Significance
The exceptional diversity of species in the coral reefs, seagrass meadows, and mangrove forests of the Coral Triangle and the many ecological functions and benefits to humans they provide have made them a high priority for conservation and fisheries management. Nevertheless, their degradation continues and calls for effective restoration. In an experimental restoration, we demonstrated that planting mixtures of diverse seagrass species improves their overall survival and growth and thus the trajectory toward successful restoration. Incorporating species diversity into restoration heralds a shift in practice from establishing a single founder species, and recognizes the widely documented positive effects that biodiversity has on ecosystem function and services. Biodiversity is often a restoration goal, but it also promises a means to improve success.
Keywords: biodiversity, Coral Triangle, restoration, seagrass, species richness
Abstract
Ecosystem restoration aims to restore biodiversity and valuable functions that have been degraded or lost. The Coral Triangle is a hotspot for marine biodiversity held in its coral reefs, seagrass meadows, and mangrove forests, all of which are in global decline. These coastal ecosystems support valuable fisheries and endangered species, protect shorelines, and are significant carbon stores, functions that have been degraded by coastal development, destructive fishing practices, and climate change. Ecosystem restoration is required to mitigate these damages and losses, but its practice is in its infancy in the region. Here we demonstrate that species diversity can set the trajectory of restoration. In a seagrass restoration experiment in the heart of the Coral Triangle (Sulawesi, Indonesia), plant survival and coverage increased with the number of species transplanted. Our results highlight the positive role biodiversity can play in ecosystem restoration and call for revision of the common restoration practice of establishing a single target species, particularly in regions having high biodiversity. Coastal ecosystems affect human well-being in many important ways, and restoration will become ever more important as conservation efforts cannot keep up with their loss.
The exceptional marine biodiversity in the Coral Triangle (1, 2) provides fisheries resources for more than 350 million humans, many of whom live in the coastal zone in close proximity to coral reefs, seagrass meadows, and mangrove forests (3, 4). In addition to fisheries resources, these coastal ecosystems provide other valuable ecological services and functions, including coastal defense against flooding; habitat and food for marine species of conservation concern, such as sharks, dugongs, and sea turtles; and carbon storage (5–7). These important functions are put at risk as coral reefs, seagrass meadows, and mangroves decline due to unrelenting coastal development in the region; fisheries overexploitation and destructive practices; and ocean warming and acidification associated with global climate change (4, 8–12). To date, conservation efforts have focused primarily on coral reef fisheries management and establishment of marine protected areas (10, 13–15). These conservation measures are critically important, but they do not address mitigation of coastal ecosystems already degraded or lost; thus, ecosystem restoration is an important complement to fisheries management and habitat protection (15). Despite this obvious need, restoration of coral reefs, seagrass meadows, and mangroves has not advanced very far in the Coral Triangle (16, 17). Of this ecosystem triad, seagrasses in particular have been understudied (18–20), despite their importance for providing food and livelihoods (21, 22). Seagrasses straddle mangroves lining the shore and coral reefs farther offshore, linking these interdependent ecosystems in an integrated coastal seascape wherein the mangroves and seagrasses intercept terrestrially derived sediments, nutrients, and pathogens, reducing their loading on sensitive corals, and provide nursery habitats for coral reef fisheries species (23–27).
The Coral Triangle hosts more seagrass species than virtually anywhere else on earth, and across the region they naturally grow together in mixed species communities (28). We investigated the role of this biodiversity in ecosystem restoration, which here refers to the deliberate establishment of a founder community. Biodiversity often determines ecosystem function and resilience (29, 30), but its role in restoration is relatively unexplored in both marine and terrestrial environments (17, 31–35). Species diversity tends to be a goal, rather than a means, of restoration, although intraspecific genetic diversity has been examined for seagrass, salt marsh, and mangrove restoration, which have focused largely on single species (36–43) (Results).
Planting monocultures in coastal vegetation restoration practice and research emanated in part from targeting a conspicuous or “climax” species, the most impacted or stress-tolerant species, or one desired for silvaculture, even in tropical regions where species grow intermixed naturally (44–49). Planting monocultures of the mangrove Rhizophora has led to failed projects (50, 51), yet the practice has not evolved (52). As part of our study, we confirmed the pervasive monospecific approach for seagrass, mangroves, and salt marshes by taking advantage of recent reviews of restoration practice and research (17, 43, 51, 53) (Results).
The monospecific rationale is understandable, but at odds with the idea that biodiversity often confers ecological benefits. Species diversity can enhance ecosystem function and services in established (29, 30), but also nascent, communities, as in a seminal study of a salt marsh restoration (54). Species diversity in the founding community can set the pace of restoration through negative (inhibition, competition) or positive (facilitation) interactions of one or more species with others, as in succession (55–59). Concern about both intra- and interspecific competition helped shape restoration practice in aquatic ecosystems (50, 60–62). However, facilitative interactions could benefit restoration early on or in fluctuating or stressful environments (34, 61), as demonstrated intraspecifically for a salt marsh species (62). Interspecific facilitation has been proposed to spread predation risk (52) or hasten colonization of a climax target species, i.e., succession could be “compressed” by planting a single nurse or pioneer species (50, 63–65). However, empirically testing the effect of species richness on restoration trajectories has remained a research gap highly relevant to restoration goals and costs (17, 66) (Results), which is particularly true in the Coral Triangle where much is at stake environmentally and socially.
To test the effect of seagrass species richness on the restoration trajectory, we conducted a field experiment using a proven restoration technique (67). We transplanted six common Indo-Pacific seagrass species (Enhalus acoroides, Thalassia hemprichii, Cymodocea rotundata, Syringodium isoetifolium, Halodule uninervis, Halophila ovalis) of ∼15 occurring in the Coral Triangle (28), at four species richness levels (monocultures, two, four, and five species). We randomly drew unique species combinations for replication at the higher richness levels (68) (Fig. 1 A and B and Tables S1 and S2). To assess differences in early restoration trajectories, we analyzed the survivorship of the initial transplants and their collective rate of expansion or contraction (percent cover) for more than a year.
Fig. 1.
Transplanting seagrasses using SCUBA (A), a mixed-species plot posttransplantation (B) (Enhalus, Cymodocea, Syringodium, Halodule), and disturbances of plots by algae (C) and marine debris (D). Photos courtesy of D. Trockel, University of California, Davis, CA (A), J.M.A. (B), C.S. (C), and K. DuBois, University of California, Davis, CA (D).
Results
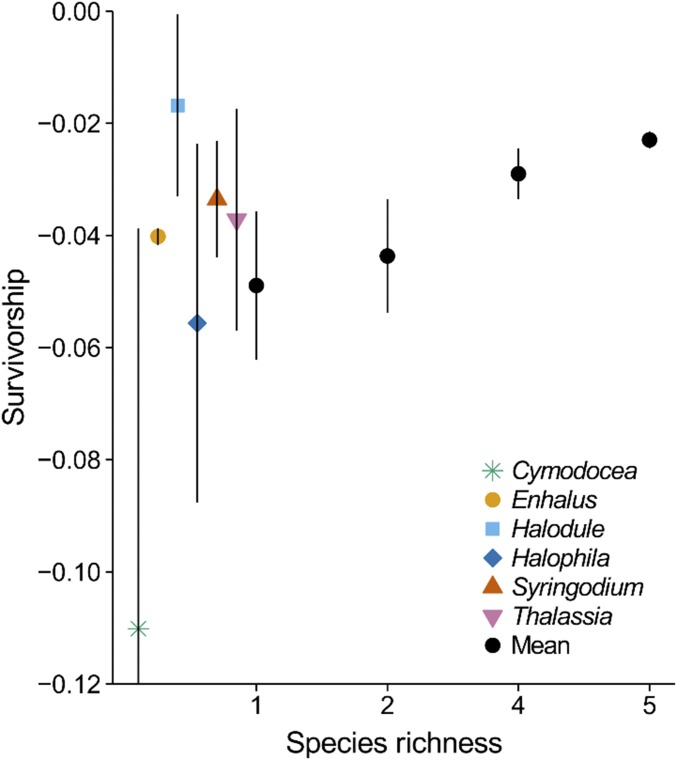
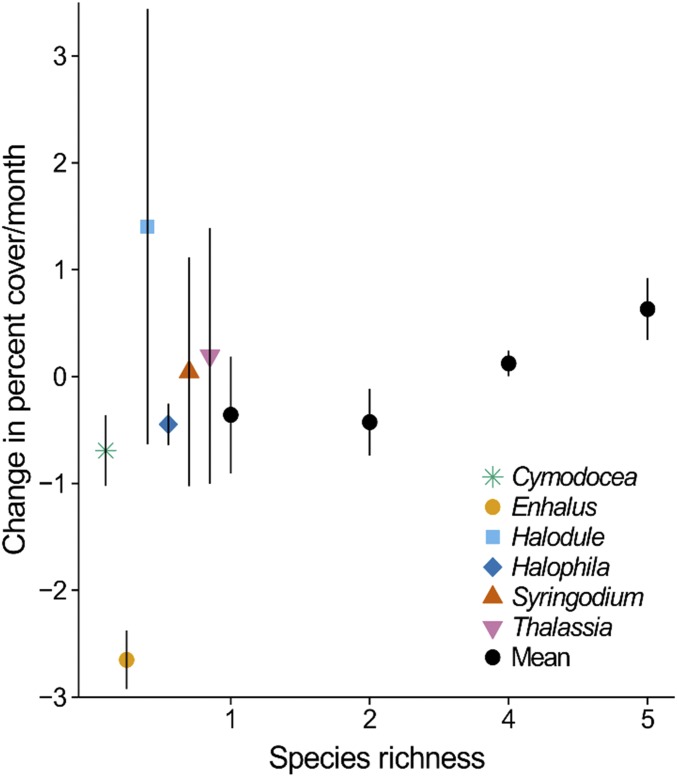
The success of the seagrass transplantations improved with the number of species planted, indicating that species richness can play a positive role in restoration. Both survivorship (Fig. 2) and the rate of change in percent cover (Fig. 3) increased with species richness (survival: Kruskal–Wallis χ2 = 7.88, P = 0.024, df = 3; percent cover: GLM, F1,30 = 5.991, P = 0.021), i.e., overyielding occurred (68–71). We also examined the variability in survival and change in cover as a measure of stability (72). Although the variances were homogeneous, i.e., they did not differ across the richness levels (survival Levene’s F = 0.904, P = 0.452, cover Levene’s F = 1.81, P = 0.169), the coefficients of variation revealed that monoculture survival, and possibly cover, was more variable (theoretically less stable) when contrasted against all mixtures (GLM survival F1,28 = 6.879, P = 0.014; cover F1,28 = 3.055, P = 0.091).
Fig. 2.
Species richness and restoration trajectories: survivorship increased with species richness (χ2 = 7.88, P = 0.024, df = 3). Mean, SE bars, n = 6 monocultures and five-species combinations, n = 10 two- and four-species mixtures; n = 32 assemblages. Individual species did not differ (χ2 = 3.68, P = 0.597, df = 5), mean and SE bars, n = 2–4.
Fig. 3.
Species richness and restoration trajectories: rate of change in percent cover increased with species richness (F1,30 = 5.99, P = 0.021). Mean, SE bars, n = 6 monocultures and five-species combinations, n = 10 two- and four-species mixtures; n = 32 assemblages. Individual species did not differ (F5,10 = 0.894, P = 0.521), mean and SE bars, n = 2–4.
Positive species richness effects are typically attributed at least statistically to either the random inclusion of a high-performing species or richness itself through complementation or facilitation among species, although these mechanisms are not exclusive (73–75). In our experiment, partitioning the variance pointed to the random inclusion of a high-performing species (species identity, sampling, selection effect, or nontransgressive overyielding), which necessarily would be in most of the five-species assemblages (68–71) (ω2; Table 1). Although Halodule uninervis, which attained the highest mean survival and increase in cover (Figs. 2 and 3 and Tables S1 and S2), was the most likely candidate for the identity effect, we detected no differences in survival (Kruskal–Wallis χ2 = 3.68, P = 0.597, df = 5) or the rate of change in cover (F5,10 = 2.41, P = 0.111) among species grown in monoculture. Despite no monoculture differences (68), we calculated commonly reported log response ratios (70, 71) to compare the five-species mixtures to the average monoculture and to Halodule. These ratios also supported an identity effect of including Halodule. The five-species mixtures survived better than the average monoculture (nontransgressive overyielding, LRmean = 0.0009, two-sided t = 16.576, P < 0.0001, df = 5) but not better than Halodule (transgressive overyielding, LRmax = −0.0002, two-sided t = −4.00, P = 0.010, df = 5; Tables S1 and S2). The cover in the five-species mixtures increased faster than the average monoculture (LRmean = 0.034, two-sided t = 3.436, P = 0.019, df = 5) but not faster than Halodule (LRmax = −0.026, two-sided t = −2.645, P = 0.046, df = 5). Neither Halodule nor any other species took over the mixtures, just as they naturally grow together (28) (Tables S3 and S4). Having replicated each unique assemblage, we contrasted changes in cover with and without Halodule among all mixtures or only the four- plus five-species mixtures and were unable to document differences (Tables S3 and S4). Altogether, the results could indicate a possible richness effect not captured by some of the statistics (68, 73–75).
Table 1.
Seagrass species diversity vs. identity effect sizes (ω2)
| Effect | SS | df | MS | P | ω2 | η2 | R2 |
| Model | 0.0873 | 86 | 0.0028 | 0.0067 | 0.2892 | 1.000 | 0.550 |
| Diversity | 0.0013 | 1 | 0.0873 | 0.0001 | 0.0002 | 0.008 | |
| Identity | 0.0860 | 30 | 0.0029 | 0.0061 | 0.2890 | 0.539 | |
| Error | 0.0723 | 55 | 0.0013 | 0.453 | |||
| Total | 0.1596 |
Partitioning of variance into species diversity and identity effects (effect size ω2) on changes in percent seagrass cover for 32 unique replicated species assemblages (Table S2). Effects of species richness vs. identity on changes in percent seagrass cover over time (linear regression coefficients) were partitioned through orthogonal planned contrasts of mixed species vs. single species (69). η2 is the proportion of the total variance attributable to each effect, R2 is proportion of the variance explained by the model.
Our experiment contrasts strongly with much restoration-oriented practice and research. Restoration databases (17, 42, 51, 53) confirmed our collective experience that monocultures are the norm in both restoration practice and research (Dataset S1). Of 253 seagrass, mangrove, and salt marsh studies, we identified 71 (28%) in which multiple species were actually planted in the field. Most of the rest were temperate studies where seagrass and salt marsh species effectively form monocultures. Of the 71 applicable studies, 30 (42%) reported planting species in mixtures in at least some part of the study. Seagrass species were mixed in only five of 29 studies (17%). Nineteen of 36 mangrove studies (53%) included species mixtures; however, Rhizophora composed 97% of the mixtures in 10 of these studies (51). Of the six applicable salt marsh studies of 42 total, species were planted in mixtures but the species richness effect was tested in only one (54, 76). Across systems, species richness was not a factor in the design of nearly all of the multispecies plantings (Dataset S1).
Discussion
Establishing a diverse founder community holds promise for enhancing restoration of seagrasses, where efforts have focused largely on a single species (48, 49), and coastal vegetation in general (Dataset S1). The mechanisms underlying the seagrass diversity effect are hypothetical, because supporting evidence in the form of ω2, log ratios, and the like are merely statistical proxies for the ecological interactions occurring among species (68–75). We found evidence for and against Halodule uninervis as driving better performance in the mixtures. Although ω2 points to Halodule, we could not document differences between mixtures with or without it, or among monocultures, and it did not dominate the plots or the naturally diverse bed (Tables S3 and S4).
Ecologically, facilitative interactions and niche partitioning to reduce competition could have been operating as the seagrasses established and grew, along with identity (selection) effects (73–75). Early facilitation followed by niche partitioning and continued coexistence is reasonable given the diverse morphologies and growth strategies within Indo-Pacific seagrasses (77, 78). For example, small, shallow-rooted species (e.g., Halodule uninervis, Halophila ovalis) colonize quickly and stabilize sediments, allowing nutrient pools to build by minimizing resuspension, thus facilitating succession (58, 79, 80). These species can then coexist within canopies of taller, slow-growing species such as Enhalus acoroides, which has roots extending ≥20 cm into the sediments. By layering their roots (79), seagrasses can partition sediment nutrients (58), thus alleviating competition as density increases. Taller canopies shade and potentially reduce photoinhibition to facilitate smaller, more-sensitive species (81). Mixtures of morphologically different species perhaps stabilize sediments and prevent uprooting and nutrient release better than single species (58, 82). Thus, mixtures could more fully utilize nutrients and light, leading to enhanced overall seagrass production (77) and potentially to higher animal diversity (83–85). This scenario is in line with the positive biodiversity effects demonstrated in many studies (29, 30, 68–71), and could imply that multispecies restoration efforts might perform better based not only on plant survival and cover but also on the ecosystem functions they provide (e.g., stabilizing sediments).
Regardless of the mechanisms for its effect, species richness clearly had a positive influence on the restoration trajectory (Figs. 2 and 3). Understanding the biological basis for the richness effect requires going beyond the statistical indicators and investigating the potential mechanism(s) proposed above. Without detailed mechanistic information, there is good reason to include multiple species in restorations of diverse communities when the best-performing species cannot be definitively identified, as in this study, and particularly in tropical regions where species coexist naturally.
Diverse transplantations certainly did better, but overall survival was low and cover increased slowly, which is typical in seagrass restorations and highlights a critical restoration issue (43). Small chronic disturbances to our plots, which were close to an inhabited shoreline, undoubtedly contributed to a slow restoration trajectory. The plots suffered from boat traffic and anchoring, trampling, mariculture installations, smothering by marine debris and sediments, and algal overgrowth, presumably stimulated by sewage inputs in the absence of a sanitation system on the island (Fig. 1 C and D). However, transplants were not destructively cropped by herbivores; sea urchins were almost absent from our study site, and we observed herbivorous fishes feeding primarily on algal epiphytes and less directly on seagrasses. Anthropogenic disturbances to seagrasses are not unique to Coral Triangle locales (6, 11, 12, 17, 19, 23, 28, 43), but the attention and resources to address them lag behind coral reef and mangrove conservation and management efforts (4, 18–20). Managing human disturbances in the Coral Triangle clearly will be necessary for the ultimate success of restoration, but meanwhile a speciose founding community offers good results in the face of such disturbances. It could very well be that the species richness effect is most evident in disturbed habitats, but verification awaits experiments deployed across a disturbance gradient.
Given the exceptional biodiversity in the Coral Triangle (1, 2) and the grave threats to its coastal ecosystems and human dependence on them (3, 4, 8, 9, 21, 22), it is imperative to begin restoration efforts in earnest to complement the existing focus on fisheries management and habitat conservation (12–15), reconnect broken linkages in the seascape (24–27), and capture and store carbon (86). Diverse founding communities can accelerate the pace of seagrass restoration, a finding that should also be tested explicitly in other ecosystems (17, 51, 53) (Dataset S1). Biodiversity is not only critical to ocean-dependent peoples, but in itself provides a means to enhance restoration results.
Methods
Transplantation Design.
We cut standardized seagrass transplants (15-cm rhizomes with terminal meristem, roots, and leaf shoots) of Enhalus acoroides, Thalassia hemprichii, Cymodocea rotundata, Syringodium isoetifolium, Halodule uninervis, and Halophila ovalis. We randomly drew unique assemblages at each richness level to control for species composition bias (68), yielding n = 6 monoculture treatments, n = 10 for two- and four-species, and n = 6 for five-species treatments (all possible combinations). We randomly assigned richness treatments to plots (60 × 60 cm, divided into 16 equal grids) separated by at least 1 m in which total transplant density (n = 16) was constant across treatments and the number of transplants of each species was equal within a plot. We standardized transplants by rhizome length and meristem, as done in restoration practice; therefore, biomass differences across species were part of random experimental error. Each monoculture and unique species combination was replicated (n = 3) except for the following replication errors during transplantation: Halodule (n = 2 plots), Halophila (n = 4), Cymodocea plus Halophila (n = 2), Syringodium plus Halophila (n = 4) (Tables S1 and S2). We anchored transplants in plots (n = 96) in unvegetated sediments in 2- to 3-m water depth within a seagrass bed growing behind the barrier reef on Pulau Badi (S 5°2′44.9–5°3′0.53′′, E 119°19′42.38′′–119°19′49.17′′), Spermonde Islands, South Sulawesi, Indonesia.
Our site was a typical Indonesian small island where inhabitants maintained traditional lifestyles and used the seagrass meadow for various purposes, including artisanal fishing, mariculture, anchorage, swimming, bathing, and waste disposal, including human. Our plots followed the seaward edge of the meadow where it abuts the reef at ∼50 m from shore. We engaged the community with outreach about seagrasses and our experiment.
We monitored plots every 2 wk for the first 4 mo and then every 4 wk for a total of 19 times over 57 wk. We estimated survival as the proportion of the original transplants (n = 16) that were alive in each plot at each census. We estimated percent cover by taking a photograph from 1 m above each plot and summing the percent cover in 320 equal-sized subdivisions. Plots invaded by other species or lost were deleted from analyses, leaving n = 87 to analyze. Because rates of change encompass variation due to seasons, storms, and other disturbances, our analyses differ from biodiversity experiments that test effects at a single point typically at the end of an experiment, which might misrepresent the overall trajectory.
Data Analysis.
Survival of the initial transplants declined exponentially (a typical survivorship curve), and we estimated survivorship as the slope of log (survival + 1) vs. time for each plot (average R2 = 0.81 ± 0.15 SD, n = 87 plots). We averaged the slopes of replicate plots (n = 2–4; see above) within each unique species assemblage (n = 32 monocultures and polycultures). The mean was used as the response variable in the nonparametric Kruskal–Wallis test for species richness effects (four levels) after transformations did not meet parametric assumptions (examination of residuals, normal Q-Q, and log-likelihood plots using R 3.0.1). Mean slopes of linear regressions of percent cover vs. time were also tested but in a general linear model; linear regressions were the best-fit function (higher R2 compared with exponential functions except for a few plots). We compared species survivorship and changes in percent cover in monocultures using Kruskal–Wallis tests and within and among species using ANOVA (after examining residual plots using SYSTAT 11), respectively (68). We used Levene’s test for homogeneity of variances and tested differences in coefficients of variation between all monocultures and all mixtures in a general linear model.
To assess whether the positive relationship between species richness and percent cover (overyielding) was likely due to species richness itself (diversity effect) or inclusion of a high-performing species in the mixtures (identity effect), we partitioned the total variance into the two effects (ω2) by performing contrasts between the poly- and monocultures to yield the diversity effect, which then yields the identity effect when subtracted from the total sum of squares (69) (Table 1). Because ANOVA was inappropriate for survivorship, we could not partition the variance. We also calculated log response ratios as a commonly reported test of species richness effects (70, 71), acknowledging the challenge of ascribing mechanisms to overyielding effects (68, 73–75). These ratios compare species mixtures to species grown alone (monocultures). The nontransgressive overyielding log ratios, LRmean, were calculated as the natural log of the ratios of the mean of replicate plots for each unique five-species mixture (n = 6) to the mean of all monocultures calculated from the average of the replicate plots for each species (Tables S1 and S2). The transgressive overyielding log ratios, LRmax, were similarly calculated based on the averages of replicated plots of each unique five-species combination (n = 6) divided by the mean of the highest-performing species in monoculture (Halodule uninervis). We performed two-sided t tests (df = 5) to assess whether LRmean and LRmax were different from zero. All data are provided in Supporting Information.
To assess multispecies approaches in seagrass, mangrove, and salt marsh restoration projects and experiments, we considered four restoration supplemental databases for mangroves, seagrass, and salt marshes (17), seagrasses (43), mangroves (51), and all ecosystems (53). After excluding freshwater and terrestrial studies (53), we culled all studies that potentially included >1 species. After reading all papers from this subset, we selected the applicable studies that provided information on transplantations or sowings in field restoration projects or experiments where the natural vegetation comprises multiple species (primarily tropical regions) and deleted those that did not, e.g., ones on natural recolonization, economic analyses, and general review papers without planting details. From the applicable papers, we calculated the percentage in which >1 species were actually planted mixed together in the field in at least some part of the study (Dataset S1).
Supplementary Material
Acknowledgments
We thank N. Asriani, B. Cheng, S. Hameed, B. Jellison, L. Komoroske, S. Mangando, J. Miller, G. Ng, E. Satterthwaite, and D. Trockel for field assistance; N. Janetski and S. Rapi for logistical support; M. E. S. Bracken and D. Trockel for analysis advice; and M. E. S. Bracken, J. J. Stachowicz, D. R. Strong, an anonymous reviewer, the editors, and, especially, J. E. Duffy for comments. This research was supported by US Agency for International Development Partnerships for Enhanced Engagement in Research Project 2-319, National Science Foundation Grant DGE0841297 (BioOce1234345), Mars Symbioscience, and the Office of Global Affairs and the Agricultural Experimental Station at the University of California, Davis.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707962114/-/DCSupplemental.
References
- 1.Reaka ML, Rodgers PJ, Kudla AU. Colloquium paper: Patterns of biodiversity and endemisms in Indo-Pacific coral reefs. Proc Natl Acad Sci USA. 2008;105:11474–11481. doi: 10.1073/pnas.0802594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanciangco JC, Carpenter KE, Etnoyer PJ, Moretzsohn F. Habitat availability and heterogeneity and the Indo-Pacific warm pool as predictors of marine species richness in the tropical Indo-Pacific. PLoS One. 2013;8:e56245. doi: 10.1371/journal.pone.0056245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asian Development Bank . Economics of Fisheries and Aquaculture in the Coral Triangle. Asian Development Bank; Manila, Philippines: 2014. [Google Scholar]
- 4.Burke L, Reytar K, Spalding M, Perry A. Reefs at Risk Revisited in the Coral Triangle. World Resources Inst; Washington, DC: 2012. [Google Scholar]
- 5.Barbier EB, et al. The value of estuarine and coastal ecosystem services. Ecol Monogr. 2011;81:169–193. [Google Scholar]
- 6.Hughes AR, Williams SL, Duarte CM, Heck KL, Jr, Waycott M. Associations of concern: Declining seagrasses and threatened dependent species. Front Ecol Environ. 2008;7:242–246. [Google Scholar]
- 7.Mcleod E, et al. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ. 2011;9:552–560. [Google Scholar]
- 8.Valiela I, Bowen JL, York JK. Mangrove forests: One of the world’s threatened major tropical environments. Bioscience. 2001;51:807–815. [Google Scholar]
- 9.Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 10.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 11.Waycott M, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA. 2009;106:12377–12381. doi: 10.1073/pnas.0905620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world’s marine ecosystems. Science. 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- 13.Mumby PJ, Steneck RS. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol. 2008;23:555–563. doi: 10.1016/j.tree.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Cinner JE, et al. Bright spots among the world’s coral reefs. Nature. 2016;535:416–419. doi: 10.1038/nature18607. [DOI] [PubMed] [Google Scholar]
- 15.Possingham HP, Bode M, Klein CJ. Optimal conservation outcomes require both restoration and protection. PLoS Biol. 2015;13:e1002052. doi: 10.1371/journal.pbio.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yap HT. The case for restoration of tropical coastal restoration. Ocean Coastal Manage. 2000;41:841–851. [Google Scholar]
- 17.Bayraktarov E, et al. The cost and feasibility of marine coastal restoration. Ecol Appl. 2016;26:1055–1074. doi: 10.1890/15-1077. [DOI] [PubMed] [Google Scholar]
- 18.Ooi JLS, Kendrick GA, Van Niel KP, Affendi YA. Knowledge gaps in tropical southeast Asian seagrass systems. Estuarine Coastal Shelf Sci. 2011;92:118–131. [Google Scholar]
- 19.Duarte CM, Dennison WC, Orth RJ, Carruthers TJB. The charisma of coastal ecosystems: Addressing the imbalance. Estuaries Coasts. 2008;31:233–238. [Google Scholar]
- 20.Unsworth RKF, Cullen LC. Recognising the necessity for Indo-Pacific seagrass conservation. Conserv Lett. 2010;3:63–73. [Google Scholar]
- 21.Unsworth RKF, Cullen LC, Pretty JN, Smith DJ, Bell JJ. Economic and subsistence values of the standing stocks of seagrass fisheries: Potential benefits of no-fishing marine protected area management. Ocean Coastal Manage. 2010;53:218–224. [Google Scholar]
- 22.Unsworth RKF, Hinder SL, Bodger OG, Cullen-Unsworth LC. Food supply depends on seagrass meadows in the coral triangle. Environ Res Lett. 2014;9:094005. [Google Scholar]
- 23.Valiela I, Cole ML. Comparative evidence that salt marshes and mangroves may protect seagrass meadows from land-derived nitrogen loads. Ecosystems. 2002;5:92–102. [Google Scholar]
- 24.McCook LJ, et al. Management under uncertainty: Guide-lines for incorporating connectivity into the protection of coral reefs. Coral Reefs. 2009;28:353–366. [Google Scholar]
- 25.McMahon KW, Berumen ML, Thorrold SR. Linking habitat mosaics and connectivity in a coral reef seascape. Proc Natl Acad Sci USA. 2012;109:15372–15376. doi: 10.1073/pnas.1206378109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagelkerken I, Grol MGG, Mumby PJ. Effects of marine reserves versus nursery habitat availability on structure of reef fish communities. PLoS One. 2012;7:e36906. doi: 10.1371/journal.pone.0036906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb JB, et al. Seagrass ecosystems reduce exposure to bacterial pathogens of humans, fishes, and invertebrates. Science. 2017;355:731–733. doi: 10.1126/science.aal1956. [DOI] [PubMed] [Google Scholar]
- 28.Short FT, et al. Extinction risk assessment of the world’s seagrass species. Biol Conserv. 2011;144:1981–1971. [Google Scholar]
- 29.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 30.Stachowicz JJ, Bruno JF, Duffy JE. Understanding the effects of marine biodiversity on communities and ecosystems. Annu Rev Ecol Evol Syst. 2007;38:739–766. [Google Scholar]
- 31.Wortley L, Hero J-M, Howes M. Evaluating ecological restoration success: A review of the literature. Restor Ecol. 2013;21:537–543. [Google Scholar]
- 32.Kettenring KM, Mercer KL, Reinhardt Adams C, Hines J. Application of genetic diversity-ecosystem function research to ecological restoration. J Appl Ecol. 2014;51:339–348. [Google Scholar]
- 33.Perring MP, et al. Advances in restoration ecology: Rising to the challenges of the coming decades. Ecosphere. 2015;6:1–25. [Google Scholar]
- 34.Maxwell PS, et al. The fundamental role of ecological feedback mechanisms for the adaptive management of seagrass ecosystems–A review. Biol Rev Camb Philos Soc. 2017;92:1521–1538. doi: 10.1111/brv.12294. [DOI] [PubMed] [Google Scholar]
- 35.Miller BP, et al. A framework for the practical science necessary to restore sustainable, resilient, and biodiverse ecosystems. Restor Ecol. 2016;25:605–617. [Google Scholar]
- 36.Williams SL. Reduced genetic diversity in eelgrass transplantations affects both individual and population fitness. Ecol Appl. 2011;11:1472–1488. [Google Scholar]
- 37.Proffitt CE, Travis SE, Edwards KR. Genotype and elevation influence Spartina alterniflora colonization and growth in a created salt marsh. Ecol Appl. 2003;13:180–192. [Google Scholar]
- 38.Hughes AR, Stachowicz JJ. Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc Natl Acad Sci USA. 2004;101:8998–9002. doi: 10.1073/pnas.0402642101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Katwijk MM, et al. Guidelines for seagrass restoration: Importance of habitat selection and donor population, spreading of risks, and ecosystem engineering effects. Mar Pollut Bull. 2009;58:179–188. doi: 10.1016/j.marpolbul.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Proffitt CE, Travis SE. Red mangrove seedling survival, growth, and reproduction: Effects of environment and maternal genotype. Estuaries Coasts. 2010;33:890–901. [Google Scholar]
- 41.Reynolds LK, McGlathery KJ, Waycott M. Genetic diversity enhances restoration success by augmenting ecosystem services. PLoS One. 2012;7:e38397. doi: 10.1371/journal.pone.0038397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes AR. Genotypic diversity and trait variance interact to affect marsh plant performance. J Ecol. 2014;102:651–658. [Google Scholar]
- 43.van Katwijk MM, et al. Global analysis of seagrass restoration: The importance of large-scale planting. J Appl Ecol. 2006;53:567–578. [Google Scholar]
- 44.Thorhaug A. Large-scale seagrass restoration in a damaged estuary. Mar Pollut Bull. 1985;16:55–62. [Google Scholar]
- 45.Fonseca MS, Kenworthy WJ, Courntey FX, Hall MO. Seagrass planting in the southeastern United States: Methods for accelerating habitat development. Restor Ecol. 1994;2:198–212. [Google Scholar]
- 46.Statton J, Dixon KW, Hovey RK, Kendrick GA. A comparative assessment of approaches and outcomes for seagrass revegetation in Shark Bay and Florida Bay. Mar Freshwater Res. 2012;63:984–993. [Google Scholar]
- 47.Tanner JE, et al. Seagrass rehabilitation off metropolitan Adelaide: A case study of loss, action, failure and success. Ecol Manage Restor. 2014;15:168–179. [Google Scholar]
- 48.Riniatsih I, Endrawati H. Pertumbuhan lamun hasil transplantasi jenis Cymodocea rotundata di Pandang lamun Teluk Awur Jepara. Bull Oseanografi Mar. 2013;2:34–40. [Google Scholar]
- 49.Lanuru M. Third International Conference on Chemical, Biological and Environmental Engineering. Vol 20. Intl Assoc Comp Sci Information Technol; Singapore: 2011. Bottom sediment characteristics affecting the success of seagrass (Enhalus acoroides) transplantation in the westcoast of south Sulawesi (Indonesia) pp. 97–102. [Google Scholar]
- 50.Lewis RR. Ecological engineering for successful management and restoration of mangrove forests. Ecol Eng. 2005;24:403–418. [Google Scholar]
- 51.Kodikara KAS, Mukhergee N, Jayaatissa LP, Dahdouh-Guebas F, Koedam N. Have mangrove restoration projects worked? An in-depth study in Sri Lanka. Restor Ecol. 2017;25:705–716. [Google Scholar]
- 52.Ellison AM. Mangrove restoration: Do we know enough? Restor Ecol. 2000;8:219–229. [Google Scholar]
- 53.Rey Benayas JM, Newton AC, Diaz A, Bullock JM. Enhancement of biodiversity and ecosystem services by ecological restoration: A meta-analysis. Science. 2009;325:1121–1124. doi: 10.1126/science.1172460. [DOI] [PubMed] [Google Scholar]
- 54.Doherty JM, Callaway JC, Zedler JB. Diversity-function relationships changed in a long-term restoration experiment. Ecol Appl. 2011;21:2143–2155. doi: 10.1890/10-1534.1. [DOI] [PubMed] [Google Scholar]
- 55.Cooper WS. The fundamentals of vegetational change. Ecology. 1926;7:391–413. [Google Scholar]
- 56.Connell JH, Slatyer RO. Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat. 1977;111:1119–1144. [Google Scholar]
- 57.Turner T. Facilitation as a successional mechanism in a rocky intertidal community. Am Nat. 1982;121:729–738. [Google Scholar]
- 58.Williams SL. Experimental studies of Caribbean seagrass bed development. Ecol Monogr. 1990;60:449–469. [Google Scholar]
- 59.Donnelly MD, Walter L. Trapping of Rhizophora mangle propagules by co-existing early successional species. Estuaries Coasts. 2014;37:1562–1571. [Google Scholar]
- 60.Fonseca MS, Kenworthy WJ, Courtney FX. Development of planted seagrass beds in Tampa Bay, Florida, USA. I. Plant components. Mar Ecol Prog Ser. 1996;132:127–139. [Google Scholar]
- 61.Halpern BS, Silliman BR, Olden JD, Bruno JP, Bertness MD. Incorporating positive interactions in aquatic restoration and conservation. Front Ecol Environ. 2007;5:153–160. [Google Scholar]
- 62.Silliman BR, et al. Facilitation shifts paradigms and can amplify coastal restoration efforts. Proc Natl Acad Sci USA. 2015;112:14295–14300. doi: 10.1073/pnas.1515297112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lord D, Paling E, Gordon D. Review of Australian rehabilitation and restoration programs. In: Butler A, editor. Seagrass in Australia: Strategic Review and Development of an R & D Plan. CSIRO; Collingwood, VIC, Australia: 1999. pp. 65–115. [Google Scholar]
- 64.McKee KL, Rooth JE, Feller IC. Mangrove recruitment after forest disturbance is facilitated by herbaceous species in the Caribbean. Ecol Appl. 2007;17:1678–1693. doi: 10.1890/06-1614.1. [DOI] [PubMed] [Google Scholar]
- 65.Uhrin AV, Hall MO, Merello MF, Fonseca MS. Survival and expansion of mechanically transplanted seagrass sods. Restor Ecol. 2009;17:359–368. [Google Scholar]
- 66.Brooker RW, et al. Facilitation in plant communities: The past, the present, and the future. J Ecol. 2008;96:18–34. [Google Scholar]
- 67.Fonseca MS, Kenworthy WJ, Julius BE, Shutler S, Fluke S. Seagrasses. In: Perrow MR, Davy AJ, editors. Handbook of Ecological Restoration. Vol 2. Cambridge Univ Press; Cambridge, UK: 2002. pp. 149–170. [Google Scholar]
- 68.Schmid B, Hector A, Saha P, Loreau M. Biodiversity effects and transgressive overyielding. J Plant Ecol. 2008;1:95–102. [Google Scholar]
- 69.Duffy JE, Richardson JP, France KE. Ecosystem consequences of diversity depend on food chain length in estuarine vegetation. Ecol Lett. 2005;8:301–309. [Google Scholar]
- 70.Cardinale BJ, et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 71.Cardinale BJ, Palmer MA, Collins SL. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature. 2002;415:426–429. doi: 10.1038/415426a. [DOI] [PubMed] [Google Scholar]
- 72.Griffin JN, et al. Biodiversity and the stability of ecosystem functioning. In: Naeem S, Bunder DI, Hector A, Loreau M, Perrings C, editors. Biodiversity, Ecosystem Function, and Human Wellbeing. Oxford Univ Press; New York: 2009. pp. 78–93. [Google Scholar]
- 73.Fargione J, et al. From selection to complementarity: Shifts in the causes of biodiversity-productivity relationships in a long-term biodiversity experiment. Proc Biol Sci. 2007;274:871–876. doi: 10.1098/rspb.2006.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petchey OL. Integrating methods that investigate how complementarity influences ecosystem functioning. Oikos. 2003;101:323–330. [Google Scholar]
- 75.Callaway RM, Walker PR. Competition and facilitation: A synthetic approach to interactions in plant communities. Ecology. 1997;78:1958–1965. [Google Scholar]
- 76.Lindig-Cisneros R, Zedler JB. Halophyte recruitment in a salt marsh restoration. Estuaries. 2002;25:1174–1183. [Google Scholar]
- 77.Vermaat JE, et al. Meadow maintenance, growth and productivity of a mixed Philippine seagrass bed. Mar Ecol Prog Ser. 1995;124:215–225. [Google Scholar]
- 78.Marbà N, Duarte CM. Rhizome elongation and seagrass clonal growth. Mar Ecol Prog Ser. 1998;174:269–280. [Google Scholar]
- 79.Brouns JJWM. Quantitative and dynamic aspects of a mixed species seagrass meadow in Papua New Guinea. Aquat Bot. 1987;29:33–47. [Google Scholar]
- 80.Rollon RN, De Ruyter Van Steveninck ED, Vierssen WV. Contrasting recolonization strategies in multi-species seagrass meadows. Mar Pollut Bull. 1999;37:8–12. [Google Scholar]
- 81.Dawson SP, Dennison WC. Effects of ultraviolet and photosynthetically active radiation on five seagrass species. Mar Biol. 1996;125:629–638. [Google Scholar]
- 82.Duarte CM, et al. Response of a mixed Philippine seagrass meadow to experimental burial. Mar Ecol Prog Ser. 1997;147:285–294. [Google Scholar]
- 83.Williams SL, Heck KL., Jr . Seagrass communities. In: Bertness M, Gaines S, Hay H, editors. Marine Community Ecology. Sinauer; Sunderland, MA: 2001. pp. 317–337. [Google Scholar]
- 84.Pogoreutz C, Kneer D, Litaay M, Asmus H, Ahnelt H. The influence of canopy structure and tidal level on fish assemblages in tropical Southeast Asian seagrass meadows. Estuar Coastal Shelf Sci. 2012;107:58–68. [Google Scholar]
- 85.Ambo-Rappe R, Nessa MN, Latuconsina H, Lajus DL. Relationship between tropical seagrass bed characteristics and the structure of the associated fish community. Open J Ecol. 2013;3:331–342. [Google Scholar]
- 86.Duarte CM, Sintes T, Marbà N. Assessing the CO2 capture potential of seagrass restoration projects. J Appl Ecol. 2013;50:1341–1349. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.