroplast peroxidases in Arabidopsis WT and mutant plants. WT and mutant lines, as indicated, were grown under long-day and growth-light conditions for 4 wk. (A) The hydrogen peroxide content in whole rosettes (n ≥ 8) was measured as indicated in Materials and Methods. Mean values ± SE from two independent experiments combined are shown, with letters indicating significant differences by Tukey test at a 99% CI. (B and C) The levels of transcripts of the indicated genes, determined by quantitative RT-PCR using oligonucleotides indicated in Table S3, were normalized against three reference genes (Materials and Methods) and referenced against the levels of each of the genes in WT plants, arbitrarily considered as 100%. Determinations were performed three times, and mean values ± SE are presented.
Discussion
The discovery that chloroplasts harbor an NADPH-dependent redox system (i.e., NTRC) in addition to the light-dependent Fd-FTR-Trxs (6) gave rise to the issue of establishing how these two redox systems interact. Based on the phenotype of Arabidopsis double mutants combining the deficiencies of NTRC and f- or x-type Trxs (12, 13) or NTRC and FTR (27), it was proposed that both redox systems act separately, but with overlapping functions. Here, we propose that these systems are integrated via the redox balance of 2-Cys Prxs as depicted in Fig. 5.
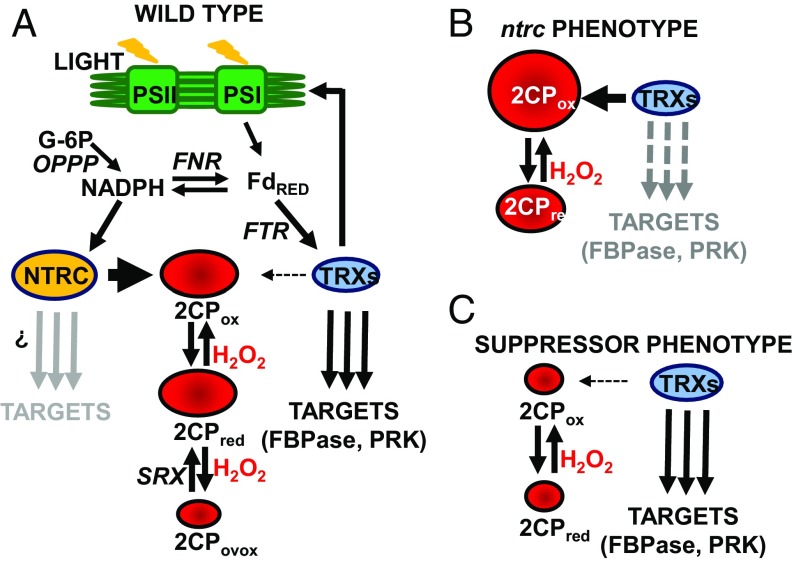
Fig. 5.
A proposed model of the control of chloroplast redox homeostasis. (A) Light-driven photochemical reactions of photosynthesis generate reduced Fd (FdRED) and NADPH by the action of Fd-NADP+ reductase (FNR). Additionally, NADPH can be produced by the oxidative pentose phosphate pathway (OPPP). Chloroplast Trxs are responsible for the light-dependent redox regulation of metabolic pathways and the feedback regulation of photochemical reactions. 2-Cys Prxs display three thiol-based redox states: oxidized (2CPox), reduced (2CPred), and overoxidized (2CPovox), which is reduced by sulfiredoxin (SRX). (B) In NTRC-deficient plants, the accumulation of oxidized 2-Cys Prxs provokes draining of reducing power from the pool of Trxs, and, consequently, redox regulation of their targets is impaired. (C) Decreasing the content of 2-Cys Prxs accordingly decreases the draining of reducing power from the pool of Trxs, and the redox regulation of their targets is thereby restored.
According to this model (Fig. 5A), NTRC maintains 2-Cys Prxs predominantly reduced in a light-independent manner in WT plants (Fig. 3 C and D). Chloroplast Trxs reduce 2-Cys Prxs (19–24) with lower efficiency than NTRC (12, 16, 28), so no or minor draining of electrons from the pool of Trxs to 2-Cys Prxs would occur in WT plants, allowing the light-dependent redox regulation of enzymes, such as those of the Calvin–Benson cycle, via the Fd-FTR-Trxs system. In NTRC-deficient plants (Fig. 5B), 2-Cys Prxs accumulate in oxidized form, as observed in leaves of the dark-adapted ntrc mutant (Fig. 3 C and D). Upon illumination, oxidized 2-Cys Prxs act as a sink of electrons in the ntrc mutant, depleting reducing power from the pool of Trxs, as shown by the light-dependent reduction of 2-Cys Prxs (Fig. 3 C and D), the impaired reduction of Trxs f (Fig. 3 A and B), and, consequently, of FBPase and PRK (Fig. 3 A and B). The deficient photosynthetic performance in the ntrc mutant (Fig. 2) shows that the NTRC-2-Cys Prxs system is also involved in feedback regulation of photochemical reactions. The mechanism of this feedback regulation is not yet clear; however, stromal Trxs are most likely involved, as suggested by the Trx f-dependent regulation of PGRL1, which transfers electrons form reduced Fd to quinones in photosynthetic cyclic electron flow (29). As the content of oxidized 2-Cys Prxs decreases in NTRC-deficient plants (Fig. 5C), withdrawal of electrons from the pool of Trxs during the dark/light transition decreases accordingly, keeping sufficiently active the Fd-FTR-Trxs system, here determined by the redox state of Trxs f, to permit the light-dependent reduction of redox-regulated enzymes such as FBPase and PRK (Fig. 3 A and B), as well as the recovery of photochemical parameters (Fig. 2).
The similar aggregation state of 2-Cys Prxs in the ntrc mutant and the ntrc-Δ2cp suppressed line (Fig. S5B) indicates that the effect of 2-Cys Prxs in NTRC-deficient plants is the result of imbalance of redox regulation rather than the possible chaperone activity of the oligomeric form of these enzymes. In addition, 2-Cys Prxs have a nonreductive stimulatory effect on chloroplast FBPase activity (30). However, the ntrc-Δ2cp and ntrc-trxf1f2-Δ2cp suppressed lines, containing lower levels of 2-Cys Prxs (Figs. 1C and 4B), showed levels of FBPase activity under oxidant and reducing conditions similar to WT (Fig. 4F), suggesting that the nonreductive effect of 2-Cys Prxs on FBPase activity is not relevant to explain the suppressor effect.
Our model is fully supported by the 2-Cys Prxs dose-dependent effect on the ntrc phenotype. The content of 2-Cys Prxs in NTRC-deficient plants would determine the extent of reducing power drainage and therefore the redox imbalance of the pool of Trxs. This is the case of the ntrc and the ntrc-2cpa mutants (Fig. 1A), which contain higher levels of 2-Cys Prxs (92% and 70% of WT level, respectively) than the ntrc-2cpaGK, ntrc-2cpb, and ntrc-Δ2cp mutants (26%, 33%, and 21% of WT level, respectively; Fig. 1C) and, to a greater extent, of the ntrc/2CPA and ntrc/2CPB transgenic lines, which contain even higher levels of 2-Cys Prxs (159–461% of ntrc level) and show a more severe phenotypic effect (Fig. S3 A and B). Moreover, overexpression of 2-Cys Prx A or B in the ntrc-Δ2cp (Fig. S3 E and F) and ntrc-trxf1f2-Δ2cp (Fig. S3 G and H) lines partially restored the growth inhibition phenotypes of ntrc and ntrc-trxf1f2, respectively. The fact that the overexpression of 2-Cys Prx A or B in NTRC-deficient plants provokes similar growth inhibition (Fig. S3 A, B, and E–H) indicates that both isoforms have indistinguishable effects.
The present results strongly suggest that the role of NTRC in chloroplast redox regulation is exerted via its functional relationship with 2-Cys Prxs, and therefore the effect of the deficiency of NTRC on plant phenotype would be indirectly produced by the redox imbalance of the pool of Trxs caused by deregulated 2-Cys Prxs. This would provide an explanation for the fact that the lack of NTRC affects such a large variety of chloroplast processes controlled by Trxs. Indeed, the redox regulation of enzymes such as FBPase, which is not reduced by NTRC (13, 27), is severely affected in mutants lacking NTRC (Figs. 3 A and B and 4 D and E and Fig. S6B). Nevertheless, it has been reported that NTRC interacts with AGPase (8), the CHLI subunit of Mg-chelatase (11, 27), and different Trxs and Calvin–Benson cycle enzymes (27, 31). Therefore, a direct effect of NTRC in redox regulation of these enzymes cannot be ruled out at the moment.
In agreement with previous reports (25, 32), mutants devoid of f-type Trxs show only a slight impairment of light-dependent FBPase or PRK reduction (Fig. 4 D and E). According to our hypothesis, in mutants lacking f-type Trxs, the redox state of FBPase and PRK remain unaltered, as NTRC is present to regulate 2-Cys Prxs (Fig. 5A). Thus, NTRC would allow other plastidial Trxs, most likely those of the m type (33), to exert a compensatory effect. The severe phenotype of the ntrc-trxf1f2 triple mutant (Fig. 4A and Fig. S7 A and B) is most probably the result of the increased depletion of reducing power from the pool of remaining Trxs provoked by the simultaneous deficiencies of NTRC and f-type Trxs, which are among the most abundant chloroplast Trxs (26). This mutant also exhibits dramatically reduced efficiency of light energy utilization, as shown by the elevated NPQ (Fig. S7C) and low ETR (Fig. 4C). Remarkably, the suppressed mutant ntrc-trxf1f2-Δ2cp recovered WT levels of CO2 fixation (Fig. S4) and a significant level of light-dependent redox regulation of FBPase and PRK (Fig. 4 D and E), in line with its growth phenotype, which resembles that of the WT (Fig. 4A and Fig. S7 A and B). However, NPQ and ETR were only partially recovered in the suppressed ntrc-trxf1f2-Δ2cp mutant (Fig. 4C and Fig. S7C), indicating that this level of efficiency of light energy utilization is sufficient to support WT growth rate.
The key function of 2-Cys Prx as a light-regulated oxidative sensor was already proposed based on the analysis of the atypical Trxs ACHT1 and ACHT4 under low light intensity (21, 23). The suppressor effect exerted by decreased levels of 2-Cys Prxs on the ntrc phenotype (Fig. 1 and Figs. S1 and S2) and, most notably, on the more severe ntrc-trxf1f2 phenotype (Fig. 4 A–E and Fig. S7), confirms the function of 2-Cys Prxs as oxidative sensor and suggests that this function affects not only the atypical Trxs ACHT1 and ACHT4 but the whole pool of chloroplast Trxs. Moreover, our results show the key function of NTRC as the major modulator of the redox balance of the 2-Cys Prxs in chloroplast redox regulation.
As 2-Cys Prxs act as H2O2 scavengers, an additional possibility to be taken into account is that the deficiency of the chloroplast redox systems analyzed here—NTRC, Trxs f, and 2-Cys Prxs—affect plant phenotype as a result of the oxidative stress caused by the accumulation of H2O2, whereas the suppressor effect might be the result of compensatory antioxidant systems. This was addressed by the analysis of nonenzymatic antioxidants (Table S1), the content of H2O2 (Fig. S8A), and the expression of genes encoding plastid-localized antioxidant enzymes (Fig. S8 B and C). The minor differences detected suggest that these parameters have very low effect, if any, on the phenotypes of the mutants analyzed here.
The redox state of chloroplast 2-Cys Prxs is maintained by the reducing activity of NTRC and, to a lesser extent, by the pool of Trxs (Fig. 5A). It should be noted, however, that reduced 2-Cys Prxs are recycled by H2O2, which is therefore an essential component of chloroplast redox regulation. Given the relevant function of the redox balance of 2-Cys Prxs, a question that arises is whether these enzymes are dispensable for plant life. It was recently shown that the Arabidopsis 2cpa-2cpb double-KO mutant showed lower photosynthetic efficiency and high sensitivity to high light (34). However, this mutant is viable, suggesting that additional backup systems operate in chloroplast redox homeostasis in the absence of 2-Cys Prxs. Although a yeast strain lacking all eight thiol peroxidases is also viable (35), clear candidates to act as backup systems are additional thiol peroxidases present in plant chloroplasts.
Materials and Methods
Detailed procedures for biological materials and growth conditions, generation of Arabidopsis transgenic plants, alkylation assays, measurements of chlorophyll fluorescence, FBPase activity assays, and CO2 fixation rates, as well as other methods, are described in SI Materials and Methods and Tables S2 and S3.
Table S2.
Arabidopsis mutants used in this study
| Mutant | Protein | KO/KD line | Reference |
| ntrc (SALK_012208) | NTRC | KO | 6 |
| 2cpa (SALK_065264) | 2-Cys Prx A | KD | 17 |
| 2cpaGK (GABI_295C05) | 2-Cys Prx A | KO | This study |
| 2cpb (SALK_017213) | 2-Cys Prx B | KO | 17 |
| ∆2cp | 2-Cys Prx A | KO | 16 |
| 2-Cys Prx B | KD | ||
| trxf1f2 | Trx f1 | KO | 25 |
| Trx f2 | KO | ||
| ntrc-2cpaGK | NTRC | KO | This study |
| 2-Cys Prx A | KO | ||
| ntrc-2cpa | NTRC | KO | This study |
| 2-Cys Prx A | KD | ||
| ntrc-2cpb | NTRC | KO | This study |
| 2-Cys Prx B | KO | ||
| ntrc-Δ2cp | NTRC | KO | This study |
| 2-Cys Prx A | KD | ||
| 2-Cys Prx B | KO | ||
| ntrc-trxf1f2 | NTRC | KO | This study |
| Trx f1 | KO | ||
| Trx f2 | KO | ||
| ntrc-trxf1f2-Δ2cp | NTRC | KO | This study |
| Trx f1 | KO | ||
| Trx f2 | KO | ||
| 2-Cys Prx A | KD | ||
| 2-Cys Prx B | KO |
KD, knockdown.
Table S3.
Arabidopsis oligonucleotides used in this study
| Gene | Locus | Sequence |
| Genotyping oligonucleotides | ||
| SALK T-DNAleft border | T-DNA | 5′-ATTTTGCCGATTTCGGAAC-3′ |
| GABI T-DNAleft border | T-DNA | 5′-ATATTGACCATCATACTCATTGC-3′ |
| ntrc (SALK_012208) | At2g41680 | 5′-TCACCAACATGTGGCCC-3′ |
| 5′-TTCTTCATCTTCACACCCGA-3′ | ||
| 2cpa (SALK_065264) | At3g11630 | 5′‐GAGAAGTTGAACACCGA‐3′ |
| 5′‐GGGGACAAAGTGAGAATC‐3′ | ||
| 2cpaGK (GABI_295C05) | At3g11630 | 5′‐CTTCCACTGGTTGGAAACAAG‐3′ |
| 5′‐AATGCCTGCAACATTGAAAAC‐3′ | ||
| 2cpb (SALK_017213) | At5g06290 | 5′‐CCACCTGAACCAAGAAAG‐3′ |
| 5′‐CCTGCAAGACAACATCAC‐3′ | ||
| trx f1 (SALK_128365) | At4g18480 | 5′-TTCATATGTGTAGCTTAGAAACCGTTAATG-3′ |
| 5′-AAGTCGACAGAGACTGGTTCATCCG-3′ | ||
| trx f2 (GK-020E05-013161) | At5g16400 | 5′-CTATTTCAACAATCGGGTCCC-3′ |
| 5′-TTCTCAATTGCCAATTCTTGC-3′ | ||
| RT-qPCR oligonucleotides | ||
| ACTIN | At3g18780 | 5′-GCACTTGCACCAAGCAGCAT-3′ |
| 5′-CCTTTCAGGTGGTGCAACGAC-3′ | ||
| AT5G25760 | At5g25760 | 5′-CTGCGACTCAGGGAATCTTCTAA-3′ |
| 5′-TTGTGCCATTGAATTGAACCC-3′ | ||
| AT4G05320 | At4G05320 | 5′-CTATTTCAACAATCGGGTCCC-3′ |
| 5′-AAAGAGATAACAGGAACGGAAACATAGT-3′ | ||
| PRX IIE | At3g52960 | 5′-GTTAGGTCAAGGAGATATGCGA-3′ |
| 5′-GAACTTTCACAACACCATCATCAG-3′ | ||
| PRX Q | At3g26060 | 5′-ATTGTTAAGCGACGAAGGGA-3′ |
| 5′-CAATGCTCCAAACAGGTCTC-3′ | ||
| 2-CYS PRX A | At3g11630 | 5′-AGGTACAATCTTTGTTATATGTGAGC-3′ |
| 5′-GACAAAGTGAGAATCAAACAAAGG-3′ | ||
| 2-CYS PRX B | At5g06290 | 5′-GCCAAAGAACCTTTGTATCTATCTC-3′ |
| 5′-TTCAATTTAAGGAGAATCACATAGCA-3′ | ||
| sAPX | At4g08390 | 5′-TCCTCCTTCACCTGCTACTC-3′ |
| 5′-TCCTTGTCATCTAATCCCATTCTG-3′ | ||
| tAPX | At1g77490 | 5′-GCCCAGATAAGCCATTACCC-3′ |
| 5′-GGAGTTGTTATTACCACCAAAGAG-3′ | ||
| GPX1 | At2g25080 | 5′-GTCAACAGTTCTAGACCTAATTCC-3′ |
| 5′-CAAGGAAGGGACGAGAAAGG-3′ | ||
| GPX7 | At4g31870 | 5′-TGCTTGCGGCTTAAATTTAGTG-3′ |
| 5′-CTGGTATACCAACAATAACCTGGA-3′ | ||
| cDNA cloning oligonucleotides | ||
| Gene | Plasmid | Sequence |
| 2-Cys Prx A | pDONR-207 | 5′-AAAAAGCAGGCTtcATGGCGTCTGTTGCTTCT-3′ |
| 5′-AGAAAGCTGGGTcCTAAATAGCTGAGAAGTACTC-3′ | ||
| 2-Cys Prx B | pDONR-207 | 5′-AAAAAGCAGGCTtcATGTCAATGGCGTCTATA-3′ |
| 5′-AGAAAGCTGGGTcCTAGATAGCTGAAAAGTATTC-3′ | ||
Additional nucleotides included to maintain the proper reading frame are underlined.
SI Materials and Methods
Biological Material and Growth Conditions.
Arabidopsis WT (ecotype Columbia) and mutant plants were routinely grown in soil in growth chambers under long-day (16-h light/8-h darkness) or short-day (8-h light/16-h darkness) at 22 °C and 20 °C during light and dark periods, respectively, and light intensity of 125 µE m−2⋅s−1. In vitro culture experiments were performed on Murashige and Skoog medium containing 0.35% Gelrite (Duchefa) and 0.5% (wt/vol) sucrose. Arabidopsis mutants ntrc, 2cpa, 2cpb, ∆2cp, and trxf1f2 were described previously (6, 16, 17, 25). The line 2cpaGK (GABI_295C05) was obtained from the Nottingham Arabidopsis Stock Centre. Multiple mutants (Table S2) were obtained by manual crossing and identified by PCR analysis of genomic DNA with oligonucleotides listed in Table S3. Escherichia coli and Agrobacterium tumefaciens were grown in liquid Miller nutrient at 37 °C and 28 °C, respectively, with the appropriate antibiotic agents.
Generation of Arabidopsis Transgenic Plants Overexpressing 2-Cys Prx A and B.
Total RNA was isolated with TRIzol reagent (Invitrogen), and cDNA was synthesized with the Maxima first-strand cDNA synthesis kit (Fermentas). The 2-Cys Prx A and B cDNAs, including the stop codons, were amplified with iProof High-Fidelity DNA Polymerase (Bio-Rad) using oligonucleotides specified in Table S3, which added attB recombination sites at the 5′ and 3′ ends, respectively. PCR products were cloned in the Gateway-compatible vector pDONR207 (Invitrogen), sequenced, and transferred to the 35S overexpression vector pEARLEYGATE100 using LR clonase (Invitrogen). Plasmids were then transformed into the A. tumefaciens strain GV301 and used for plant transformation (36).
Protein Extraction, Alkylation Assays, and Western Blot Analysis.
Plant tissues were ground under liquid nitrogen. For SDS/PAGE, extraction buffer [50 mM Tris⋅HCl, pH 8.0, 0.15 M NaCl, 0.5% (vol/vol) Nonidet P-40] was immediately added, mixed on a vortex, and centrifuged at 16,100 × g at 4 °C for 20 min. For native gel electrophoresis, extraction buffer was supplemented with 1% (vol/vol) digitonin, and samples were incubated on ice for 15 min before centrifugation. Protein was quantified by using the Bradford reagent (Bio-Rad). Alkylation assays were performed as previously described (25) using MM(PEG)24 (Thermo Scientific). Protein samples were subjected to native PAGE (4–16%) or SDS/PAGE under reducing or nonreducing conditions using acrylamide gel concentration of 9.5% (FBPase and PRK), 12% (2-Cys Prxs and NTRC), and 14% (2-Cys Prxs in alkylation assays and Trxs f). Resolved proteins were transferred to nitrocellulose membranes and probed with the indicated antibody. Specific antibodies for NTRC, 2-Cys Prx, and Trx f were previously raised in our laboratory (6, 14, 25). PRK antibody was purchased from Agrisera. The anti-FBPase antibody was provided by M. Sahrawy, Estación Experimental del Zaidín, Granada, Spain.
Determination of Chlorophylls and Measurements of Chlorophyll a Fluorescence.
Chlorophyll levels were measured as previously described (14). Chlorophyll a fluorescence was measured by using a pulse-amplitude modulation fluorometer (DUAL-PAM-100; Walz). Measurements of relative linear ETRs, ETR(II), were based on chlorophyll fluorescence of preilluminated plants applying stepwise increasing actinic light intensities of as much as 2,000 μmol E m−2⋅s−1. The maximum PSII quantum yield, determined as Fv/Fm ratio, and Y(NPQ) were determined during an induction/recovery curve in dark-adapted leaves as previously reported (18, 25), applying 75 μE m−2⋅s−1 of actinic light during the induction period.
Determination of the Rate of CO2 Fixation and FBPase Activity.
CO2 fixation (AN) was determined by the Service for Photosynthesis, Instituto de Recursos Naturales y Agrobiología, Seville, Spain. Leaves from plants grown under long-day conditions for 4 wk were dark-adapted and then illuminated with PAR of 125 μE m−2⋅s−1 until the rate of CO2 assimilation, determined with an open gas exchange system (Li-6400; LI-COR) equipped with the chamber head (Li-6400–40), was stabilized. FBPase activity was determined on leaf extracts from plants grown under long-day conditions for 4 wk and harvested at 12 h of the day period using the coupled assay with glucose 6-phosphate dehydrogenase (G6PDH) and phosphoglucose isomerase (PGI). Briefly, frozen leaf powder (75–130 mg) was extracted with extraction buffer [50 mM Tris acetate, pH 7.9, 100 mM potassium acetate, 1 mM EDTA, 20% (vol/vol) glycerol], mixed on a vortex, and centrifuged at 16,100 × g at 4 °C for 20 min. Protein content was determined by using the Bradford reagent (Bio-Rad). For determining the contribution of redox-regulated isoforms to maximal FBPase activity, 20 µL of the supernatant (75–135 µg of protein) was incubated for 10 min in the presence of oxidizing (100 µM CuCl2) or reducing (10 mM DTT) reagents and added to 180 µL of FBPase activity buffer, final reaction mix containing 100 mM Tris, pH 8.0, 2 mM MgCl2, 0.3 mM NADP+, 4 mM d-fructose 1,6-bisphosphate, 0.28 U of G6PDH, and 0.7 U PGI. The formation of NADPH was monitored spectrophotometrically at 340 nm for 8 min.
Determination of H2O2 and Nonenzymatic Antioxidants.
H2O2 was determined as previously reported (14) on whole rosettes of long-day grown plants. GSH and GSSG were determined by reverse-phase HPLC (37), and AsA and DHA by the iron (III) reduction method (38), on whole rosettes of short-day grown plants.
Quantitative RT-PCR Analysis.
Gene expression analyses were performed using an IQ5 system (Bio-Rad) with oligonucleotides listed in Table S3 and a standard thermal profile (95 °C, 3 min; 40 cycles at 95 °C for 10 s and 60 °C for 30 s). After the PCR, a melting curve analysis (55–94 °C at 0.5 °C for 30 s) was performed to confirm the specificity of the amplicon. Relative contents of transcripts were normalized by using AT4G05320, AT5G25760 (39), and ACTIN as reference genes.
Supplementary Material
Acknowledgments
This work was supported by European Regional Development Fund-cofinanced Grant BIO2013-43556-P from the Spanish Ministry of Innovation and Competitiveness (MINECO) and predoctoral fellowships from MINECO (to B.N. and V.O.). The anti-FBPase antibody was provided by Dr. M. Sahrawy, Estación Experimental del Zaidín, Granada, Spain.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706003114/-/DCSupplemental.
References
- 1.Schmitt FJ, et al. Reactive oxygen species: Re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim Biophys Acta. 2014;1837:835–848. doi: 10.1016/j.bbabio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan BB, Holmgren A, Jacquot JP, Scheibe R. Fifty years in the thioredoxin field and a bountiful harvest. Biochim Biophys Acta. 2012;1820:1822–1829. doi: 10.1016/j.bbagen.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Michelet L, et al. Redox regulation of the Calvin-Benson cycle: Something old, something new. Front Plant Sci. 2013;4:470. doi: 10.3389/fpls.2013.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrato AJ, Fernández-Trijueque J, Barajas-López JD, Chueca A, Sahrawy M. Plastid thioredoxins: A “one-for-all” redox-signaling system in plants. Front Plant Sci. 2013;4:463. doi: 10.3389/fpls.2013.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schürmann P, Buchanan BB. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid Redox Signal. 2008;10:1235–1274. doi: 10.1089/ars.2007.1931. [DOI] [PubMed] [Google Scholar]
- 6.Serrato AJ, Pérez-Ruiz JM, Spínola MC, Cejudo FJ. A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana. J Biol Chem. 2004;279:43821–43827. doi: 10.1074/jbc.M404696200. [DOI] [PubMed] [Google Scholar]
- 7.Kirchsteiger K, Ferrández J, Pascual MB, González M, Cejudo FJ. NADPH thioredoxin reductase C is localized in plastids of photosynthetic and nonphotosynthetic tissues and is involved in lateral root formation in Arabidopsis. Plant Cell. 2012;24:1534–1548. doi: 10.1105/tpc.111.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P. NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci USA. 2009;106:9908–9913. doi: 10.1073/pnas.0903559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepistö A, et al. Deletion of chloroplast NADPH-dependent thioredoxin reductase results in inability to regulate starch synthesis and causes stunted growth under short-day photoperiods. J Exp Bot. 2013;64:3843–3854. doi: 10.1093/jxb/ert216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter AS, et al. Posttranslational influence of NADPH-dependent thioredoxin reductase C on enzymes in tetrapyrrole synthesis. Plant Physiol. 2013;162:63–73. doi: 10.1104/pp.113.217141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Ruiz JM, Guinea M, Puerto-Galán L, Cejudo FJ. NADPH thioredoxin reductase C is involved in redox regulation of the Mg-chelatase I subunit in Arabidopsis thaliana chloroplasts. Mol Plant. 2014;7:1252–1255. doi: 10.1093/mp/ssu032. [DOI] [PubMed] [Google Scholar]
- 12.Thormählen I, et al. Thioredoxin f1 and NADPH-dependent thioredoxin reductase C have overlapping functions in regulating photosynthetic metabolism and plant growth in response to varying light conditions. Plant Physiol. 2015;169:1766–1786. doi: 10.1104/pp.15.01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojeda V, et al. NADPH thioredoxin reductase C and thioredoxins act concertedly in seedling development. Plant Physiol. 2017;174:1436–1448. doi: 10.1104/pp.17.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Ruiz JM, et al. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell. 2006;18:2356–2368. doi: 10.1105/tpc.106.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins A, Nelson KJ, Parsonage D, Poole LB, Karplus PA. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci. 2015;40:435–445. doi: 10.1016/j.tibs.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulido P, et al. Functional analysis of the pathways for 2-Cys peroxiredoxin reduction in Arabidopsis thaliana chloroplasts. J Exp Bot. 2010;61:4043–4054. doi: 10.1093/jxb/erq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchsteiger K, Pulido P, González M, Cejudo FJ. NADPH thioredoxin reductase C controls the redox status of chloroplast 2-Cys peroxiredoxins in Arabidopsis thaliana. Mol Plant. 2009;2:298–307. doi: 10.1093/mp/ssn082. [DOI] [PubMed] [Google Scholar]
- 18.Naranjo B, et al. The chloroplast NADPH thioredoxin reductase C, NTRC, controls non-photochemical quenching of light energy and photosynthetic electron transport in Arabidopsis. Plant Cell Environ. 2016;39:804–822. doi: 10.1111/pce.12652. [DOI] [PubMed] [Google Scholar]
- 19.Broin M, Cuiné S, Eymery F, Rey P. The plastidic 2-cysteine peroxiredoxin is a target for a thioredoxin involved in the protection of the photosynthetic apparatus against oxidative damage. Plant Cell. 2002;14:1417–1432. doi: 10.1105/tpc.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collin V, et al. The Arabidopsis plastidial thioredoxins: New functions and new insights into specificity. J Biol Chem. 2003;278:23747–23752. doi: 10.1074/jbc.M302077200. [DOI] [PubMed] [Google Scholar]
- 21.Dangoor I, Peled-Zehavi H, Wittenberg G, Danon A. A chloroplast light-regulated oxidative sensor for moderate light intensity in Arabidopsis. Plant Cell. 2012;24:1894–1906. doi: 10.1105/tpc.112.097139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng F, et al. Chloroplastic thioredoxin-f and thioredoxin-m1/4 play important roles in brassinosteroids-induced changes in CO2 assimilation and cellular redox homeostasis in tomato. J Exp Bot. 2014;65:4335–4347. doi: 10.1093/jxb/eru207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eliyahu E, Rog I, Inbal D, Danon A. ACHT4-driven oxidation of APS1 attenuates starch synthesis under low light intensity in Arabidopsis plants. Proc Natl Acad Sci USA. 2015;112:12876–12881. doi: 10.1073/pnas.1515513112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochmal AK, et al. Calredoxin represents a novel type of calcium-dependent sensor-responder connected to redox regulation in the chloroplast. Nat Commun. 2016;7:11847. doi: 10.1038/ncomms11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naranjo B, Diaz-Espejo A, Lindahl M, Cejudo FJ. Type-f thioredoxins have a role in the short-term activation of carbon metabolism and their loss affects growth under short-day conditions in Arabidopsis thaliana. J Exp Bot. 2016;67:1951–1964. doi: 10.1093/jxb/erw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietz KJ. Peroxiredoxins in plants and cyanobacteria. Antioxid Redox Signal. 2011;15:1129–1159. doi: 10.1089/ars.2010.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida K, Hisabori T. Two distinct redox cascades cooperatively regulate chloroplast functions and sustain plant viability. Proc Natl Acad Sci USA. 2016;113:E3967–E3976. doi: 10.1073/pnas.1604101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthuramalingam M, et al. Multiple redox and non-redox interactions define 2-Cys peroxiredoxin as a regulatory hub in the chloroplast. Mol Plant. 2009;2:1273–1288. doi: 10.1093/mp/ssp089. [DOI] [PubMed] [Google Scholar]
- 29.Hertle AP, et al. PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol Cell. 2013;49:511–523. doi: 10.1016/j.molcel.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Caporaletti D, et al. Non-reductive modulation of chloroplast fructose-1,6-bisphosphatase by 2-Cys peroxiredoxin. Biochem Biophys Res Commun. 2007;355:722–727. doi: 10.1016/j.bbrc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Nikkanen L, Toivola J, Rintamäki E. Crosstalk between chloroplast thioredoxin systems in regulation of photosynthesis. Plant Cell Environ. 2016;39:1691–1705. doi: 10.1111/pce.12718. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida K, Hara S, Hisabori T. Thioredoxin selectivity for thiol-based redox regulation of target proteins in chloroplasts. J Biol Chem. 2015;290:14278–14288. doi: 10.1074/jbc.M115.647545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okegawa Y, Motohashi K. Chloroplastic thioredoxin m functions as a major regulator of Calvin cycle enzymes during photosynthesis in vivo. Plant J. 2015;84:900–913. doi: 10.1111/tpj.13049. [DOI] [PubMed] [Google Scholar]
- 34.Awad J, et al. 2-cysteine peroxiredoxins and thylakoid ascorbate peroxidase create a water-water cycle that is essential to protect the photosynthetic apparatus under high light stress conditions. Plant Physiol. 2015;167:1592–1603. doi: 10.1104/pp.114.255356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fomenko DE, et al. Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proc Natl Acad Sci USA. 2011;108:2729–2734. doi: 10.1073/pnas.1010721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 37.Dominguez-Solís JR, Gutierrez-Alcalá G, Vega JM, Romero LC, Gotor C. The cytosolic O-acetylserine(thiol)lyase gene is regulated by heavy metals and can function in cadmium tolerance. J Biol Chem. 2001;276:9297–9302. doi: 10.1074/jbc.M009574200. [DOI] [PubMed] [Google Scholar]
- 38.Kampfenkel K, Van Montagu M, Inzé D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem. 1995;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- 39.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]