Significance
How eating animals affect the evolution of fruit-producing plants is still poorly understood. This is important because large vertebrates are being overhunted in ecosystems around the world, which might force plants to alter phenotypes in response to the loss of these animals. I used a natural experiment in which many of the same plant lineages in a large and megadiverse tropical archipelago have been exposed to very different suites of vertebrates on different islands. Statistical analysis that accounts for species’ shared evolutionary histories revealed that average fruit sizes across more than 400 plant species were positively related to the diversity and size of the fruit-eating birds and mammals in each area. Fruit color, however, was not affected by the vertebrate assemblage.
Keywords: defaunation, hunting, seed dispersal, species interactions, Wallace Line
Abstract
Large, fruit-eating vertebrates have been lost from many of the world’s ecosystems. The ecological consequences of this defaunation can be severe, but the evolutionary consequences are nearly unknown because it remains unclear whether frugivores exert strong selection on fruit traits. I assessed the macroevolution of fruit traits in response to variation in the diversity and size of seed-dispersing vertebrates. Across the Indo-Malay Archipelago, many of the same plant lineages have been exposed to very different assemblages of seed-dispersing vertebrates. Phylogenetic analysis of >400 plant species in 41 genera and five families revealed that average fruit size tracks the taxonomic and functional diversity of frugivorous birds and mammals. Fruit size was 40.2–46.5% smaller in the Moluccas and Sulawesi (respectively), with relatively depauperate assemblages of mostly small-bodied animals, than in the Sunda Region (Borneo, Sumatra, and Peninsular Malaysia), with a highly diverse suite of large and small animals. Fruit color, however, was unrelated to vertebrate diversity or to the representation of birds versus mammals in the frugivore assemblage. Overhunting of large animals, nearly ubiquitous in tropical forests, could strongly alter selection pressures on plants, resulting in widespread, although trait-specific, morphologic changes.
Defaunation, the loss of large vertebrates from natural ecosystems, is increasingly recognized as a threat to global biodiversity that must be addressed with the same urgency as habitat loss and climate change (1–3). Defaunation is nearly ubiquitous: In much of the world, including many areas that still retain natural habitat, large animals have been nearly or completely extirpated (1, 3, 4). Because many vertebrates play key ecological roles, their loss can have widespread impacts on the ecosystem. For example, many large mammals and birds are important dispersers of plant seeds, and in defaunated areas, plants can suffer recruitment decline, local extinction, and shifts in species composition (5–7). In extreme cases, plants can potentially be “orphaned” by complete loss of the large frugivores upon which they had formerly relied for seed transport (8, 9).
While the ecological impacts of defaunation are becoming clearer, the evolutionary implications of vertebrate loss for plants are all but unknown (1, 2, 7). Our inability to predict the evolutionary implications of defaunation is largely due to our limited understanding of the importance of animals for the evolution of plant reproductive traits. A large and contentious body of scientific work has assessed whether frugivorous vertebrates have shaped the evolution of fruit traits (e.g., refs. 10–13). Various fruit traits such as size and color tend to be correlated, forming what are known as “dispersal syndromes” that may have arisen to attract particular dispersers. Bird-dispersed fruits, for example, are thought to be generally small, red or black, and borne in leaf axils, whereas mammal-dispersed fruits are larger, dull in coloration, and borne on large branches or tree trunks (10, 12). Diet breadths of frugivore species can be quite large (14), and the composition of the animal assemblage dispersing a given plant can be inconsistent in space and time (15, 16). Thus, many “bird-dispersed” and “bat-dispersed” fruits are actually consumed by both birds and bats, as well as a number of other animals (14). Nevertheless, there do appear to be at least some consistent patterns whereby particular frugivore species prefer particular constellations of fruit traits (12, 17).
The problem with dispersal syndromes, however, lies in determining the direction of causality. Because nearly all studies on dispersal syndromes have been correlative, we have very little understanding of whether different frugivores induce different selection pressures, and whether this generates or maintains variation in fruit traits. On the one hand, it could be that dispersal syndromes have arisen in response to differential selection imposed by (for example) frugivory from birds versus mammals (10, 12, 17). On the other hand, fruit traits could be correlated because of physiological constraints (18, 19) or plant life-history tradeoffs (14, 20), and it could be that different types of frugivorous animals simply prefer to feed on different types of fruits, whose correlated traits evolved for reasons unrelated to selection from seed-dispersing animals. Indeed, fruit traits may be under selection from a number of sources other than seed dispersers, as fruit and seed morphology can be important to seedling growth (21), rot resistance (22), and seed predation (23).
In a recent natural experiment, seed size of the Neotropical palm Euterpe edulis was estimated to have declined ∼30% in areas that had lost large-bodied frugivorous birds but where smaller-bodied species remained extant (24, 25). (Smaller animals have narrower gape widths and tend to be restricted to consuming smaller fruits.) So, on the one hand, a shift in the frugivore assemblage from a wide size range to only small species may induce directional selection leading to smaller fruits. On the other hand, a number of Neotropical plants thought to be adapted to having their seeds dispersed by large Pleistocene mammals retained their “megafaunal” fruit traits for ∼10 millennia after the extinction of their putative seed vectors (9). Dispersal of these megafaunal fruits may have been replaced by scatter-hoarding rodents or humans (26), but without knowing whether traits such as seed size were affected by megafauna loss (26), we cannot assess the evolution of fruit traits in response to altered selection.
To assess the general importance of frugivorous vertebrates for plant evolution, two critical questions remain. First, how common are responses to defaunation, such as those described above for the palm E. edulis (24, 25), across plant taxa? Second, what is the extent to which traits might change over longer time periods (e.g., moving from centuries to millennia)? Indeed, understanding the ubiquity and significance of long-term “evolutionary cascades” is critical, given the widespread nature of defaunation (1, 3).
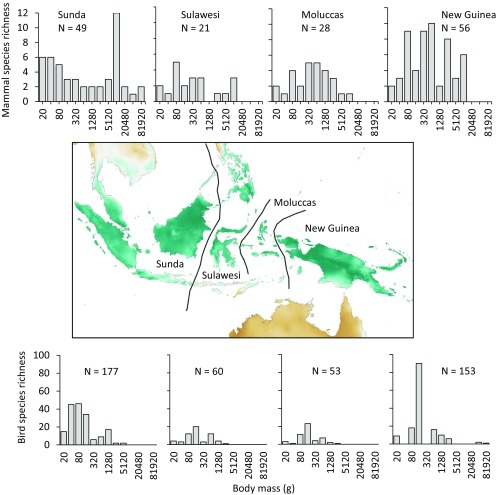
I used a natural experiment to assess macroevolutionary changes in fruit traits in response to long-term natural variation in frugivore taxonomic and functional diversity. This is not meant to be a literal assessment of the implications of ongoing overhunting in tropical forests but, rather, an examination of long-term plant trait evolution in response to dramatic variation in the number and size of fruit-eating vertebrates. The study system used here, the Indo-Malay Archipelago (excluding the Lesser Sunda Islands), has several strong zoogeographic barriers, including the famous Wallace Line, that mark dramatic turnovers in vertebrate composition and diversity (27) across island groups that are broadly similar in climatic conditions (Fig. 1 and SI Appendix, Fig. S1). However, these biogeographic barriers are much weaker for plants: while there is turnover at the species level, at higher taxonomic levels, the archipelago is fairly homogenous botanically (SI Appendix, Table S1). Thus, myriad plant lineages evolved in Asia (the western end of the archipelago) or New Guinea (the eastern end), where they were exposed to diverse assemblages of frugivorous mammals and birds across a range of body sizes. When these plants then spread across the archipelago to Sulawesi and the Moluccas (island groups that have not been connected to continental land masses), they became sympatric with much more depauperate assemblages of mostly small-bodied birds (Fig. 1). Analogously, in contemporary defaunation, overhunting and habitat fragmentation (for example) drive the erosion of vertebrate diversity, preferentially driving the near or complete loss of large-bodied species (1–4, 7).
Fig. 1.
Zoogeographic subregions of the Indo-Malay Archipelago. Histograms show taxonomic diversity (number of species, N) and body size diversity (in log2 bins) for the frugivorous mammal and bird groups. Map color shows the gradient in rainfall seasonality from aseasonal (blue) to seasonal (brown).
Though clearly not a controlled manipulation, this natural experimental approach is distinct from a purely observational approach (28), with inference based on replication (i.e., across plant linages) and explicit hypothesis testing (i.e., to explain differences in fruit traits) based on analysis of variation in the factors hypothesized to affect fruit traits (i.e., frugivore diversity) (29).
I assessed, in a phylogenetically explicit analysis, fruit traits of 442 species (representing 41 genera in five families) in the Sapindales. This taxonomic order is pantropical and is noted for its high proportion of animal-dispersed (often mammal-dispersed) species (30). It contains many species that provide food resources for frugivorous animals, as well as wild relatives of human food plants such as mangoes (Mangifera spp.), pistachios (Pistacia spp.), rambutan (Nephelium spp.), lychee (Litchi chinensis), and citrus (Citrus spp.). If declines in frugivore taxonomic and functional diversity, as occurs with defaunation, importantly alter the selective pressures on plants, then fruits in Sulawesi and the Moluccas should be smaller than those in the Sunda Region and New Guinea. Fruits may also have changed color in response to frugivores assemblages that differ in their proportions of birds versus mammals; these two vertebrate groups use different visual and olfactory cues for foraging (17, 31).
Results
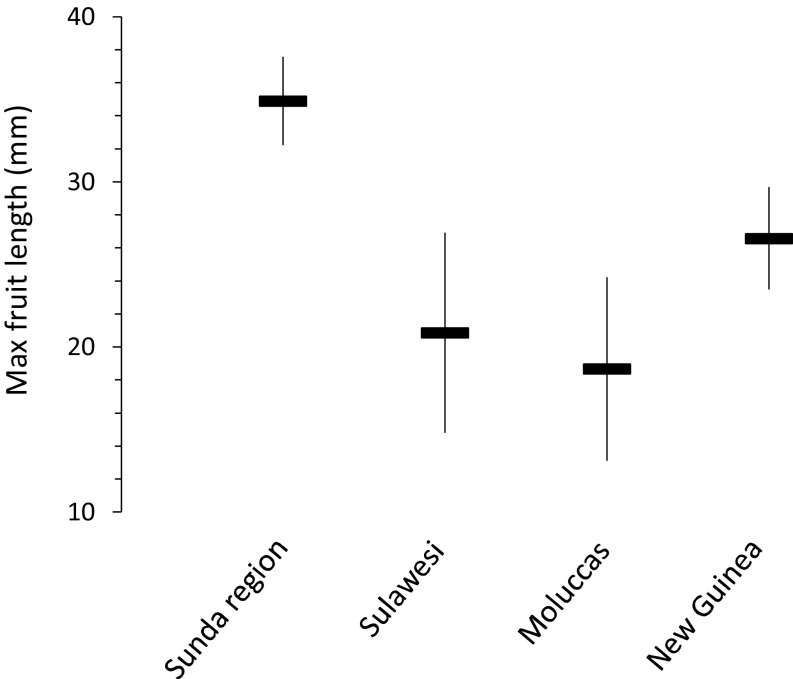
Phylogenetic generalized least-squares analysis (PGLS; ref. 32) revealed that fruits in the Sunda Region were significantly larger than those in Sulawesi and the Moluccas, based on regression coefficients (Table 1 and SI Appendix, Table S5) and comparison against an ecological null model in which traits were randomized across the phylogeny (SI Appendix, Fig. S5). Predictions from the best-fit Ornstein-Uhlenbeck model suggest that average maximum fruit lengths were 40.2% and 46.5% smaller in Sulawesi and the Moluccas, respectively, than in the Sunda Region (Fig. 2 and SI Appendix, Tables S4 and S5). Fruit size differences across the subregions of the Archipelago could be consistent with frugivore-induced selection or else, potentially, with area effects. Sulawesi and the Moluccas are smaller than the Sunda Region and New Guinea, and changes in size-related traits are common on islands. But recent evidence suggests that seed size, across plants with different dispersal modes, actually tends to increase on islands relative to mainland areas (33). The opposite pattern that I observed is, therefore, more consistent with altered selection pressure driven by the absence of large frugivores. Moreover, in a follow-up analysis, I used PGLS to assess geographic changes in two other size-related traits available in the Flora Malesiana, and found that variation in neither leaf length (P > 0.17) nor flower length (P > 0.05) was significantly related to subregion.
Table 1.
Coefficients (β), SEs, and P values from the most parsimonious phylogenetically explicit regression models assessing differences in each fruit trait across the subregions of the Indo-Malay Archipelago
| Fruit trait | Model | No. of species | Sundaland | Sulawesi | Moluccas | New Guinea | ||||
| β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | |||
| Maximum length, mm | Ornstein-Uhlenbeck | 385 | 13.61 (3.44) | <0.01 | −0.42 (4.32) | 0.92 | −2.61 (4.00) | 0.52 | 5.30 (3.48) | 0.13 |
| Color (red or black vs. other colors) | Logistic | 265 | −0.01 (0.16) | 0.97 | −0.14 (0.16) | 0.38 | 0.07 (0.17) | 0.66 | −0.27 (0.17) | 0.11 |
Full model-selection results are shown in SI Appendix, Table S4. A total of 442 species were included in the analysis, but not all species have both fruit length and fruit color information available.
Fig. 2.
Phylogenetically independent average (±SE) maximum fruit lengths in the subregions of the Indo-Malay Archipelago that differ in taxonomic and body size diversity of frugivorous vertebrates (see Fig. 1).
I assessed changes in fruit color across the subregions of the archipelago, using phylogenetic generalized linear models (PGLM; ref. 34). The categorization scheme that distinguished “red” or “black” fruits from all other colors (SI Appendix, Table S2) received overwhelming support from Akaike Information Criterion (AIC)-based model selection analysis (SI Appendix, Table S3). The proportion of fruits that were red or black did not differ significantly among subregions of the archipelago (SI Appendix, Table S5).
Discussion
Galetti et al. (24) observed a reduction in mean seed size in a Neotropical palm species relatively quickly (within ∼200 y) following the loss of large-bodied frugivores in habitat fragments. Fruit size and seed size are often highly correlated (phylogenetically explicit regression of fruit length vs. seed length in the data used here: β = 0.29; P < 0.001). The analysis of fruit size here, across five families, suggests that such declines in size may be widespread across plant lineages. Interestingly, the estimated magnitude of the change over the course of millennia that I detected (40.2–46.5% reduction in fruit length) is not substantially higher than what Galetti et al. (24) estimated (∼30% reduction) after just 2 centuries. This could suggest that, for some species at least, fruit trait changes occur quickly following alterations to the frugivore assemblage, but then much more slowly or not at all for long periods afterward, a form of punctuated equilibrium (cf. ref. 35).
The discrimination of red and black fruits from other colors is consistent with empirical studies, which frequently suggest that red and black fruits are preferred by birds (36–38). The fact that fruit color was not related to variance in frugivore diversity also supports previous research that has suggested that fruit or seed size may be the only trait that responds to selection from frugivores (11). While many fruit characteristics are correlated (10, 12), evolution of traits such as color in response to selection from frugivores can be constrained by physiology (19) or diluted by selection on other traits (with potentially pleiotropic gene control) such as fruit placement (14) or leaf reflectance (20).
These results demonstrate an association between frugivore diversity (taxonomic and functional) and fruit size. Several lines of evidence suggest that fruit size is responding to frugivores, rather than the other way round. First, empirical evidence suggests that the expectation would be for seed (and therefore fruit) size to increase on small islands (33), but I found the opposite result in this system. Second, Sulawesi and the Moluccas have fewer large-bodied vertebrates in guilds besides just frugivores; there are many more large species of granivore, carnivore, and nonvolant insectivore in Sundaland and New Guinea than on the islands in between (SI Appendix, Figs. S6–S8). Colonization of oceanic islands can be a strong filter, and has likely limited the diversity of vertebrates on Sulawesi and the Moluccas. The distribution of body sizes in these depauperate assemblages is therefore narrower (SI Appendix, Figs. S6–S8), with large bodied (and often very small bodied) species missing in a range of feeding guilds. In contrast, volant insectivores and frugivores (which clearly have greater colonization abilities) do not necessarily have fewer large-bodied species on the oceanic islands than in Sundaland and New Guinea (SI Appendix, Figs. S9 and S10). Having fewer and smaller frugivores could then have altered the selection pressure on plants that had originated in areas with diverse and large seed-dispersing animals.
Fruit traits could reflect selection not just from extant frugivores but also from extinct species as well (8, 9). All of the subregions of the Indo-Malay Archipelago had species of megafauna that could have been important seed dispersers into the late Pleistocene (39, 40): elephants formerly lived in Sulawesi; elephant-like stegodons and giant tortoises in Sundaland, Sulawesi, and the Moluccas; and large marsupials (diprotodontids and kangaroos) in New Guinea (SI Appendix, Table S6). But as shown here, Sapindales fruits today are, on average, smaller in Sulawesi and the Moluccas than they are in Sundaland, where frugivorous megafauna remain extant and were probably always more diverse. It might be that fruits in Sulawesi and the Moluccas were larger in the late Pleistocene than they are today and underwent relatively rapid reductions in size following megafauna loss. Alternatively, megafauna might have less impact on fruit trait evolution than do smaller but more abundant vertebrates; indeed, the most important frugivore groups in tropical East Asia are thought to be birds, primates, and bats (41). Asian elephants (Elephas maximus) seem to have had relatively little influence on vegetation traits, at least in comparison with African forest elephants (Loxodonta cyclotis) (42).
In an increasingly defaunated world, both the ecology of natural communities and the evolutionary trajectories of lineages may be altered. Many of the large frugivores in Southeast Asia, such as orangutans (Pongo spp.), elephants, rhinoceroses, and certain hornbills, are highly threatened with extinction. Loss of these seed-dispersing mutualists could potentially drive an unknown number of plant species extinct (1). For those plants that remain, we might expect dramatic reductions in fruit size to maintain seed dispersal by the fewer and smaller frugivores in the forests of the future. This could lead to a homogenization of fruit sizes across the region (or, indeed, across tropical forests). Such a loss of functional diversity over ecological or evolutionary timescales could even, in turn, alter the global carbon cycle because trees with seeds dispersed by large animals tend to hold more biomass (43, 44).
Materials and Methods
I assembled a database of fruit traits of the Sapindales from the Flora Malesiana project (45–49) and a phylogeny of the order based on rbcL and atpB genes and the trnL-trnLF spacer region (50). Species-level phylogenies are not available for most plant taxa in Southeast Asia. I used the Sapindales phylogeny (50) as a genus-level backbone. For most of the genera with fruit trait information, the phylogeny only contained one species, which I replaced with a polytomy consisting of all of the species in that genus for which I had fruit trait data. For the few genera that had more than one species in the phylogeny, I randomly selected one species to replace with a polytomy and ensured that the remaining species in the phylogeny were not duplicates of any of those that I had inserted. I transformed the resulting phylogeny to make it ultrametric, using a penalized maximum likelihood method that allows evolutionary rates (and therefore branch lengths) to vary across lineages (51). I examined a range of values for the rate-smoothing parameter and chose the value (λ = 0) with the maximum log-likelihood (SI Appendix); the final phylogeny is shown in SI Appendix, Fig. S13. I performed PGLS (for fruit length) or PGLM (fruit color) analysis on both ultrametric and nonultrametric phylogenies (full model results shown in SI Appendix, Tables S4 and S5).
For the PGLS analysis, I compared models with different assumptions about evolutionary rates including Brownian motion (52) and Ornstein-Uhlenbeck (53) models. For the PGLM logistic analyses, I used two estimators: one based on maximized penalized likelihood and the other on generalized estimating equations approximations to the penalized likelihood. Full model selection results are shown in SI Appendix, Table S4.
In addition to the linear models described here, I also assessed whether subregional variation in fruit length could have exhibited threshold effects such that the subregions differed in the mean size of, for example, “large” but not “small” fruits. I examined a range of size thresholds from 18 to 45 mm in maximum length, using segmented linear model analysis. Based on AIC model selection, the most parsimonious thresholds were 26–28 mm. But the segmented linear analysis was vastly outperformed by the nonsegmented linear analysis (ΔAIC > 20), so only the latter are reported.
Fruit color information in Flora Malesiana (45–49) is descriptive rather than quantitative: I tested several schemes by which to convert the information into binary data for phylogenetically explicit generalized linear model analysis. In the first (“Fruit_color1” in SI Appendix, Table S2), fruits that were qualitatively “bright” (e.g., red, orange, yellow, or white) were assigned to 1 and “dull” fruits (e.g., black, brown, green) assigned to 0. In the next three categorization schemes, fruits that were red or black were assigned a 1, and other fruits were assigned 0; this is based on empirical measurements of fruit preference in birds (36–38); indeed, Schmidt et al. (ref. 54, p. 551) noted a “global prevalence” of red and black fruits, likely driven by conspicuousness of those colors to avian frugivores. These three binary classification schemes differ in their strictness: “Fruit_color2” includes only fruits labeled “red” or “black,” “Fruit_color3” includes fruits with hyphenated color descriptions but where “red” or “black” is in the first position (e.g., “red-orange”), “Fruit_color4” includes fruits with hyphenated color descriptions where “red” or “black” appear anywhere (e.g., “orange-red”). The next three color categorization schemes assign 1 to red, black, or blue fruits, because blue fruits may also be selected by some bird species (18, 37). Classification schemes “Fruit_color5,” “Fruit_color6,” and “Fruit_color7” again differ in their strictness. Note, however, that there were no fruits classified simply as “blue,” so “Fruit_color2” and “Fruit_color5” are identical. Therefore, only the former was explicitly analyzed.
These different color categorization schemes (SI Appendix, Table S2) were then compared by using each, separately, as the response variable in a phylogenetically explicit generalized linear model with a logistic link and a penalized maximum likelihood estimator.
Supplementary Material
Acknowledgments
A. Longakit, J. Yangel, and D. Holinda assisted with data preparation. J. Maron and several anonymous reviewers provided valuable feedback on previous versions of the manuscript. Funding was provided by the University of British Columbia and the University of Montana.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission. P.J. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710172114/-/DCSupplemental.
References
- 1.Dirzo R, et al. Defaunation in the anthropocene. Science. 2014;345:401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 2.Galetti M, Dirzo R. Ecological and evolutionary consequences of living in a defaunated world. Biol Conserv. 2013;163:1–6. [Google Scholar]
- 3.Young HS, McCauley DJ, Galetti M, Dirzo R. Patterns, causes, and consequences of anthropocene defaunation. Annu Rev Ecol Evol Syst. 2016;47:333–358. [Google Scholar]
- 4.Milner-Gulland EJ, Bennett EL. Wild meat: The bigger picture. Trends Ecol Evol. 2003;18:351–357. [Google Scholar]
- 5.Peres CA, Emilio T, Schietti J, Desmoulière SJM, Levi T. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc Natl Acad Sci USA. 2016;113:892–897. doi: 10.1073/pnas.1516525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terborgh J, et al. Tree recruitment in an empty forest. Ecology. 2008;89:1757–1768. doi: 10.1890/07-0479.1. [DOI] [PubMed] [Google Scholar]
- 7.Brodie JF, et al. Secondary extinctions of biodiversity. Trends Ecol Evol. 2014;29:664–672. doi: 10.1016/j.tree.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Federman S, et al. Implications of lemuriform extinctions for the Malagasy flora. Proc Natl Acad Sci USA. 2016;113:5041–5046. doi: 10.1073/pnas.1523825113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janzen DH, Martin PS. Neotropical anachronisms: The fruits the gomphotheres ate. Science. 1982;215:19–27. doi: 10.1126/science.215.4528.19. [DOI] [PubMed] [Google Scholar]
- 10.Janson CH. Adaptation of fruit morphology to dispersal agents in a neotropical forest. Science. 1983;219:187–189. doi: 10.1126/science.219.4581.187. [DOI] [PubMed] [Google Scholar]
- 11.Jordano P. Angiosperm fleshy fruits and seed dispersers: A comparative analysis of adaptation and constraints in plant-animal interactions. Am Nat. 1995;145:163–191. [Google Scholar]
- 12.Lomáscolo SB, Levey DJ, Kimball RT, Bolker BM, Alborn HT. Dispersers shape fruit diversity in Ficus (Moraceae) Proc Natl Acad Sci USA. 2010;107:14668–14672. doi: 10.1073/pnas.1008773107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer KE, Chapman CA. Frugivores and fruit syndromes: Differences in patterns at the genus and species level. Oikos. 1993;66:472–482. [Google Scholar]
- 14.Harrison RD, Rønsted N, Xu L, Rasplus JY, Cruaud A. Evolution of fruit traits in Ficus subgenus Sycomorus (Moraceae): To what extent do frugivores determine seed dispersal mode? PLoS One. 2012;7:e38432. doi: 10.1371/journal.pone.0038432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman CA, Chapman LJ. Seed Dispersal and Frugivory: Ecology, Evolution, and Conservation. CABI Publishing; Wallingford, UK: 2002. Plant–animal coevolution: Is it thwarted by spatial and temporal variation; pp. 275–290. [Google Scholar]
- 16.Herrera CM. Long-term dynamics of Mediterranean frugivorous birds and fleshy-fruits: A 12-year study. Ecol Monogr. 1998;68:511–538. [Google Scholar]
- 17.Amico GC, Rodriguez-Cabal MA, Aizen MA. Geographic variation in fruit colour is associated with contrasting seed disperser assemblages in a south-Andean mistletoe. Ecography. 2011;34:318–326. [Google Scholar]
- 18.Valenta K, et al. It’s not easy being blue: Are there olfactory and visual trade-offs in plant signalling? PLoS One. 2015;10:e0131725. doi: 10.1371/journal.pone.0131725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitney KD. Comparative evolution of flower and fruit morphology. Proc Biol Sci. 2009;276:2941–2947. doi: 10.1098/rspb.2009.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns KC, Cazetta E, Galetti M, Valido A, Schaefer HM. Geographic patterns in fruit colour diversity: Do leaves constrain the colour of fleshy fruits? Oecologia. 2009;159:337–343. doi: 10.1007/s00442-008-1227-3. [DOI] [PubMed] [Google Scholar]
- 21.Moles AT, Westoby M. Seed size and plant strategy across the whole life cycle. Oikos. 2006;113:91–105. [Google Scholar]
- 22.Cipollini ML, Bohs L, Mink K, Paulk E, Böhning-Gaese K. Secondary metabolites of ripe fleshy fruits. Ecology and phylogeny in genus Solanum. In: Levey DJ, Silva WR, Galetti M, editors. Seed Dispersal and Frugivory. Ecology, Evolution and Conservation. CABI Publishing; Wallingford, UK: 2002. pp. 111–128. [Google Scholar]
- 23.Tewksbury JJ, Nabhan GP. Seed dispersal. Directed deterrence by capsaicin in chilies. Nature. 2001;412:403–404. doi: 10.1038/35086653. [DOI] [PubMed] [Google Scholar]
- 24.Galetti M, et al. Functional extinction of birds drives rapid evolutionary changes in seed size. Science. 2013;340:1086–1090. doi: 10.1126/science.1233774. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho CS, Galetti M, Colevatti RG, Jordano P. Defaunation leads to microevolutionary changes in a tropical palm. Sci Rep. 2016;6:31957. doi: 10.1038/srep31957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guimarães PR, Jr, Galetti M, Jordano P. Seed dispersal anachronisms: Rethinking the fruits extinct megafauna ate. PLoS One. 2008;3:e1745. doi: 10.1371/journal.pone.0001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace AR. The Geographical Distribution of Animals. Macmillan; London: 1876. [Google Scholar]
- 28.Diamond J, Robinson JA. Natural Experiments of History. Harvard Univ Press; Cambridge, MA: 2010. [Google Scholar]
- 29.Dawson MN. Natural experiments and meta‐analyses in comparative phylogeography. J Biogeogr. 2014;41:52–65. [Google Scholar]
- 30.Fleming TH, Kress WJ. A brief history of fruits and frugivores. Acta Oecol. 2011;37:521–530. [Google Scholar]
- 31.Lomáscolo SB, Schaefer HM. Signal convergence in fruits: A result of selection by frugivores? J Evol Biol. 2010;23:614–624. doi: 10.1111/j.1420-9101.2010.01931.x. [DOI] [PubMed] [Google Scholar]
- 32.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. GEIGER: Investigating evolutionary radiations. Bioinformatics. 2008;24:129–131. doi: 10.1093/bioinformatics/btm538. [DOI] [PubMed] [Google Scholar]
- 33.Kavanagh PH, Burns KC. The repeated evolution of large seeds on islands. Proc Biol Sci. 2014;281:20140675. doi: 10.1098/rspb.2014.0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho Ls, Ané C. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst Biol. 2014;63:397–408. doi: 10.1093/sysbio/syu005. [DOI] [PubMed] [Google Scholar]
- 35.Gould SJ, Eldredge N. Shaking the Tree: Readings from Nature in the History of Life. Univ Chicago Press; Chicago: 2000. Punctuated equilibrium comes of age. [Google Scholar]
- 36.Duan Q, Goodale E, Quan RC. Bird fruit preferences match the frequency of fruit colours in tropical Asia. Sci Rep. 2014;4:5627. doi: 10.1038/srep05627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan Q, Quan RC. The effect of color on fruit selection in six tropical Asian birds. Condor. 2013;115:623–629. [Google Scholar]
- 38.Gagetti BL, Piratelli AJ, Piña-Rodrigues FCM. Fruit color preference by birds and applications to ecological restoration. Braz J Biol. 2016;76:955–966. doi: 10.1590/1519-6984.05115. [DOI] [PubMed] [Google Scholar]
- 39.Corlett RT. Megafaunal extinctions and their consequences in the tropical Indo-Pacific. Terra Australis. 2010;32:117–131. [Google Scholar]
- 40.Corlett RT. The shifted baseline: Prehistoric defaunation in the tropics and its consequences for biodiversity conservation. Biol Conserv. 2013;163:13–21. [Google Scholar]
- 41.Corlett RT. The Ecology of Tropical East Asia. Oxford Univ Press; Oxford: 2009. [Google Scholar]
- 42.Corlett RT, Primack R. Tropical Rain Forests. 2nd Ed Wiley-Blackwell; West Sussex, UK: 2011. [Google Scholar]
- 43.Bello C, et al. Defaunation affects carbon storage in tropical forests. Sci Adv. 2015;1:e1501105. doi: 10.1126/sciadv.1501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brodie JF. How monkeys sequester carbon. Trends Ecol Evol. 2016;31:414–416. doi: 10.1016/j.tree.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Leenhouts PW, Kalkman C, Lam HJ. 1958. Burseraceae. Flora Malesiana, Series I, Spermatophyta: Flowering Plants, ed Van Steenis C (Noordhoff-Kolff N.V., Jakarta, Indonesia), Vol 5, pp 209–296.
- 46.Nooteboom HP. 1972. Simaroubaceae. Flora Malesiana, Series I, Spermatophyta: Flowering Plants, ed Van Steenis C (Wolters-Noordhoff Publishing, Groningen, The Netherlands), Vol 6, pp 193–226.
- 47.Hou D. 1978. Anacardiaceae. Flora Malesiana, Series I, Spermatophyta: Flowering Plants, ed Van Steenis C (Sijthoff & Noordhoff International Publishers, Alphen Aan Den Rijn, The Netherlands), Vol 8, pp 395–548.
- 48.Adema F, Leenhouts PW, van Welzen PC. 1994. Sapindaceae. Flora Malesiana, Series I, Spermatophyta: Flowering Plants, ed Malesiana FF (Rijksherbarium/Hortus Botanicus, Leiden University, Leiden, The Netherlands), Vol 11, pp 419–756.
- 49.Mabberley DJ, Pannell CM, Sing AM. 1995. Meliaceae. Flora Malesiana, Series I, Spermatophyta: Flowering Plants, ed Malesiana FF (Rijksherbarium/Hortus Botanicus, Leiden University, Leiden, The Netherlands), Vol 12, pp 1–388.
- 50.Muellner-Riehl AN, et al. Molecular phylogenetics and molecular clock dating of Sapindales based on plastid rbcL, atpB and trnL-trnF DNA sequences. Taxon. 2016;65:1019–1036. [Google Scholar]
- 51.Sanderson MJ. Estimating absolute rates of molecular evolution and divergence times: A penalized likelihood approach. Mol Biol Evol. 2002;19:101–109. doi: 10.1093/oxfordjournals.molbev.a003974. [DOI] [PubMed] [Google Scholar]
- 52.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 53.Martins EP, Hansen TF. Phylogenies and the comparative method: A general approach to incorporating phylogenetic information into the analysis of interspecific data. Am Nat. 1997;149:646–667. [Google Scholar]
- 54.Schmidt V, Schaefer HM, Winkler H. Conspicuousness, not colour as foraging cue in plant-animal signalling. Oikos. 2004;106:551–557. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.