Significance
Insulin resistance is a metabolic disorder in which target cells fail to respond to physiological levels of circulating insulin, leading to hyperinsulinemia and glucose intolerance. The molecular mechanism underlying insulin resistance is still largely unknown. Here, we found that intracellular Ca2+ overloading in obesity attenuates insulin-stimulated phosphorylation of protein kinase B and its downstream signaling by preventing membrane localization of various pleckstrin homology (PH) domains. When at high intracellular levels, Ca2+ binds tightly with phosphoinositides to yield Ca2+-phosphoinositides (PIPs), abrogating the membrane targeting of PH domains and disrupting insulin signaling. Thus, we identified a previously unknown physiological function of intracellular Ca2+ as a critical negative regulator of insulin signaling, especially through the formation of Ca2+-PIPs.
Keywords: membrane localization, PH domain, Ca2+-phosphoinositides, intracellular Ca2+ concentration, insulin resistance
Abstract
Insulin resistance, a key etiological factor in metabolic syndrome, is closely linked to ectopic lipid accumulation and increased intracellular Ca2+ concentrations in muscle and liver. However, the mechanism by which dysregulated intracellular Ca2+ homeostasis causes insulin resistance remains elusive. Here, we show that increased intracellular Ca2+ acts as a negative regulator of insulin signaling. Chronic intracellular Ca2+ overload in hepatocytes during obesity and hyperlipidemia attenuates the phosphorylation of protein kinase B (Akt) and its key downstream signaling molecules by inhibiting membrane localization of pleckstrin homology (PH) domains. Pharmacological approaches showed that elevated intracellular Ca2+ inhibits insulin-stimulated Akt phosphorylation and abrogates membrane localization of various PH domain proteins such as phospholipase Cδ and insulin receptor substrate 1, suggesting a common mechanism inhibiting the membrane targeting of PH domains. PH domain-lipid overlay assays confirmed that Ca2+ abolishes the binding of various PH domains to phosphoinositides (PIPs) with two adjacent phosphate groups, such as PI(3,4)P2, PI(4,5)P2, and PI(3,4,5)P3. Finally, thermodynamic analysis of the binding interaction showed that Ca2+-mediated inhibition of targeting PH domains to the membrane resulted from the tight binding of Ca2+ rather than PH domains to PIPs forming Ca2+-PIPs. Thus, Ca2+-PIPs prevent the recognition of PIPs by PH domains, potentially due to electrostatic repulsion between positively charged side chains in PH domains and the Ca2+-PIPs. Our findings provide a mechanistic link between intracellular Ca2+ dysregulation and Akt inactivation in insulin resistance.
Insulin resistance is a systemic metabolic disorder that manifests as decreased insulin-stimulated glucose transport and metabolism in adipocytes and skeletal muscles and as impaired suppression of hepatic gluconeogenesis (1–3). These functional defects may result from impaired insulin signaling in the peripheral tissues. Although the underlying molecular mechanisms of these signaling defects are not completely understood, the dysregulation of Ca2+ homeostasis in intracellular organelles such as cytosol, endoplasmic reticulum (ER), and mitochondria has emerged as a key pathophysiological event in insulin resistance, obesity, and type 2 diabetes (3–10). In animal models of obesity and insulin resistance, saturated fatty acids have been shown to inhibit the ER calcium importer, the sarco/ER calcium pump, which subsequently leads to elevated cytoplasmic Ca2+ levels (7, 9–11). Chronically elevated intracellular Ca2+ has extreme negative effects on the functions of subcellular organelles such as the ER and mitochondria, leading to impaired metabolic homeostasis (5, 9, 10). In contrast, interventions that block Ca2+ entry into cells not only improved insulin sensitivity and glucose homeostasis in obese subjects and diabetic patients (12, 13), but also restored autophagy (4, 9) and insulin sensitivity in obese mouse models (14). However, the molecular mechanisms that link intracellular Ca2+ overload to insulin resistance have not been completely elucidated.
Insulin-stimulated phosphoinositide 3-kinase (PI3K) catalyzes the phosphorylation of phosphoinositides (PIPs) at the 3-position to produce PI(3,4)P2 or PI(3,4,5)P3, which recruit a variety of signaling proteins with pleckstrin homology (PH) domains, including phosphoinositide-dependent kinase 1 (PDK1) and protein kinase B (Akt) (1, 15). In turn, Akt acts as a key merge point of the PI(3,4,5)P3-mediated insulin signaling system by phosphorylating the enzyme glycogen synthase kinase 3 beta (GSK3β), the forkhead transcription factors, the 160-kDa substrate of Akt (AS160), and cAMP response element-binding protein (CREB) (1). The activity of the insulin signaling pathway is transiently attenuated by dephosphorylation of PI(3,4,5)P3 via phosphoinositide phosphatases such as PTEN and SHIP2, altering its binding specificity and affinity to PH domains (15). Thus, the binding of PH domains to PI(3,4,5)P3 has a critical role in regulating Akt function (16). Aside from enzymatic dephosphorylation of PI(3,4,5)P3 by phosphoinositide phosphatases, however, other regulatory mechanisms of the binding of PH domains to PI(3,4,5)P3 have not been reported.
PH domains are small protein modules that occur in a large variety of ∼250 proteins, including Akt/Rac family serine/threonine kinases, Btk/Itk/Tec subfamily tyrosine kinases, phosphoinositide-specific phospholipase C (PLC), the Rho family of GTPases, insulin receptor substrates (IRSs), and cytoskeletal proteins (17), suggesting their broad and important roles in cell signaling and regulation. PH domains play essential roles in recruiting proteins to the plasma membrane by binding to their phosphoinositides with a broad range of specificity and affinity. PH domains of Akt, Bruton’s tyrosine kinase (BTK), and general receptor for phosphoinositides-1 (GRP1) are known to recognize highly specific PI3K products of PI(3,4)P2 and PI(3,4,5)P3 (17). Mutations disrupting PH domain function that abolish PI(3,4,5)P3 binding cause severe signaling defects such as X-linked agammaglobulinemia in humans and X-linked immunodeficiency in mice (18, 19). In contrast, mutations that promote constitutive membrane localization of Akt PH domains at the plasma membrane can cause cancer (20). These findings imply that membrane targeting of PH domains through PI(3,4,5)P3 recognition is essential for Akt activity.
In this study, we provide evidence that phosphoinositides tightly bind with Ca2+, forming Ca2+-PIPs under obesity-associated intracellular Ca2+ overload. These Ca2+-PIPs prevent membrane recruitment of PH domains by inhibiting their binding to PI(3,4,5)P3, leading to abnormal subcellular localization of PH domains. Our results demonstrate a molecular mechanism of Ca2+-mediated inhibition of the recruitment of various PH domain-containing molecules to the plasma membrane, providing insights into diseases associated with abnormal subcellular localization of signaling proteins.
Results
High-Fat Diets and Palmitate Treatment Increase Intracellular Ca2+ Levels and Attenuate Insulin Signaling.
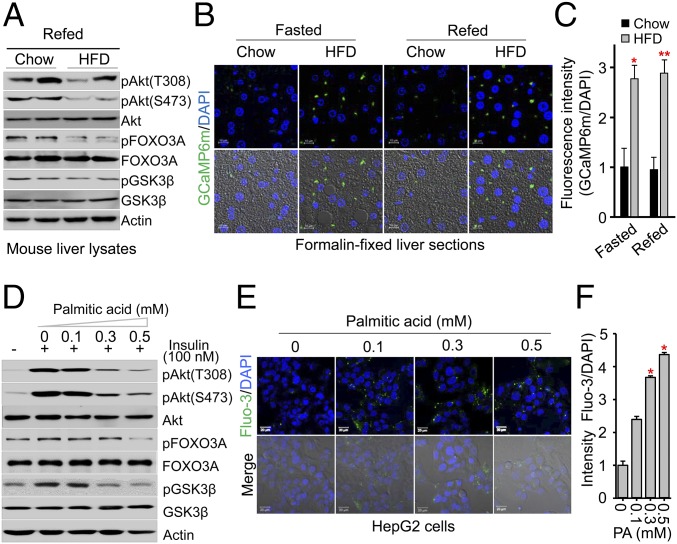
To investigate the molecular mechanisms of insulin resistance, we fed mice a high-fat diet (HFD) or normal chow for 8 wk, then fasted the mice overnight and subsequently refed them with normal chow or a HFD for 4 h. We then analyzed the effects of a HFD on the phosphorylation of key insulin signaling molecules, Akt, and its downstream signaling molecules GSK3β and FOXO3. Interestingly, postprandial phosphorylation of Akt at T308 and S473 and the phosphorylation of GSK3β and FOXO3 were dramatically decreased in mice livers after refeeding with a HFD (Fig. 1A), suggesting that insulin signaling was impaired in the livers of mice fed a HFD for 8 wk. Based on recent findings that dysregulation of intracellular Ca2+ plays an important role in insulin resistance (5, 9, 10, 21), we next analyzed in vivo levels of intracellular Ca2+ in the liver of mice fed a HFD or normal chow diet for 10 wk using adenoviral vectors to express calmodulin-based genetically encoded fluorescent calcium indicators (GCaMP6m) (22). This method results in robust expression of adenoviral GCaMP6m in the hepatocytes of mice fed a HFD (Fig. 1B), where we observed that the hepatocytes expressing GCaMP6m were significantly elevated in the livers of HFD-fed mice compared with controls (Fig. 1B). Quantification of fluorescent signals showed that the intracellular Ca2+ level was almost threefold higher in the hepatocytes of HFD-fed mice than in control mice regardless of feeding status (Fig. 1C), demonstrating that intracellular Ca2+ was highly elevated in the hepatocytes of HFD-fed mice.
Fig. 1.
High-fat diet (HFD) and palmitate treatment increase intracellular Ca2+ levels and attenuate insulin signaling. (A) Immunoblot analysis of mouse liver extracts after overnight fasting and subsequent refeeding with normal chow or a HFD for 4 h. (B) Representative confocal images of cytosolic free Ca2+ in the hepatocytes expressing adenoviral GCaMP6m from mice fed normal chow or a HFD for 10 wk following 7 d of adenoviral infection. Ex vivo hepatocytes expressing adenoviral GCaMP6m were visualized using confocal microscopy from formalin-fixed liver sections of mice following overnight fasting and subsequent refeeding with normal chow or a HFD for 4 h. (Scale bars: 10 μm.) (Bottom) Images merged with 4,6-diamidino-2-phenylindole (DAPI) staining of nuclei and differential interference contrast (DIC) microscopy. (C and F) Fluorescence intensities of GCaMP6m images (C) and Fluo-3 AM images (F) of cytosolic Ca2+ were quantified with low power field images using ImageJ software. Data represent means ± SEM (n = 3–5, *P < 0.05, **P < 0.01). (D) Immunoblot analysis of HepG2 cells treated with the indicated concentrations of palmitic acid for 24 h followed by treatment with 100 nM insulin for 15 min. (E) Representative Fluo-3 AM images of cytosolic Ca2+ in HepG2 cells treated with the indicated concentrations of palmitic acid for 24 h. Intracellular Ca2+ visualized using confocal microscopy. (Scale bars: 10 μm.)
To assess whether impaired insulin signaling in mice fed a HFD is associated with increased intracellular Ca2+ levels, we treated human HepG2 hepatoma cells for 24 h with palmitic acid, a long-chain saturated fatty acid that causes insulin resistance in animals (23). Similar to our in vivo findings, palmitic acid treatment markedly attenuated the insulin-stimulated phosphorylation of Akt at T308 and S473 and the phosphorylation of GSK3β and FOXO3 in a dose-dependent manner (Fig. 1D and SI Appendix, Fig. S1), indicating that palmitic acid impairs insulin signaling in vitro. Next, we examined the effects of palmitic acid on intracellular Ca2+ levels in HepG2 cells using the fluorescent dye Fluo-3 acetoxymethyl (AM). We found that palmitic acid significantly elevated intracellular Ca2+ levels in the HepG2 cells in a dose-dependent manner (Fig. 1E), showing that intracellular Ca2+ levels were almost threefold higher in HepG2 cells treated with palmitic acid (Fig. 1F). These results indicated that exposure to a HFD elevated palmitic acid levels, leading to increased intracellular Ca2+ levels, suggesting a mechanism responsible for impaired insulin signaling. To further assess the importance of intracellular Ca2+ overload, we measured intracellular Ca2+ concentrations in HepG2 cells with Fura-2 AM after palmitic acid treatment for 24 h. Treatment with high concentrations of palmitic acid significantly elevated the baseline intracellular Ca2+ concentrations (approximately threefold, SI Appendix, Fig. S2A). Strikingly, high concentrations of palmitic acid led to irregular patterns of sustained intracellular Ca2+ overload in HepG2 cells (SI Appendix, Fig. S2B). Thus, we hypothesized that the attenuation of insulin-stimulated Akt phosphorylation by a HFD is potentially driven by elevated intracellular Ca2+ levels.
Akt Phosphorylation Is Modulated by Intracellular Ca2+ Concentration.
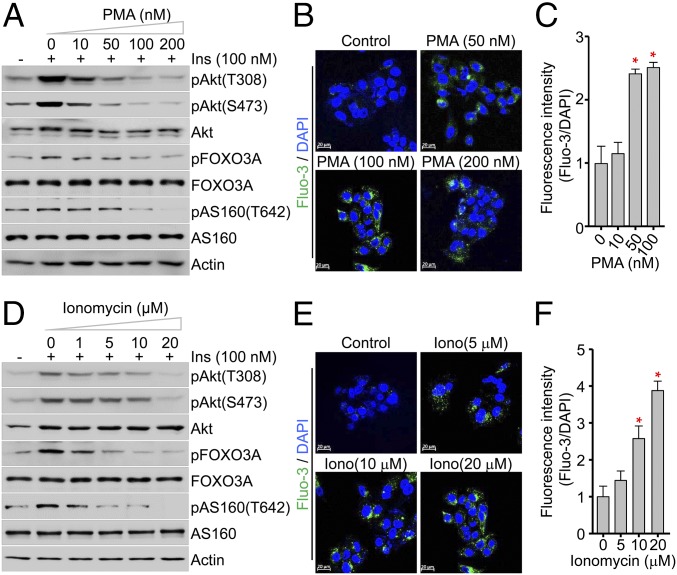
To investigate the direct effects of elevated intracellular Ca2+ on Akt phosphorylation, we evaluated the effects of phorbol myristate acetate (PMA) and ionomycin on Akt phosphorylation, both of which are used to trigger intracellular calcium influx. After pretreating the HepG2 cells with PMA or ionomycin for 30 min to induce sustained intracellular Ca2+ overload, we examined insulin-stimulated phosphorylation of Akt after stimulating with insulin (100 nM) for 15 min. Immunoblotting clearly showed that PMA dramatically inhibited insulin-stimulated phosphorylation of Akt at T308 and S473 and its substrates AS160 and FOXO3 in a dose-dependent manner (Fig. 2A and SI Appendix, Fig. S3). Confocal images using the fluorescent dye Fluo-3 AM showed that PMA treatment dramatically increased the levels of intracellular free Ca2+, which distribution was distinct from that of mitochondria (SI Appendix, Fig. S4). This increase was almost fivefold higher compared with controls, indicating that elevated intracellular Ca2+ is highly correlated with decreased phosphorylation of Akt and its key downstream signaling proteins (Fig. 2 B and C). Consistently, ionomycin also significantly decreased insulin-stimulated phosphorylation of Akt, AS160, and FOXO3 (Fig. 2D and SI Appendix, Fig. S5). Indeed, the levels of intracellular Ca2+ in HepG2 cells treated with ionomycin were markedly higher than in control cells (Fig. 2 E and F), suggesting that decreased phosphorylation of Akt is driven by sustained intracellular Ca2+ overload. Together, these results indicated that intracellular Ca2+ overload attenuates insulin signaling in HepG2 cells, as well as other cells such as Chinese hamster ovary-insulin receptor (CHO-IR) cells (SI Appendix, Fig. S6).
Fig. 2.
The catalytic activity of Akt is modulated by intracellular Ca2+ concentration. (A and D) Immunoblot analysis of the phosphorylation states of Akt, FOXO3A, and AS160, and the total amounts of the indicated proteins in HepG2 cells. Cells were incubated for 30 min with the indicated concentrations of PMA (A) or ionomycin (D), followed by treatment with 100 nM insulin for 15 min. (B, C, E, and F) Representative Fluo-3 AM images (B) and quantification (C) of intracellular Ca2+ in HepG2 cells treated with PMA. Data represent means ± SEM (n = 5, *P < 0.05).
Consistent with a recent study showing that verapamil, a Ca2+ channel blocker that inhibits calcium entry into intracellular stores, improves hepatic steatosis in mice fed a HFD (9), we found that pretreatment with verapamil increased the sensitivity of insulin-stimulated phosphorylation of Akt at T308 and S473 after 15 min of insulin (10 nM) treatment (SI Appendix, Fig. S7A). Indeed, treatment with verapamil substantially reversed palmitic acid-induced decreases in Akt phosphorylation at T308 and S473 (SI Appendix, Fig. S7 B and C), suggesting that a calcium channel blocker might reverse or improve impaired insulin signaling.
High Intracellular Ca2+ Concentration Prevents Membrane Localization of PH Domains.
Given that intracellular Ca2+ concentration in cells transiently increases up to 10−4 M (24) and high intracellular Ca2+ concentration inhibits the phosphorylation of Akt and its downstream signaling proteins, we asked whether intracellular Ca2+ leaks can modulate the subcellular localization of PH domains required for kinase activity. For the experiment, we selected two different PH domains, Akt-PH and PLCδ-PH. To examine the effects of intracellular Ca2+ on the membrane localization of these two PH domains, we transiently expressed Akt-PH domain mCherry (Akt-PH mCherry) or PLCδ-PH domain GFP (PLCδ-PH GFP) fusion proteins in CHO cells that stably express the IR (CHO-IR cells) (25).
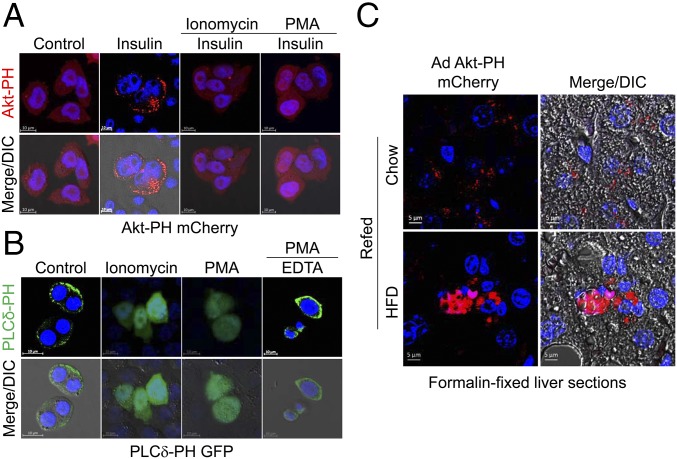
The Akt-PH domain recognizes the highly specific PI3K products of PI(3,4)P2 and PI(3,4,5)P3, which are generated transiently upon activation of almost all surface receptors such as insulin and growth factors (1). We treated CHO-IR cells with or without insulin (100 nM). The localization of Akt-PH mCherry was primarily cytoplasmic in unstimulated cells (Fig. 3A). After stimulation with insulin, Akt-PH mCherry was preferentially localized to the plasma membrane (Fig. 3A). In contrast, pretreatment with PMA/ionomycin inhibited insulin-stimulated membrane recruitment of Akt-PH mCherry (Fig. 3A). Similarly, pretreatment with ionomycin led to the inhibition of insulin-stimulated membrane localization of endogenous Akt in CHO-IR cells (SI Appendix, Fig. S8A), suggesting that intracellular Ca2+ overload prevents membrane translocation of Akt, potentially by inhibiting PH domain interactions with PI(3,4)P2 or PI(3,4,5)P3.
Fig. 3.
Higher intracellular Ca2+ concentrations prevent membrane localization of PH domain proteins. (A) Fluorescence images of Akt-PH mCherry. CHO-IR cells were transfected with Akt PH domain-mCherry fusion vector, serum starved for 3 h, and treated with or without ionomycin(10 μM)/PMA (100 nM) for 30 min before a 15-min stimulation with 100 nM insulin. (B) Fluorescence images of PLCδ-PH GFP. CHO-IR cells were transfected with PLCδ-PH GFP fusion vector, followed by incubation with 10 μM ionomycin or 100 nM PMA for 15 min. After 100 nM PMA for 15 min treatment, the cells were incubated with 1 mM EDTA for 5 min to chelate Ca2+. (Scale bars: 10 μm.) (C) Representative fluorescence images of adenoviral Akt-PH mCherry from mice fed normal chow or a HFD for 10 wk following 7 d of adenoviral infection. Ex vivo hepatocytes expressing adenoviral Akt-PH mCherry were visualized using confocal microscopy from formalin-fixed liver sections of mice following overnight fasting and subsequent refeeding with normal chow or a HFD for 4 h. (Scale bars: 5 μm.)
Because the PLCδ-PH domain recognizes PI(4,5)P2, which is present at 10- to 20-fold higher levels than those of PI3K-dependent products PI(3,4)P2 and PI(3,4,5)P3 (17), we monitored the subcellular localization of PLCδ-PH GFP in CHO-IR cells. As shown in Fig. 3C, PLCδ-PH GFP was localized to the plasma membrane when transiently expressed in CHO-IR cells. However, PLCδ-PH GFP was rapidly moved from the plasma membrane to the cytosol after simulation with ionomycin or PMA (Fig. 3C), which was consistent with a previous study (26). Interestingly, the inhibitory effects of PMA on membrane localization of PLCδ-PH GFP was completely reversed by subsequent chelation of intracellular Ca2+ by EDTA (Fig. 3C), implying that higher intracellular Ca2+ is a negative regulator for membrane targeting of PH domains. Next, we also monitored the subcellular localization of endogenous IRS1 protein containing a PH domain with broad substrate specificity in CHO-IR cells pretreated with ionomycin before insulin stimulation. Again, pretreatment with PMA/ionomycin completely blocked insulin-stimulated membrane localization of endogenous IRS1 protein (SI Appendix, Fig. S8B), indicating that intracellular Ca2+ overload prevents membrane translocation of various PH domains by a common mechanism, potentially inhibiting interactions with PIPs.
Finally, to investigate whether physiological elevation of intracellular Ca2+ in mice fed a HFD inhibits membrane localization of the Akt-PH domain, we examined the subcellular localization of Akt-PH domains in mice fed normal chow or a HFD using adenovirus-mediated overexpression of Akt-PH mCherry. Adenoviral Akt-PH mCherry was mostly localized to the plasma membrane in the hepatocytes of normal chow-fed mice in response to refeeding (insulin stimulation). Concurrent with the increased intracellular Ca2+ levels in mice fed a HFD (Fig. 1 B and C), however, adenoviral Akt-PH mCherry did not translocate to the plasma membrane in the hepatocytes of HFD-fed mice (Fig. 3C). This provides direct evidence for the inhibition of PH domain localization to the plasma membrane via physiological elevation of intracellular Ca2+ in HFD-fed mice. Taken together, these results demonstrate that sustained intracellular Ca2+ overload in mice fed a HFD prevents membrane localization of Akt in vivo by inhibiting membrane localization of the PH domain.
Ca2+ Inhibits the Binding of PH Domains to PIPs with Two Adjacent Phosphate Groups.
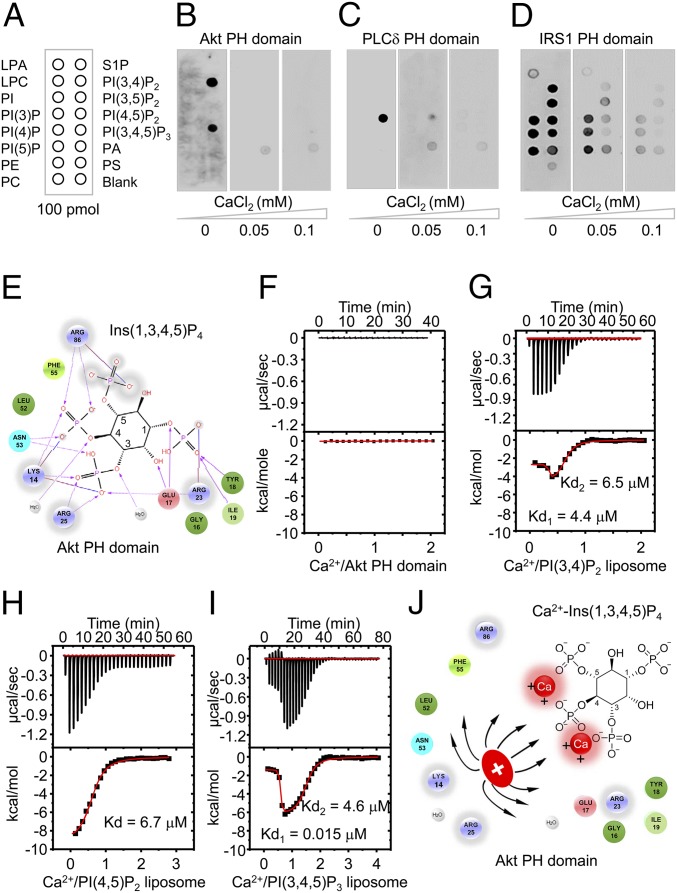
The PH domains of Akt, BTK, and GRP1 recognize highly specific PI3K products PI(3,4)P2 and PI(3,4,5)P3, which are generated transiently upon stimulation of almost all cell surface receptors (17). Because the activation and phosphorylation of Akt are regulated by direct interactions of PI(3,4)P2 or PI(3,4,5)P3 with PH domains (16), we reasoned that PH domains play an important role in Ca2+-mediated inhibition of Akt phosphorylation. To address this question, we expressed and purified the PH domain of Akt and examined its binding properties toward various PIPs (Fig. 4A). Protein-lipid overlay experiments showed selective binding of Akt PH domain to PI(3,4)P2 and PI(3,4,5)P3 in the absence of Ca2+ (Fig. 4B). However, increasing the Ca2+ concentration inhibited the binding of Akt PH domain to PI(3,4)P2 and PI(3,4,5)P3, suggesting that high concentrations of intracellular Ca2+ inhibit electrostatic interactions between PH domains and PIPs.
Fig. 4.
High Ca2+ concentrations abolishes the electrostatic interactions between PH domains and PIPs through the formation of Ca2+-PIPs. (A) Schematic representation of the various biological phospholipids (PIP strips, Echelon Biosciences), including LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; and S1P, sphingosine 1-phosphate. (B–D) Binding of the PH domains of Akt (B), PLCδ (C), and IRS1 (D) to immobilized phospholipids under the indicated Ca2+ concentrations. (E) Schematic representation of electrostatic interactions between the PH domain of Akt (Protein Data Bank accession code:1H10) and Ins(1,3,4,5)P4. (F–I) ITC results for Ca2+ binding to the PH domain of Akt (F), PI(3,4)P2 (G), PI(4,5)P2 (H), or PI(3,4,5)P3 (I) liposomes. Kd values were determined by curve fitting. (J) Schematic representation of electrostatic repulsion between basic residues in the Akt PH domain and Ca2+-Ins(1,3,4,5)P4. Positive charges of basic residues in the PH domain will repel the positively charged Ca2+-Ins(1,3,4,5)P4 and thus inhibit the electrostatic interactions in E.
This result also raises the possibility that high intracellular Ca2+ concentrations may inhibit the binding of other PH domains to various PIPs. We purified PH domains from phospholipase C-δ1(PLC-δ1), which binds most tightly to PI(4,5)P2 (27), and other PH domains from adapter proteins for several members of the tyrosine kinase receptor family, such as IRS1 (25). Consistent with previous findings (27), protein-lipid overlay experiments showed that the PLC-δ1 PH domain (PLCδ-PH) bound tightly to PI(4,5)P2 only in the absence of Ca2+ (Fig. 4C). However, increasing the Ca2+ concentration completely abolished the binding of PLCδ-PH to PI(4,5)P2. Interestingly, the IRS1-PH domain bound to all of the PIPs, including PI(3)P, PI(4)P, PI(5)P, PI(3,4)P2, PI(4,5)P2, PI(3,5)P2, and PI(3,4,5)P3 in the absence of Ca2+, suggesting that IRS1 has a broad binding specificity for various PIPs (28). Consistently, higher Ca2+ concentrations abolished the binding affinity of the IRS1 PH domain to PIPs, including PI(3,4)P2, PI(4,5)P2, and PI(3,4,5)P3 (Fig. 4D), suggesting that Ca2+ inhibits the binding of PH domains to PIPs with two adjacent phosphate groups. Thus, these results demonstrate that higher intracellular Ca2+ prevents the binding of PIPs to the PH domains of Akt, PLC-δ1, and IRS1.
Elevated Ca2+ Causes the Formation of Ca2+-PIPs, Which Abolish Electrostatic Interactions Between PH Domains and PIPs.
The crystal structure of Akt PH domain bound to inositol-1,3,4,5-tetraphosphate (Ins(1,3,4,5)P4), a head group of PI(3,4,5)P3, provides mechanistic clues to the Ca2+-mediated inhibition of PH domain binding to PIPs (29). The PH domain of Akt anchors the phosphates at the 3, 4, and 5 positions of PI(3,4,5)P3 through electrostatic interactions with positively charged side chains of K14, K23, R25, and R86 (Fig. 4E), signifying that Ca2+ may inhibit the electrostatic interactions by binding to either the PH domain of Akt or PI(3,4,5)P3.
To distinguish between these two possibilities, we used isothermal titration calorimetry (ITC), the gold standard for measuring binding affinity, to analyze whether Ca2+ binds to either the PH domain of Akt or PIPs. We examined the thermodynamics of Ca2+ binding to the PH domain of Akt at 25 °C. ITC analysis showed that Ca2+ does not bind to the PH domain of Akt (Fig. 4F), suggesting that Ca2+ may directly interact with PIPs, including PI(3,4)P2 and PI(3,4,5)P3. For the ITC analysis of Ca2+ binding to PIPs, we made liposomes composed of di-palmitoyl-sn-glycero-3-phosphocholine (POPC)/PI(3,4)P2 or PI(3,4,5)P3 (molar ratio of 80:20) (30). ITC analysis showed that PI(3,4)P2 bound two molecules of Ca2+ with strong affinity (Kd1 = 4.6 ± 0.7 μM, Kd2 = 6.5 ± 0.4 μM) (Fig. 4G). Ca2+ also bound PI(4,5)P2 liposomes with a very high affinity (Kd = 6.7 ± 0.12 μM) (Fig. 4H). Interestingly, PI(3,4,5)P3 tightly bound two molecules of Ca2+, one with high affinity (Kd = 15.1 ± 1.5 nM) and the second with low affinity (Kd = 4.6 ± 0.14 μM) (Fig. 4I). These results indicate that Ca2+ has a high affinity for PIPs with two adjacent phosphate groups and forms Ca2+-PIPs, which are highly compatible with physiological concentrations of elevated intracellular Ca2+ (24). Importantly, these results are consistent with a well-known property of inositol phosphate, which mediates the formation of a bidentate (P-Ca2+-P) between Ca2+ and the two acidic phosphate groups of inositol phosphates (31, 32). Consistent with this observation, previous computational modeling studies (33) have suggested that Ca2+ can form Ca2+-induced PI(4,5)P2 clusters through electrostatic interactions. Furthermore, Bilkova et al. (34) showed that Ca2+ directly interacts with the head group phosphates of PI(4,5)P2, which further blocks the interactions of the PLCδ-PH domain to PI(4,5)P2. Taken together, we demonstrated that intracellular Ca2+ overload causes the formation of Ca2+-PIPs, which prevent the recognition of PIPs by PH domains, likely due to electrostatic repulsion between positively charged side chains of PH domains and Ca2+-PIPs (Fig. 4J).
Discussion
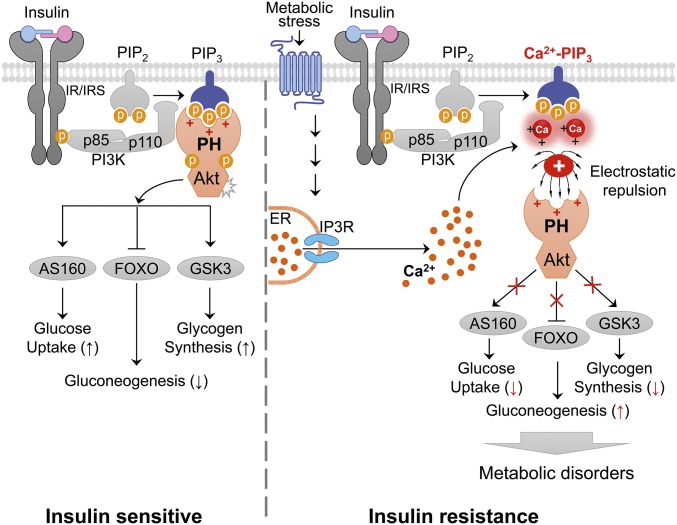
Dysregulation of intracellular Ca2+ homeostasis is one of the primary causes of insulin resistance in obesity and type 2 diabetes (5, 9, 10), although the molecular mechanisms that underlie these associations are not completely elucidated. Here, we provide evidence that an increased intracellular Ca2+ concentration in obesity inhibits the phosphorylation of Akt and its critical downstream signaling events by preventing membrane translocation of PH domains to the plasma membrane (Fig. 5). Thus, we propose the role of Ca2+-PIPs as critical negative regulators of the translocation of PH domain-containing molecules to the plasma membrane.
Fig. 5.
A proposed model of intracellular Ca2+-mediated inhibition of insulin signaling in obesity. Models showing that, under normal physiological conditions, PI(3,4,5)P3 recruits the PH domain of Akt to the plasma membrane, where Akt mediates insulin signaling by phosphorylating GSK3β, FOXO, and AS160. In pathological conditions, such as obesity, however, metabolic stress may lead to elevated intracellular Ca2+ levels, thereby impairing insulin action on carbohydrate and lipid metabolism by blocking membrane localization of the PH domain of Akt through the formation of Ca2+-phosphoinositides.
In addition, acute induction of intracellular Ca2+ flux triggered by ionomycin and PMA markedly suppressed insulin-stimulated Akt phosphorylation, while Ca2+ channel blockers increased the sensitivity of insulin-stimulated phosphorylation of Akt at T308 and S473, in accordance with earlier studies (9, 35) although PMA cannot be regarded as a specific modulator of Ca2+ concentration. Moreover, immunofluorescence analysis showed that PMA/ionomycin-induced increases in intracellular Ca2+ concentration prevented insulin-stimulated membrane localization of either Akt-PH domain mCherry fusion protein or endogenous Akt that recognizes PI(3,4)P2/PI(3,4,5)P3, confirming that high intracellular Ca2+ level inhibits interactions between the PH domain and either PI(3,4)P2 or PI(3,4,5)P3 on the plasma membrane. Consistent with previous findings (26), we also found that ionomycin-induced intracellular Ca2+ overload also rapidly dissociated other PH domains of proteins such PLC-δ1 and IRS1 from the plasma membrane, suggesting that high intracellular Ca2+ concentration is a negative regulator of PH domain translocation to the plasma membrane. PH domain-lipid overlay experiments further demonstrated that Ca2+ abolished the binding of Akt PH domains as well as other PH domain-containing molecules such as PLC-δ1 and IRS1 to their specific membrane PIPs. Finally, the crystal structure of Akt PH domain with Ins(1,3,4,5)P4 (29) and ITC studies verified that Ca2+-mediated inhibition of targeting PH domains to the membrane resulted from the tight binding of Ca2+ rather than PH domains to PIPs, so that Ca2+-PIPs eventually abrogated the binding of PH domains to the membrane due to electrostatic repulsion.
Ca2+ is one of the most versatile and universal signaling components, and it exerts allosteric regulatory effects on many enzymes and proteins (36). Intracellular Ca2+ signaling is initiated by a hormone or other agonist binding to a G protein-coupled receptor (GPCR) and subsequent signaling cascades, including the activation of inositol trisphosphate receptor (IP3R) (37). At physiological levels, glucagon and catecholamine transiently raises intracellular Ca2+ levels through the activation of IP3R, whereby the elevated Ca2+ antagonizes insulin signaling by complexing with Ca2+-phosphoinositides and inhibiting the membrane recruitment of proteins containing PH domains to phosphoinositides. However, at the pathological conditions such as obesity or type 2 diabetes (38), activation of GPCRs may lead to sustained elevation of cytosolic Ca2+ levels in hepatocytes through IP3R (37). Thus, dysregulation of intracellular Ca2+ homeostasis in obesity may disrupt insulin action and mediate insulin resistance by inhibiting membrane localization and activation of proteins with PH domains through sustained formation of Ca2+-phosphoinositides (Fig. 5). Alternatively, sustained high intracellular Ca2+ in obesity may also activate several Ca2+-responsive proteins, such as CaMK (21), NFAT transcription factors (39), and PKCs (40) that contribute to the development of insulin resistance.
Conversely, Akt is frequently hyperactivated in human cancer (41). Mutations that lead to either constitutive membrane localization of PH domains or disruption of the inhibitory interactions between PH domain and kinase domain promote oncogenesis in vivo (42), suggesting that the Akt PH domain acts as an inhibitor of kinase activation. Although many gaps remain in our understanding of the inhibitory functions of the PH domain in Akt, our findings suggest that Ca2+-PIPs in elevated intracellular Ca2+ conditions may act as negative regulators that eventually block the dissociation of the inhibitory interactions between the PH domain and the kinase domain in Akt. Interestingly, pretreatment of PMA or ionomycin inhibited both EGF-stimulated membrane localization of endogenous Akt and EGF-stimulated phosphorylation of Akt and its downstream signaling molecules in HaCaT cells (SI Appendix, Fig. S9). This suggested that intracellular Ca2+ overload prevents membrane localization of PH domains by other growth factors. These results may explain why increased intracellular Ca2+ levels induce apoptosis in multiple cell types, including thymocytes (43), neurons (44), and various cancer cells (45). Therefore, drugs that inhibit membrane localization of PH domain in Akt may be effective against many human cancers. Further elucidation of the role of Ca2+-PIPs in cell biology and physiology may require additional studies to provide new potential targets for pharmacological interventions for major human diseases, including cancer and diabetes. In conclusion, dysregulation of intracellular Ca2+ homeostasis may contribute to the pathogenesis of insulin resistance, obesity, and type 2 diabetes by preventing the localization of PH domains to the plasma membrane by coupling Ca2+-PIPs.
Materials and Methods
C57BL/6 male mice from Orient Bio, Inc. were studied under protocols approved by the animal ethics committee of Gachon University, Lee Gil Ya Cancer and Diabetes Institute (LCDI-2014-0080). For full details of all these processes, see SI Appendix.
Supplementary Material
Acknowledgments
We thank Drs. Steven E. Shoelson (Harvard Medical School) and Jae Young Park (Korea University) for helpful discussions. This work was supported by grants from the Korea Health Technology R&D Project, Korea Ministry of Health & Welfare (HI14C1135, A111345), and the Basic Science Research Program (2017R 1D 1A 1B03031094) of the National Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706489114/-/DCSupplemental.
References
- 1.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: Insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 3.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park HW, Lee JH. Calcium channel blockers as potential therapeutics for obesity-associated autophagy defects and fatty liver pathologies. Autophagy. 2014;10:2385–2386. doi: 10.4161/15548627.2014.984268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arruda AP, et al. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byyny RL, LoVerde M, Lloyd S, Mitchell W, Draznin B. Cytosolic calcium and insulin resistance in elderly patients with essential hypertension. Am J Hypertens. 1992;5:459–464. doi: 10.1093/ajh/5.7.459. [DOI] [PubMed] [Google Scholar]
- 7.Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab. 2009;296:E690–E701. doi: 10.1152/ajpendo.90525.2008. [DOI] [PubMed] [Google Scholar]
- 8.Standley PR, Ali S, Bapna C, Sowers JR. Increased platelet cytosolic calcium responses to low density lipoprotein in type II diabetes with and without hypertension. Am J Hypertens. 1993;6:938–943. doi: 10.1093/ajh/6.11.938. [DOI] [PubMed] [Google Scholar]
- 9.Park HW, et al. Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nat Commun. 2014;5:4834. doi: 10.1038/ncomms5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu S, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustavo Vazquez-Jimenez J, et al. Palmitic acid but not palmitoleic acid induces insulin resistance in a human endothelial cell line by decreasing SERCA pump expression. Cell Signal. 2016;28:53–59. doi: 10.1016/j.cellsig.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Beer NA, Jakubowicz DJ, Beer RM, Nestler JE. The calcium channel blocker amlodipine raises serum dehydroepiandrosterone sulfate and androstenedione, but lowers serum cortisol, in insulin-resistant obese and hypertensive men. J Clin Endocrinol Metab. 1993;76:1464–1469. doi: 10.1210/jcem.76.6.8501151. [DOI] [PubMed] [Google Scholar]
- 13.Xu G, Chen J, Jing G, Shalev A. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61:848–856. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, et al. The effects of calcium channel blockade on agouti-induced obesity. FASEB J. 1996;10:1646–1652. [PubMed] [Google Scholar]
- 15.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 16.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 17.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 18.Rawlings DJ, et al. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 19.Lindvall JM, et al. Bruton’s tyrosine kinase: Cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling. Immunol Rev. 2005;203:200–215. doi: 10.1111/j.0105-2896.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 20.Carpten JD, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 21.Ozcan L, et al. Activation of calcium/calmodulin-dependent protein kinase II in obesity mediates suppression of hepatic insulin signaling. Cell Metab. 2013;18:803–815. doi: 10.1016/j.cmet.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benoit SC, et al. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-θ subcellular localization in rodents. J Clin Invest. 2009;119:2577–2589. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu N, Francis M, Cioffi DL, Stevens T. Studies on the resolution of subcellular free calcium concentrations: A technological advance. Focus on “detection of differentially regulated subsarcolemmal calcium signals activated by vasoactive agonists in rat pulmonary artery smooth muscle cells.”. Am J Physiol Cell Physiol. 2014;306:C636–C638. doi: 10.1152/ajpcell.00046.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem. 2004;279:35298–35305. doi: 10.1074/jbc.M405203200. [DOI] [PubMed] [Google Scholar]
- 26.Várnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: Calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavran JM, et al. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem. 1998;273:30497–30508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- 28.Dhe-Paganon S, Ottinger EA, Nolte RT, Eck MJ, Shoelson SE. Crystal structure of the pleckstrin homology-phosphotyrosine binding (PH-PTB) targeting region of insulin receptor substrate 1. Proc Natl Acad Sci USA. 1999;96:8378–8383. doi: 10.1073/pnas.96.15.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milburn CC, et al. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem J. 2003;375:531–538. doi: 10.1042/BJ20031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerrero-Valero M, Marín-Vicente C, Gómez-Fernández JC, Corbalán-García S. The C2 domains of classical PKCs are specific PtdIns(4,5)P2-sensing domains with different affinities for membrane binding. J Mol Biol. 2007;371:608–621. doi: 10.1016/j.jmb.2007.05.086. [DOI] [PubMed] [Google Scholar]
- 31.Kim OH, et al. β-propeller phytase hydrolyzes insoluble Ca(2+)-phytate salts and completely abrogates the ability of phytate to chelate metal ions. Biochemistry. 2010;49:10216–10227. doi: 10.1021/bi1010249. [DOI] [PubMed] [Google Scholar]
- 32.Oh BC, et al. Ca(2+)-inositol phosphate chelation mediates the substrate specificity of beta-propeller phytase. Biochemistry. 2006;45:9531–9539. doi: 10.1021/bi0603118. [DOI] [PubMed] [Google Scholar]
- 33.Wang YH, Slochower DR, Janmey PA. Counterion-mediated cluster formation by polyphosphoinositides. Chem Phys Lipids. 2014;182:38–51. doi: 10.1016/j.chemphyslip.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilkova E, et al. Calcium directly regulates phosphatidylinositol 4,5-bisphosphate headgroup conformation and recognition. J Am Chem Soc. 2017;139:4019–4024. doi: 10.1021/jacs.6b11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiwa M, et al. Distinct time course of the decrease in hepatic AMP-activated protein kinase and Akt phosphorylation in mice fed a high fat diet. PLoS One. 2015;10:e0135554. doi: 10.1371/journal.pone.0135554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berridge MJ, Bootman MD, Lipp P. Calcium:A life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 37.Arruda AP, Hotamisligil GS. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015;22:381–397. doi: 10.1016/j.cmet.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brozinick JT, Jr, Roberts BR, Dohm GL. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: Potential role in insulin resistance. Diabetes. 2003;52:935–941. doi: 10.2337/diabetes.52.4.935. [DOI] [PubMed] [Google Scholar]
- 39.Yang TT, et al. Role of transcription factor NFAT in glucose and insulin homeostasis. Mol Cell Biol. 2006;26:7372–7387. doi: 10.1128/MCB.00580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang W, Bansode R, Mehta M, Mehta KD. Loss of protein kinase Cbeta function protects mice against diet-induced obesity and development of hepatic steatosis and insulin resistance. Hepatology. 2009;49:1525–1536. doi: 10.1002/hep.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 42.Parikh C, et al. Disruption of PH-kinase domain interactions leads to oncogenic activation of AKT in human cancers. Proc Natl Acad Sci USA. 2012;109:19368–19373. doi: 10.1073/pnas.1204384109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youn H-D, Sun L, Prywes R, Liu JO. Apoptosis of T cells mediated by Ca2+-induced release of the transcription factor MEF2. Science. 1999;286:790–793. doi: 10.1126/science.286.5440.790. [DOI] [PubMed] [Google Scholar]
- 44.Wang H-G, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 45.Furuya Y, Lundmo P, Short AD, Gill DL, Isaacs JT. The role of calcium, pH, and cell proliferation in the programmed (apoptotic) death of androgen-independent prostatic cancer cells induced by thapsigargin. Cancer Res. 1994;54:6167–6175. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.