Significance
Pathological cardiac hypertrophy, characterized by heart growth in response to pressure or volume overload, such as in the setting of hypertension, is the main risk factor for heart failure (HF). The identification of therapeutic strategies to prevent or reverse cardiac hypertrophy is therefore a priority for curing HF. It is known that growth hormone-releasing hormone (GHRH) displays cardioprotective functions; however, its therapeutic potential in hypertrophy and HF is unknown. Here we show that GHRH reduces cardiomyocyte hypertrophy in vitro through inhibition of hypertrophic pathways. In vivo, the GHRH analog MR-409 attenuates cardiac hypertrophy in mice subjected to transverse aortic constriction and improves cardiac function. These findings suggest therapeutic use of GHRH analogs for treatment of pathological cardiac hypertrophy and HF.
Keywords: growth hormone-releasing hormone, cardiac hypertrophy, heart failure
Abstract
It has been shown that growth hormone-releasing hormone (GHRH) reduces cardiomyocyte (CM) apoptosis, prevents ischemia/reperfusion injury, and improves cardiac function in ischemic rat hearts. However, it is still not known whether GHRH would be beneficial for life-threatening pathological conditions, like cardiac hypertrophy and heart failure (HF). Thus, we tested the myocardial therapeutic potential of GHRH stimulation in vitro and in vivo, using GHRH or its agonistic analog MR-409. We show that in vitro, GHRH(1-44)NH2 attenuates phenylephrine-induced hypertrophy in H9c2 cardiac cells, adult rat ventricular myocytes, and human induced pluripotent stem cell-derived CMs, decreasing expression of hypertrophic genes and regulating hypertrophic pathways. Underlying mechanisms included blockade of Gq signaling and its downstream components phospholipase Cβ, protein kinase Cε, calcineurin, and phospholamban. The receptor-dependent effects of GHRH also involved activation of Gαs and cAMP/PKA, and inhibition of increase in exchange protein directly activated by cAMP1 (Epac1). In vivo, MR-409 mitigated cardiac hypertrophy in mice subjected to transverse aortic constriction and improved cardiac function. Moreover, CMs isolated from transverse aortic constriction mice treated with MR-409 showed improved contractility and reversal of sarcolemmal structure. Overall, these results identify GHRH as an antihypertrophic regulator, underlying its therapeutic potential for HF, and suggest possible beneficial use of its analogs for treatment of pathological cardiac hypertrophy.
Cardiac hypertrophy is initially an adaptive response of the heart to pathophysiologic stimuli, in an attempt to counterbalance ventricular wall stress and preserve cardiac function. However, sustained hypertrophy in response to cardiac insults, such as hypertension or myocardial infarction (MI), can eventually lead to arrhythmias, dilated cardiomyopathy, and heart failure (HF), leading causes of cardiac morbidity and mortality (1). At a cellular level, pathological hypertrophy is characterized by myocyte enlargement and increased protein synthesis, gene-expression reprogramming, and modifications in the sarcomere organization (1, 2). Many pathways are implicated in the transduction of hypertrophic signals, and the initiating stimuli include biomechanical stress and neurohumoral factors, associated with the release of hormones, cytokines, and growth factors by cardiomyocytes (CMs) themselves (2–4). Thus, a major therapeutic strategy in HF is the use of pharmacologic agents aimed at limiting the progression of cardiac hypertrophy. In fact, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or β-adrenergic blockers impact on cardiac function by acting on hypertrophy, together with other effects, including those on inotropism (2). These drugs inhibit neuroendocrine signaling, acting also on mechanisms responsible for myocyte growth, cardiac hypertrophy, and HF; however, HF progression is still unavoidable, with a mortality of 50% within 5 y in advanced stages (2, 3). Therefore, identifying new therapeutic targets to prevent or reverse cardiac hypertrophy may have an impact on survival.
In addition to regulating the release of pituitary growth hormone (GH), GH-releasing hormone (GHRH) exerts many peripheral effects (5, 6), displaying autocrine and paracrine actions and regulating the survival and proliferation of different cell types. Accordingly, the expression of both GHRH and GHRH receptors has been demonstrated in extrapituitary sites (5), including CMs (7–9), skeletal myoblasts, and myotubes (10). We have recently demonstrated the antiapoptotic action of GHRH in H9c2 cardiac cells and adult rat ventricular myocytes (ARVMs), through signaling mediated by the GHRH receptor (GHRH-R) and activation of cAMP/protein kinase A (PKA) (7). GHRH also improved heart function and reduced MI in isolated rat hearts subjected to ischemia/reperfusion (7, 11). Furthermore, synthetic GHRH analogs, with greater activity and metabolic stability compared with native GHRH, were found to promote cardiac repair, improve cardiac function, and reverse ventricular remodeling in rat models of ischemic injury (8, 12, 13), and to reduce MI in swine with ischemic cardiomyopathy (14). Recently, highly potent new GHRH analogs, such as MR-356 and MR-409, have been shown in vitro to reduce calcium influx and promote the survival of H9c2 cardiac cells, and in vivo to reduce rat MI and inflammatory response (9).
These findings suggest that GHRH and its analogs could be useful for therapy of cardiovascular diseases; however, the role of GHRH in cardiac hypertrophy and HF in vivo has not been investigated to date. Hence, in the present study we tested the hypothesis that GHRH would attenuate cardiac hypertrophy. The role of GHRH was assessed in different in vitro models of phenylephrine (PE)-induced hypertrophy, along with the mechanisms involved in these effects. The results obtained from the in vitro experiments were further validated in vivo by investigating the therapeutic action of the GHRH analog MR-409 in a mouse model of pressure overload hypertrophy and HF induced by transverse aortic constriction (TAC).
Results
GHRH Attenuates PE-Induced Hypertrophy in Cardiac Cells.
To test the impact of GHRH on hypertrophy, we initially used H9c2 cardiac cells that possess biochemical and electrophysiological characteristics of CMs and similar hypertrophic responses (15, 16). The cells were cultured for 24 h with GHRH at a concentration of 0.5 μM, previously observed to be the most protective against apoptosis (7), alone or with PE (10 and 50 μM). PE, but not GHRH, increased cell surface area, whereas pretreatment with GHRH blocked this effect (Fig. S1A). GHRH also lowered the expression of hypertrophic markers NPPA and MYH7, encoding atrial natriuretic peptide and β-myosin heavy chain (β-MHC), respectively, both up-regulated by PE (Fig. S1 B and C).
The GHRH-R antagonist JV-1-36, used at 50 nM, in line with our previous studies (7), had no effect alone; however, it blocked the antihypertrophic action of GHRH in PE-treated cells, suggesting receptor-mediated mechanisms (Fig. S1 D and E).
GHRH Inhibits Gq Signaling.
Activation of Gq protein by receptors, such as α1-adrenercic receptors (α1-AR), promotes cardiac hypertrophy in vivo and in isolated CMs (4). Hence, we tested the capacity of GHRH to inhibit PE-induced Gq/phospholipase Cβ (PLCβ) signaling. The PLC inhibitor U-73122 blocked the increase in NPPA and MYH7 mRNA in PE-treated H9c2 cells, whereas GHRH maintained its antihypertrophic action, suggesting Gq mediated signaling only for PE (Fig. S2 A and B). Although both GHRH and PE alone increased PLCβ protein, their coadministration reduced PLCβ to control levels (Fig. S2C). Similar results were obtained for phosphorylation of PKCε at Ser729 and for calcineurin, downstream targets of PLCβ (Fig. S2 D and E). GHRH also counteracted the effect of PE on phosphorylation of phospholamban (PLN) at Thr17, dependent on Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Fig. S2F).
GHRH Activates Adenylyl Cyclase/cAMP/PKA.
It is known that GHRH-R activates Gαs and adenylyl cyclase (AC)/cAMP/PKA (5–7); moreover, β-AR signaling, mediated by cAMP/PKA, may antagonize α1-AR and CM hypertrophy (17), whereas α1-AR agonists also stimulate β-AR signaling (18, 19). Here, both GHRH and PE alone increased PKA activity; however, this effect was potentiated in cardiac cells costimulated with PE and GHRH. Moreover, the PKA activator, N6-benzoyladenosine-3′-5′-cAMP (6-Bnz-cAMP), and the AC activator forskolin (FSK) increased, whereas the PKA inhibitor, KT5720, reduced PKA activity (Fig. S3A). Accordingly, GHRH also promoted the PKA-dependent phosphorylation of PLN at Ser16, whereas PE had no effect, both alone and in combination with GHRH (Fig. S3B). FSK inhibited the increase in NPPA and MYH7 by PE, but potentiated the antihypertrophic effect of GHRH (Fig. S3 C and D); this, in turn, was inhibited by KT5720, which had no effect on PE alone (Fig. S3 E and F). Thus, the antihypertrophic action of GHRH involves AC/cAMP/PKA signaling and, although elevated by PE, PKA is not implicated in the hypertrophic effect of PE.
GHRH Counteracts PE-Induced Increase in Epac1.
Increase in exchange protein directly activated by cAMP1 (Epac1) is involved in cardiac hypertrophy, HF, and arrhythmogenesis through β-ARs signaling (20–23). Here, PE, but not GHRH, increased Epac1 mRNA in H9c2 cells, an effect abolished by GHRH (Fig. S4A). The PKA activator 6-Bnz-cAMP, ineffective on Epac1 (24), blocked PE-induced increase in Epac1 and potentiated the inhibitory action of GHRH on Epac1 reduction (Fig. S4B). Both the inhibitor of Epac1, ESI-09, with no activity on PKA (22), and the β1-AR antagonist metoprolol, blocked the PE-induced elevation of Epac1, NPPA, and MYH7, with no effect on the antihypertrophic action of GHRH (Fig. S4 C–H). These results suggest that GHRH blocks the increase in Epac1 and counteracts the hypertrophic action of PE through mechanisms mediated by cAMP and PKA.
GHRH Attenuates Hypertrophy in ARVMs and Human Induced Pluripotent Stem Cell-Derived CMs.
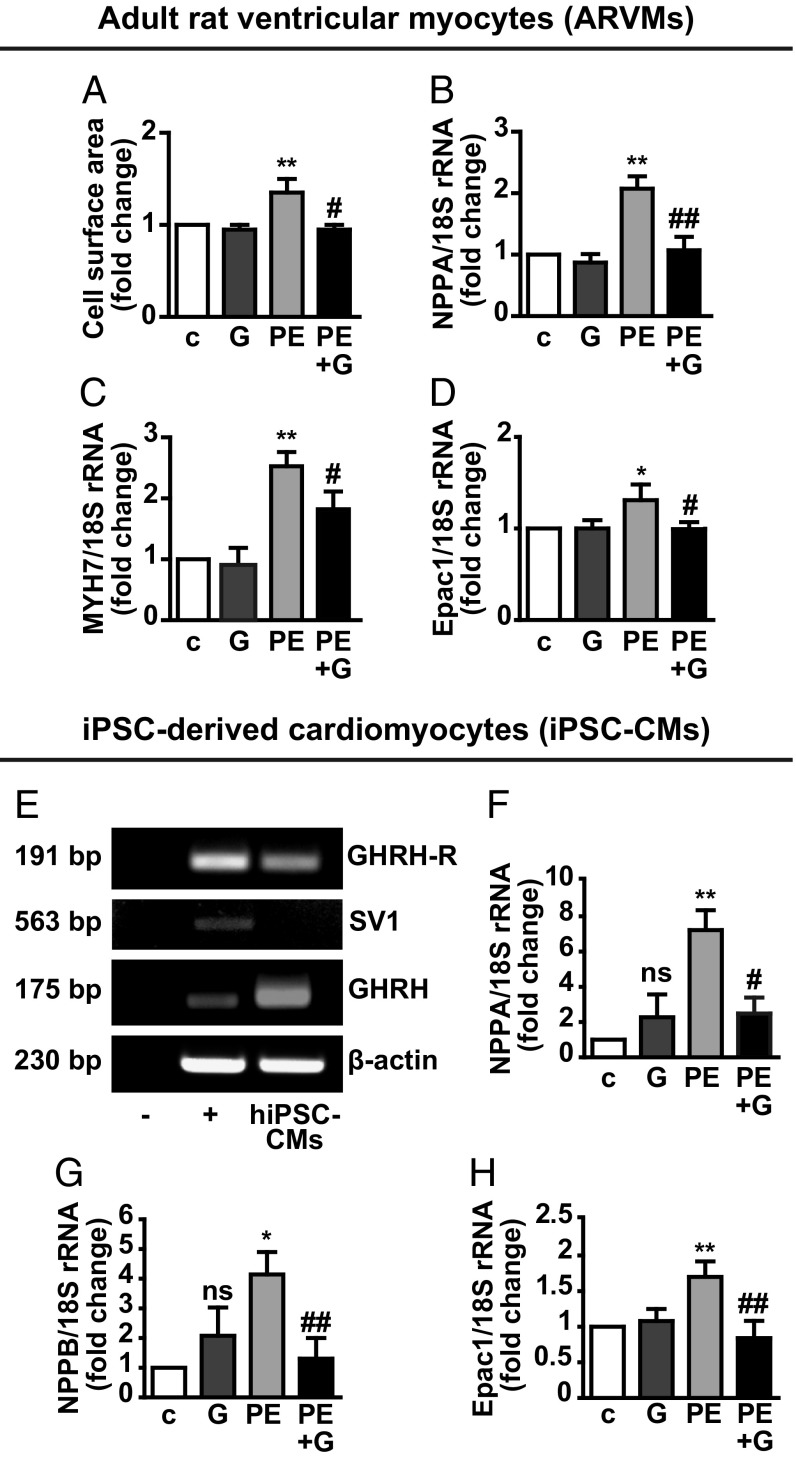
The antihypertrophic effect of GHRH was then tested in ARVMs and human induced pluripotent stem cell-derived CMs (iPSC-CMs). ARVMs, cultured for 24 h with PE, showed increased cell surface area. This effect was attenuated by pretreatment with GHRH (Fig. 1A), which also reduced the increase in NPPA and MYH7 mRNA (Fig. 1 B and C). Moreover, GHRH counteracted the up-regulation in Epac1 by PE, while having no effect alone (Fig. 1D). Human iPSC-CMs showed expression for pituitary GHRH-R and GHRH, but not for the receptor splice variant SV1 (Fig. 1E). Treatment with PE for 24 h increased mRNA levels of NPPA, NPPB (encoding brain natriuretic peptide), and Epac1; these effects were abolished by cotreatment with GHRH (Fig. 1 F–H).
Fig. 1.
Antihypertrophic effect of GHRH in ARVMs and human iPSC-CMs. Serum-starved ARVMs were treated with GHRH (0.5 µM) and PE (10 µM) for 24 h, alone or with GHRH for 40 min, then with PE for 24 h. (A) Cell surface area measured on α-actinin–stained cells. The relative area, normalized to the control, was analyzed in at least 50 cells for each condition in three different fields. (B–D) Real-time PCR for NPPA, MYH7, and Epac1 normalized to 18S rRNA. For A–D, results, expressed as fold-change of control (c), are mean ± SEM *P < 0.05 and **P < 0.01 vs. c; #P < 0.05 and ##P < 0.01 vs. PE; n = 3. (E) Representative RT-PCR showing mRNA for GHRH-R, SV1, and GHRH in human iPSC-CMs. Buffer alone was used as negative control (−). LNCaP human prostate cancer cells were used as positive control (+) and β-actin as internal control. NPPA (F), NPPB (G), and Epac1 (H) mRNA assessed by real-time PCR in cells treated for 24 h with 0.5 µM GHRH and 100 µM PE, alone or in combination. Results, normalized to 18S rRNA, are expressed as fold-change of control (c) and are mean ± SEM *P < 0.05 and **P < 0.01 vs. c; #P < 0.05 and ##P < 0.01 vs. PE; ns, not significant vs. c; n = 3.
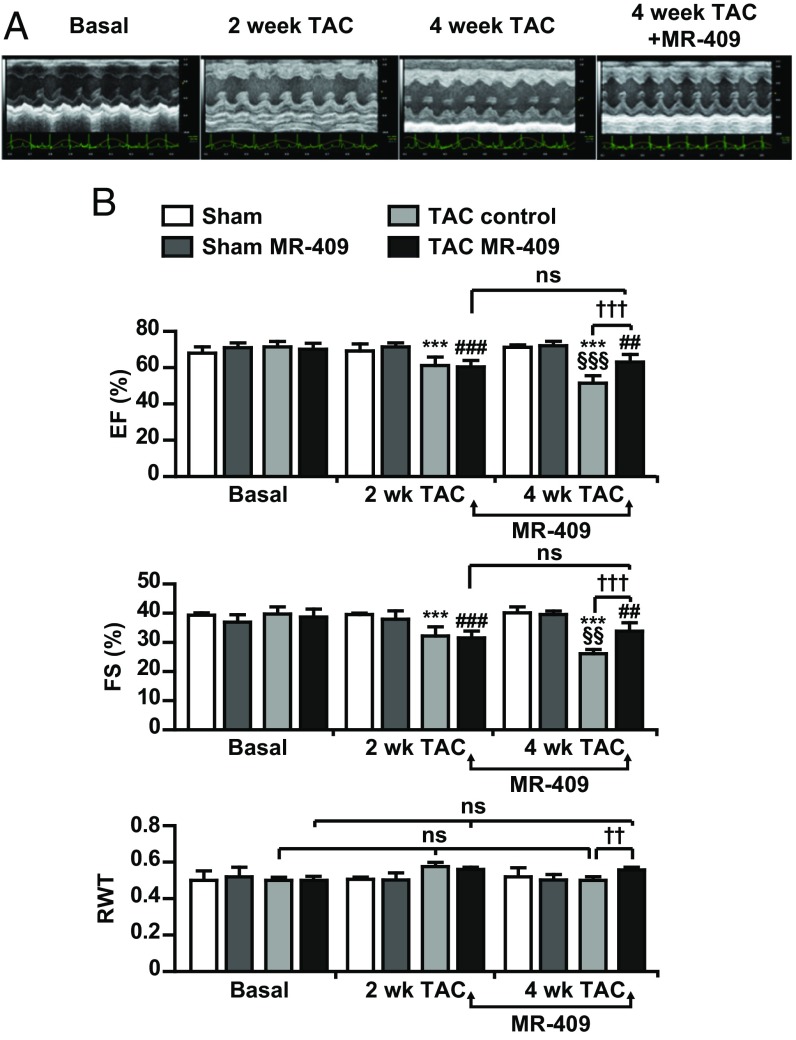
MR-409 Improves Cardiac Function and Remodeling in Pressure Overload-Induced HF.
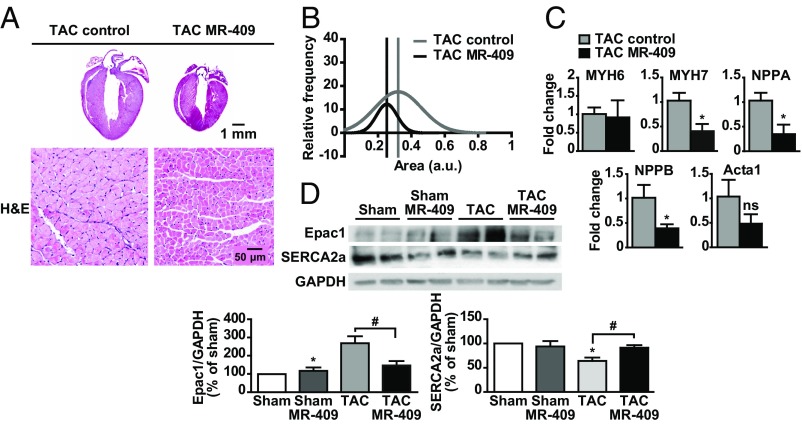
The effect of the synthetic agonistic analog of human GHRH, MR-409, was tested in the TAC-induced pressure overload model of HF. At 4-wk, TAC mice displayed a significant depression of cardiac function, as evaluated by echocardiographic parameters [i.e., ejection fraction and fractional shortening (FS)]. MR-409 was given 2 wk after TAC, when mice had developed left ventricular hypertrophy and showed a decrease in cardiac function (Fig. 2). Four weeks after TAC, the decrease of FS, the progressive left ventricular dilation, and the transition from concentric to dilated hypertrophy in left ventricles were hindered by the treatment with MR-409 (Fig. 2B and Table 1). At 4-wk after TAC, H&E staining showed that the hearts from MR-409–treated mice were smaller compared with those of TAC control (Fig. 3A, Upper). Cross-sectional area analysis in isolated CMs revealed a reversal in myocyte enlargement in TAC mice treated with MR-409 compared with untreated (Fig. 3A, Lower, and 3B), whereas expression of markers of fibrosis and apoptosis was unchanged (Fig. S5 A–E). mRNA for NPPA and NPPB was reduced in CMs of TAC mice treated with MR-409, whereas MYH6 (encoding α-MHC) and Acta1 (encoding skeletal α-actin) levels were unchanged (Fig. 3C). MR-409 also counteracted the effect of TAC on increase in Epac1, and up-regulated the expression of sarco/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a), that was reduced by TAC (Fig. 3D). The serum levels of GH and IGF-I, measured at the end of 4-wk TAC, were unchanged in both sham- and TAC-operated mice treated with MR-409 (Fig. S5 F and G).
Fig. 2.
Antihypertrophic effect of MR-409 in vivo. After 14 d, mice subjected to sham operation or TAC underwent echocardiography analysis for heart function and pressure gradient. Only mice with gradients between 60 and 90 mmHg were included in the experiment. Two weeks (wk) after the operation, mice were subcutaneously injected with MR-409 (500 μg/kg/d for 14 d) or vehicle. (A) Representative M-mode left ventricular echocardiographic recording at baseline, 2 wk post-TAC, 4 wk post-TAC, and 4 wk post-TAC with MR-409. (B) Echocardiographic data: EF%, percent ejection fraction and FS (%), percent fractional shortening, as parameters of left ventricle contractile function; RWT, relative wall thickness. Arrows indicate the time of MR-409 administration (from 2 to 4 wk after TAC). Results are mean ± SEM ***P < 0.001 vs. TAC Basal; ##P < 0.01 and ###P < 0.001 vs. TAC MR-409 basal; §§P < 0.01 and §§§P < 0.001 vs. TAC control 2 wk; ††P < 0.01 and †††P < 0.001; ns, not significant (Sham, n = 5; Sham MR, n = 5; TAC, n = 8; Tac MR, n = 9).
Table 1.
Echocardiographic analysis at basal, 2-wk, and 4-wk Sham and TAC mice treated with MR-409
| Basal | 2 wk | 4 wk | ||||||||||
| Parameter | Sham | Sham MR | TAC control | TAC MR | Sham | Sham MR | TAC control | TAC MR | Sham | Sham MR | TAC control | TAC MR |
| BW, g | 25.6 ± 2.1 | 25.8 ± 1.095 | 25.9 ± 2.2 | 26.4 ± 2.1 | 24.7 ± 2.6 | 26.6 ± 1.14 | 26.1 ± 2.0 | 26.6 ± 1.14 | 25.4 ± 2.9 | 28.2 ± 1.1 | 26.1 ± 1.7 | 27.1 ± 2.0 |
| HR M-mode, bpm | 601.8 ± 17.6 | 588 ± 30.34 | 629.3 ± 158.3 | 593.6 ± 42.3 | 597.2 ± 53.7 | 588.8 ± 30.34 | 577.6 ± 55.8 | 523.0 ± 138.0 | 606.6 ± 46.9 | 627.2 ± 35.2 | 560.0 ± 24.6 | 630.1 ± 21.4††† |
| LVIDd, mm | 3.3 ± 0.1 | 3.312 ± 0.233 | 3.4 ± 0.1 | 3.3 ± 0.2 | 3.22 ± 0.07 | 3.312 ± 0.23 | 3.67 ± 0.28** | 3.54 ± 0.13 | 3.3 ± 0.2 | 3.296 ± 0.14 | 4.1 ± 0.3*** | 3.7 ± 0.2††† |
| LVIDs, mm | 2.0 ± 0.1 | 2.092 ± 0.225 | 2.0 ± 0.1 | 2.1 ± 0.2 | 1.95 ± 0.04 | 2.058 ± 0.219 | 2.49 ± 0.27*** | 2.43 ± 0.15 | 2.0 ± 0.2 | 1.992 ± 0.07 | 3.0 ± 0.4*** | 2.4 ± 0.3††† |
| IVSd, mm | 0.9 ± 0.1 | 0.854 ± 0.053 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.0 | 0.824 ± 0.05 | 1.0 ± 0.0** | 1.0 ± 0.1 | 0.8 ± 0.0 | 0.87 ± 0.07 | 1.02 ± 0.07*** | 1.02 ± 0.06 |
| IVSs, mm | 1.3 ± 0.1 | 1.232 ± 0.072 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.0 | 1.21 ± 0.053 | 1.3 ± 0.1*** | 1.3 ± 0.1 | 1.26 ± 0.06 | 1.222 ± 0.03 | 1.39 ± 0.06*** | 1.40 ± 0.04 |
| PWTd, mm | 0.8 ± 0.0 | 0.858 ± 0.08 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.834 ± 0.053 | 1.0 ± 0.1** | 1.0 ± 0.1 | 0.82 ± 0.10 | 0.862 ± 0.01 | 1.01 ± 0.08** | 1.02 ± 0.09 |
| PWTs, mm | 1.2 ± 0.1 | 1.218 ± 0.036 | 1.2 ± 0.1 | 1.2 ± 0.0 | 1.2 ± 0.0 | 1.276 ± 0.043 | 1.4 ± 0.1*** | 1.4 ± 0.0 | 1.26 ± 0.04 | 1.256 ± 0.06 | 1.38 ± 0.13*** | 1.47 ± 0.07 |
Statistical significance was measured by two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Basal TAC control; †††P < 0.001 vs. 4 wk TAC control. bpm, beats per minute; BW, body weight; HR, heart rate; IVSDd, interventricular septal end diastole; IVSDs, interventricular septal end systole; LVIDd, left ventricular internal diameter end diastole; LVIDs, left ventricular internal diameter end systole; MR, MR-409; PWTd, posterior wall thickness in end diastole; PWTs, posterior wall thickness in end systole. (Sham, n = 5; Sham MR, n = 5; TAC, n = 8; TAC MR, n = 9.)
Fig. 3.
Histological analysis and hypertrophic signaling in TAC mice treated with MR-409. (A) Representative mouse heart sections (Upper) and CM cross-sectional area (Lower) 4 wk after TAC (Left, TAC control) or TAC with MR-409 (Right) (H&E staining). (B) Cell size of single adult CMs from TAC Control and TAC MR-409 mice at 4-wk after TAC (n = 250, three mice per group). (C) Expression of the indicated genes in CMs from TAC MR-409 and TAC control mice (4-wk). Results, normalized to 18S rRNA, are presented as fold-change vs. TAC control (n = 3 mice per group) and are mean ± SEM *P < 0.05. (D) Representative Western blot for Epac1 and SERCA2a in left ventricles from TAC mice treated with MR-409. Results, normalized to GAPDH and expressed as percent of Sham, are mean ± SEM *P < 0.05 vs. Sham; #P < 0.05; n = 5.
MR-409 Promotes the Normalization of Contractile Responses in TAC CMs ex Vivo.
A feature of the reverse-remodeled heart is the recovery of contractile responses in isolated CMs, which are blunted during HF (25). Thus, the effect of MR-409 was next studied on the contractility of CMs isolated from TAC mice. Chronic treatment with MR-409 improved both cell shortening at all stimulation frequencies (Fig. S6A) and velocity of cell relaxation (Fig. S6B). Similarly, the magnitude of calcium transients ameliorated the rising phase (calculated as percentage from baseline-to-peak) increased for all stimulation frequencies, suggesting an improved release of calcium ions from the sarcoplasmic reticulum (Fig. S6C). Consequently, the “falling phase” required more time-to-reuptake calcium ions and store them in the sarcoplasmic reticulum (calculated as percentage from peak to baseline at the 90% of time) at 1, 2, and 3 Hz (Fig. S6D). Overall, CMs exposed to MR-409 denoted a decrease in the time of mechanical relaxation but an increase in the time of calcium reuptake (Fig. S6 B and D).
In HF, CMs show a typical alteration in morphology and organization of cell membrane transverse tubules (T-tubules) (26) that can be studied by hopping probe scanning ion-conductance microscopy (HPICM), for topographical evaluation of cell membrane organization at nanoscale resolution (27, 28). HPICM revealed a recovery of the alternating z-groove and crest morphology on the surface of CMs from TAC mice treated with MR-409, compared with CMs from TAC control mice, which showed less-striated membrane topography. An increase, from 0.43 ± 0.02 to 0.57 ± 0.05, in the z-groove index was observed in CMs from TAC MR-409 compared with TAC control mice (Fig. S7).
Discussion
Our results demonstrate that GHRH attenuates cardiac hypertrophy under pathological conditions. Indeed, GHRH inhibited PE-induced hypertrophy in H9c2 cardiac cells, as well as in ARVMs and human iPSC-CMs, while its analog, MR-409, recapitulates the same rescuing in vivo.
Of note, although our groups have recently demonstrated the cardioprotective role of GHRH, both in vitro and in vivo (7–9, 11–14), the potential protective effect of the hormone in the context of HF has not been previously investigated. We found that in vitro, GHRH reduced the increase in expression of fetal genes by PE, which are classically associated with the development of pathological, but not physiological hypertrophy (29). The antihypertrophic action of GHRH, dependent on activation of GHRH-R, also included blockade of Gq signaling, induced by PE. In fact, GHRH abolished the increase in PLCβ expression and phosphorylation of PKCε by PE, as well as elevation of calcineurin, and inhibited the CaMKII-dependent phosphorylation of PLN at Thr17. Moreover, although GHRH alone increased PLCβ signaling, its antihypertrophic action was not affected by the PLCβ inhibitor, suggesting that Gq signaling is implicated in other effects of the hormone.
In addition to Gq, our results show that the antihypertrophic action of GHRH involved its canonical pathway, namely the GHRH-R–dependent Gαs/cAMP/PKA (5, 7, 10). GHRH enhanced PKA activity, an effect also observed with PE, in line with studies showing that high concentrations of PE may activate β-AR signaling in CMs (18, 19). Interestingly, the combination of GHRH and PE further increased PKA activity, suggesting that PKA is implicated in the antihypertrophic activity of GHRH. Accordingly, β-AR–induced elevation of cAMP/PKA was previously shown to reduce the fetal gene response to α1-AR agonists, including PE, in CMs (17). Moreover, our results show that GHRH, but not PE, promoted the PKA-dependent phosphorylation of PLN at Ser16. In line with these findings, activation of cAMP/PKA by FSK blocked the hypertrophic effect of PE, but potentiated the antihypertrophic action of GHRH. Conversely, inhibition of PKA reduced the antihypertrophic effect of GHRH, while being ineffective on PE, indicating a PKA-mediated effect for GHRH, but not for PE.
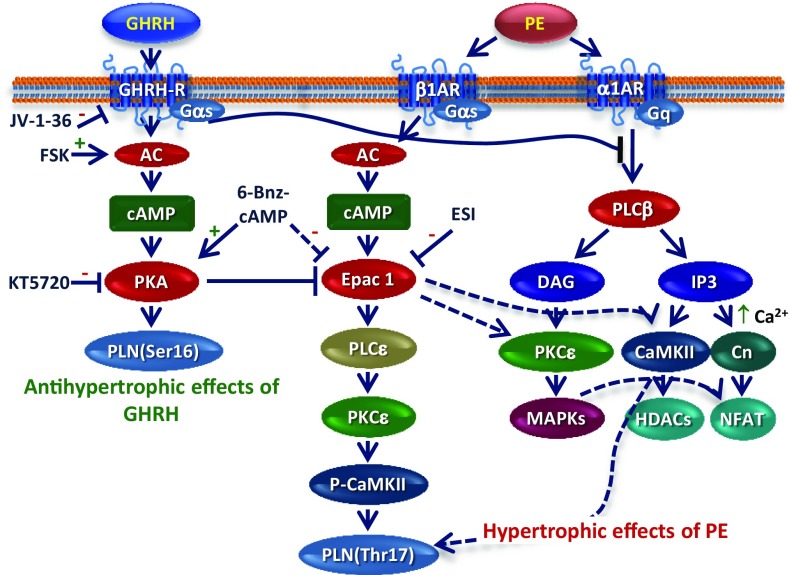
To further clarify these mechanisms, we focused on Epac, a direct PKA-independent target of cAMP and a mediator of cardiac hypertrophy, HF, and arrhythmogenesis (20–23), whose expression of the main cardiac isoform, Epac1, has been demonstrated in H9c2 cells as well (20). Epac1 induces hypertrophy in vitro and in vivo through mechanisms mediated by the PKA-independent phosphorylation of PLN on Thr17, as well as Ca2+ release, activation of PLCε/PKCε/CaMKII signaling (22, 30), and elevation of calcineurin and NFAT (21–23). We found that PE increased Epac1 mRNA, an effect abolished by GHRH. Moreover, the PKA activator 6-Bnz-cAMP blocked the increase in Epac1 by PE and potentiated the inhibitory effect of GHRH, consistent with the involvement of PKA in the antihypertrophic action of the hormone. To the best of our knowledge, no studies have previously demonstrated that an α1-AR agonist, such as PE, up-regulates Epac1, which is typically activated by β-AR agonists (23). Accordingly, the increase in Epac1 and the hypertrophic effect of PE, but not the antihypertrophic action of GHRH, were blocked by the Epac1 inhibitor ESI-09, and by the β1-AR antagonist metoprolol. Collectively, these findings indicate that, in addition to inhibiting Gq, GHRH blocks hypertrophy in CMs through activation of receptor-mediated cAMP/PKA and inhibition of Epac1, suggesting cross-talk mechanisms between α1- and β1-AR pathways (Fig. 4).
Fig. 4.
Signaling pathways involved in the antihypertrophic effects of GHRH in vitro. Antihypertrophic actions of GHRH include GHRH-R–dependent activation of the AC/cAMP/PKA pathway (Left). GHRH inhibits PE-induced signaling by blocking the α1-AR/Gq pathway and its downstream effectors (Right). GHRH also counteracts the increase in Epac1 by PE through activation of PKA and inhibition of Epac1-induced hypertrophic pathways (Center). Cross-talk mechanisms between β1-AR/Gαs/Epac1 and α1-AR/Gq signaling are shown. “+” and “−” indicate stimulatory and inhibitory effects, respectively; interrupted lines indicate indirect effects. Abbreviations: Cn, calcineurin; DAG, diacylglycerol; Epac1, exchange protein directly activated by cAMP; GSK-3β, glycogen synthase kinase-3 β; HDACs, class II histone deacetylases; IP3, inositol trisphosphate; MAPKs, mitogen-activated protein kinases; NFAT, nuclear factor of activated T cells; PLN(Ser16) phospholamban at serine 16; PLN(Thr17) phospholamban at threonine 17.
The antihypertrophic effect of GHRH was demonstrated also in ARVMs and human iPSC-CMs, along with inhibition in Epac1 expression, suggesting a potential antihypertrophic action for GHRH in the human heart. In fact, although displaying ultrastructural and electrophysiological properties of immature CMs, human iPSC-CMs are being intensively investigated because of their potential therapeutic applications, specifically in cardiac regeneration and patient-specific cell therapy, representing promising tools for drug discovery and the identification of novel therapeutic molecules (31).
In vivo experiments using the stable agonistic GHRH analog, MR-409, confirmed the potent antihypertrophic properties of GHRH in vitro. Interestingly, as the effect of GHRH(1-44)-NH2 could be reproduced with the analog of GHRH(1-29), MR-409 (13), the active cardioprotective sequence—that is, the part responsible for the cardiac effects—is likely contained in the first 29 amino acids of GHRH.
Importantly, treatment of TAC mice with MR-409 did not just halt disease progression, but rather reversed dysregulated cardiac function. MR-409 even counteracted the effect of TAC on increase in Epac1 and on reduction of SERCA2a expression. Of note, SERCA2a plays an essential role in the contraction and relaxation of cardiac muscle and SERCA2a expression has been shown to be reduced in left hypertrophic ventricles (32). Moreover, in agreement with our previous findings (8, 12, 14), we could not observe elevation of serum GH and IGF-I after administration of MR-409 for 2 wk, suggesting direct cardiac activation. In fact, subcutaneous administration of MR-409 should increase GH levels after 15 and 30 min and last for 30–60 min (13); thus, we cannot exclude an initial raise in GH. However, the beneficial role of GH on cardiac function is controversial (8, 33), whereas the receptor-mediated cardioprotective effects of GHRH and its analogs have been clearly demonstrated, in support of a direct antihypertrophic action of MR-409. Furthermore, it cannot be excluded that in our model of cardiac hypertrophy MR-409 may stimulate the regeneration of cardiac stem cells, since it has been recently demonstrated that GHRH-R agonists, including MR-409, promote the proliferation and survival of cardiac stem cells in vivo and in vitro (8, 12).
The effects on cardiac function in vivo were confirmed by ex vivo experiments in isolated CMs, where MR-409 potently increased inotropic and lusotropic parameters. A similar beneficial effect on signaling and, consequently, on cardiac function has been described for β-AR normalization induced by β-AR blockers in HF (34). From the morphological side, we observed, in the same treated CMs, a plasticity of the cell surface in terms of an increment in z-groove ratio compared with untreated TAC cells. These results recapitulated what was recently demonstrated through AAV9.SERCA2a gene therapy for HF by our group: that is, reappearance of z-grooves and T-tubules and β2-AR relocalization in the reverse remodeled hearts (35).
In summary, our study demonstrates that GHRH and its analog MR-409 not only counteract maladaptive hypertrophy under pathological conditions, but also improve cardiac function in a model of HF with reduced ejection fraction. These findings have important therapeutic implications for disorders characterized by cardiac hypertrophy and HF, since they establish GHRH agonists as novel regulators of hypertrophy and strongly support further translational testing for the treatment of HF.
Methods
Cell Lines and Reagents.
H9c2 cardiac cells were cultured as described previously (7). Rat, human, mouse GHRH(1-44)-NH2, and JV-1-36 were from Phoenix Pharmaceuticals. Synthesis and purification of MR-409 [N-Me-Tyr1, D-Ala2, Orn12, Abu15, Orn21, Nle27, Asp28)-hGHRH(1-29)NH-CH3)] in the laboratory of A.V.S. has been described previously (13).
Isolation of ARVMs.
ARVMs were obtained from young adult rats (1–3 mo) by enzymatic dissociation and cultured as described previously (7). All procedures were approved by the Animal Care and Use Committee of the University of Turin, in accordance with the European Directive 2010/63/EU.
Generation of iPSC-CMs.
iPSC-CMs were differentiated from human iPSCs previously generated from skin fibroblasts of healthy individuals, as described previously (36).
Cell Surface Area.
ARVMs were fixed with paraformaldehyde and stained with α-actinin antibody (1:800), then with Alexa Fluor-546 antibody (1:450; Life Technologies). Analysis was performed using an Olympus Fluoview 200 confocal head with an Ar/Kr laser (488 and 568 nm; magnification, 60×) and area calculated using ImageJ software.
RT-PCR and Real-Time PCR.
RT-PCR and real-time PCR analysis were performed as described previously (7, 10). The primer sequences and amplification products are described in Tables S1 and S2.
Western Blot Analysis.
Western blotting was performed as described previously (10, 37).
Animals.
All procedures were performed according to institutional guidelines in compliance with national (D.L. N.26, 04/03/2014) and international law and policies (new directive 2010/63/EU). The protocol was approved by the Italian Ministry of Health. TAC or sham surgery was performed in 8-wk-old C57BL/6J male mice as described previously (38). Fourteen days after the operation, mice were subcutaneously injected with MR-409 (500 μg/kg/day for 14 d) or vehicle, and killed 14 d after treatment.
In Vivo Cardiac Physiology.
Transthoracic echocardiography was performed at baseline, 14 d after the operations, and at the end of treatments in mice anesthetized with 1% isoflurane, as described previously (39). The pressure gradient was measured by Doppler echocardiography.
Isolation of Adult Mice CMs and Contractility.
CMs were isolated from adult male mice using standard enzymatic techniques and contractility was measured as described previously (38, 40).
Supplementary Material
Acknowledgments
We thank the Neuroscience Institute of Turin. This work was supported by a European Research Council Advanced Grant (CardioEpigen, 294609), the Italian Ministry of Health (PE-2013-02356818), PRIN (Italian Ministry of Research and Education) 2015583WMX, and Fondazione CARIPLO Grant 2015-0573 (all to G.C.); PRIN 2010-B5B2NL (to E.G.); Fondazione CRT 2015/273 and University of Turin Ex-60% 2014 and 2015 (to R.G.); and NIH Grants R01 HL107110, R01 HL084275, UM1 HL113460, and R01 HL110737 (to J.M.H.). The work in the laboratory of A.V.S. was supported by the Medical Research Service of the Veterans Affairs Department and University of Miami Miller School of Medicine.
Footnotes
Conflict of interest statement: A.V.S. is a coinventor on the Patent for growth hormone-releasing hormone agonists, assigned to the University of Miami, FL, and the Veterans Affairs Medical Center, Miami, FL. J.M.H. owns equity in Biscayne Pharmaceuticals Inc. (Miami, FL). Biscayne Pharmaceuticals did not provide funding for this study.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712612114/-/DCSupplemental.
References
- 1.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Bisping E, Wakula P, Poteser M, Heinzel FR. Targeting cardiac hypertrophy: Toward a causal heart failure therapy. J Cardiovasc Pharmacol. 2014;64:293–305. doi: 10.1097/FJC.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 3.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 5.Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV. Growth hormone-releasing hormone: Not only a neurohormone. Trends Endocrinol Metab. 2011;22:311–317. doi: 10.1016/j.tem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Granata R. Peripheral activities of growth hormone-releasing hormone. J Endocrinol Invest. 2016;39:721–727. doi: 10.1007/s40618-016-0440-x. [DOI] [PubMed] [Google Scholar]
- 7.Granata R, et al. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res. 2009;83:303–312. doi: 10.1093/cvr/cvp090. [DOI] [PubMed] [Google Scholar]
- 8.Kanashiro-Takeuchi RM, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:2604–2609. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanashiro-Takeuchi RM, et al. New therapeutic approach to heart failure due to myocardial infarction based on targeting growth hormone-releasing hormone receptor. Oncotarget. 2015;6:9728–9739. doi: 10.18632/oncotarget.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallo D, et al. GH-releasing hormone promotes survival and prevents TNF-α-induced apoptosis and atrophy in C2C12 myotubes. Endocrinology. 2015;156:3239–3252. doi: 10.1210/EN.2015-1098. [DOI] [PubMed] [Google Scholar]
- 11.Penna C, et al. GH-releasing hormone induces cardioprotection in isolated male rat heart via activation of RISK and SAFE pathways. Endocrinology. 2013;154:1624–1635. doi: 10.1210/en.2012-2064. [DOI] [PubMed] [Google Scholar]
- 12.Kanashiro-Takeuchi RM, et al. Activation of growth hormone releasing hormone (GHRH) receptor stimulates cardiac reverse remodeling after myocardial infarction (MI) Proc Natl Acad Sci USA. 2012;109:559–563. doi: 10.1073/pnas.1119203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai R, et al. Synthesis of new potent agonistic analogs of growth hormone-releasing hormone (GHRH) and evaluation of their endocrine and cardiac activities. Peptides. 2014;52:104–112. doi: 10.1016/j.peptides.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagno LL, et al. Growth hormone-releasing hormone agonists reduce myocardial infarct scar in swine with subacute ischemic cardiomyopathy. J Am Heart Assoc. 2015;4:e001464. doi: 10.1161/JAHA.114.001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hescheler J, et al. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 16.Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim. 2011;47:125–131. doi: 10.1007/s11626-010-9368-1. [DOI] [PubMed] [Google Scholar]
- 17.Patrizio M, et al. cAMP-mediated beta-adrenergic signaling negatively regulates Gq-coupled receptor-mediated fetal gene response in cardiomyocytes. J Mol Cell Cardiol. 2008;45:761–769. doi: 10.1016/j.yjmcc.2008.09.120. [DOI] [PubMed] [Google Scholar]
- 18.Valks DM, et al. Phenylephrine promotes phosphorylation of Bad in cardiac myocytes through the extracellular signal-regulated kinases 1/2 and protein kinase A. J Mol Cell Cardiol. 2002;34:749–763. doi: 10.1006/jmcc.2002.2014. [DOI] [PubMed] [Google Scholar]
- 19.Markou T, Hadzopoulou-Cladaras M, Lazou A. Phenylephrine induces activation of CREB in adult rat cardiac myocytes through MSK1 and PKA signaling pathways. J Mol Cell Cardiol. 2004;37:1001–1011. doi: 10.1016/j.yjmcc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Ulucan C, et al. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H1662–H1672. doi: 10.1152/ajpheart.00159.2007. [DOI] [PubMed] [Google Scholar]
- 21.Morel E, et al. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res. 2005;97:1296–1304. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- 22.Lezoualc’h F, Fazal L, Laudette M, Conte C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ Res. 2016;118:881–897. doi: 10.1161/CIRCRESAHA.115.306529. [DOI] [PubMed] [Google Scholar]
- 23.Métrich M, et al. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res. 2008;102:959–965. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- 24.Christensen AE, et al. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- 25.Davies CH, et al. Reduced contraction and altered frequency response of isolated ventricular myocytes from patients with heart failure. Circulation. 1995;92:2540–2549. doi: 10.1161/01.cir.92.9.2540. [DOI] [PubMed] [Google Scholar]
- 26.Lyon AR, et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci USA. 2009;106:6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miragoli M, et al. Microtubule-dependent mitochondria alignment regulates calcium release in response to nanomechanical stimulus in heart myocytes. Cell Rep. 2016;14:140–151. doi: 10.1016/j.celrep.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miragoli M, et al. Scanning ion conductance microscopy: A convergent high-resolution technology for multi-parametric analysis of living cardiovascular cells. J R Soc Interface. 2011;8:913–925. doi: 10.1098/rsif.2010.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: Fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oestreich EA, et al. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem. 2009;284:1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priori SG, Napolitano C, Di Pasquale E, Condorelli G. Induced pluripotent stem cell-derived cardiomyocytes in studies of inherited arrhythmias. J Clin Invest. 2013;123:84–91. doi: 10.1172/JCI62838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi M, Shannon TR, Euler DE, Bers DM, Samarel AM. Downregulation of sarcoplasmic reticulum Ca(2+)-ATPase during progression of left ventricular hypertrophy. Am J Physiol. 1997;272:H2416–H2424. doi: 10.1152/ajpheart.1997.272.5.H2416. [DOI] [PubMed] [Google Scholar]
- 33.Marleau S, Mulumba M, Lamontagne D, Ong H. Cardiac and peripheral actions of growth hormone and its releasing peptides: Relevance for the treatment of cardiomyopathies. Cardiovasc Res. 2006;69:26–35. doi: 10.1016/j.cardiores.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 35.Lyon AR, et al. Plasticity of surface structures and β(2)-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circ Heart Fail. 2012;5:357–365. doi: 10.1161/CIRCHEARTFAILURE.111.964692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Pasquale E, et al. CaMKII inhibition rectifies arrhythmic phenotype in a patient-specific model of catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis. 2013;4:e843. doi: 10.1038/cddis.2013.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penna C, et al. Overexpression of the muscle-specific protein, melusin, protects from cardiac ischemia/reperfusion injury. Basic Res Cardiol. 2014;109:418. doi: 10.1007/s00395-014-0418-9. [DOI] [PubMed] [Google Scholar]
- 38.Greco CM, et al. DNA hydroxymethylation controls cardiomyocyte gene expression in development and hypertrophy. Nat Commun. 2016;7:12418. doi: 10.1038/ncomms12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rusconi F, et al. Peptidomimetic targeting of Cavβ2 overcomes dysregulation of the L-type calcium channel density and recovers cardiac function. Circulation. 2016;134:534–546. doi: 10.1161/CIRCULATIONAHA.116.021347. [DOI] [PubMed] [Google Scholar]
- 40.Di Mauro V, et al. Bioinspired negatively charged calcium phosphate nanocarriers for cardiac delivery of MicroRNAs. Nanomedicine (Lond) 2016;11:891–906. doi: 10.2217/nnm.16.26. [DOI] [PubMed] [Google Scholar]
- 41.Lian X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakahama H, Di Pasquale E. Generation of cardiomyocytes from pluripotent stem cells. Methods Mol Biol. 2016;1353:181–190. doi: 10.1007/7651_2014_173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.