Significance
The enteric nervous system of vertebrates arises mostly from a rostral portion of the neural crest, encapsulated by the term “vagal.” We show that the “vagal crest” is in fact a juxtaposition of two completely different types of cells: Schwann cell precursors associated with the vagus nerve, which provide esophageal neurons, and the rostral-most trunk crest, which also forms sympathetic ganglia and locally overshoots the aorta to colonize most of the gut. Moreover, in line with the known dependency of both Schwann cell precursors and trunk crest on the ErbB3 tyrosine receptor kinase and its ligand Neuregulin1, we discover that the enteric nervous system is also atrophic in ErbB3 mutants, with potential relevance to Hirschsprung disease, a congenital hypoganglionosis.
Keywords: enteric nervous system, neural crest, chicken, mouse, Neuregulin1
Abstract
Most of the enteric nervous system derives from the “vagal” neural crest, lying at the level of somites 1–7, which invades the digestive tract rostro-caudally from the foregut to the hindgut. Little is known about the initial phase of this colonization, which brings enteric precursors into the foregut. Here we show that the “vagal crest” subsumes two populations of enteric precursors with contrasted origins, initial modes of migration, and destinations. Crest cells adjacent to somites 1 and 2 produce Schwann cell precursors that colonize the vagus nerve, which in turn guides them into the esophagus and stomach. Crest cells adjacent to somites 3–7 belong to the crest streams contributing to sympathetic chains: they migrate ventrally, seed the sympathetic chains, and colonize the entire digestive tract thence. Accordingly, enteric ganglia, like sympathetic ones, are atrophic when deprived of signaling through the tyrosine kinase receptor ErbB3, while half of the esophageal ganglia require, like parasympathetic ones, the nerve-associated form of the ErbB3 ligand, Neuregulin-1. These dependencies might bear relevance to Hirschsprung disease, with which alleles of Neuregulin-1 are associated.
The enteric nervous system (ENS) is, for the most part, formed by one rostro-caudal wave of migrating neural crest-derived precursors that originate in the “vagal neural crest,” lying from the levels of somites 1–7 (refs. 1 and 2 and references therein). The progression of enteric precursors through the postgastric digestive tract has been extensively studied (3, 4), in particular with respect to its dependency on Glial-derived neurotrophic-factor (GDNF) signaling through the tyrosine kinase receptor Ret and its dimerization partner GFRα1. In contrast, the inception of the invasive process (i.e., the events that bring the vagal neural crest in the walls of the esophagus) remain controversial. Early observations inspired the hypothesis that enteric precursors were nerve-associated cells that followed the vagus (Xth) cranial nerve (which provides extrinsic innervation to the gut) (5). However, these studies ignored the neural crest as such and were evinced from the corpus of accepted knowledge once the neural crest origin of enteric neurons was firmly established (6, 7) and are now long forgotten. Moreover, enteric precursors were later spotted ahead of the incipient vagus nerve, which has thus been viewed as following and “overtaking” them (8). An ensuing paradox is that the adjective “vagal” has stuck to the enteric crest after the vagus nerve was no longer assigned any role. In mouse embryos, it was proposed that the vagal crest, defined as spanning somites 1–5 (9), colonizes most of the gut in addition to forming the superior cervical ganglion (and was hence called “sympatho-enteric”), while an adjacent “anterior trunk” (cervical) crest would populate the esophagus exclusively. This dichotomy, however, was never fully integrated in the canonical narrative of ENS development (e.g., ref. 10) and remains at odds with the situation in chicken, where the most-caudal vagal crest (corresponding to the anterior trunk crest of ref. 9) colonizes not the most rostral but the most caudal part of the digestive tract (11). More recently, the vagal crest was proposed as a transitional entity between the cranial and trunk region, where both a dorsal and a ventral migration pathway would take place in temporal succession (12). Finally, several mutations, while they completely block the rostro-caudal invasion of the gut mesenchyme by enteric precursors past the stomach, respect, to an extent or for a while, the colonization of the esophagus and stomach (see below). Altogether, this slim body of data, some of them contradictory, shows that foregut colonization by enteric precursors obeys rules different from the rest of the digestive tract, and is still poorly understood.
Results
Schwann Cell Precursors of the Vagus Nerve Contribute Neurons to the Foregut.
Null mutations in the genes for GDNF, its receptor GFRα1, its coreceptor Ret (9, 13–16), and for the pan-autonomic homeodomain transcription factor Phox2b (17), partially spare enteric neuronal precursors in a region that, strikingly, is coextensive with the stretch of the vagus nerve that travels alongside the digestive tract (Fig. S1): from the larynx down to the stomach, where the left vagus arborizes terminally and the right vagus veers off to join the prevertebral sympathetic plexi. This suggests that the vagus nerve itself could guide enteric precursors to the esophagus and stomach, independently of Phox2b or GDNF signaling, as it guides—and other cranial nerves guide—parasympathetic ganglionic precursors (18, 19). Evocative of such a mechanism was the fact that, at embryonic day (E) 11.5, the vagus nerve was covered with Sox10+, Phox2b+ cells coexpressing the Schwann cell precursor markers PLP-1 and Cadherin 19 (Fig. S2).
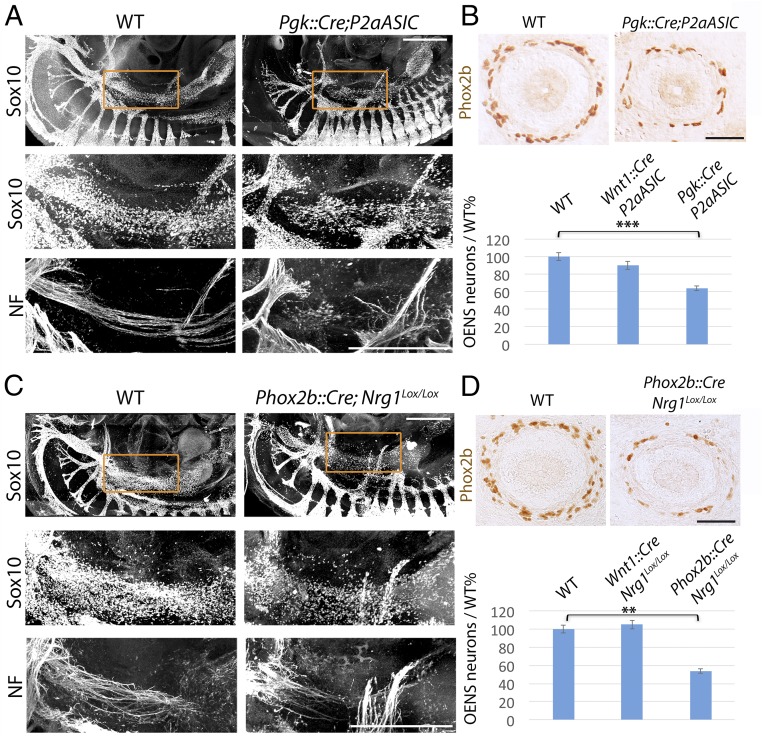
We investigated a role for the vagus nerve in the formation of esophageal ganglia in two ways. First, we prevented the formation of the nerve by deleting most neurons that project into it: viscerosensory neurons born in epibranchial placodes, as well as branchial and visceral motor neurons of the hindbrain were killed using a toxic variant of the sodium channel ASIC2a conditionally expressed from the promoter of Phox2a (18), the paralogue of Phox2b expressed in all these cell types (20). In Pgk:Cre;Phox2aASIC2a embryos, where Cre-mediated recombination occurs in the egg—thus where all Phox2a+ cells are killed by ASIC2a—the vagus nerve was reduced to a vestigial ramus, most likely composed of somatosensory fibers emanating from its proximal ganglion (Fig. 1A). Consequently, Sox10+ cells in the esophageal region were fewer at E11.5 (Fig. 1A) and, 2 days later, 36% of Phox2b+ neuronal precursors were missing in the wall of the esophagus (Fig. 1B). Second, we hampered signaling by the vagus nerve to its Schwann cell precursors through the epidermal growth factor family protein Neuregulin-1 (Nrg1) (21) by partnering a floxed allele of Nrg1 with a Cre recombinase driven by the Phox2b promoter, thus expressed in all cranial visceral sensory and motor neurons (20). Phox2b::Cre;Nrg1lox/lox embryos lacked Schwann cell precursors associated with the facial and glossopharyngeal nerves, which moreover appeared defasciculated (Fig. S3). Concordantly all parasympathetic ganglia appended to these nerves were missing 2 days later (Fig. S3), phenocopying the constitutive knockouts for the receptor of Nrg1, the tyrosine kinase receptor ErbB3, which has been documented after birth (19). Similarly, the vagus nerve was depleted of Schwann cell precursors and, concomitantly, the esophageal ganglia were atrophic by 46% (Fig. 1 C and D). The effect was noncell-autonomous, as shown by the lack of phenotype of Wnt1::Cre;Nrg1lox/lox embryos (Fig. 1D) and the lack of expression of Nrg1 by enteric precursors (Fig. S5). Thus, about half of the esophageal nervous system (or more if compensatory mechanisms take place in the mutants) derives from Schwann cell precursors of the vagus nerve.
Fig. 1.
Genetic damage to the vagus nerve depletes the esophageal nervous system. (A and C) Lateral views of whole-mount E11.5 (A) or E10.5 (C) embryos stained for Sox10 and Neurofilament, in the indicated genotypes. For each genotype, the Middle and Bottom panels are a magnified view of the area boxed in the Top panel. Atrophy of the vagus nerve (A), or deletion of Neuregulin-1 from vagal fibers (C), leads to depletion of the pool of Sox10+ cells (Middle) along the vagus path (Bottom) and defasciculation of the nerve (C). (B and D) (Upper) Cross-sections through the esophagus at E13.5 in the indicated genotypes, stained for Phox2b. (Lower) Count of Phox2b+ neuronal precursors in the esophagus at E13.5, in the indicated genotypes. Esophageal precursors were depleted in Pgk:Cre;Phox2aASIC2a (64 ± 1.6%/wild-type; P = 0.001, n = 4) and Phox2b::Cre;Nrg1lox/lox (54 ± 2.6%/wild-type; P = 0.004, n = 3) embryos, but were not significantly affected in Wnt1::Cre;Phox2aASIC2 a (90 ± 4.2%/wild-type; P = 0.898, n = 4) or in Wnt1::Cre;Nrg1lox/lox (105 ± 4.77%/wild-type; P = 0.842, n = 3) embryos. Error bars indicate SEM. **P < 0.005, ***P < 0.001. The Wnt1::Cre;Phox2aASIC2a and Wnt1::Cre;Nrg1lox/lox genetic backgrounds serve as controls, in which the expression of ASIC2a or the recombination of Nrg1 respectively, is targeted to the neural-crest derived enteric precursors, rather than all Phox2a+ or Phox2b+ cells. The lack of phenotype—most likely because only a small subset of enteric precursors express Phox2a (20), and none express Nrg1 at this stage (Fig. S5)—ensures that in Pgk:Cre;Phox2aASIC2aand Phox2b::Cre;Nrg1lox/lox embryos the enteric phenotype is noncell‐autonomous, and due to the damage of the Phox2a- or Phox2b-expressing components of the vagus nerve. (Scale bars: A and B, 500 µm; B and D, 50 µm.)
The Cervical Sympathetic Crest Contributes Most of the ENS.
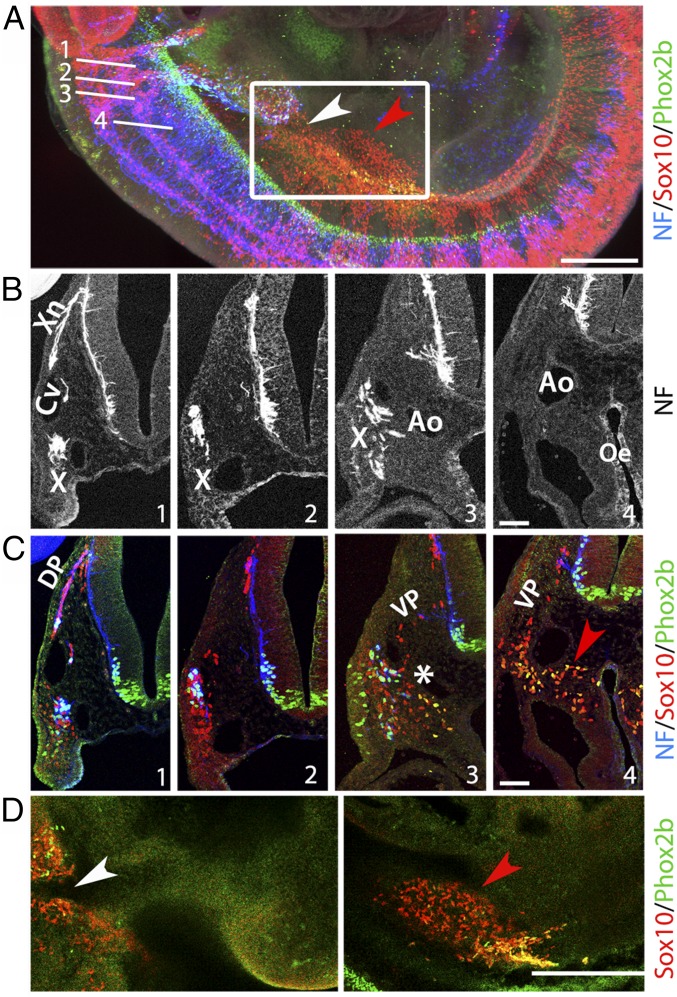
In contrast, the postgastric ENS was not affected in Pgk:Cre;Phox2aASIC2a and only mildly so in Phox2b::Cre;Nrg1lox/lox mutants (Fig. S4). Therefore, either a compensatory mechanism acts during the migration of enteric precursors to mitigate cell loss downstream of the stomach, or a second, nerve-independent population of cells invades the postgastric digestive tract (as well as the esophagus, since only half of its resident neurons are missing when the vagus nerve is damaged). Consistent with the latter hypothesis, we spotted a contingent of Sox10+ cells at E10 in continuity with the incipient cervical sympathetic chain at the level of the esophagus (red arrowhead in Fig. 2 A and D and Movie S1). On transverse sections (Fig. 2 B and C), these cells formed a stream that followed the ventral neural crest pathway (Fig. 2C, sections 3, 4, and compatible images in refs. 9, 12, and 22), passed by the lateral edges of the dorsal aortas, overshot them, and invaded the walls of the digestive tract (red arrowhead in Fig. 2C, section 4). The bulk of these “postvagal” (i.e., cervical) cells were mostly segregated from the rostrally situated vagus-associated ones (Fig. 2 A and D, white arrowhead), both populations mingling only at a narrow junction (white asterisk in Fig. 2C, section 3).
Fig. 2.
Two distinct streams of cells migrate to the esophagus in mouse embryos. (A) Lateral view of a whole mount at E10 stained with the indicated markers and showing the four planes of sections displayed in B and C and a boxed area enlarged in D. (B) Oblique transverse sections through an E10 wild-type mouse embryo along the four planes indicated in A, stained for neurofilament (NF, the signal in the lining of the esophagus is due to cross-reactivity of the secondary antibody), with indications of anatomical landmarks: Ao, dorsal aorta; Cv, cardinal vein; X, nodose ganglion; Xn, vagus nerve. (C) Same sections as in B costained for Phox2b, NF, and Sox10. At the more rostral levels (sections 1, 2) Sox10+ cells migrate along a dorsal pathway (DP), following the nascent vagus nerve, lateral to the anterior cardinal vein, and joining the forming nodose ganglion; at more caudal levels (sections 3, 4), a stream of Sox10+ cells follows the ventral migratory pathway (VP) and colonizes the sides of the dorsal aortas and the lateral walls of the esophagus (red arrowhead, also in A and D). The two populations are connected only at a narrow junction (white asterisk in section 3). (D) Sagittal optical sections at the level of the boxed area in A, along a lateral (Left) and more medial (Right) plane. The white arrowhead marks the discontinuity between the vagal-associated cell population and the postvagal one (red arrowhead). (Scale bars: A and D, 500 µm; B and C, 50 µm.)
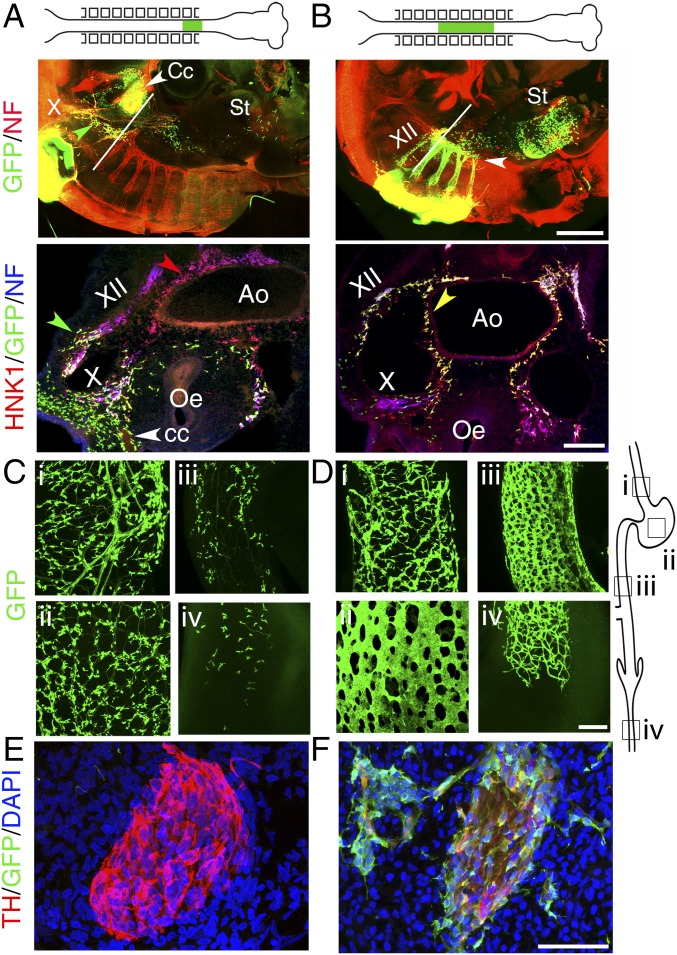
To directly demonstrate the contrasting migratory behavior of the two types of enteric crest—vagal proper and postvagal—we turned to the chicken embryo, where enteric neurons develop in a similar manner as in mouse (1, 2). We performed isotopic grafts of neural tubes from GFP transgenic chicken embryos into wild-type hosts. Grafts of neural tubes adjacent to somites 1 and 2 produced—in addition to the circumpharyngeal crest (marked Cc in Fig. 3A) that contributes to the heart and third branchial arch (23)—a neural crest that was intimately associated with the fibers of the vagus nerve at E3.5 (Fig. 3A). These cells correspond to the contingent of enteric precursors previously described as following a “dorsolateral” migration path (ref. 23 and references therein). At E7, these grafts had seeded mostly the esophagus and stomach, although a few cells could be found all of the way to the colon (Fig. 3C). In contrast, grafts adjacent to somites 3–7 produced exclusively a neural crest that followed the ventral path and invaded the digestive tract ahead of, and at a right angle to, the descending vagus nerve and its associated cells (Fig. 3B). At E7, cells arising from such grafts had contributed to the entire ENS (Fig. 3D). These different rostro-caudal extents of enteric colonization are compatible with previous reports using grafts with different limits, which blurred the sharp dichotomy (24).
Fig. 3.
Two distinct streams of cells migrate into the esophagus in chicken embryos. (A) E3.5 chicken embryo after a stage 10 isotopic graft of the neural tube facing somites 1 and 2, from a GFP transgenic donor to a wild-type host. (Upper) Whole-mount lateral view showing that the graft has produced circumpharyngeal crest (Cc) and cells associated with fibers, mostly in the vagus nerve (X) but also in a connecting meshwork between the hypoglossal nerve and the nodose ganglion (green arrowhead); a few cells have migrated ahead of the vagus nerve, all of the way to the stomach (St). (Lower) Transverse section at the level indicated on the Upper panel, showing that the crest produced by the graft reaches the esophagus (Oe) by following the vagus, not the sides of the aorta (Ao) which is populated only by HNK1+;GFP− cells (red arrowhead). (B, Upper) Same as above but with a graft facing somites 3–7. The graft has produced cells associated with spinal nerves—and the hypoglossal (XII)—the nascent sympathetic chain (white arrowhead), a few cells in the esophagus and many more cells in the stomach than for the somite1-2 grafts. (Lower) Transverse section at the level indicated on the Upper panel, showing that the crest from the graft, apart from colonizing the hypoglossal (XII), follows the ventral path and reaches the esophagus by circumnavigating the dorsal aorta (yellow arrowhead), not by following the vagus. (C and D) Whole-mount views of the digestive tube at E7 showing the presence of graft-derived cells, after somite1–2 grafts (C) versus somite 3–7 grafts (D), at the rostro-caudal levels indicated in the schematic on the right: (i) esophagus; (ii) gizzard; (iii) preumbilical intestine; (iv) colon. At this stage the colon is still incompletely colonized (level iv). (E and F) Sagittal sections through the superior cervical ganglion at E5.5, stained with the indicated markers. [Scale bars: A and B (Upper), 500 µm; A and B (Lower), 100 µm; C–F, 50 µm.]
An additional contrast between the vagal grafts (facing somites 1–2) and postvagal, cervical grafts (facing somites 3–7), is that the latter colonized the sympathetic chain (Fig. 3F and Fig. S6), while the former did not (Fig. 3E and Fig. S6). Even grafts restricted to the level of somite 3 or 4 contributed many cells to the superior cervical ganglia, mostly glia [as was previously observed with S1–S3 grafts (25)] and an occasional Th+ cell in the case of S4 grafts (Fig. S6). Thus, the sympathetic and enteric crest overlap, not only between somites 5 and 7, as previously recognized (26), but all of the way to somite 3 (but no further rostrally), and nothing distinguishes their ventral migratory paths toward the aorta.
Nrg1/ErbB3 Signaling Is Required for the Formation of the ENS.
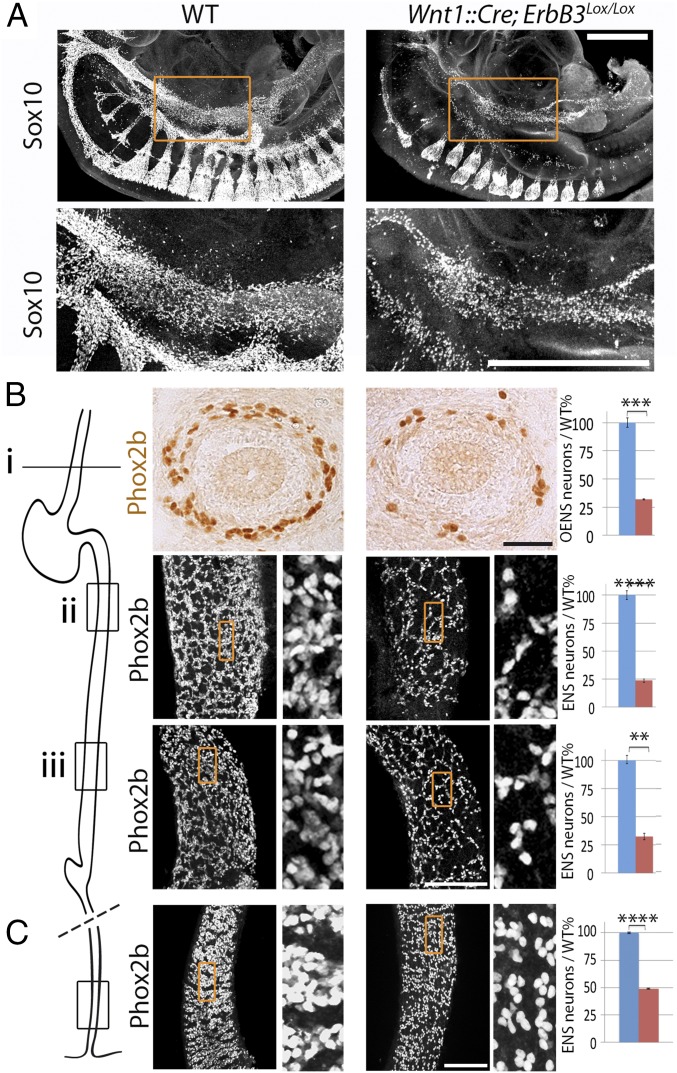
Schwann cell precursors colonize nerves by responding to axonal Nrg1 (21) (Fig. 1 and Fig. S3) through the tyrosine kinase receptor ErbB3. Sympathetic precursors also express ErbB3, through which they respond to mesenchymal Nrg1 that directs their ventral migration toward the aorta (27, 28). Consequently, the sympathetic chains are massively atrophic in ErbB3 knockouts (28) (white arrowheads in Fig. S7). Because at cervical levels the sympathetic crest is also the source of most of the ENS, we surmised that the latter should likewise depend on Erbb3. Following deletion of ErbB3 from the neural crest—in a Wnt1::Cre;ErbB3lox/lox background—the cervical sympatho-enteric population was depleted at E10 (red arrowheads in Fig. S7) and the corresponding region of the foregut contained fewer Sox10+ cells at E10.5 (Fig. 4A). At E13.5, the esophageal ganglia were atrophic by 68% (Fig. 4B) [i.e., more than in Phox2b::Cre;Nrg1lox/lox embryos (Fig. 1), likely due to the depletion of both the vagal and cervical contributions]. In addition, the postgastric ENS displayed a gradient of atrophy, from 76% just caudal to the stomach to 68% rostral to the cecum (Fig. 4B). To test whether the atrophy also affects the rectal aspect of the digestive tube (typically aganglionic in Hirschsprung disease), we examined the ENS at E17.5 and found a 51% atrophy in Wnt1::Cre;ErbB3lox/lox embryos (Fig. 4C). The diminishing rostro-caudal gradient of atrophy of the ENS suggests that the digestive tract provides compensatory proliferative signals.
Fig. 4.
The ENS is atrophic in the absence of the tyrosine kinase receptor ErbB3. (A) Lateral views of wholemount E10.5 embryos stained for Sox10, in the indicated genotypes. In mutants where ErbB3 is deleted from the neural crest, Sox10+ cells are partially depleted in the foregut. (Lower) Magnified views of the area boxed in the Upper panels. (B) Sections through the esophagus (Upper) and whole mounts of the midgut at two rostro-caudal levels [(Lower) low magnifications at Left, enlarged views (7× zoom) of the boxed area at Right] indicated on the schematic on the Left, in E13.5 wild-type (Left column) and conditional ErbB3 mutants (Right column), stained for Phox2b. The atrophy of the enteric ganglia is quantified on the graphs. Wnt1::Cre;ErbB3lox/lox mutants showed fewer esophageal precursors (level i: 32 ± 0.6%/wild-type; P = 0.003, n = 3) and postgastric precursors (level ii: 24 ± 1.3%/wild-type; P = 0.0001, n = 4; level iii: 32 ± 2.8%/wild type; P = 0.007, n = 4). (C). Whole mounts of the distal hindgut (enlarged views of the boxed area on the Right), in E17.5 wild-type (Left column) and conditional ErbB3 mutants (Right column) stained for Phox2b (49 ± 0.41%; P = 0,00001, n = 5. (Scale bars: A, 500 µm; B and C, 100 µm.). Error bars indicate SEM. **P < 0.01, ***P < 0.005, ****P < 0.0005.
Discussion
In sum, our data substantiate the proposal that the vagal crest is a pseudo or “hybrid” entity (12, 23). More precisely, we show that it is a juxtaposition of three radically different cell populations, two of them precursors for the ENS: on the one hand, emerging from somites 1 and 2, (i) the circumpharyngeal crest (destined to the heart and third branchial arch) and (ii) Schwann-cell precursors of the vagus nerve; on the other hand, emerging from somites 3–7, (iii) the cervical (upper cervical in chicken) region of the trunk crest.
Schwann cell precursors destined to the ENS behave like parasympathetic precursors (18): they migrate along a nerve and form autonomic ganglia at their final destination, here, enteric ganglia in the walls of esophagus and stomach. They derive from a crest that is vagal indeed, and more literally than the original term intended, since it populates the vagus nerve. Of note, vagus nerve-derived enteric precursors have been proposed back in the 1910s based on histological descriptions (5), but have been overlooked since then.
In contrast, the cervical crest migrates along the classically described ventral pathway toward the dorsal aorta—where it contributes to the sympathetic chain—and part of it, possibly the major part at that level, overshoots the dorsal aorta to invade the nearby esophageal mesenchyme. It remains to be explored what determines some cells to home close to the aorta and others to continue their voyage to the gut. This crest is thus sympatho-enteric and has nothing vagal about it, not even a registration with the vagal motor roots, as evidenced by the failure of isotopic grafts of the neural tube at that level to contribute fibers to the vagus nerve (Fig. 3B). [The term sympatho-enteric, which we repurpose, was originally coined to describe the crest from somites 1–5 (9), which straddles the two populations that we identify here, and therefore obscures their contrasted nature].
A third source of enteric precursors is the sacral crest, which contributes 20% of the neurons in the descending colon and rectum (29, 30). Since the major contribution of the sacral crest to the autonomic nervous system, the pelvic ganglion, is entirely sympathetic (31), the trunk crest is sympatho-enteric at both ends (Fig. 5). Given that thoracic crest will produce enteric neurons when transposed rostrally (32) and, more generally, that neural crest cells are not specified before migration (33, 34), the restriction of the dual sympatho-enteric fate to the cervical and sacral levels of the trunk crest is likely to stem, less from cell-intrinsic fate restriction than from topological factors, such as the continuity of the peri-aortic and foregut mesenchymes at one end, and the contiguity of the pelvic ganglion—a “staging site” for the enteric sacral crest (30, 35)—to the rectum at the other end.
Fig. 5.
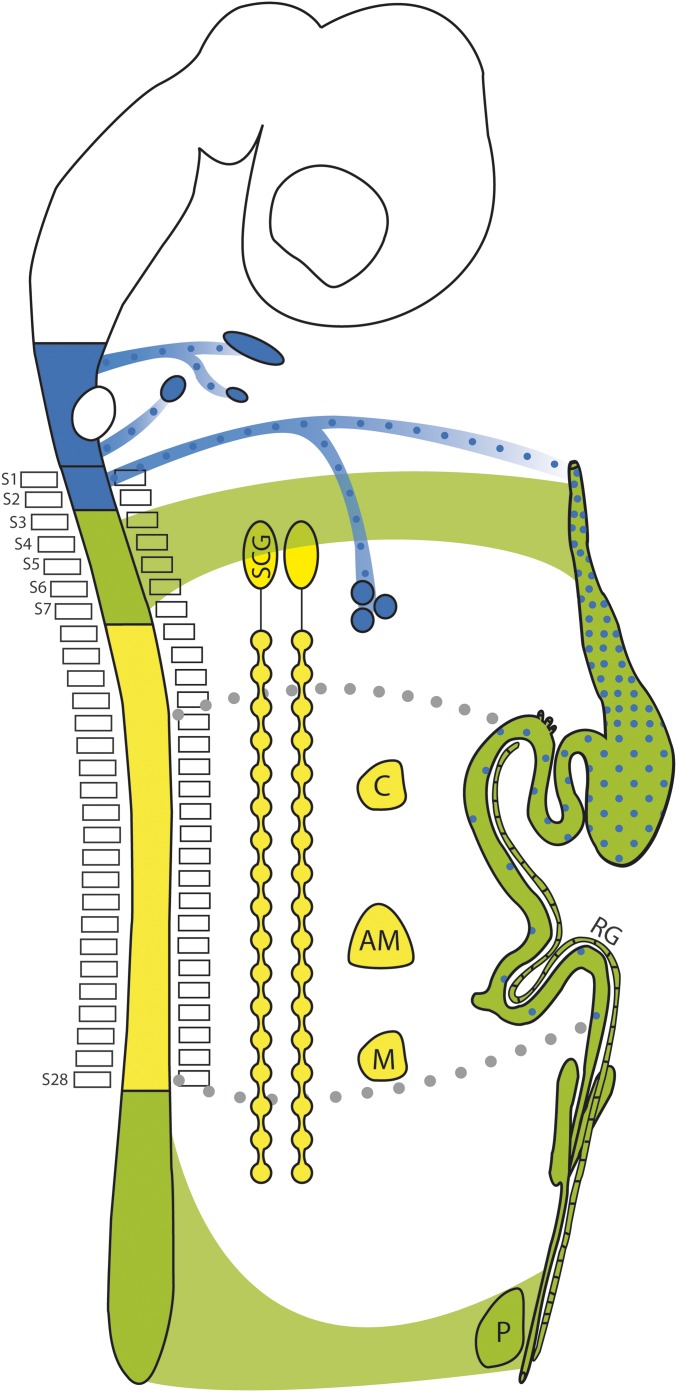
Rostro-caudal levels of the neural crest contribution to the ENS. Schematic of the central and autonomic nervous systems of a tetrapod showing the three types of neural crest cells that can be distinguished according to their fates: (i) sympathetic and sympathoadrenal (yellow) from somite 8–28, that contributes to most para- and prevertebral sympathetic ganglia (C: celiac; M: mesenteric) and the adrenal medulla (AM); (ii) sympatho-enteric (green) from somite 3–7 and caudal to somite 28, that contributes to the superior cervical ganglion (SCG) and forms the pelvic ganglion (P) [as well as the ganglion of Remak in chicken (RG)], and most of the ENS; and (iii) parasympatho-enteric (blue), from preotic levels to somite 2, that forms parasympathetic ganglia and contributes to the foregut nervous system. Gray dots represent postnatal contribution of Schwann cell precursors of enteric extrinsic nerves to the ENS (36).
A fourth, recently discovered source of enteric neurons are Schwann cell precursors at a later differentiation stage than the vagal ones we describe here, which travel along the mesenteric and pelvic nerves and become enteric neurons after birth (36). Many of them are presumably born in the thoracolumbar neural crest and it thus appears that the trunk crest has been co-opted in all of its regions (cervical, thoracolumbar, and sacral) to invade the gut, but at different stages of development, according to a variety of mechanisms and intermediates, in a seemingly opportunistic fashion. It will be interesting to explore the evolutionary history of this complicated and presumably stepwise assemblage. A recent study in lamprey (37) showed a contribution of the trunk crest but not of the vagal crest to the ENS. Since agnathans are suggested to have no sympathetic neural crest derivative (38), the absence of vagal contribution to the ENS fits with our data that the formerly called “vagal” crest of gnathostomes is for the most part cervical and sympathetic; but it also entails the surprising notion that the vagus nerve itself does not carry Schwann cell precursors-like enteroblasts to the gut of lampreys, when trunk nerves do.
Finally, after an early suggestion (39), we conclusively demonstrate a role for ErbB-mediated signaling during the embryonic development of the ENS. Better than the previous implication of ErbB/Nrg1 signaling in postnatal enteric ganglia in vivo (40) or in vitro (41), it could explain that common variants of Neuregulin-1, the ErbB3 ligand, are associated with Hirschsprung’s disease, which results from a partial agenesis of enteric ganglia (42). Given the missing heritability in Hirschprung disease, our results are also a suggestion to look for ErbB3 variants. Another possible clinical relevance is to neuropathic cases of chronic intestinal pseudo-obstruction (43).
Materials and Methods
In situ hybridization and immunochemistry have been described previously (44). Immunofluorescence on cryostat or vibratome sections was performed as previously described (18). Whole-mount immunofluorescent staining using the 3DISCO method was adapted from ref. 45, as previously described (31). Whole mounts of chicken embryos were treated as described in SI Materials and Methods. Transgenic chicken expressing the GFP reporter ubiquitously (46) were obtained from the Roslin Institut (University of Edinburgh). Chicken chimeras were generated via transplantation of discrete segments of the neural tube, including the neural crest, from GFP+ donors to wild-type hosts, as previously described (47). References for all antibodies and mouse lines are in SI Materials and Methods. Quantification of esophageal neurons and measurements of the surface occupied by enteric neuron nuclei in the postgastric ENS were performed by use of FIJI software, as described in more details in SI Materials and Methods. All animal studies were done in accordance with the guidelines issued by the French Ministry of Agriculture and have been approved by the Direction Départementale des Services Vétérinaires de Paris.
Supplementary Material
Acknowledgments
We thank the Imaging Facility of Institut de Biologie de l’Ècole Normale Supèrieure (IBENS); the animal facility of IBENS; C. Goridis for helpful comments on the manuscript; and all the members of the J.-F.B. laboratory for discussions. The imaging facility of IBENS is supported by grants from the Fédération pour la Recherche sur le Cerveau, Région Ile de France DIM NeRF 2009 and 2011 and France-BioImaging. This study was supported by the CNRS, the Ecole normale supérieure, INSERM, Agence Nationale de la Recherche Award ANR-12-BSV4-0007-01 (to J.-F.B.), Fondation pour la Recherche Médicale Award DEQ2000326472 (to J.-F.B.), the ‘Investissements d’Avenir’ program of the French Government implemented by the Agence Nationale de la Recherche (referenced ANR-10-LABX-54 MEMO LIFE and ANR-11-IDEX-0001-02 PSL* Research University). I.E.-M. received a fellowship from the French Ministry of Higher Education and Research and from the Fondation pour la Recherche Médicale (FDT20160435297).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710308114/-/DCSupplemental.
References
- 1.Uesaka T, Young HM, Pachnis V, Enomoto H. Development of the intrinsic and extrinsic innervation of the gut. Dev Biol. 2016;417:158–167. doi: 10.1016/j.ydbio.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Heanue TA, Shepherd IT, Burns AJ. Enteric nervous system development in avian and zebrafish models. Dev Biol. 2016;417:129–138. doi: 10.1016/j.ydbio.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366:64–73. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Lake JI, Heuckeroth RO. Enteric nervous system development: Migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol. 2013;305:G1–G24. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuntz A. The development of the sympathetic nervous system in mammals. J Comp Neurol Psychol. 1910;20:211–258. [Google Scholar]
- 6.Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–541. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- 7.Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- 8.Baetge G, Gershon MD. Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: Relationship to the development of enteric neurons. Dev Biol. 1989;132:189–211. doi: 10.1016/0012-1606(89)90217-0. [DOI] [PubMed] [Google Scholar]
- 9.Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- 10.Nagy N, Goldstein AM. Enteric nervous system development: A crest cell’s journey from neural tube to colon. Semin Cell Dev Biol. 2017;66:94–106. doi: 10.1016/j.semcdb.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns AJ, Delalande J-MM, Le Douarin NM. In ovo transplantation of enteric nervous system precursors from vagal to sacral neural crest results in extensive hindgut colonisation. Development. 2002;129:2785–2796. doi: 10.1242/dev.129.12.2785. [DOI] [PubMed] [Google Scholar]
- 12.Kuo BR, Erickson CA. Vagal neural crest cell migratory behavior: A transition between the cranial and trunk crest. Dev Dyn. 2011;240:2084–2100. doi: 10.1002/dvdy.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan H, et al. Neural cells in the esophagus respond to glial cell line-derived neurotrophic factor and neurturin, and are RET-dependent. Dev Biol. 2004;272:118–133. doi: 10.1016/j.ydbio.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Cacalano G, et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sánchez MP, et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 16.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: Advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 17.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- 18.Espinosa-Medina I, et al. Neurodevelopment. Parasympathetic ganglia derive from Schwann cell precursors. Science. 2014;345:87–90. doi: 10.1126/science.1253286. [DOI] [PubMed] [Google Scholar]
- 19.Dyachuk V, et al. Neurodevelopment. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science. 2014;345:82–87. doi: 10.1126/science.1253281. [DOI] [PubMed] [Google Scholar]
- 20.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- 21.Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol. 2010;21:922–928. doi: 10.1016/j.semcdb.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson RB, Stewart AL, Young HM. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 2006;323:11–25. doi: 10.1007/s00441-005-0047-6. [DOI] [PubMed] [Google Scholar]
- 23.Kuratani SC, Kirby ML. Initial migration and distribution of the cardiac neural crest in the avian embryo: An introduction to the concept of the circumpharyngeal crest. Am J Anat. 1991;191:215–227. doi: 10.1002/aja.1001910302. [DOI] [PubMed] [Google Scholar]
- 24.Burns AJ, Champeval D, Le Douarin NM. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Dev Biol. 2000;219:30–43. doi: 10.1006/dbio.1999.9592. [DOI] [PubMed] [Google Scholar]
- 25.Verberne ME, Gittenberger-De Groot AC, Van Iperen L, Poelmann RE. Contribution of the cervical sympathetic ganglia to the innervation of the pharyngeal arch arteries and the heart in the chick embryo. Anat Rec. 1999;255:407–419. doi: 10.1002/(SICI)1097-0185(19990801)255:4<407::AID-AR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Le Douarin N, Kalcheim C. The Neural Crest. Cambridge Univ Press; Cambridge, UK: 1999. [Google Scholar]
- 27.Saito D, Takase Y, Murai H, Takahashi Y. The dorsal aorta initiates a molecular cascade that instructs sympatho-adrenal specification. Science. 2012;336:1578–1581. doi: 10.1126/science.1222369. [DOI] [PubMed] [Google Scholar]
- 28.Britsch S, et al. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns AJ, Douarin NM. The sacral neural crest contributes neurons and glia to the post-umbilical gut: Spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- 30.Kapur RP. Colonization of the murine hindgut by sacral crest-derived neural precursors: Experimental support for an evolutionarily conserved model. Dev Biol. 2000;227:146–155. doi: 10.1006/dbio.2000.9886. [DOI] [PubMed] [Google Scholar]
- 31.Espinosa-Medina I, et al. The sacral autonomic outflow is sympathetic. Science. 2016;354:893–897. doi: 10.1126/science.aah5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Douarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974;41:162–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- 33.Baggiolini A, et al. Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell. 2015;16:314–322. doi: 10.1016/j.stem.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Bronner-Fraser M, Fraser SE. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature. 1988;335:161–164. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- 35.Nagy N, Brewer KC, Mwizerwa O, Goldstein AM. Pelvic plexus contributes ganglion cells to the hindgut enteric nervous system. Dev Dyn. 2007;236:73–83. doi: 10.1002/dvdy.20933. [DOI] [PubMed] [Google Scholar]
- 36.Uesaka T, Nagashimada M, Enomoto H. Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J Neurosci. 2015;35:9879–9888. doi: 10.1523/JNEUROSCI.1239-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green SA, Uy BR, Bronner ME. Ancient evolutionary origin of vertebrate enteric neurons from trunk-derived neural crest. Nature. 2017;544:88–91. doi: 10.1038/nature21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Häming D, et al. Expression of sympathetic nervous system genes in lamprey suggests their recruitment for specification of a new vertebrate feature. PLoS One. 2011;6:e26543. doi: 10.1371/journal.pone.0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson SL, et al. ErbB3 is required for normal cerebellar and cardiac development: A comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 40.Crone SA, Negro A, Trumpp A, Giovannini M, Lee K-F. Colonic epithelial expression of ErbB2 is required for postnatal maintenance of the enteric nervous system. Neuron. 2003;37:29–40. doi: 10.1016/s0896-6273(02)01128-5. [DOI] [PubMed] [Google Scholar]
- 41.Barrenschee M, et al. Expression and function of Neuregulin 1 and its signaling system ERBB2/3 in the enteric nervous system. Front Cell Neurosci. 2015;9:360. doi: 10.3389/fncel.2015.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang CS-M, et al. Trans-ethnic meta-analysis of genome-wide association studies for Hirschsprung disease. Hum Mol Genet. 2016;25:5265–5275. doi: 10.1093/hmg/ddw333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burns AJ, et al. White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies. Dev Biol. 2016;417:229–251. doi: 10.1016/j.ydbio.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coppola E, et al. Epibranchial ganglia orchestrate the development of the cranial neurogenic crest. Proc Natl Acad Sci USA. 2010;107:2066–2071. doi: 10.1073/pnas.0910213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ertürk A, et al. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc. 2012;7:1983–1995. doi: 10.1038/nprot.2012.119. [DOI] [PubMed] [Google Scholar]
- 46.McGrew MJ, et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 2004;5:728–733. doi: 10.1038/sj.embor.7400171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delalande J-M, Thapar N, Burns AJ. Dual labeling of neural crest cells and blood vessels within chicken embryos using Chick(GFP) neural tube grafting and carbocyanine dye DiI injection. J Vis Exp. 2015;99:e52514. doi: 10.3791/52514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hama H, et al. Scale: A chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci. 2011;14:1481–1488. doi: 10.1038/nn.2928. [DOI] [PubMed] [Google Scholar]
- 49.Dubreuil V, et al. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Autréaux F, Coppola E, Hirsch M-R, Birchmeier C, Brunet J-F. Homeoprotein Phox2b commands a somatic-to-visceral switch in cranial sensory pathways. Proc Natl Acad Sci USA. 2011;108:20018–20023. doi: 10.1073/pnas.1110416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagashimada M, et al. Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expression. J Clin Invest. 2012;122:3145–3158. doi: 10.1172/JCI63401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 53.Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- 54.Sheean ME, et al. Activation of MAPK overrides the termination of myelin growth and replaces Nrg1/ErbB3 signals during Schwann cell development and myelination. Genes Dev. 2014;28:290–303. doi: 10.1101/gad.230045.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riethmacher D, et al. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- 56.Li L, et al. The breast proto-oncogene, HRGalpha regulates epithelial proliferation and lobuloalveolar development in the mouse mammary gland. Oncogene. 2002;21:4900–4907. doi: 10.1038/sj.onc.1205634. [DOI] [PubMed] [Google Scholar]
- 57.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 58.Meyer D, et al. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.